Abstract
It has been well known that smoking alters the property and functionality of a wide range of immune cells including dendritic cells (DCs). However, a great deal of effort in the past has been mainly devoted to dissect the effect of smoking on pulmonary DCs, while its exact impact on circulating DCs remains to be fully addressed. Therefore, in the present report we particularly examined the impact of smoking on the number and subset of DCs in the peripheral blood by multi-parametric flow cytometry analysis. A significant increase for peripheral blood mononuclear cells (PBMCs) was noted in the smoking subjects. Subsequent studies revealed that the percentage for plasmacytoid DCs (pDCs) and total DCs in PBMCs was significantly higher in the smoking subjects as compared with that of control subjects, while the percentage for myeloid DCs (mDCs) did not differ between two groups. It was also found that the absolute number for total DCs, mDCs and pDCs were significantly higher in the smoking subjects than that of control subject. However, the mDC/pDC ratio was significantly reduced, suggesting that smoking impairs the balance of DC subsets. Given that pDCs are in favor of tolerogenic function, our data support that smoking could induce the production of pDCs to manifest immunosuppressive properties in the chronic smokers.
Keywords: Smoking, dendritic cells, plasmacytoid dendritic cells, myeloid dendritic cells
Introduction
Cigarette smoking is a worldwide epidemic, and is one of main health problems affecting multiple organ systems associated with death and disability. Particularly, smoking alters a wide range of immunological functions including innate and adaptive immune responses. For example, maternal smoking alters both the adaptive and innate immune arms of newborns [1]. Smoking is also found to be associated with the release of both proinflammatory and anti-inflammatory mediators [2,3], and cigarette smokers have been shown with increased numbers for neutrophils, macrophages and T lymphocytes in the airway and peripheral blood [4-6].
Dendritic cells (DCs) are key regulators of immune responses, in which they promote the activation of T cells to orchestrate the polarized immune responses. DCs are comprised of a heterogeneous group of cells. In humans, two main circulating subsets, namely myeloid (m) and plasmacytoid (p) DCs, have been described according to their origin, phenotype and functional property [7,8]. To date, myeloid DCs (mDCs) are regarded as the main antigen presenting cells (APCs) to drive cell-mediated immune responses [9], while the plasmacytoid DCs (pDCs) exert potent antiviral activity due to their capability to produce type-I interferons (IFNs) [10], and are also thought to be critically involved in immune tolerance by acting on regulatory T cells (Tregs) [11].
Previously, a great deal of effort has been devoted to dissect the impact of cigarette smoking on the alteration for the number and functionality of pulmonary DCs [12,13]. However, to our knowledge, few studies have been conducted to investigate the exact effect of cigarette smoking on circulating DCs and their subsets. Therefore, the aim of this pilot study was to clarify and to characterize the number and subset of circulating DCs in the smoking subjects by multi-parametric flow cytometry analysis.
Materials and methods
Human subjects
A total of 53 subjects were recruited from the physical examination center at the Renmin Hospital of Wuhan University. Laboratory examination included routine blood test, blood biochemical full set, urine routine test, electrocardiogram and abdomen ultrasound. All subjects were free of inflammatory conditions for at least 6 weeks and no evidence of allergic disease and diabetes. Twenty six subjects were defined as regular smokers manifested by consuming more than five cigarettes per day and more than five pack years of smoking history, while the other 27 subjects were normal controls (NC) without smoking history and free of passive smoking environment. Prior informed consent was obtained from all subjects and the study was approved by the Ethical Committee of Renmin Hospital, Wuhan University. Demographics and clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Demographics and Clinical characteristics for the smoking and control subjects
| variable | smokers | controls |
|---|---|---|
| Total number | 26 | 27 |
| Age (years) | 52 | 47 |
| Sex (male/female) | 26/0 | 27/0 |
| Pack-year | 23.6 | N/A |
| aTotal WBCs/mm3 | 6798 | 5703 |
Age, Pack-year and Total WBCs/mm3 are presented as means.
The mean WBCs/mm3 is significantly higher in the smoking subjects as compared with that of control subject(p < 0.002).
Peripheral DC staining and subset analysis
Fresh heparinized blood was obtained through venipuncture and then subjected to DC staining within 2h. An aliquot (0.3ml) of the blood sample was stained with 20μl of anti-BDCA cocktail (containing monoclonal antibodies specific for DC markers BDCA1, BDCA2 along with CD14 and CD19 antibodies) or 20μl of control cocktail containing the appropriate isotype controls (Miltenyi Blood DC Enumeration kit, Miltenyi Biotec Inc., Auburn, CA). Dead cell detector (10μl) was then added into the mixtures, followed by incubating on ice for lysis of erythrocytes for 10min. After washes with PBS containing 1% BSA, 0.1% sodium azide, 2% FCS and 1% human serum, the cells were next fixed and analyzed by three color flow cytometry (Epics Altre II, Beckman Coulter) with the exclusion of B cells, monocytes and dead cells. One million events in a mononuclear gate were collected. With this protocol, the viability of the cells is about 99% and the percentages of the DC subsets are consistent when the samples are processed within the first few hours of sampling. The relative proportion and total number of two DC subsets (mDC and pDC) were calculated. The total count of each DC subsets was also assessed for those subjects with less than 1 million peripheral blood mononuclear cells (PBMCs) in 0.3ml of blood. Results were expressed as percentage of mDCs or pDCs or DCs among total PBMCs.
Absolute enumeration of blood dendritic cells
The absolute number per mm3 of any DC subset was calculated as follows: [percent of a given DC subset x total number of white blood cells (WBCs) per mm3]/100. The mDC/pDC ratio was calculated as follows: absolute number of type-1/absolute number of pDCs. An aliquot of blood from each subject was used to determine the number of WBCs/mm3 using a hemocytometer (XT-1800i Sysmex Europe, Norderstedt, Germany).
Statistical analysis
The Kolmogorov–Smirnov test was used to confirm normality distribution assumption for both mDC and pDC ratio. Data were summarized as mean ± standard deviation. Comparison between groups was carried out using unpaired Student’s t test. In all cases, p < 0.05 was considered with statistical significance.
Results
The smoking subjects show higher percentage of total DCs and pDCs in PBMCs
We first sought to determine the percentage for total DCs, and mDC/pDC subset among total PBMCs. For this purpose, we employed the Miltenyi Blood DC Enumeration kit for flow cytometry analysis as described using the whole blood samples, by which it only requires small blood volume (0.3ml) and minimizes sample handling. We first gated total PBMCs in R1 to exclude cell debris and platelets (Figure 1A). Total DCs among PBMCs were next gated to R2 to exclude B cells, monocytes and dead cells (Figure 1B). The two blood DC subsets, mDCs and pDCs, were next respectively gated into R3 and R4 as shown in Figure 1C.
Figure 1.
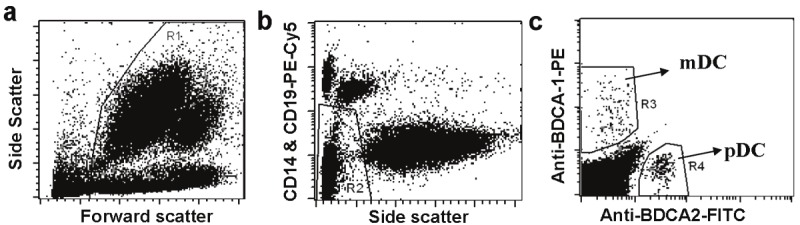
Strategies for gating total DCs. mDCs, and pDCs from total PBMCs. A. PBMCs were gated into R1 to exclude debris and platelets. B. Total DCs were gated into R2 to exclude B cells, monocytes and dead cells. C. By combing R1 and R2, mDCs and pDCs were gated into R3 and R4, respectively.
Interestingly, a significant increase for total DCs was noted in the smoking subjects as compared with that of normal controls (0.95 ± 0.20 vs. 0.82 ± 0.24, p = 0.012, Figure 2A). This result promoted us to further examine the proportions for mDCs and pDCs. It was noted that the percentage for pDCs in total PBMCs was significantly higher in smokers than that of normal controls (0.44 ± 0.13 vs. 0.32 ± 0.13, p = 0.0009, Figure 2B). Particularly, the proportion of pDCs in PBMCs was beyond 0.4% in about 73% of smoking subjects, and 5 of which showed pDC proportion higher than 0.6%. In sharp contrast, only 41% of control subjects manifested pDC proportion higher than 0.4%, and only one of which manifested pDC proportion higher than 0.6% (Figure 2B). Unexpectedly, we failed to detect a significant difference for the proportion of mDCs between smoking subjects and control subjects (0.52 ± 0.17 vs. 0.50 ± 0.15, Figure 2C). This result allowed us to assume that the increase of pDCs could be the main contributor to the higher total DCs observed in the smoking subjects.
Figure 2.
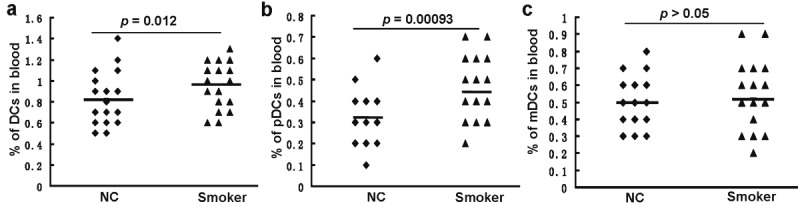
Comparison of peripheral DC percentages between smoking subjects (Smoker) and control subjects (NC). A. The Percentages for total DCs. B. The percentages for pDCs. C. The percentages for mDCs. Horizontal bars represent mean values.
Next, a regression analysis was conducted in the smoking subjects to examine the correlation between the percentage of DC subsets and the pack-year of smoking history. To our surprise, we failed to detect a significant correlation between the pack-year of smoking history and the percentages for total DCs, mDCs and pDCs examined (data not shown).
Peripheral mDCs and pDCs are elevated in the smoking subjects
To further determine the impact of smoking on peripheral DCs, we examined the differences of absolute peripheral DC numbers between smoking subjects and normal controls in total PBMCs. To this end, an aliquot of blood from each subject was first employed to count the number of white blood cells (WBCs) within each mm3 of blood, and the absolute number per mm3 of mDCs, pDCs and total DCs were next calculated as described. Unlike the percentage of mDCs in total PBMCs, a significant increase for the absolute number of mDCs was noted in the smoking subjects as compared with that of control subjects (34.44 ± 12.29 vs. 28.06 ± 8.57, p = 0.016, Figure 3A). Of importantly note, an approximately 50% increase for the absolute pDC number was found in the smoking subject as compared with that of control subjects (29.73 ± 10.94 vs. 17.93 ± 6.68, p < 0.00001, Figure 3B). Similarly, the absolute number for total DCs in the smoking group was significant higher than the control group (64.17 ± 18.11 vs. 45.99 ± 15.52, p < 0.0001, Figure 3C). In line with these observations, smoking subjects showed significantly higher PBMCs than that of control subjects (Table 1).
Figure 3.
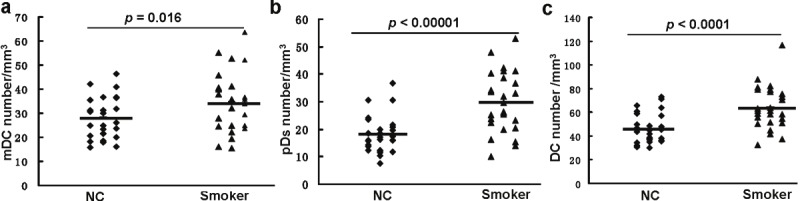
Comparison of absolute DC numbers between smoking subjects (Smoker) and control subjects (NC). A. Comparison for mDC absolute numbers. B. Comparison for absolute pDC numbers. C. Comparison for absolute total DC numbers. Horizontal bars represent mean values.
Peripheral mDC/pDC ratio is reduced in the smoking subjects
Finally, we examine the differences for the mDC/pDC ratio between smoking subjects and control subjects. As shown in Figure 4, the mDC/pDC ratio is significantly reduced in the smoking subjects (1.34 ± 0.83; range, 0.5-4.5) as compared with that of control subjects (1.72 ± 0.75; range,1-4) (p = 0.041), indicating that smoking impairs the balance of DC subset.
Figure 4.
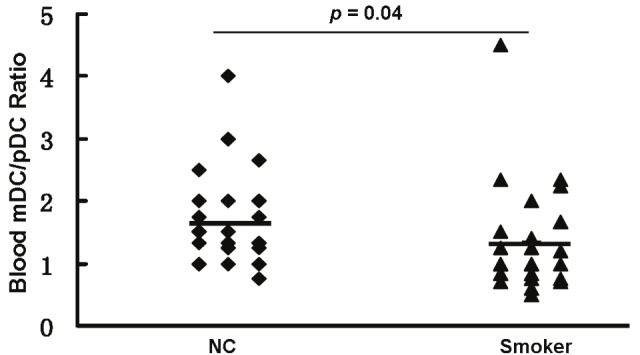
Comparison of peripheral mDC/pDC ratio between smoking subjects (Smoker) and control subjects (NC). Horizontal bars represent mean values.
Discussion
Cigarette smoke (CS) is a mixture of thousands of chemicals generated from the burning or heating of tobacco. Other than its impact on the development of lung and other cancers, CS also predisposes individuals with increased risk to tuberculosis [14], and invasive pneumococcal infection [15]. A number of studies have consistently revealed that nicotine in cigarettes acts as a potent suppressor for the functionality of macrophages, DCs, and T cells [16-18]. Therefore, chronic smoking is commonly relevant to the presence of immunosuppressive properties in the airways along with chronic inflammation.
DCs play a central role in the initiation and regulation of immune responses and are thereby essential for the protection against infectious pathogens and neoplasm [19,20]. Other than the critical role played in adaptive immune responses, DCs are also found contributing to the mechanisms of immune tolerance. In humans, mDCs and pDCs are known for years as the two main circulating DC subsets. As aforementioned, mDCs are the major APCs to drive immune response. In contrast, pDCs are considered with tolerogenic function, in which they are not only capable of anergizing effector T cells, but also endowed with the capacity to drive the differentiation and/or proliferation of FoxP3+ regulatory T cells (Tregs) [21-23]. Given that DCs in favor of tolerogenic rather than immunogenic function is believed contributing to infectious disease and neoplasm, we thus designed current study to demonstrate whether cigarette smoking with potential to alter the proportion of circulating DC subsets, and through which to impair immune defense. Our results showed that the proportion of pDCs in PBMCs is significantly higher in the smoking subjects as compared with that of control subjects (Figure 2B). We further calculated the absolute number of pDCs in PBMCs, and a 50% increase of pDCs was noted in the smoking subjects as compared with that of control subjects (Figure 3B).
Previously, two studies have also investigated the endogenous DC subset in the peripheral blood of smoking subjects, but their results are contrary to our conclusion, in which the authors only analyzed 17 smoking subjects and found lower proportion of pDCs in the peripheral blood [24,25]. In contrast, we included 26 smoking subjects in the study, and our data are likely more conclusive, as the increase of pDCs is consistent with the observation of immunosuppressive properties found in the smoking subjects. Indeed, the increased peripheral pDCs in the smoking subjects are highly likely to foster the generation and/or promotion of Tregs, which appears to be an essential mechanism employed by pathogens and cancer cells to generate immunosuppressive Tregs, and by which they can be escaped from anti-infection and anti-tumor immune responses. Furthermore, pDCs are also known with the capacity to produce copious amounts of type I interferons in response to viruses and other stimuli, while cigarette extracts are found with potency to suppress anti-viral cytokine generation from pDCs activated by TLR9 signaling [26]. Therefore, it is speculated that smoking directly increases the proportion and number of peripheral pDCs, and through which it decreases immunogenic functions and predisposes smoking subjects with increased risk for the development of cancer [27] and infectious disease [14,15].
It has been reported that chronic cigarette smokers manifest a typical increase for the number of Langerhans cells and mDCs in the airway [12,28]. Although our studies revealed that the percentage of mDCs did not differ between smoking subjects and control subjects, but the absolute number of mDCs was increased in the smoking subjects as compared with that of control subjects (Figure 3A), which was likely caused by the increase of total PBMCs in the smoking subjects (Table 1). The increase for mDC numbers in the peripheral blood may exacerbate mDC infiltration in the airway in response to smoking. Unfortunately we do not have the information on the airway DC profiles in our studied subjects to allow us to address this question. Given that mDCs exposed to CS display an immature phenotype in mice along with diminished T-cell stimulatory capability [26], we speculate that mDCs in the smoking subjects are probably functionally impaired although their absolute number is increased.
In summary, we demonstrated for the first time that the percentage and absolute number of circulating pDCs are significantly increased in the smoking subjects. The increase for pDCs leads to a significant decrease for the peripheral mDC/pDC ratio, suggesting that smoking alters the balance and functionality of DC subtypes. Given the fact that mDC/pDC ratio is critical to the outcome of immune response, our results support that the immunosuppressive effect resulted from smoking is probably caused by the induction of tolerogenic pDCs. Future studies are necessary to clarify whether the higher number of peripheral pDCs in the smoking subjects is due to the increase of their generation at the medulla levels.
Acknowledgement
The authors would like to thank the personnel of the physical examination center at the Renmin Hospital of Wuhan University for their help to select patients and collect blood. This work was supported by a grant from the National Natural Science Foundation of China (81070032) to Dr. Xue-Qin Chen.
References
- 1.Noakes PS, Holt PG, Prescott SL. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy. 2003;58:1053–1058. doi: 10.1034/j.1398-9995.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 3.Hagiwara E, Takahashi KI, Okubo T, Ohno S, Ueda A, Aoki A, Odagiri S, Ishigatsubo Y. Cigarette smoking depletes cells spontaneously secreting Th(1) cytokines in the human airway. Cytokine. 2001;14:121–126. doi: 10.1006/cyto.2001.0860. [DOI] [PubMed] [Google Scholar]
- 4.Hoser G, Domagala-Kulawik J, Droszcz P, Droszcz W, Kawiak J. Lymphocyte subsets differences in smokers and nonsmokers with primary lung cancer: a flow cytometry analysis of bronchoalveolar lavage fluid cells. Med Sci Monit. 2003;9:BR310–315. [PubMed] [Google Scholar]
- 5.Smith MR, Kinmonth AL, Luben RN, Bingham S, Day NE, Wareham NJ, Welch A, Khaw KT. Smoking status and differential white cell count in men and women in the EPIC-Norfolk population. Atherosclerosis. 2003;169:331–337. doi: 10.1016/s0021-9150(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 6.Tanigawa T, Araki S, Nakata A, Kitamura F, Yasumoto M, Sakurai S, Kiuchi T. Increase in memory (CD4+CD29+ and CD4+CD45RO+) T and naive (CD4+CD45RA+) T-cell subpopulations in smokers. Arch Environ Health. 1998;53:378–383. doi: 10.1080/00039899809605724. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 8.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, Lommatzsch M. Function-associated surface molecules on airway dendritic cells in cigarette smokers. Am J Respir Cell Mol Biol. 2008;38:655–660. doi: 10.1165/rcmb.2007-0400OC. [DOI] [PubMed] [Google Scholar]
- 13.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol. 2005;175:2684–2691. doi: 10.4049/jimmunol.175.4.2684. [DOI] [PubMed] [Google Scholar]
- 14.den Boon S, van Lill SW, Borgdorff MW, Verver S, Bateman ED, Lombard CJ, Enarson DA, Beyers N. Association between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence area. Thorax. 2005;60:555–557. doi: 10.1136/thx.2004.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 17.Mortaz E, Lazar Z, Koenderman L, Kraneveld AD, Nijkamp FP, Folkerts G. Cigarette smoke attenuates the production of cytokines by human plasmacytoid dendritic cells and enhances the release of IL-8 in response to TLR-9 stimulation. Respir Res. 2009;10:47. doi: 10.1186/1465-9921-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Petro TM. The effect of nicotine on murine CD4 T cell responses. Int J Immunopharmacol. 1996;18:467–478. doi: 10.1016/s0192-0561(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 19.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 21.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 22.Ouabed A, Hubert FX, Chabannes D, Gautreau L, Heslan M, Josien R. Differential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid versus conventional spleen dendritic cells. J Immunol. 2008;180:5862–5870. doi: 10.4049/jimmunol.180.9.5862. [DOI] [PubMed] [Google Scholar]
- 23.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 24.Galgani M, Fabozzi I, Perna F, Bruzzese D, Bellofiore B, Calabrese C, Vatrella A, Galati D, Matarese G, Sanduzzi A, Bocchino M. Imbalance of circulating dendritic cell subsets in chronic obstructive pulmonary disease. Clin Immunol. 2010;137:102–110. doi: 10.1016/j.clim.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Spears M, McSharry C, Donnelly I, Jolly L, Brannigan M, Thomson J, Lafferty J, Chaudhuri R, Shepherd M, Cameron E, Thomson NC. Peripheral blood dendritic cell subtypes are significantly elevated in subjects with asthma. Clin Exp Allergy. 2011;41:665–672. doi: 10.1111/j.1365-2222.2010.03692.x. [DOI] [PubMed] [Google Scholar]
- 26.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol. 2008;180:6623–6628. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 28.Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res. 2010;11:45. doi: 10.1186/1465-9921-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]


