Abstract
Cholangiocarcinomas (CCs) show intraluminal spread to bile ducts and periductal infiltration associated with vascular, lymphatic and perineural invasion in addition to direct penetration. Recently, the peribiliary gland networks located around the hilar and extrahepatic bile ducts have been reportedly to be involved in a variety of biliary diseases. However, the pathological features and roles of these networks in the carcinogenesis and progression of CCs remain to be explored. Recently, we experienced two cases of hilar CC showing a nodular sclerosing growth grossly and histologically well-differentiated adenocarcinomas, with an extensive involvement of the peribiliary gland networks. In situ like spread of carcinoma cells in the bile duct lumen was focal in one case and not identifiable in the other. In contrast, other 4 cases of ordinary hilar CC which were also well-differentiated adenocarcinomas, showed variable intraductal luminal spread and also extensive periductal infiltration irrespective of the peribiliary gland network. In conclusion, the present study showed a unique form of periductal spread of CCs with preferential and extensive involvement of the peribiliary gland networks.
Keywords: Biliary tree, peribiliary glands, hilar cholangiocarcinoma
Introduction
Cholangiocarcinoma (CC), a malignant neoplasm originating from the cholangiocytes lining the bile duct at any antatomical level of the bile duct, remains an intractable disease because a majority of CCs are inoperable at the time of diagnosis mainly due to their ductal and periductal infiltration [1,2]. These CCs are usually of the nodular sclerosing type. They grow along the luminal surface and also invade into the duct wall and spread to the periductal tissue. The bile ducts are supplied by a delicate vascular system known as the peribiliary vascular plexus (PBP), and by lymphatic and nerve fibers [3-5]. These anatomical structures generally serve as routes for the periductal spread of carcinoma cells with frequent lymphatic and vascular invasion and also perineural invasion [1].
CCs differ in pathological features and biological behavior according to the anatomical level of the biliary tree from which they arise. Those arising in the upper part of common hepatic ducts, the right and left hepatic bile ducts and their major branches, are generally called hilar or perihilar CCs [6,7]. In addition to the vascular and neural networks around these hilar bile ducts, peribiliary gland networks are attached to these ducts [8,9]. Peribiliary glands are the glandular components within the duct walls and periductal tissue, and drain into the duct lumen via their own conduits [5,8,10,11]. Recently, the peribiliary gland networks located around the hilar and extrahepatic bile ducts have been implicated in a variety of biliary diseases [9,12]. These glands are also supplied by the PBP, and the lymphatic and nervous systems, and such anatomical complexes have been called the peribiliary gland network [3,5,8,10]. It seems quite possible that hilar CCs may infiltrate the periductal tissue along the peribiliary glands. However, the involvement of the peribiliary glands remains to be clarified.
Recently, we experienced two cases of hilar CC showing preferential involvement of the peribiliary glands. Herein, we report these two cases with an emphasis on pathologic changes in comparison with ordinarly hilar CCs (4 cases) as a control.
Report of two cases of CCs
Neoplastic and non-neopalstic liver tissues of the 6 cases of CCs were fixed in 10% neutral buffered formalin and embedded in paraffin. More than 10 consecutive 4-μm-thick sections were cut from each paraffin block, and some of which were then stained with H&E, Azan-Mallory stain and mucicarmine stain for histological characterizations of the CCs and their relation to the bile duct and peribiliary glands. The remaining sections were used for the immunohistochemistry of cytokerain-7 (CK7) which is known to be expressed in biliary epithelial cells including CC cells, by using a primary monoclonal antibody against CK7. The sections were then treated with secondary antibodies conjugated to a peroxidase- labeled polymer, the EnVision system (DakoCytomation). Color development was performed using DAB or TSA Plus Fluorescence (Tyramide Signal Amplification (TSA) PLUS Fluorescein/TMR Kits, PerkinElmerR).
Case 1
The patient was a 63-year-old female who was admitted to hospital with epigastralgia. Radiologically, stenosis of the left hepatic duct and its major branches, particularly, the beginning of branches of B2 and B3, was evident. Under a diagnosis of hilar CC, a left lobectomy was performed. Grossly, the tumor presented as a nodular sclerosing carcinoma of the left hepatic lobe, mainly involving the hilar and major portal tracts (Figure 1A). Histologically, there were areas of well differentiated adenocarcinoma, mainly extending into the pre-existing peribiliary glands and the wall of bile duct, though the lumen of bile duct was largely spared (Figure 1B and 2C). Such prefrenital involvement of the peribiliary glands was clear in comparison with the non-involved peribiliary gland network of the uninvolved regions from the same case (Figure 1D). CK7 immunostaining which was positive in carcinoma cells and also non-neoplastic biliary cells, showed such preferential growth of carcinoma cells along the peribiliary gland network (Figure 1E). In addition, there was infiltration of carcinomas cells into the adjacent hepatic parenchyma and portal tracts.
Figure 1.
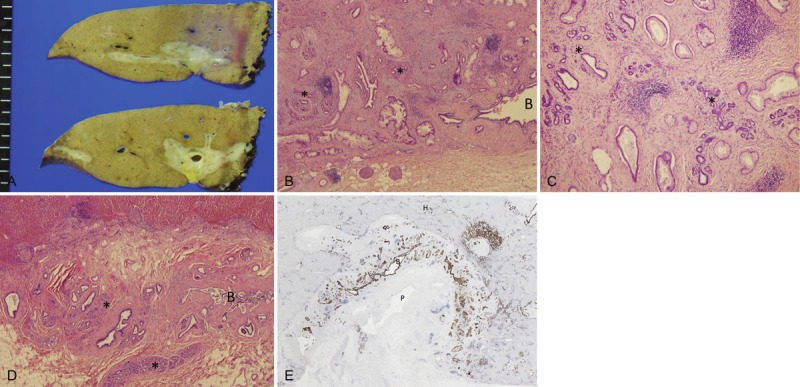
A: Grossly, the tumor presents as a nodular sclerosing carcinoma of the left hepatic lobe, mainly involving the hilar and perihilar area; B, C, D. Histologically, there were areas of well differentiated adenocarcinoma, mainly extending into pre-existing peribiliary glands. HE. B: Carcinoma mainly involved the pre-existing peribiliary gland network (*). Carcinoma infiltrated the duct wall but not the lumen of perihilar bile duct (B). C: Carcinoma spread into the pre-existing peribiliary glands (*). D: Un-involved peribiliary glands (*) network and bile duct (B) in the same case; E: CK7 immunostaining which was positive in carcinoma cells and also non-neoplastic biliary cells, showed the preferential growth of carcinoma along the peribiliary glands. B, perihilar bile duct; P, portal vein; H, hepatic parenchyma. CK7 immunostaining with hematoxylin counterstaining.
Figure 2.
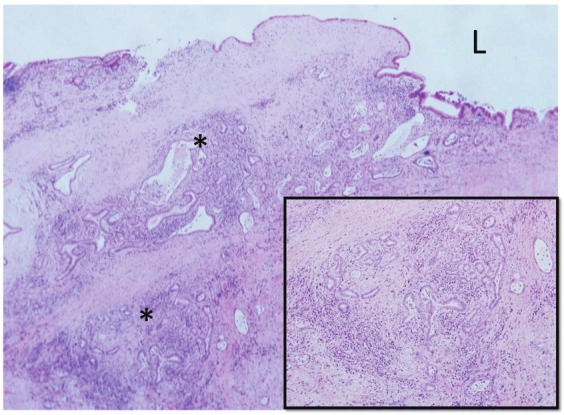
In the major branch of the left hepatic duct, adenocarcinoma cells spread prererenitally along the peribiliary glands (*). L, bile duct lumen. HE. Inset: Higher magnification of one lobule of peribiliary gland invaded by carcinoma cells. HE.
Case 2
The patient was a 70-year-old male who was admitted to hospital complaining of abdominal pain and vomiting. Radiologically, a nodular mass with stenosis of the left hepatic bile duct and portal vein branches was detected. Under a diagnosis of CC, a left lobectomy was performed. This case showed a nodular sclerosing CC involving the left hepatic lobe with stenotic bile ducts containing biliary sludges, and the CC was histologically a well-differentiated adenocarcinoma. The carcinoma showed micropapillary lesions on the luminal surface, focally. In addition, the adenocarcinoma had spread along the peribiliary glands in many of the major branches of the left hepatic duct (Figure 2), while in some areas it showed direct infiltration into the bile duct wall of the hilar bile duct and perineural invasion.
Controls
Four cases of hilar CC (age ranging from 65 to 75 years, and two males and two females) showing grossly a nodular sclerosing growth involving the hepatic hilar region of the left hepatic lobe and histologically a well-differentiated adenocarcinoma, were collected from the hepatobiliary disease file of our department (all were surgically resected cases). These cases showed variable intraluminal spread of carcinoma cells along the affected bile ducts. Interestingly, all showed periductal spread of carcinoma cells associated with direct infiltration of carcinoma cells with desmoplastic reactions and frequent perineural and vascular and lymphatic invasion (Figure 3), but failed to show any preferential invasion to the pre-existing peribiliary gland network as seen in cases 1 and 2. However, 2 of these 4 cases disclosed focal glandular involvement of carcinoma cells in association with other types of invasion.
Figure 3.
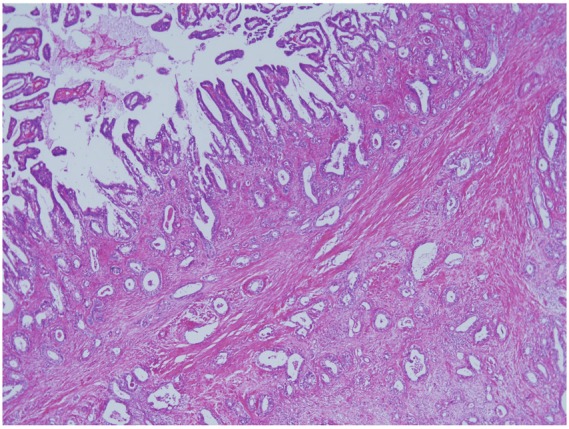
Carcinoma showed periductal spread associated with intraluminal growth, direct infiltration, desmopalstic reactions and perineural, vascular and lymphatic invasion, but failed to show preferential invasion of the pre-existing peribiliary gland network. HE.
Discussion
CCs remain an intractable malignant tumor, and surgical resection is currently the only curable therapeutic approach [1,2]. CCs arising from the hilar bile ducts usually present grossly as a nodular sclerosing growth. These carcinoma cells are known to invade the ductal wall and periductal tissue and also to spread along the luminal surface of the affected bile ducts [2]. They are associated with vascular and lymphatic invasion and also perineural infiltration of carcinoma cells in addition to their direct penetration. Such infiltration is not only an important route for the spread of CCs but usually means a poor outcome or prognosis. Taken together, ductal and periductal invasion may have significant clinical implications in the progression of CC.
It was found for the first time in this study that there is another route of periductal spread for CC. The two cases reported here showed preferential spread along the peribiliary gland networks and this could be regarded as a unique pathway of infiltration of hilar CCs arising from the hilar bile ducts annexed by the peribiliary glands [3,9,10]. The peribiliary glands are the glandular components within the duct walls and periductal tissue, and drain into the duct lumen via their own conduits. These glands are supplied by a delicate vascular system, the PBP, and the lymphatic and nervous systems. These anatomical complexes have been called the peribiliary gland network [3,5,10]. It seems highly possible that hilar CCs invade the peribiliary glands and then infiltrate the periductal tissue around them. In fact, the two cases presented here seem to have demonstrated such a possibility.
Carcinoma cells can spread as an intraepithelial, in situ-like, replacement growth within the peribiliary glands and their conduits and also penetrate the connective tissue including the lymphatics, vasculatures, and nerves within or adjacent to these networks. Such invasion can lead to further periductal involvement and could be another challenge to clinical treatment and also research field into the spread of CCs. In addition, some CCs may actually arise from the peribiliary glands [13]. Taken together, the peribiliary glands are an important anatomical structure in the development and spread of CCs arising in the hilar bile ducts.
In conclusion, two cases of hilar CC showing a nodular sclerosing growth and extensive involvement of the networks of peribiliary glands were reported. The peribiliary gland involvement may be important for further periductal spread of CCs. In the four CC cases used as a control, the carcinomas showed extensive periductal infiltration associated with direct penetrating infiltration, and vascular, lympathtic and neural invasion which seem to be unrelated to the pre-existing peribiliary glands. Pathological changes to the peribiliary glands seem to be important to the development and spread of CCs arising in the perihilar bile ducts in some cases of CC. Furhter study of these gland networks is warranted.
Conflict of interest statement
All authors have no conflict of interest.
References
- 1.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakanuma Y, Curabo MP, Franceschi S, Gores G, Paradis V, Sripa B, Tsui WMS, Wee A. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. Who Classification of Tumours of the Digestive System; World Health Organization of Tumours. 4th edition. Lyon: IARC; 2010. pp. 217–224. [Google Scholar]
- 3.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: a lectin-histochemical and immunohistochemical study. Hepatology. 1993;18:529–36. [PubMed] [Google Scholar]
- 4.Terada T, Nakanuma Y. Innervation of intrahepatic bile ducts and peribiliary glands in normal human livers, extrahepatic biliary obstruction and hepatolithiasis. An immunohistochemical study. J Hepatol. 1989;9:141–8. doi: 10.1016/0168-8278(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 5.Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–70. doi: 10.1002/(SICI)1097-0029(19970915)38:6<552::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Hiriart JB, Laurent C, Blanc JF. Perihilar cholangiocarcinoma, the need for a new staging system. Clin Res Hepatol Gastroenterol. 2011;35:697–8. doi: 10.1016/j.clinre.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Gandou C, Harada K, Sato Y, Igarashi S, Sasaki M, Ikeda H, Nakanuma Y. Hilar cholangiocarcinoma and pancreatic ductal adenocarcinoma share similar histopathologies, immunophenotypes, and development-related molecules. Human Pathol. 2012 doi: 10.1016/j.humpath.2012.08.004. in press. [DOI] [PubMed] [Google Scholar]
- 8.Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419–29. doi: 10.1111/j.1440-1827.2010.02543.x. [DOI] [PubMed] [Google Scholar]
- 9.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–99. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanuma Y, Katayanagi K, Terada T, Saito K. Intrahepatic peribiliary glands of humans. I. Anatomy, development and presumed functions. J Gastroenterol Hepatol. 1994;9:75–9. doi: 10.1111/j.1440-1746.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanuma Y, Zen Y, Portman BC. Diseases of the bile ducts. In: Burt A, Portman B, Ferrell L, editors. MacSween’s Pathology of the Liver. 6th ed. Edinburg, London, New York, Oxford, Philadelphia, St Louis, Sydney, Toronto: Churchill Livingstone; 2011. pp. 491–562. [Google Scholar]
- 13.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers. II. A possible source of cholangiocarcinoma. Hepatology. 1990;12:92–7. doi: 10.1002/hep.1840120115. [DOI] [PubMed] [Google Scholar]


