Abstract
Background: Merkel cell carcinoma is a high-grade neuroendocrine carcinoma of skin that is characterized by immature cells which, because of its striking morphologic similarity, may be confused with other small round blue cell tumors such as pulmonary small cell carcinoma or lymphoblastic leukemia/lymphoma. Immunohistochemistry is therefore paramount to ensuring accurate diagnostic distinction between these tumors. The aim of our study was to evaluate and compare the expression of PAX5 and Terminal deoxynucleotidyl transferase (TdT), in Merkel cell carcinoma and pulmonary small cell carcinoma. Design: PAX5 and TdT immunohistochemical stains were performed on 27 Merkel cell carcinomas and 10 pulmonary small cell carcinomas. Results: PAX5 was expressed in 24/27 (89%) Merkel cell carcinomas and 0/10 (0%) pulmonary small cell carcinomas. TdT was expressed in 21/27 (78%) Merkel cell carcinomas and 9/10 (90%) pulmonary small cell carcinomas. Conclusions: Our study confirms that PAX5 and TdT expression can be expressed in a high percentage of Merkel cell carcinomas and so when positive are not diagnostic of lymphoblastic leukemia/lymphoma. When dealing with metastatic lesions, PAX5 negativity would favor a diagnosis of pulmonary small cell carcinoma over Merkel cell carcinoma. In addition, TTF-1 negative pulmonary small cell carcinoma is to be differentiated from Merkel cell carcinoma.
Keywords: Merkel cell carcinoma, PAX5, TdT, small cell carcinoma, lung
Introduction
Merkel cell carcinoma (MCC) of the skin is an aggressive cutaneous neuroendocrine carcinoma which was first reported in 1972 by Toker, who coined the term “trabecular carcinoma” of the skin [1]. MCC predominantly affects elderly Caucasians and has a propensity for local recurrence and regional lymph node metastasis. These tumors are most commonly found on sun-exposed areas of the head and neck or extremities, but can occur at virtually any site. In addition to ultraviolet radiation, immunosuppression has been suggested as a likely cause of Merkel cell carcinoma, with reports of up to a 13-fold increase in patients with human immunodeficiency virus (HIV) infection [2]. Patients with Merkel cell carcinoma typically present with rapidly growing, painless, red to purple colored cutaneous nodules or plaques.
Like other neuroendocrine neoplasms, Merkel cell carcinoma shares histologic features with cutaneous small blue round cell tumors in that they are characterized by a dermal and subcutaneous infiltrate of monotonous cells that have high nuclear to cytoplasmic ratio, fine stippled chromatin and high proliferation rate. The differential diagnosis for Merkel cell carcinoma includes other small blue round cell tumors such as neuroblastoma, metastatic carcinoid, amelanotic melanoma, Ewing sarcoma, pulmonary small cell carcinoma (PSCC) and lymphomas including lymphoblastic lymphoma (LBL). Immunohistochemical stains are extremely helpful in distinguishing Merkel cell carcinoma from both lymphoblastic lymphoma and pulmonary small cell carcinoma with a panel of markers including, cytokeratin 7 (CK7), cytokeratin 20 (CK20), thyroid transcription factor-1 (TTF-1), and lymphocyte common antigen (CD45/LCA).
PAX5, a member of the paired box gene family, is located on chromosome 9q13 and plays an important role in B-cell ontogeny. In addition to this primary function, PAX5 has been detected in the spinal cord and mesencephalon-rhombencephalon boundary by in situ hybridization [3]. Earlier reports have demonstrated PAX5 expression in MCC and a subset of PSCC [4]. Terminal deoxynucleotidyl transferase (TdT), a DNA polymerase, catalyzes the elongation of nucleotide chains with its actions being most pronounced during the early development of B and T cells during immunoglobulin and T-cell receptor gene rearrangements. TdT expression has recently been reported in both MCC as well as PSCC [5]. To our knowledge no single study has reported the expression patterns of PAX5 and TdT in MCC and PSCC, which is the goal of this paper.
Materials and methods
We retrieved 27 cases of Merkel cell carcinoma and 10 cases of pulmonary small cell carcinoma from the pathology files of Georgia Health Sciences University, Augusta, GA, Charlie Norwood Veterans Affairs Medical Center (VAMC), Augusta, GA and Emory University Hospital, Atlanta, GA between 1999 and 2009. The study was approved by the Human Assurance Committee of each institution. Original slides were reviewed and the diagnoses confirmed by two reference pathologists.
Neuroendocrine markers (synaptophysin, chromogranin, CD56), keratin markers (pancytokeratin, low-molecular-weight cytokeratin, CK7, CK20), TTF-1 and CD45 were variably performed at the time of initial diagnosis. These stains were also reviewed for the purpose of the study.
Seven cases of Merkel cell carcinoma and 10 pulmonary small cell carcinoma from Georgia Health Sciences University and Charlie Norwood VAMC were stained with PAX5 (Clone-SP-34, 1:100, Biocare Medicals, Walnut Creek, CA.) and TdT (Clone- SP-3, 1:100, DAKO, Carpentaria, CA). Formalin-fixed paraffin-embedded blocks were sectioned at 4μm and sections mounted on treated slides. Immunohistochemistry was performed using the standard avidin-biotin complex method on a DAKO autostainer (DAKO, Carpinteria, CA). Antigen retrieval consisted of pretreatment with target retrieval solution, pH 6.0. Bound antibody was detected with DAB+ substrate kit (DAKO Carpinteria, CA). Positive controls for PAX5 (tonsil) and TdT (cervical lymph node involved by lymphoblastic lymphoma) and negative controls with primary antibody replaced with Tris buffer were simultaneously run.
The cases of Merkel cell carcinoma (n=20) from Emory University were stained using TdT (polyclonal antibody, 1:40 dilution, DAKO, Carpinteria, CA) and PAX5 (clone 24, Cell Marque, Rocklin, CA, RTU). After antigen retrieval, sections (5 micron) of formalin-fixed paraffin-embedded tissue were incubated with TdT for 15 minutes, with post primary polymer for 8 minutes, blocked with 3% hydrogen peroxide for 5 minutes (Bonded Polymer Refine Detection Kit (DAB chromogen), (Leica Microsystems, Bannockburn, IL) with 3,3-diaminobenzidine 9DAB, brown chromogen, and hematoxylin as counterstain for 5 minutes. All steps were performed on the Leica Bond Max III automated system. Incubations were performed at room temperature; between incubations, sections were washed with Tris-buffered saline (Bond wash solution). Positive controls for PAX5 (tonsil) and TdT (case of lymphoblastic lymphoma) and negative controls with primary antibody replaced with Tris buffer were simultaneously run.
All slides were assessed for extent of nuclear staining of PAX5 and TdT in the neoplastic cells as follows: 0: no staining, 1+: 1% to 25%, 2+: 26% to 50%, 3+: greater than 50%. Nuclear staining intensity was also evaluated and was scored as: negative (-), weak (1+), moderate (2+) or strong (3+).
Results
PAX5 was expressed in 24/27 (89%) Merkel cell carcinoma and 0/10 (0%) of pulmonary small cell carcinoma. TdT was expressed in 21/27 (78%) Merkel cell carcinomas and 9/10 (90%) pulmonary small cell carcinoma (Table 1). In the Merkel cell carcinoma cohort, diffuse (>50%), strong (2-3+) PAX5 positivity was seen in 11 of 27 (40%) cases, and diffuse, strong TdT staining was seen in 12 of 27 (44%) cases. Eight of 10 (80%) of pulmonary small cell carcinoma showed diffuse, strong positivity for TdT. Immunohistochemical results for pancytokeratin, CK20, synaptophysin, chromogranin, and TTF-1 are summarized in Table 2 and Figure 1.
Table 1.
Expression of PAX5 and TdT in Merkel cell carcinoma and pulmonary small cell carcinoma
| Diagnosis | PAX5 | TdT |
|---|---|---|
| Merkel cell carcinoma | 24/27 (89%) | 21/27 (78%) |
| Pulmonary small cell carcinoma | 0/10 (0%) | 9/10 (90%) |
TdT, Terminal deoxynucleotidyl transferase.
Table 2.
Expression of immunohistochemical stains performed for the diagnosis of Merkel cell carcinoma and pulmonary small cell carcinoma
| Diagnosis | Pancytokeratin | CK20 | Synaptophysin | Chromogranin | TTF-1 |
|---|---|---|---|---|---|
| Merkel cell carcinoma | 5/6 (83%) | 15/16 (94%) | 14/14 (100%) | 9/9 (100%) | 0/10 (0%) |
| Pulmonary small cell carcinoma | 2/2 (100%) | NP | 5/5 (100%) | 6/6 (100%) | 2/2 (100%) |
NP, not performed.
Figure 1.
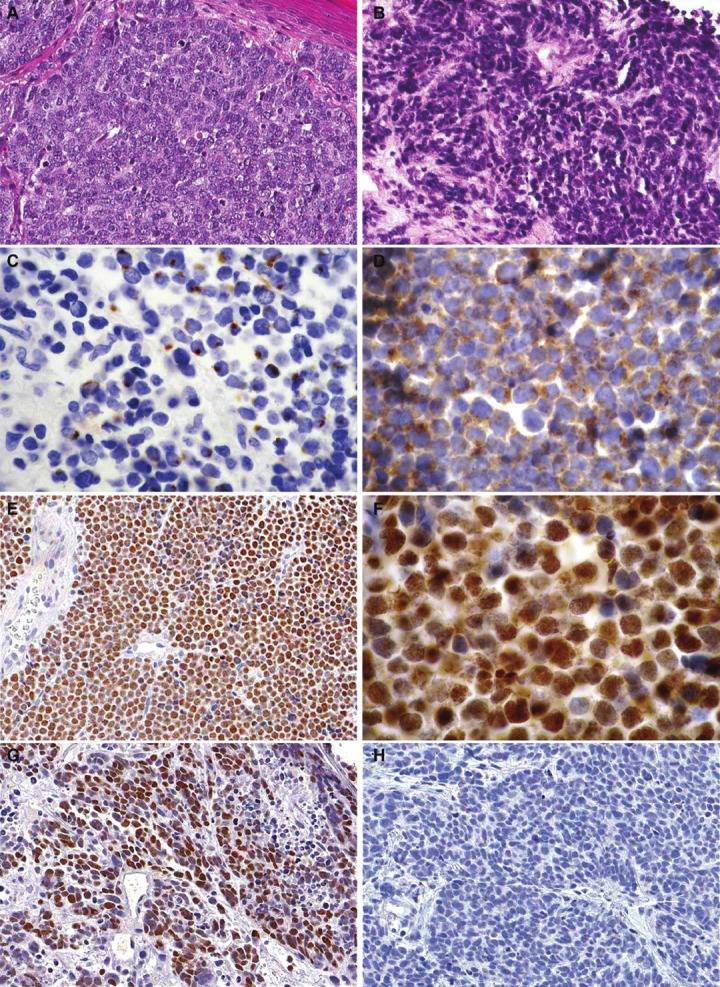
A. Merkel cell carcinoma showing neoplastic cells with finely stippled chromatin, indistinct nucleoli and high mitotic activity (Hematoxylin & Eosin, magnification x200). B. Pulmonary small cell carcinoma showing malignant cells with high N/C ratio, “salt-and-pepper” chromatin and characteristic crush artifact (Hematoxylin & Eosin, magnification x200). C. Cytokeratin 20 positivity in Merkel cell carcinoma with typical paranuclear dot-like staining pattern (magnification x400). D. Diffuse positivity for synaptophysin in Merkel cell carcinoma (magnification x400). E. Merkel cell carcinoma showing diffuse, strong nuclear staining for PAX5 (magnification x200). F. Merkel cell carcinoma showing strong nuclear staining for TdT (magnification x400). G. Pulmonary small cell carcinoma with diffuse positive nuclear staining for TdT (magnification x200). H. Pulmonary small cell carcinoma demonstrating negative staining for PAX5 (magnification x200).
Discussion
In this study, we report the expression of PAX5 and TdT in a cohort of Merkel cell carcinomas and pulmonary small cell carcinomas. Both markers are typically associated with B- lymphoblastic lymphoma. TdT is an intranuclear template-independent DNA polymerase that catalyzes the elongation of nucleotide chains by repetitive addition of deoxyribonucleotides to the 3’-OH-terminated segment of DNA in mammalian cells. TdT has been found to span at least 65 kb and is located on chromosome 10 q23-q25 [6]. It has greatest activity in early B cells and T cells during the phase of immunoglobulin and T-cell receptor gene rearrangements and is expressed in normal thymic T cells.
The most notable example of expression of TdT in human tumors is in lymphoblastic lymphoma, but nuclear expression has also been described in acute myeloid leukemia, some cases of medulloblastoma, CD56+/CD4+ hematodermic neoplasm, rare cases of pediatric rhabdomyosarcoma, and Ewing sarcoma [7-10]. Sur et al reported strong nuclear TdT expression in 53% (8/15) of Merkel cell carcinomas. Six of their cases morphologically resembled blastic hematologic malignancies with large- to intermediate-sized cells showing vesicular nuclei and one or two prominent nucleoli. This morphology in conjunction with TdT positivity could result in an erroneous diagnosis of a blastic hematological malignancy in the skin. A subsequent study by Bernd et al reported similar nuclear TdT immunoreactivity in 10 of 28 (36%) cases of Merkel cell carcinoma [11]. In the present study, we found that TdT was positive in the majority (89%) of Merkel cell carciomas. The expression of TdT in both Merkel cell carcioma and pulmonary small cell carcinoma is particularly notable because its uniformly strong nuclear staining was frequently at intensities mimicking those seen in lymphoblastic lymphoma. The latter observation emphasizes the caution that must be exercised when interpreting PAX5 and TdT positivity in the setting of any small round blue cell tumor.
Differentiating Merkel cell carcinoma from metastatic pulmonary small cell carcinoma can sometimes be challenging as both tumors have a neuroendocrine phenotype. Other discriminatory immunohistochemical stains can be helpful in this differential such as CK20 and TTF1. Merkel cell carcinomas are typically positive for CK20 (~ 97%) and show a characteristic paranuclear dot-like positivity but are negative for TTF-1. The converse is typically true of pulmonary small cell carcinoma; rare positivity has been reported [12]. Furthermore, up to a third of Merkel cell carcinomas can be negative for CK20, and CK7 has been reported to be positive in up to 25% of Merkel cell carcinomas [13-16]. Thyroid transcription factor -1 (TTF-1) is typically negative in Merkel cell carcinoma, but up to 20% of pulmonary small cell carcinomas are also negative for TTF-1 [5,17], thus limiting its usefulness in differentiating these tumors.
PAX5, as a marker of B cells, was initially reported by Torlakovic et al in 2002 [18]. He also reported PAX5 expression in non-hematopoietic tumors such as neuroendocrine tumors, Merkel cell carcinoma, and mesonephric tumors [18]. Dong et al [4] later also reported the expression of PAX5 in neuroendocrine carcinomas such as Merkel cell carcinoma (93.5%) and small cell carcinoma (73.3%). An interesting finding in our study was that pulmonary small cell carcinomas were mostly positive for TdT (90%) but none were positive for PAX5. Dong et al [4] found PAX5 reactivity in greater than two thirds (~74%) of PSCC. In their study, they confirmed the presence of PAX5 mRNA by real time RT-PCR in 8 cases of Merkel cell carcinoma, but no cases of pulmonary small cell carcinoma were tested. The antibody clones used in our and Dong et al’s studies are not the same (monoclonal anti-PAX5 antibody, clone 24, BD Biosciences versus monoclonal anti-PAX5 antibody, clone SP-34, Biocare Medicals, Walnut Creek, CA, respectively) and this could possibly explain the difference in staining patterns that were observed. Given this significant discrepancy, additional studies using different PAX5 clones are needed to determine the true incidence of PAX5 positivity in pulmonary small cell carcinoma.
In conclusion, co-expression of TdT and PAX5 by Merkel cell carcinoma may result in its misdiagnosis as B- lymphoblastic lymphoma, particularly since the latter is often negative for CD45, a frequently used lymphoid marker. When confronted with a cutaneous small round blue cell tumor in which an immature hematologic malignancy such as B- lymphoblastic lymphoma is in the differential diagnosis, the use of an immunohistochemical panel including CD34, CD79a, CD10, CK20 as well as neuroendocrine markers is strongly recommended. In our hands, PAX5 and TdT positivity in conjunction with the expression of neuroendocrine markers, suggests a diagnosis of Merkel cell carcinoma rather than pulmonary small cell carcinoma, when evaluating cutaneous small round blue cell tumors. PAX5 may therefore represent an additional marker to facilitate the distinction between Merkel cell carcinoma and pulmonary small cell carcinoma.
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. [PubMed] [Google Scholar]
- 2.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 3.Gerard M, Abitbol M, Delezoide AL, Dufier JL, Mallet J, Vekemans M. PAX-genes expression during human embryonic development, a preliminary report. C R Acad Sci III. 1995;318:57–66. [PubMed] [Google Scholar]
- 4.Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol. 2005;29:687–692. doi: 10.1097/01.pas.0000155162.33044.4f. [DOI] [PubMed] [Google Scholar]
- 5.Sidiropoulos M, Hanna W, Raphael SJ, Ghorab Z. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831–838. doi: 10.1309/AJCPLCB2Q9QXDZAA. [DOI] [PubMed] [Google Scholar]
- 6.Isobe M, Huebner K, Erikson J, Peterson RC, Bollum FJ, Chang LM, Croce CM. Chromosome localization of the gene for human terminal deoxynucleotidyltransferase to region 10q23-q25. Proc Natl Acad Sci U S A. 1985;82:5836–5840. doi: 10.1073/pnas.82.17.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzumiya J, Ohshima K, Kikuchi M, Takeshita M, Akamatsu M, Tashiro K. Terminal deoxynucleotidyl transferase staining of malignant lymphomas in paraffin sections: a useful method for the diagnosis of lymphoblastic lymphoma. J Pathol. 1997;182:86–91. doi: 10.1002/(SICI)1096-9896(199705)182:1<86::AID-PATH821>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Budke H, Orazi A, Neiman RS, Cattoretti G, John K, Barberis M. Assessment of cell proliferation in paraffin sections of normal bone marrow by the monoclonal antibodies Ki-67 and PCNA. Mod Pathol. 1994;7:860–866. [PubMed] [Google Scholar]
- 9.Orazi A, Cattoretti G, John K, Neiman RS. Terminal deoxynucleotidyl transferase staining of malignant lymphomas in paraffin sections. Mod Pathol. 1994;7:582–586. [PubMed] [Google Scholar]
- 10.Braziel RM, Keneklis T, Donlon JA, Hsu SM, Cossman J, Bollum FJ, Jaffe ES. Terminal deoxynucleotidyl transferase in non-Hodgkin’s lymphoma. Am J Clin Pathol. 1983;80:655–659. doi: 10.1093/ajcp/80.5.655. [DOI] [PubMed] [Google Scholar]
- 11.Bernd HW, Krokowski M, Feller AC, Bartsch S, Thorns C. Expression of terminal deoxynucleotidyl transferase in Merkel cell carcinomas. Histopathology. 2007;50:676–678. doi: 10.1111/j.1365-2559.2007.02630.x. [DOI] [PubMed] [Google Scholar]
- 12.Bobos M, Hytiroglou P, Kostopoulos I, Karkavelas G, Papadimitriou CS. Immunohistochemical distinction between merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28:99–104. doi: 10.1097/01.dad.0000183701.67366.c7. [DOI] [PubMed] [Google Scholar]
- 13.Beer TW. Merkel cell carcinomas with CK20 negative and CK7 positive immunostaining. J Cutan Pathol. 2009;36:385–386. doi: 10.1111/j.1600-0560.2008.01062.x. author reply 387. [DOI] [PubMed] [Google Scholar]
- 14.Calder KB, Coplowitz S, Schlauder S, Morgan MB. A case series and immunophenotypic analysis of CK20-/CK7+ primary neuroendocrine carcinoma of the skin. J Cutan Pathol. 2007;34:918–923. doi: 10.1111/j.1600-0560.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen K, Kohler S, Rouse RV. Cytokeratin staining in Merkel cell carcinoma: an immunohistochemical study of cytokeratins 5/6, 7, 17, and 20. Appl Immunohistochem Mol Morphol. 2000;8:310–315. [PubMed] [Google Scholar]
- 16.Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125:228–231. doi: 10.5858/2001-125-0228-IFTTFA. [DOI] [PubMed] [Google Scholar]
- 17.Torlakovic E, Torlakovic G, Nguyen PL, Brunning RD, Delabie J. The value of anti-pax-5 immunostaining in routinely fixed and paraffin-embedded sections: a novel pan pre-B and B-cell marker. Am J Surg Pathol. 2002;26:1343–1350. doi: 10.1097/00000478-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Torlakovic E, Slipicevic A, Robinson C, DeCoteau JF, Alfsen GC, Vyberg M, Chibbar R, Florenes VA. Pax-5 expression in nonhematopoietic tissues. Am J Clin Pathol. 2006;126:798–804. doi: 10.1309/XEC7-JMW9-YRM7-4RNO. [DOI] [PubMed] [Google Scholar]


