Abstract
α-Naphthoflavone (α-NF) is a synthetic flavonone derivative and is well known as a potent inhibitor of aromatase in a variety of systems. However, its role in lipid metabolism remains far from understood. The aim of current study was to investigate the effects of α-NF on 3T3-L1 pre-adipocytes differentiation and the mechanism through which it acts. Treatment of 3T3-L1 cells with α-NF in conjunction with a hormone cocktail resulted in α-NF mediated suppression of adipocyte differentiation in a dose dependent manner. At the molecular level, our findings demonstrated that α-NF inhibited the mid and late phase, but not the early phase of adipogenic markers expression during 3T3-L1 adipogenesis. The phosphorylation of p38 was activated upon adipogenic stimulation, yet was substantially suppressed by α-NF treatment. α-NF also synergistically inhibited expression of the adipogenic marker peroxisome proliferator-activated receptor gamma (PPARγ) expression together with p38 selective inhibitor, SB203580. Our study demonstrated for the first time that α-NF is capable of suppressing 3T3-L1 adipocyte differentiation and that this effect likely occurs through repression of the p38MAPK signaling pathway.
Keywords: α-Naphthoflavone, adipogenesis, PPARγ, p38MAPK
Introduction
The rising prevalence of obesity is a worldwide health concern, especially for children and young adults because it can lead to serious health complications later in life [1]. Increased weight gain is a major contributor to multiple health outcomes including type 2 diabetes, cardiovascular disease, metabolic disorder and cancers [2]. Excessive fat deposition is an imbalance between energy intake and expenditure that results in an increase in either adipocyte number or size, or both. Adipocytes can be generated from pre-adipocytes (precursors) or enlarged by excessive lipid accumulation. The essential step of adiocyte differentiation is the commitment from pre-adipocytes and this process is regulated by an elaborate network of transcription factors that coordinate multiple genes expression. Peroxisome proliferator-activated receptor gamma (PPARγ) is a key regulator of adipogenesis among transcription factors in particular [3]. The CCAAT/enhancer-binding protein(C/EBP) family also plays a vital role in promoting adipocyte differentiation [4]. C/EBPα and PPARγ coordinately drive downstream genes expression such as cluster of differentiation 36, fatty acid binding protein 4, lipoprotein lipase and glucose transport protein 4 which are responsible for adipocytes phenotype [5].
The clinical importance of herbal drugs for treatment of obesity has received considerable attention during the past decades [6]. Flavonoids are groups of more than 6,000 polyphenolic compounds [7] that are present in medical plants, herbal remedies, fruits, vegetables and grains, and have been used in folk medicine around the world [8]. The human daily intake of all flavonoids is about hundreds of milligrams [9]. Generally, flavonoids are not considered as nutritive elements, however, these compounds have a wide arrange of biological activities including anti-oxidative [10], anti-inflammatory [11], gastroprotective [12], cardioprotective [13], anti-cancer [8], and modulation of enzymatic activities [14]. Evidences from epidemiological studies revealed that there is an inverse correlation between higher daily consumption of flavonoids and the incidence of several chronic diseases [15,16]. A number of flavonoids, including anthocyanins, resveratrol, curcuma [17-19], have been identified as potential modulators of adipocyte differentiation.
Alpha-Naphthoflavone (α-NF) is a flavonoid that is acts as an inhibitor of certain types of cytochrome p450 enzymes and as an antagonist at the aromatic hydrocarbon receptor (AhR) [20] (Figure 1). α-NF also possesses vasorelaxation induction and anti-platelet properties [21,22]. Currently, the synthetic α-NF is used as an AhR antagonist or a selective Cyp1a inhibitor in a variety of systems. There is a paucity of data regarding the role of α-NF in adipocyte differentiation. A deeper understanding of its actions on pre-adipocyte differentiation will aid in the evaluation of its potential for the treatment and prevention of obesity. Therefore, our present studies explore the consequences and possible mechanism ofaction of α-NF in a pre-adipocyte cell culture model, 3T3-L1 cells.
Figure 1.
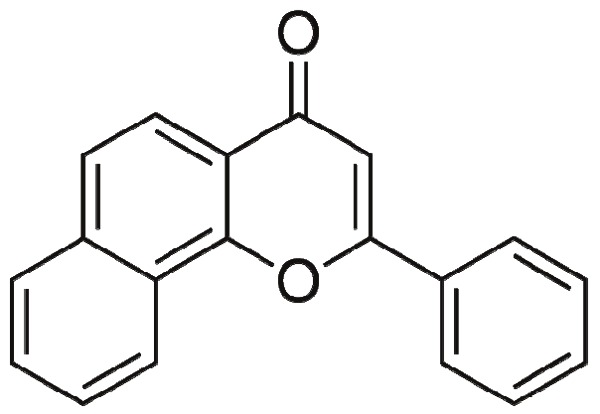
Structure of α-Naphthoflavone.
Materials and methods
Reagents
α-NF, Oil Red O, SB203580, insulin, dihydroxyacetone-3-phosphate, 3-isobutyl-1-methylxanthine and dexamethasone were obtained from Sigma. Trizol was purchased from Invitrogen. Fetal calf serum and fetal bovine serum were purchased from Hyclone.
Cell culture and differentiation
3T3-L1 mouse pre-adipocytes were purchased from the Chinese Academy of Science (Shanghai, China) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum and were maintained at 37°C in a humidified, 5% CO2 incubator. All media contained 100IU/ml penicillin-streptomycin. To differentiate the cells, two days after confluence, they were stimulated with hormone cocktail consisting of 10mg/ml insulin, 0.5 mM isobutylmethylxanthine, and 1 mM dexamethasone in 10% FBS-DMEM medium for 2 days. On day 3, the cocktail was replaced with 10mg/ml insulin only. This step was repeated every two days until the appearance of mature adipocytes. A schematic of the procedure is presented in Figure 3A.
Figure 3.
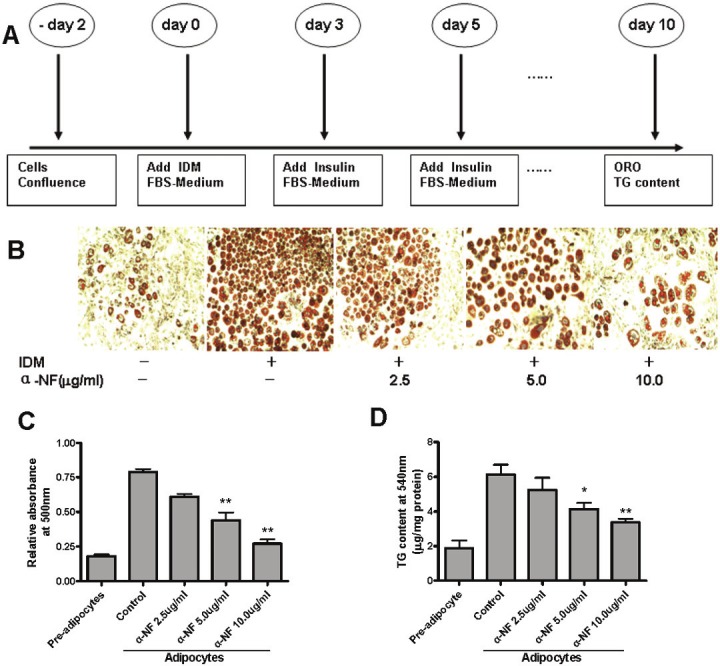
α-NF suppresses 3T3-L1 pre-adipocyte differentiation. Differentiation was initiated by adding 10mg/ml Isulin, 1mM Dexamethasone, and 0.5mM Isobutylmethylxanthine, (IDM) with presence of 10% FBS in postconfluenct 3T3-L1 pre-adipocytes. A. Schematic diagram indicates that α-NF was added on day0,3,5,7,9 during differentiation. B. Intracellular lipid was stained by Oil Red O (ORO) on day 10. C. Quantification of ORO dye by spectorphotometer at 500nm. D. Quantification of TG content by spectorphotometer at 540nm.
Cell viability by CCK8 assay
Cell viability was evaluated by conversion of Dojindo’s highly water-soluble tetrazolium salt WST-8 to a yellow-colored culture media soluble formazan dye. The amount of formazan dye generated by the activity of dehydrogenases in cells is directly proportional to the number of living cells. 3T3-L1 cells were trypsinized and seeded at 1 × 104 cells/well in 96 well plates. After 24 hr, various concentrations of α-NF were added, followed by incubation for another 24 or 48 hr. Then, 10 μL CCK8 (Dojindo, Japan) solution was added to each well. Plates were incubated for an additional 2 hr. The optical density of each well was measured using a microplate reader at a 450nm.
Oil red o staining
Cells in 6-well plates were washed twice with PBS and fixed for 15 min with 4% paraformaldehyde in PBS (pH 7.4). To prepare Oil Red O (ORO) working solution, ORO sock solution (0.5% in isopropanol, Sigma) was diluted with distilled water (3 parts of ORO stock + 2 parts of distilled water) and filtered through a 0.45mm filter. Fixed cells were then stained for 60 min with freshly prepared ORO working solution at room temperature and then washed with distilled water until the water was clear. The stained lipodroplets in the cells were visualized by light microscopy and photographed. ORO dye retained in the cell was quantified by elution into isopropanol and measured at 500nm with a spectophotometer.
Quantification of triglycerides
On day 10 after differentiation, 3T3-L1 cells were washed twice with PBS, scraped on ice in 100ml of saline solution, sonicated to homogenize and assayed for total triglyceride (GPO-Trinder, Sigma) according to the method of Norris AW [23].
Real-time RT PCR
Cells were harvested in 1 ml of Trizol reagent (Invitrogen) and RNA was extracted according to the manufacturer’s instructions. cDNA synthesis was performed with 1 μg of total RNA by MMLV reverse transcriptase (Promega). qPCR was performed in 96-well plates with the SYBR Green kit (ABI) in a Stepone Plus real-time PCR detection system, and the PCR baseline-subtracted data were computer generated as described by the manufacturer (ABI). Cyclophilin and β-actin were used as reference genes for normalization according to amplification efficiency of the target genes. The efficiency of PCR amplification for each gene was calculated by the standard curve m ethod ( E = 102 (1/log slope)). Gene expression was quantified by the comparative cycle threshold method. To calculate the relative mRNA abundance of target genes (C/EBPα C/EBPβ, PPARγ, FABP4, Glut4, and p38) in response to α-NF, samples were compared to untreated adipocytes after normalizaton to reference gene. Details of Primer sets are summarized in Table 1.
Table 1.
qRT-PCR primer sets for markers of adipogenesis
| Gene name | Forward primer | Reverse primer | Amplicon (bp) |
|---|---|---|---|
| PPARγ | ACC CCC TGC TCC AGG AGA T | TGC AAT CAA TAG AAG GAA CAC GTT | 84 |
| C/EBPα | CGC AAG AGC CGA GAT AAA GC | GCG GTC ATT GTC ACT GGT CA | 81 |
| C/EBPβ | CGG GGT TGT TGA TGT TTT TGG | CCG AAA CGG AAA AGG TTC TCA | 151 |
| FABP4 | ACG ACA GGA AGG TGA AGA GC | ACT CTT GTG GAA GTC ACG CC | 154 |
| GLUT4 | TAC ATA CCT GAC AGG GCA AGG | TTC GGG TTT AGC ACC CTT C | 131 |
| P38β | CCT TGA CCA AGA AGA AAT G | ACA GAC GAA CAG ACA GAC AC | 200 |
| P38β | ACC AAG AAG TCC TTA GCT TC | GTA GAG TTT CTC AAG GCA AG | 200 |
| JNK | AAT GGT TTG CCA CAA AAT CC | GAG TCA GCT GGG AAA AGC AC | 201 |
| ERK | GCT CAC CCT TAC CTG GAA CA | GGA CCA GAT CCA AAA GGA CA | 201 |
Immunoblot analysis
Right after treatments, 3T3-L1 cells were collected and lysed in ice-cold RIPA lysis buffer with protease inhibitor cocktail (Sigma) and PMSF (Sigma). Supernatants were collected by centrifugation at 12,000xg for 20 minutes and stored in -80°C until use. Protein concentration was measured by Bradford Assay. A total of 40-60 mg protein were separated with 10% or 12% SDS-PAGE gel electrophoresis, transferred onto a PVDF membrane (Millipore), blocked with 5% nonfat dry milk in TBST for 1 hour at room temperature, and incubated with primary antibodies at 4°C overnight. After incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature, immunoreactive proteins were detected with chemiluminescent ECL assay. Antibodies specific for PPARγ (1:500), phospho-p38 MAPK (Thr180/Tyr182) (1:500), p38 MAPK (1: 500) were from Cell Signaling Technology, and antibodies specific for C/EBPα (1:500), C/EBPβ (1:500) were from Santa Cruz. Antibody β-actin (1:5000) was from Sigma, and all the secondary antibodies (1:5000) were from Promega.
Statistics
Results are expressed as the mean ± SEM unless otherwise mentioned. PRISM was used for statistical analysis. All statistical data were the average of three independent experiments. Two-tailed Student t test was performed to obtain P values. Statistical significance was established at * P<0.05.
Results
Effect of α-NF on 3T3-L1 pre-adipocytes viability
To determine whether α-NF treatment affects cell viability in 3T3-L1 preadipocytes, we performed a CCK8 assay. The results revealed that only subtle changes in preadipocytes viability at 1.25, 2.5, 5.0, 10mg/ml of α-NF compared to control, respectively. However, we found that 20mg/ml α-NF significantly decreased 3T3-L1 cell viability compared with untreated control cells (Figure 2).
Figure 2.
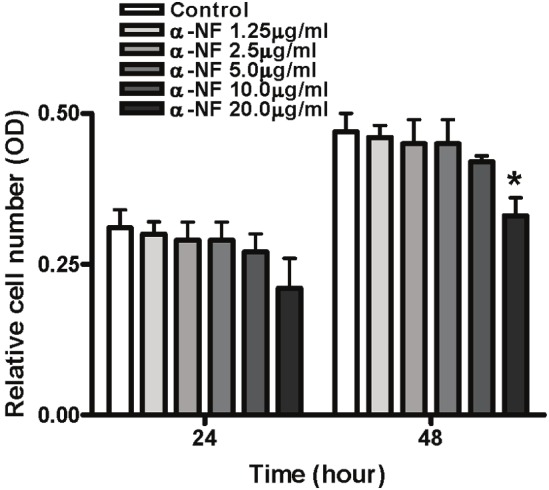
Effect of α-NF on cell viability. 3T3-L1 pre-adipocytes were treated for 24 and 48 hr with various concentrations of α-NF(mg/ml). Cell viability was determined by CCK8 assay kit. Values are presented as OD at 450nm (mean ± SEM, n=3).
α-NF inhibits adipogenic differentiation in 3T3-L1 pre-adipocyte
Accumulation of intracellular lipid droplets is a phenotypic character of committed adipocytes which can be detected by Oil Red O (ORO). To explore the effect of α-NF on pre-adipocyte differentiation, we first assessed lipid deposition in hormone cocktail treated 3T3-L1 cells by ORO. Two days after confluence, 3T3-L1 preadipocytes were treated with hormone cocktail---Insulin (I), dexamethasone (D), and isobutyl methyl xanthine (M), in the presence of FBS. Accumulated lipid droplets could be observed under high resolution microscopy as early as 12 hr after induction; however, a more pronounced phenotype was found after 6 days. 3T3-L1 cells treated with different levels of α-NF in conjunction with hormone cocktail during adipogenesis suppressed the intensity of ORO staining (Figure 3B), which indicates less lipdroplets deposition with α-NF administration. This effect was dose dependent and could be obtained as low as 1.25ug/ml of α-NF. To further evaluate the extent of differentiatial suppression by α-NF, we assessed cellular triglyceride (TG) content via ORO dye and TG concentration (Figure 3C and D). Results from both experiments were correlated with ORO intensity. Of note, α-NF is better able to to inhibit 3T3-L1 pre-adipocyte differentitation than to reverse it. Adding α-NF 48 hr after hormone cocktail administration could not effectively inhibit adipocyte differentiation.
α-NF inhibits mid- and late-phase adipogenic genes expression during 3T3-L1 differentiation
To investigate the mechanism involved in α-NF mediated inhibition of adipocyte differentiation, q-RT-PCR and western blot were performed to measure the effects of α-NF on gene expression during adipocyte differentiation. To this end, we assessed mRNA expression of the key genes C/ EBPα, C/EBPβ, PPARγ, Glut4, and FABP4, which are involved in early-, mid-, and late-phase differentiation of adipocytes, respectively. As expected (Figure 4A, upper and middle panels), α-NF suppressed the mRNA expression of C/EBPα, PPARγ, Glut4, and FABP4, which are involved mid- and late stage of differentiation. However, the expression of C/EBPβ, which is known to modulate mitotic clonal expansion (MCE) in early stage of differentiation, is remained unchanged. Protein expression of these adipogenic markers, C/EBPα, PPARγ and Glut4, correlated well with mRNA levels (Figure 4B, lower panel).
Figure 4.
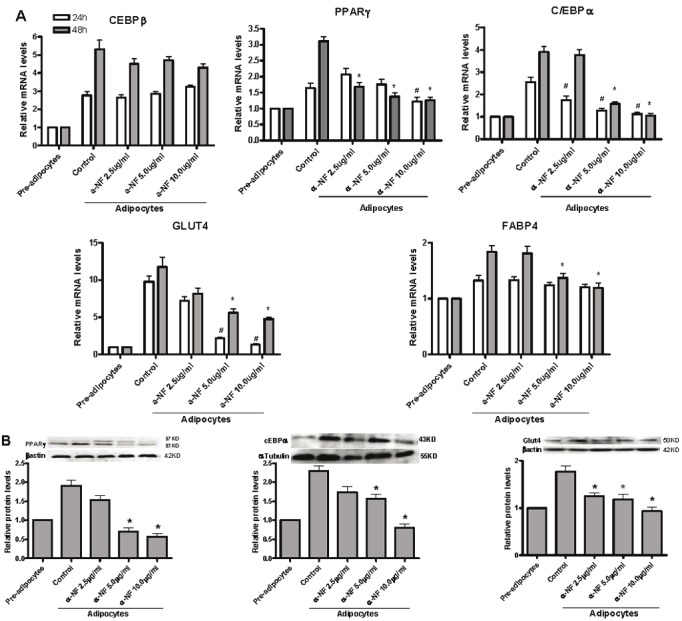
α-NF inhibits the expression of adipogenic markers duiring 3T3-L1 pre-adipocyte differentiation. Two-day postconfluent 3T3-L1 preadipocytes (day 0) were treated with the indicated concentrations of α-NF for 24 and 48 hr. Cells were collected for genes expression in both mRNA and protein levels. A. qRT-PCR was performed for the mRNA expressions of adipogenic markers. B. The protein expression of adipogenic genes were evaluated by western blot. All the experiments are triplicated, * P< 0.05 vs. 48 hr controls. # P< 0.05 vs. 24 hr controls.
α-NF inhibits the expression of adipogenic markers by modulating the p38MAPK pathway
Intracellular MAPK signalings plays a vital role in the regulation of cell proliferation and differentiation [24]. There are three groups of kinases (ERKs, JNKs and p38MAPK) in the MAPK Family. To investigate the signaling pathway which involved in α-NF inhibition of 3T3-L1 differentiation, we first measured the mRNA expression of several molecules which involved in MAPK signaling and found that α-NF decreased the expression of both p38 α and β isoforms significantly (Figure 5A and B). Further results indicated that α-NF also substantially suppressed phosphorylation of p38 (Figure 5D) although total protein levels were only slightly decreased. Phosporylation of p38 regulates diverse biological processes [25-27], many of which could contribute to the role of p38 in driving cell differentiation. Indeed, the p38 pathway has been shown to regulate adipocyte differentiation by controlling PPARγ expression [28]. To further explore the role of p38 pathway involving in α-NF modulation of PPARγ expression, we measued PPARγ expression in response to the p38 antagonist, SB203580. We observed synergistic effect of α-NF on reduction of PPARγ and Glut4 expression with SB203580 during 3T3-L1 differentiation (Figure 6A and B). These results suggested that α-NF inhibits 3T3-L1 pre-adipocytes differentiation through PPARγ via p38MAPK signaling.
Figure 5.
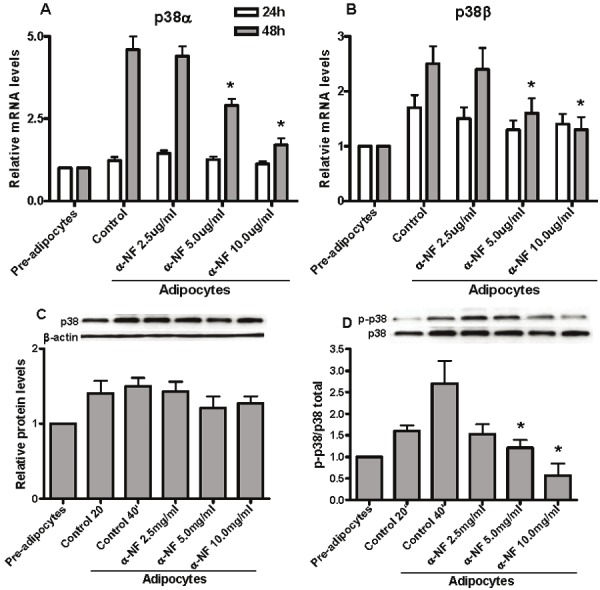
α-NF inhibits 3T3-L1 pre-adipocyte differentiation involving p38 signaling pathway. A and B. The mRNA expressions of both p38α and p38β isoforms were down-regulated by α-NF during adipogenesis; C. α-NF slightly suppressed total p38 protein expression but not reached significantly level; D. After 20 and 40 minutes of hormone cocktail administration, phosphorylation of p-p38(thr180/tyr182) was significantly induced and was substainly inhibited by α-NF.
Figure 6.
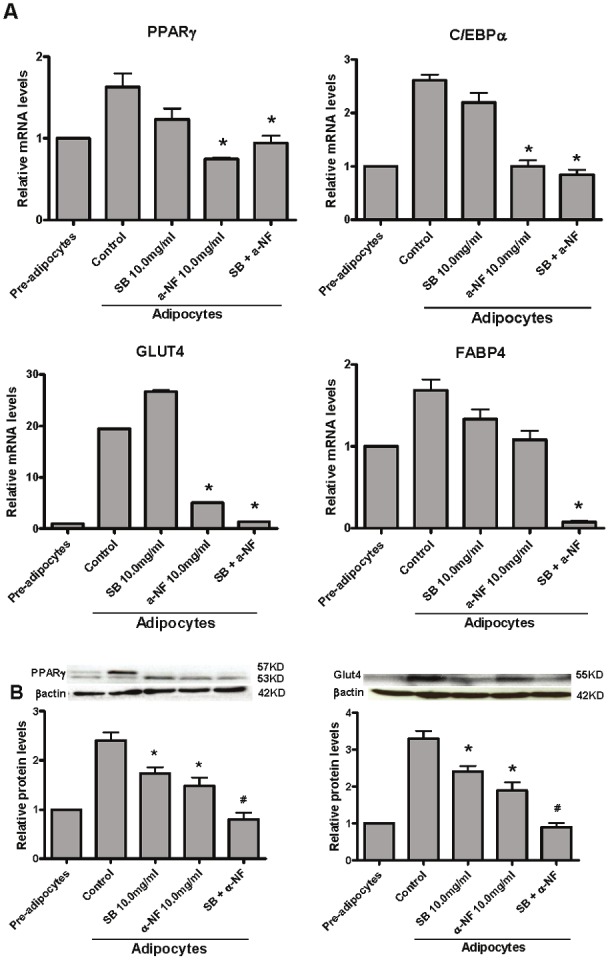
α-NF exerted synergistic suppressive effects with SB203580, a selective p38 inhibitor, on adipogenicgenes protein expression during 3T3-L1 cells differentiation. A. SB203580, a p38 selective inhibitor, has slightlynegative effects on mRNA expression of mid-phase differentiation genes, PPARγ, C/EBPα, but exhibited synergisticlyeffects with α-NF on late phase differentiation marker—FABP4 and Glut4. B. α-NF exerted substainly inhibitory effectson PPARγ and Glut4 protein expression and synergisticaly with SB203580.
Discussion
Obesity is a consequence of an imbalance between energy intake and energy expenditure, which results in an excessive fat mass deposition. Adipose tissue expansion involves both increased adipocyte cell number (hyperplasia) and increased adipocyte cell size (hypertrophy) [29]. Therefore, treatments that regulate either the number or size of adipocytes or both may provide therapeutic options for treating obesity.
Due to the high costs and potential harmful side effects of pharmaceuticals, more and more people today seek the plant-based medicinal approaches to manage their body weights than ever before [30,31]. Flavonoids are prevalent in dietary vegetables, fruits and grains [7,8]. The physiological concentrations of dietary flavonoids in human have been reported in the range about 0.5-4.4uM in plasma. α-NF is a synthetic flavone and has been intensively studied as an aromatase antagonist in various systems. However, its role in lipid metabolism remains largely unknown. In this study, we evaluated the effects of α-NF on adipogenesis in mouse 3T3-L1 pre-adipocytes.
Our study demonstrated for the first time that α-NF inhibits 3T3-L1 pre-adipocyte differentiation by down-regulating the mid- and late-phase of adipogenic genes expression. The genes involved include C/ EBPα, PPARγ, Glut4, and FABP4, but not C/EBPβ (Figure 4), an early stage of adipogenic marker which is responsible for MCE. Our results also demonstrate that the viability of differentiating 3T3-L1 cells is not significantly influenced by α-NF treatment (Figure 2). Therefore, the possibility that α-NF exerts its inhibition on 3T3-L1 differentiation by inducing cellular cytotoxicity can be ruled out. We focused our study on the effects of α-NF on pre-adipocyte differentiation.
At the molecular level, differentiation of preadipocyte into adipocyte is regulated by a complex network of transcription factors that involves the sequential activation of hundreds of genes responsible for adipocyte phenotype [32,33]. An understanding of the molecular and cellular biology of the adipocytes will be required to fully understand the causes and consequence of obesity, and to develop therapeutic strategy for prevention and treatment. Generally, the initial event will be the rapid induction of C/EBPβ when cells undergo the differentiation process in response to adipogenic signals [32,33]. And sequentially, C/EBPβ is responsible for the induction of PPARγ, a master regulator of adipogenesis. PPARγ and C/ EBPα induce the expression of multiple adipocyte specific genes, such as FABP4, Perilipin, and Glut4, which are important for adipocyte phenotypic development. Our findings confirm that α-NF suppresses cellular differentiation of 3T3-L1 cells not only at the mRNA and protein levels of PPARγ and C/EBPα, but also by decreasing expression of several target genes of PPARγ including FABP4 and GLUT4. However, our results demonstrate that exposing 3T3-L1 pre-adipocytes to α-NF during adipogenesis decreases both mRNA and protein expressions of C/EBPα, PPARγ, but not C/EBPβ (Figure 4). Therefore,α-NF induced suppression of C/EBPα and PPARγ induced by adipogenic stimulation occurs independently of C/EBPβ expression.
Intracellular MAPK signalings play a vital role in the regulation of cell proliferation and differentiation. Numerous studies have verified that the ERK pathway regulates multiple steps of adipogenesis from stem cells to mature adipocyte [34], but the JNK pathway is not directly implicated in adipocyte differentiation. The role of p38MAPK in adipocyte differentiation remains controversial. Takenouchi et al demonstrated that p38 activation is required for adipocyte differentiation in 3T3-L1 [35] and inhibition of p38 early in 3T3-L1 differentiation decreased adipocyte formation [36]. However, Aouadi et al provided evidence that suppressing p38 pathway increases adipogenesis [37]. In the present study, p38 phosphorylation was observed during the early phase of differentiation upon adipogenic stimulation. Treatment with α-NF inhibited p38 phosphorylation effectively along with inhibition of PPARγ and C/EBPα expression, which demonstrated that α-NF suppressed adipocyte differentiation partially by suppressing the phosphorylation of p38 (Figure 5). It was also found that SB203580, a specific inhibitor of p38, inhibited PPARγ expression and also exhibited synergistic effects on PPARγ expression together with α-NF, which indicated that the effect of α-NF on 3T3-L1 differentiation, at least, was partially due to the inhibition of p38 signaling.
In summary, our study suggests a new role of α-NF in inhibition of adipogenesis through targeting the mid- and late-stage biochemical and cellular events of cell differentiation including PPARγ, C/EBPα and GLUT4, FABP4 expression. Although α-NF was unable to suppress C/EBPβ expression in the early stage of differentiation, we can not rule out the possibility that α-NF has a potential effect on early adipogenesis. Because the anti-adipogenic effects of α-NF were observed only when α-NF was added to the 3T3-L1 cell on day 0, but not day 3 after differentiation was initiated, our data suggests a complicated mechanism that requires further study.
Taken together, our findings provide the first evidence that α-NF is capable of suppressing 3T3-L1 adipogenesis and also suggests a possible mechanism involving p38MAPK signaling, which may open a novel avenue for the development of new treatments for obesity.
Acknowledgement
The authors would like to thank Dr. Cara J Westmark for help with proofreading and critical suggestions. This work was supported by National Science Foundation of China (30972463) and financial support for China Scholar Council.
References
- 1.Barton M. Childhood obesity: a life-long health risk. Acta Pharmacol Sin. 2012;33:189–193. doi: 10.1038/aps.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flie JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 3.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBP beta and/or C/EBP delta gene. Embo J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasudeva N, Yadav N, Sharma SK. Natural Products: A safest Approach for Obesity. Chin J of Integr Med. 2012;18:473–480. doi: 10.1007/s11655-012-1120-0. [DOI] [PubMed] [Google Scholar]
- 7.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Ren WY, Qiao ZH, Wang HW, Zhu L, Zhang L. Flavonoids: Promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 9.Hollman PCH, Katan MB. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 10.Riceevans CA, Miller NJ, Bolwell GP, Bramley PM, Pridham JB. Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radical Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 11.Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH, Kim JW, Song H, Kim SH. Anti-inflammatory activity of fisetin in human mast cells (HMC-1) Pharmacol Res. 2007;55:31–37. doi: 10.1016/j.phrs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Mojzis J, Hviscova K, Germanova D, Bukovicova D, Mirossay L. Protective effect of quercetin on ischemia/reperfusion-induced gastric mucosal injury in rats. Physiol Res. 2001;50:501–506. [PubMed] [Google Scholar]
- 13.Rimbach G, Boesch-Saadatmandi C, Frank J, Fuchs D, Wenzel U, Daniel H, Hall WL, Weinberg PD. Dietary isoflavones in the prevention of cardiovascular disease - A molecular perspective. Food Chem Toxicol. 2008;46:1308–1319. doi: 10.1016/j.fct.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Androutsopoulos VP, Papakyriakou A, Vourloumis D, Tsatsakis AM, Spandidos DA. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol Ther. 2010;126:9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Craggs L, Kalaria RN. Revisiting dietary antioxidants, neurodegeneration and dementia. Neuroreport. 2011;22:1–3. doi: 10.1097/WNR.0b013e328342741c. [DOI] [PubMed] [Google Scholar]
- 16.Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J. 2010;31:2583–U32. doi: 10.1093/eurheartj/ehq332. [DOI] [PubMed] [Google Scholar]
- 17.Chen SF, Li ZL, Li WX, Shan ZM, Zhu W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can J Physiol Pharmacol. 2011;9:793–799. doi: 10.1139/y11-077. [DOI] [PubMed] [Google Scholar]
- 18.Meydani M, Hasan ST. Dietary Polyphenols and Obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overman A, Chuang CC, Mcintosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond) 2011;5:1165–1172. doi: 10.1038/ijo.2010.272. [DOI] [PubMed] [Google Scholar]
- 20.Cheon H, Woo YS, Lee JY, Kim HS, Kim HJ, Cho S, Won NH, Sohn J. Signaling pathway for 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med. 2007;39:524–34. doi: 10.1038/emm.2007.58. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YW, Li CH, Lee CC, Kang JJ. Alpha-naphthoflavone induces vasorelaxation through the induction of extracellular calcium influx and NO formation in endothelium. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:377–385. doi: 10.1007/s00210-003-0820-6. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao G, Chang CY, Shen MY, Chou DS, Tzeng SH, Chen TF, Sheu JR. alpha-Naphthoflavone, a potent antiplatelet flavonoid, is mediated through inhibition of phospolipase C activity and stimulation of cyclic GMP formation (vol 53, pg 5179, 2005) J Agr Food Chem. 2005;53:6954–6954. doi: 10.1021/jf0500738. [DOI] [PubMed] [Google Scholar]
- 23.Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPARgamma deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–18. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–6. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Turesson I, Book M, Gerwins P, Claesson-Welsh L. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J Cell Biol. 2002;156:149–160. doi: 10.1083/jcb.200103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc Natl Acad Sci U S A. 2004;101:147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuge L, Hide I, Kumagai T, Kumei Y, Takeda S, Kanno M, Sugiyama M, Kataoka K. Cell differentiation and p38(MAPK) cascade are inhibited in human osteoblasts cultured in a three-dimensional clinostat. In Vitro Cell Dev Biol Anim. 2003;39:89–97. doi: 10.1290/1543-706x(2003)039<0089:cdapca>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Wang JJ, Li JM, Park K, Qian XX, Ma JX, Zhang SX. Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes. Am J Physiol Endocrinol Metab. 2009;297:E1378–E1387. doi: 10.1152/ajpendo.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couillard C, Mauriège P, Imbeault P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord. 2000;24:782–788. doi: 10.1038/sj.ijo.0801227. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao G, Chang CY, Shen MY, Chou DS, Tzeng SH, Chen TF, Sheu JR. alpha-naphthoflavone, a potent antiplatelet flavonoid, is mediated through inhibition of phospholipase C activity and stimulation of cyclic GMP formation. J Agr Food Chem. 2005;53:5179–5186. doi: 10.1021/jf0500738. [DOI] [PubMed] [Google Scholar]
- 31.Rondanelli M, Klersy C, Iadarola P, Monteferrario F, Opizzi A. Satiety and amino-acid profile in overweight women after a new treatment using a natural plant extract sublingual spray formulation. Int J Obes (Lond) 2009;33:1174–1182. doi: 10.1038/ijo.2009.155. [DOI] [PubMed] [Google Scholar]
- 32.Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010;31:384–90. doi: 10.1016/j.it.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leff T, Granneman JG. Adipose Tissue in Health and Disease Preface. Adipose Tissue in Health and Disease. 2010:Xix–Xx. [Google Scholar]
- 34.Bost F, Aouadi M, Caron L, Binetruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Takenouchi T, Takayama Y, Takezawa T. Co-treatment with dexamethasone and octanoate induces adipogenesis in 3T3-L1 cells. Cell Biol Int. 2004;28:209–16. doi: 10.1016/j.cellbi.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 37.Aouadi M, Laurent K, Prot M, Marchand-Brustel YL, Binetruy B, Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 2006;55:281–289. doi: 10.2337/diabetes.55.02.06.db05-0963. [DOI] [PubMed] [Google Scholar]


