Abstract
Still today, the status of the cervical lymph nodes is the most important prognostic factor for head and neck cancer. So the individual treatment concept of the lymphatic drainage depends on the treatment of the primary tumor as well as on the presence or absence of suspect lymph nodes in the imaging diagnosis. Neck dissection may have either a therapeutic objective or a diagnostic one. The selective neck dissection is currently the method of choice for the treatment of patients with advanced head and neck cancers and clinical N0 neck. For oncologic reasons, this procedure is generally recommended with acceptable functional and aesthetic results, especially under the aspect of the mentioned staging procedure. In this review article, current aspects on pre- and posttherapeutic staging of the cervical lymph nodes are described and the indication and the necessary extent of neck dissection for head and neck cancer is discussed. Additionally the critical question is discussed if the lymph node metastasis bears an intrinsic risk of metastatic development and thus its removal in a most possible early stage plays an important role.
Keywords: head and neck cancer, lymphogenic metastasis, lymph node metastasis, neck dissection
1 Introduction
The different questions on lymphogenic metastasis of head and neck cancers were summarized in a previous review published by Werner in 1997 at the occasion of the annual meeting of the German Society of Otolaryngology, Head & Neck Surgery, in Nürnberg [1]. Since then several current developments took place also in this field so that the current president of the society, Prof. N. Stasche, decided to work up this topic again and to update the information. In the following, the single details of the topic are defined before starting the discussion of perspectives.
Every year, between 10,000 and 15,000 people are newly diagnosed with cansers of the upper aerodigestive tract in Germany. Among the therapeutic options of those tumors, which are in more than 90% squamous cell carcinomas, radio- and chemotherapy and latterly so-called biotherapy beside surgical measures are mentioned. By those treatments, it is possible to treat the primary tumor in the majority of the affected patients. However, cancers of the mentioned region are characterized by very poor prognosis with a 5-year survival rate of about 50–60%. The discrepancy between a successful treatment of the primary tumor on the one hand and the poor long-term prognosis on the other hand, results from an often early and advanced lymphogenic metastasis, the distant metastases possibly occurring in the further course of the disease, and the development of second primaries. While cancers in an early stage (stage I and II) have a 5-year survival rate of about 80% in total, the 5-year survival of cancers of stage III, IVA, and IVB (locoregional metastasis) amounts to about 50% and in patients staged IVC (distant metastasis) to about 25% [2].
The lymphogenic metastasis represents the most important independent prognostic factor for squamous cell carcinomas of the upper aerodigestive tract. Especially the presence of lymph node metastases is associated with a dramatic reduction of the survival rate. Different pathologic characteristics of lymph node metastases also have a prognostic relevance. Those are the size, the number, and the location of lymph node metastases. Lymph node metastases of the levels IV and V are generally associated with a poorer prognosis [3], [4]. In this context, the presence of extracapsular spread of the cervical metastasis represents the most important prognostic factor and is associated with a significantly higher locoregional recurrence rate and with distant metastases [5]. Thus, the consideration of the locoregional lymphatic drainage and the histopathologic examination of the cervical lymph nodes plays a major role in the treatment concept of head and neck cancer beside the treatment of the primary tumor.
During history, the neck dissection underwent considerable changes since its first description documented in 1888 by Jawdynski. The radical neck dissection that was later described by Crile in 1905 [6] had been the standard therapy of the cervical lymph nodes for many decades. Since the middle of the last century, concepts for preservation of non-lymphatic structures are discussed. A better understanding of the lymphatic drainage of the head and neck region as well as technical progress in radiotherapy allow more individual and gentle types of neck dissection with local control rates comparable to radical therapeutic options.
2 Nomenclature of the cervical lymph nodes and classification of the neck dissection types
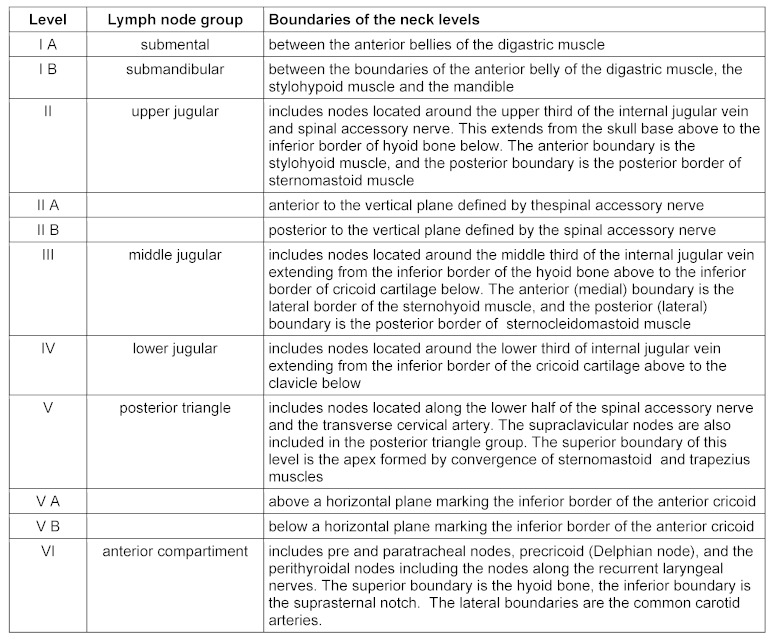
The lymphatic drainage of the upper aerodigestive tract is effectuated via a total of about 300 lymph nodes that are linked by a complex lymphatic system. It is confirmed that apart from some variations, the lymph fluid is drained along relatively predictable and constant lymph vessels into certain lymph node groups. This information is the basis for the division of the lymph nodes in the head and neck area. Further there is the classification of the cervical lymph nodes from 2000, issued by the committee of classification of neck dissection of the American Head and Neck Society [7]. This classification aimed at simplifying the nomenclature of selective neck dissection as well as improving the assessment of lymph node regions by means of imaging procedures (Table 1 (Tab. 1), Figure 1 (Fig. 1)).
Table 1. Topography and nomenclature according to Robbins [7].
Figure 1. Topography of the cervical lymph node regions with description of the neck muscles relevant for the classification. In the preauricular region the parotid gland and in level I the submandibular gland is revealed. The accessory nerve delineates the limit between the levels IIA and B and is part of the level V.
George W. Crile who published his experience with 105 operated patients in 1905 is considered as the one who first described the today called radical neck dissection [6]. This procedure that includes the dissection of the ipsilateral levels I–V and at the same time the resection of the internal jugular vein, the sternocleidomastoid muscle, and the accessory nerve, was applied mostly in the first part of the 20th century. However, as this technique is associated with a significant postoperative morbidity and life-threatening complications for the patients, function preserving procedures were propagated since the beginning of the 1960s. The aim of the this technique, first called functional neck dissection was to preserve a maximum of the function by sparing at least on extra-lymphatic structure that would have been resected during radical neck dissection, without worsening the prognosis of the patient. Opponents of the functional neck dissection stated that this procedure would not be radical enough in cases of metastasized tumors, on the other hand patients with clinical N0 neck would undergo overtreatment.
The basis for the performance of the type of surgery that is called today selective neck dissection is the identification of lymph node regions that are mostly affected by metastases, in dependence of the location of the primary tumor. With the selective dissection of single lymph node regions, the postoperative morbidity of the patients was intended to be further reduced and the functional as well as aesthetic results were intended to be improved in comparison to modified radical neck dissection. Supporters of this technique also state that the preservation of non-affected, immunologically intact lymph nodes could avoid further tumor spread. Beside the application as therapeutic procedure, selective neck dissection serves for tumor staging and is performed electively in cases of clinical N0 neck as well as after primary radiotherapy.
Since the introduction of neck dissection, there are various types and nomenclatures which are sometimes also based on different nomenclatures of the cervical lymph node regions. A new proposal for classification the neck dissection types originated from the Japan neck dissection Study Group (JNSG). Their aim was to standardize different aspects of non-radical neck dissection [8]. Among those are the extent of lymph node dissection and the resection of non-lymphatic structures. This system divides the cervical lymph nodes into three regions which are then subdivided into different sub-regions. According to the extent of lymph node dissection, the difference is made between total and selective neck dissection. The description of the resected structures is performed by means of a special combination of letters. This should allow a precise and simple description of the intervention. The disadvantage of such a classification system, however, is that it is based on a lymph node classification that differs from the terminology acknowledged up to now. A modification of this classification system was presented recently [9]. It must be observed if the advantage and the possible introduction of this new staging system may be realized.
The currently acknowledged and mostly used classification of the different neck dissection types refers to the classification system established by Robbins that was updated lastly in 2008 [10]. In this context, the limit between level IB and IIA formed by the stylohyoid muscle was defined as a vertical surface dorsally to the submandibular gland for better radiologic mapping. Similarly, also the limit between the levels III and IV to the level VI formed by the sternohyoid muscle is described in the axial plain as medial side of the common carotid artery. This classification represents a simple and well-established division of the cervical lymph nodes for head and neck surgeons, oncologists, and radiologists and is supposed to be used internationally [11]. It can however be assumed that also in the future further updates and changes of this nomenclature will be published.
3 Bases of lymphogenic metastasis
The lymphangiogenesis is a significant step of lymphogenic metastasis of squamous cell carcinomas of the upper aerodigestive tract. The exact molecular mechanism of this multistep process is not known in detail. Different lymphangiogenetic cytokines of squamous cell carcinoma, especially VEGF-C and -D that are mostly expressed in the area of the tumor invasion front stimulate the genesis of lymphatic vessels in different ways [12]. It could be confirmed that the increased expression of the last-mentioned cytokines is associated with a high density of lymph vessels and lymphogenic metastasis [13].
In squamous cell carcinomas where lymphogenic metastasis had occurred, a significantly higher number of intra- and peritumoral lymphatic vessels could be detected while those vessels were more numerous and with greater lumens in the peritumoral area thank directly located within the tumor tissue [14]. It could be shown that lymph vessel endothelia induced by cancer cells of the tongue had a high proliferation; they are able to form capillary structures [15]. Some evaluations could reveal that the density of the lymph vessels in the tumor area are associated with a high metastatic rate and a poor prognosis [16], [17]. So the tumor induced lymphangiogenesis is supposed to be an important step in the lymphogenic metastasis.
The lymphatic endothelium does not only represent the limit area of the lymphatic vessels but also an interactive surface for tumor cells. Also the lymph vessel endothelia and the tumor cells show a high degree of phenotypic changes. Different receptors of the lymph vessel endothelia, such as CLEVER-1 (common lymphatic endothelial and vascular endothelial receptor-1), mannose receptor, LYVE-1 (lymphatic vascular endothelial protein-1), play an important role in the tumor cell adhesion process at the lymphatic endothelium and the migration of the cells into the lymphatic system. Recently, the expression of the chemokines CXCL1, CXCL5, CXCL6, CCL2, CCL7, CCL17, and CCL20 could be found in tongue cancer in induced lymphatic endothelia. Those chemokines are expected to facilitate the migration of tumor cells [18]. It could be revealed that an inhibition of the lymphangiogenetic characteristics of the lymphatic endothelia leads to a significant reduction of the metastatic rate. The interaction of the tumor cells with tumor induced lymphatic endothelia that secrete different chemokines and form receptors at their surface is the decisive step of migration of the tumor cells into the lymphatic vessels [19].
In another step tumor cells, that change their phenotype during the whole process, disconnect from their united structure and follow the chemotactic gradients in the tissue in the direction of the lymphatic vessels. Disconnected tumor cells secrete proteolytic enzymes such as MMP-9 (metallo-proteinase 9) for local tumor invasion and express specific adhesion molecules [20]. After binding the tumor cells with lymphatic endothelia and their entrance into the vascular lumen, they are draind via an afferent lymph vessel into the sentinel lymph node.
The tumor cells drained into the lymph nodes first form small foci of 2–3 mm as micrometastases in the marginal sinus (Figure 2 (Fig. 2)). Those micrometastases are located far from the hilus and can only be identified by immuno-histochemistry or molecular genetics. With the growth of the metastasis the internal architecture and the blood supply of the lymph node change because of the formation of new blood vessels around the metastatic area. At the same time, a growth of the affected lymph node occurs. Those changes can partly be pursued by ultrasound examination and Doppler sonography. When the whole lymph node is affected by tumor cells and the lymph node increases to a size of up to 20 mm, generally the lymph node hilus can no longer be identified. At this size, however, necrotic areas can be revealed by ultrasound [21] (Figure 3 (Fig. 3)). At this time, the lymph node capsula may still be respected by the tumor cells. A further growth, however, shows infiltration of the capsula and the extracapsular detection of tumor cells. Those changes are described schematically in Figure 4 (Fig. 4). An extracapsular growth occurs in about 70% of the lymph nodes larger than 3 cm while lymph nodes smaller than 3 cm show an extracapsular spread in about 40% of the cases [22]. In a prospective examination 96 neck dissection specimens of 63 patients with clinical N0 neck who had undergone selective neck dissection were examined histologically [23]. Occult lymph node metastases were found in 19 patients (30.2%). Among those patients, extracapsular growth was detected in 12 cases. Those are 19% of the patients with clinical N0 neck and 63.2% of the patients with occult metastases. The exact mechanism of the extracapsular spread in particular of smaller lymph nodes is still unclear.
Figure 2. Histological description of a micrometastasis of squamous cell carcinomas in the marginal sinus of a cervical lymph node, HE 200x. K = lymph node capsula. S = marginal sinus.
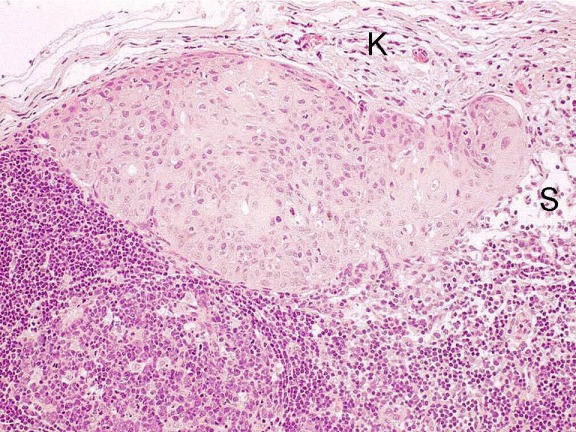
Figure 3. Sonographic description of a cervical lymph node metastasis with maximal diameter of 18 mm and hypoechoic central necrosis.
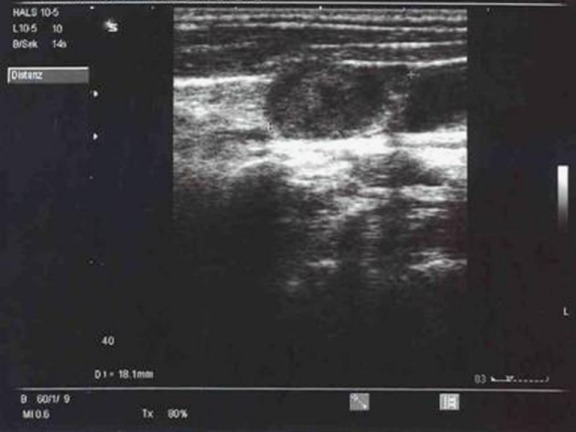
Figure 4. Schematic description of more-step development of cervical lymph node metastasis.
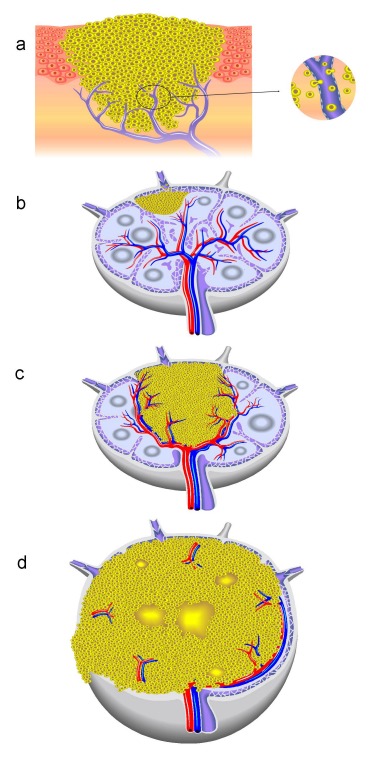
a) Tumor-induced lymphangiogenesis in the intra- and peritumoral and invasion of the tumor cells in the lymphatic system, b) Development of micrometastasis in the sinus area, c) Enlarged lymph node metastases and the related change of the angio-architecture of the affected lymph node by curved displacement of the vessels near the metastasis and genesis of an avascular zone in the metastatic area, d) Extracapsular spread with description of aberrant and subcapsular vessels as well as necrotic areas in the metastatic region.
4 Diagnosis of cervical lymph node metastases
The secure diagnosis of cervical lymph node metastases in head and neck cancer still represents a clinical problem. This is mainly due to the anatomic particularities of the lymph nodes in the head and neck area. In this area the close neighbourhood of the primary tumor and the draining lymph nodes are characteristic as well as the dense lymphatic system and the high number of cervico-facial lymph nodes. Further there is the problem of micrometastases and the fact that a high number of cervical metastases have a size of less than one centimeter.
The sensitivity of the exclusive inspection and palpation for detection of the cervical lymph nodes amounts to about 60–70% while the according values for MRT and CT scan vary between 65 and 88% in the literature [24], [25]. The most significant procedure for detection of lymph node metastases is currently B mode sonography, completed by Doppler sonography in combination with sonographically guided aspiration cytology. According to the results of a comparative meta-analysis it disposes of a sensitivity of 80% and a specificity of 98% and is thus superior to CT scan and to MRI [26]. This examination procedure is widely available and economic and can be repeated without important timely or organisational efforts. Further, sonography allows detailed examination of the intranodal architecture while the diagnosis of the lymph node metastases by means of computed tomography is mainly based on measures of the nodal size. An important limitation of sonography is especially the missing identification of the deep cervical soft parts such as the retropharyngeal lymph nodes. However, as in 15–25% of all occult metastases micrometastases may be present none of the mentioned diagnostic procedures achieves a higher sensitivity than about 80% with simultaneous reduction of the specificity. In this context, it must also be mentioned that the significance of the imaging diagnosis depends largely on the experience of the examining physician.
The significance of PET for the diagnosis of cervical lymph node metastases is discussed controversially. The investigations performed up to now do not support the routinely clinical application of PET for pretherapeutic evaluation of the lymph node status in patients with head and neck cancer, among those also patients with clinical N0 neck [27]. A meta-analysis showed that PET examination may achieve a sensitivity of up to 80% and a specificity of 86%, however, only half of the patients with clinical N0 neck and histologically identified metastasis could be identified by means of PET [28].
Even for patients with previously irradiated necks, the lymph node status is significant. The indication for possible planning of neck dissection after radiochemotherapy depends on the detection of intranodal residual tumor tissue. Further it remains unclear how the response of lymph node metastases after irradiation can be verified securely. Clinical and radiological examinations are not able to reveals with certainty the pathologic changes in the area of the irradiated lymph nodes. Although the significance of the sonographically guided fine needle aspiration cytology for determination of the cervical response rate has not been analysed intensively up to now, a sensitivity of about 80% is reported [29].
Based on the combination of anatomic and metabolic data, PET-CT has a higher sensitivity and specificity than CT scan and MRI for diagnosis of nodal residual tissue after radiochemotherapy [30]. Because of the comparably high negative predictive value of PET-CT this examination is recommended for the detection of residual tumors after radiochemotherapy. Retrospective evaluations could show a correlation of PET-CT examination with the pathological results of neck dissection specimens [31], [32]. Prospectively the significance of PET-CT for determination of the nodal residual tissue after radiochemotherapy was analysed in 30 patients by histological examinations or follow-up examinations [33]. The positive predictive value amounted to 100% and the negative predictive value to 50%. In this evaluation the median follow-up time was 28 months and PET-CT was performed 3.2 months on the average after radiochemotherapy.
Despite the relatively limited availability, PET-CT is considered as cost-effectively for posttherapeutic evaluation of lymph node status [34]. For detection of residual tumors after radiochemotherapy by means of CT examination a complete response rate of 63% in N2 necks and about 40% in N3 necks could be revealed [35]. The application of PET-CT instead of CT scan may increase the diagnosed response rate to up to 30% and so neck dissection may be avoided in a relevant number of patients and cost may be saved.
To summarize the previous statements it can be said that the gold standard for the diagnosis of lymph node metastasis is still the histological examination of the neck dissection specimen. The histological examination of the neck dissection specimen especially for the detection of occult lymph node metastases, the number, location, and size of the metastases as well as the presence of possible perinodal growth is of highest clinical significance. The standard is the making of one histological slice per paraffin block and the light-microscopic examination of the slice after staining with hematoxylin/eosin [36]. However, it is confirmed that the routinely performed histological examination of the cervical lymph nodes is not able to detect all occult metastases. In about 10–15% of the patients with pN0 neck late cervical lymph node metastases may occur after neck dissection. In this context we indicate chapter 9. Further immunohistochemical examinations of the neck nodes on cytokeratines for occult subpathological metastases can be revealed in about 15% of the cases. Those would not have been identified in routinely performed histology [37]. The application of molecular genetic procedures such as quantitative RT-PCR for cytokeratines 5 or 14 may further improve the detection of subpathological metastases [38]. One problem in this context is the specificity of the positive results without morphological correlation and of course the practicability of this procedure.
Finally it must be mentioned that the prognostic relevance of the micrometastases in head and neck cancer is further not clarified although some few investigations report of a prognostic relevance [39].
5 Direction and extent of lymphogenic metastasis of squamous cell carcinomas of the upper aerodigestive tract
The different density and distribution pattern of the lymph vessels in the field of the primary tumor and different aspects of lymphogenic invasion of tumor cells are the morphologic basis of the preferred metastatic direction of squamous cell carcinomas of the head and neck depending on the location of the primary tumor. It must also be mentioned that the given directions of lymphatic drainage may show a great variability and must be understood as preferred drainage directions [40].
5.1 Nasal cavity and sinuses
Sinonasal squamous cell carcinomas represent about 60% of the head and neck cancer and have a metastatic rate of about 10%. This rate increases with an infiltration of the nasal floor, the columella, and the upper lip. Lymph nodes of the levels I, II and the parotid and retropharyngeal lymph nodes are the preferred metastatic areas. An infiltration of the floor of the maxillary sinus based on the extended lymphatic network in the area of the nasal floor and the hard palate in comparison to other paranasal sinuses. So the metastatic rate of T2 maxillary sinus cancers is higher than the one of T3 and T4 cancers [41].
5.2 Lips and oral cavity
The lymph fluid from the upper lip flows into the lymph nodes of level I, while the cancers of the upper lip may affect buccal and parotid lymph nodes. cancers of the lower lip that represent about 95% of the cancers of the lips show a relatively low metastatic tendency. The size of the cancer correlates with the metastatic rate. For T1 to T2 cancers it is given with values of up to 30% and for T3 to T4 cancers of more than 60%.
The lymph fluid from the anterior oral cavity flows mainly to the lymph nodes of level I while the posterior parts may also drain to the lymph nodes of level II. In contrast to this well-known metastatic behaviour, tongue cancers as most frequent cancers of the oral cavity (25–40%) may affect exclusively the lymph nodes of level IV in up to 10% of the cases. A retrospective evaluation of 277 cancers of the tongue showed metastases in level III and IV in 15.8% of the cases without lymph node affection of level I and II [42].
Part of the cancers of the anterior oral cavity may affect the so-called lingual lymph nodes of the floor of the mouth that are located superior to the mylohyoid muscle. As those lymph nodes are not removed in the context of neck dissection, they may be the origin of local recurrences. The significance of those lymph nodes is still not exactly known, however, if preoperative imaging leads to the suspect of affection of the mentioned lymph nodes the dissection of those nodes is indicated [43].
About 50% of the patients suffering from oral cancer reveal lymph node metastases while the cancers of the tongue show the highest metastatic rate due to the high lymphatic density and the muscular structure of the tongue. The incidence of occult lymph node metastases of T1 and T2 cancers of the oral cavity amounts to about 30–40%.
The metastatic tendency of oral cancer is directly related to the tumor size and especially to its infiltration depth. A multivariate analysis of the clinical and histopathological tumor characteristics of tongue cancers showed that only the tumor infiltration depth has a predictive value for cervical metastasis [44]. A cut-off value of 4 mm for the tumor infiltration depth was defined as predictor for cervical metastasis in a meta-analysis of metastasized cancers of the oral cavity [45]. The preoperative MRI examination is able to identify the tumor infiltration depth with a high correlation with the histological measurements while the values of MRI measurements are about 10% higher than the histologically revealed values of the infiltration depth [46], [47]. This difference is supposed to the due to the tissue shrinking in the context of tissue fixation.
5.3 Nasopharynx
Because radiotherapy is the primary treatment of nasopharyngeal cancers the metastatic pattern of nasopharyngeal cancers that were formerly evaluated by palpation of the neck are determined by tomographic examination. So it could be revealed that the retropharyngeal lymph nodes are the first station of metastasis of nasopharyngeal cancers and may be affected in 94% of the metastasized cancers of the nasopharynx [48]. In an evaluation of 786 patients with metastasized nasopharyngeal cancer, 13% of the patients with lymph node metastases in level II showed no retropharyngeal affection [49]. As only a low percentage of patients with metastasize nasopharyngeal cancers have lymph node metastases in other cervical regions without affection of lymph nodes of the retropharyngeal area and level II [50], [51], the lymphatic drainage of the nasopharynx is supposed to be into the retropharyngeal lymph nodes and lymph nodes of level II.
Nasopharyngeal cancers show a higher metastatic rate than other head and neck cancers and already at first diagnosis they may reveal a metastatic rate of up to 90%. In a retrospective evaluation of 4,768 patients with a nasopharyngeal cancer, enlarged cervical lymph nodes were clinically detected in 75% of the patients while it was the first symptom in 37% of the cases [52].
5.4 Oropharynx
The lymphatic drainage of the oropharyngeal region is effectuated mainly into the lymph nodes of the levels II, III, and retropharyngeal lymph nodes. The cancers in the area of the posterior and lateral wall metastasize preferably into the retropharyngeal lymph nodes and lymph nodes of level II.
The affection of the retropharyngeal lymph nodes of oropharyngeal cancers are reported with a frequency of about 15–50%. In an evaluation of 77 patients with oropharyngeal cancers the dissection of retropharyngeal lymph nodes was performed in addition to the tumor resection and neck dissection. Histologically, retropharyngeal lymph node metastases could be detected in 29% (11/38) of the patients suffering from cancers of the posterior and lateral wall [53]. In another investigation retropharyngeal lymph node metastases could be detected histopathologically in 26% of oropharyngeal cancers [54]. Radiologically retropharyngeal lymph node metastases could be identified in about 21% of oropharyngeal cancers by means of PET-CT [55]. Criticism on primary surgical therapy of oropharyngeal cancers is the routinely performed resection of retropharyngeal lymph nodes in the context of neck dissection and the morbidity associated with the resection of the mentioned lymph nodes. While in some evaluations it was shown that the occurrence of retropharyngeal metastases per se has no prognostic relevance [53], [54], however, another retrospective evaluation of 208 patients revealed that the retropharyngeal lymph node metastases determine significantly the locoregional recurrence rate and the may influence negatively the prognosis of the patients [56].
During the last years it could be revealed that the infection with HPV, especially type 16 plays an important role for the development of a subgroup of oropharyngeal cancers. Patients with HPV positive oropharyngeal cancers are generally younger patients with reduced tobacco or alcohol abuse that seem to have a better prognosis than patients with HPV negative oropharyngeal cancers. Despite the better prognosis, HPV associated oropharyngeal cancers show a higher metastatic rate [57]. A significant correlation between the positive HPV status and cervical metastasis of oropharyngeal cancers could be revealed [58]. A possible explanation for the better prognosis of HPV associated cancers is the comparably lower perineural and perivascular infiltration of cancers [57] and especially tumor-associated immunologic reactions of the HPV positive cells [59].
5.5 Larynx
The lymphatic fluid of supraglottic and glottic areas flows mainly into the lymph nodes of level II and III. The subglottic lymph fluid is drained to the lymph nodes of level III and IV. Lymph nodes of level I and V are only rarely affected in cases of laryngeal cancers. The occurrence of the so-called delphian lymph node in level VI depends on the age and in half of the adult patients. This prelaryngeal lymph node has an afflux from the area of the petiolus, the anterior commissure and the subglottis and may also be affected in cases of hypopharyngeal or thyroid cancer. Metastatic affection of those nodes may be associated with a poor prognosis and a high rate of locoregional recurrences [60].
The metastatic frequency of laryngeal cancers varies according to the tumor location and its extent. About 40% of all supraglottic cancers show locoregional metastasis at the time of diagnosis. For the supraglottic region it is given for T1 cancers with values between 6 an 25%, for T2 cancers between 30 and 70%, and for T3 and T4 cancers between 65 and 80%. Despite modern staging procedures the incidence of occult cervical metastasis of supraglottic cancers amounts to more than 20%.
Glottic cancers show a comparably low metastatic rate that is probably related to the lower lymphatic density in the glottic region. The highest density of lymph collectors of the larynx is found in the triangle formed by epiglottis, false vocal fold, and aryepiglottic fold.
There is a significant correlation between the motility of the vocal folds and the incidence of lymph node metastases in glottic cancers. While the mobile vocal folds represent a dynamic barrier for the lymphatic drainage the increasing impairment of the mobility of the vocal folds (T2–T3) leads to an unhindered lymphatic flow into the locoregional lymph nodes. The extralaryngeal growth of those cancers is associated with a significantly increased metastatic tendency (T4). So no lymphogenic metastasis may be expected in T1a cancers of the vocal cords while for T2 cancers values between 5 and 10% are assumed, 10–20% for T3 cancers, and 25–40% for T4 cancers [40].
5.6 Hypopharynx
From the hypopharynx, the lymph fluid flows via the collectors to the lymph nodes of the levels II, III, and more rarely IV. The lymphatic drainage of the posterior wall of the hypopharynx is effectuated first into the retropharyngeal lymph nodes, whose lymph fluid is forwarded via collectors to the levels II and III. An affection of the lymph nodes of level I or level V occurs rarely in hypopharyngeal cancer, even in cases of N+ necks, and it is mostly associated with lymph node metastases of other neck regions [61], [62].
The incidence of lymph node metastases of hypopharyngeal cancers amounts to about 65–80% and the one of occult lymph node metastases is about 30–40%. There is no direct relationship between the tumor size and the incidence of lymph node metastases in cancers of the hypopharynx. The incidence of lymphogenic metastases is mainly determined by the maximal infiltration depth of the cancer. Further, the metastatic rate is influenced by the degree of differentiation of the primary tumor. Poorly or undifferentiated cancers show more often locoregional metastases than better differentiated cancers.
Based on histopathological and radiological findings, retropharyngeal metastases were found in about 13% of the patients suffering from hypopharyngeal cancers [63]. Radiologically, retropharyngeal lymph node metastases were identified in about 11% of the hypopharyngeal cancers by means of PET-CT [64]. Retropharyngeal metastases especially occur in cancers of the retro-cricoid area and the posterior wall of the pharynx. The survival rate of patients with hypopharyngeal cancers seems to be independent from the occurrence of retropharyngeal metastases [63], [65].
6 Cervical lymph node metastases of unknown primary tumor
In about 2–5% of head and neck cancers, the cervical lymph node metastasis originates from an unknown primary tumor (CUP syndrome). Those metastases are mainly located in the area of the jugulo-digastric and medio-jugular lymph nodes. Caudo-jugular metastases are generally metastases of a primary tumor outside the head and neck area. The vast majority of patients with CUP syndrome have unknown squamous cell carcinomas in the area of the tonsils, the base of tongue, and the nasopharynx so that the location of the primary tumor must be excluded by ipsilateral tonsillectomy and blind excisional biopsies from the base of the tongue and the nasophaynx. Beside the ENT-specific examination, it is recommended that patients with CUP syndrome further undergo CT scan or MRT of the head and neck before planned panendoscopy.
A recently published prospective study could show that PET-CT may significantly improve the detection rate of primary tumors in CUP syndrome [66]. A current meta-analysis of 11 investigations on PET-CT shows a detection rate of 37% for primary tumors with a sensitivity and specificity of 84% [67]. Different limitations of the investigations performed up to now, i.e., inconsistent examination protocols, non-standardized evaluation, or relatively high false-positive results in the oropharynx, impede the routine application of this procedure for CUP diagnosis [68].
The primary objective of the therapy in CUP syndrome is the local control of the ipsilateral metastatic cervical lymph node affection, the occult metastases of the contralateral neck side, and the unknown primary tumor as origin of metastasis. Retrospective evaluations report about different procedures such as neck dissection, radiotherapy, and chemotherapy for treatment of the neck while chemotherapy seems only to be appropriate in cases of advanced metastasis.
The significance of neck dissection in CUP syndrome cases is still controversially discussed. Similarly high local control rates may be achieved in patients with pN1 neck without extracapsular growth exclusively by performing neck dissection of radiotherapy; however there are only retrospective investigations on this topic reporting about a low number of patients [69]. In this context it must be said that an extracapsular growth especially of smaller lymph nodes can only be excluded by performing surgical therapy. In cases of isolated lymph node metastasis without extracapsular spread the therapy of the neck should be completed by neck dissection or radiotherapy. This procedure emphasized the dependence of the therapeutic options from the exactness of the performed diagnosis and the identified extent of the lymphogenic metastasis.
In all other patients apart from pN1 neck and patients with extracapsular spread a combined treatment modality is required. Neck dissection is recommended in combination with radiotherapy. There are reports on a better locoregional control after performed neck dissection in combination with radiotherapy compared to exclusively performed radiotherapy [70]. In contrast to this, another article evaluates the significance of neck dissection before planned radiotherapy in case of CUP syndrome [71]. It could be detected that the survival rate as well as the locoregional control rate did not depend on neck dissection. The 8-year survival rate did not show a significant difference in patients with or without neck dissection.
A comparison of the data from the literature shows that neck dissection in combination with radiotherapy of both neck sides and possible mucosal tumor areas lets expect the best results regarding the local control rate and the survival rate in comparison to ipsilateral neck irradiation or exclusive irradiation [72], [73]. More recent investigations could show comparable oncologic results with IMRT with lower toxicity in comparison to conventional radiotherapy for patients with CUP syndrome, also in long-term courses [74], [75]. In a current review article on different therapeutic options of CUP syndrome similar results could be confirmed after neck dissection and radiotherapy in comparison to primary radio(chemo)therapy followed by neck dissection for possible presence of residual metastases [69].
7 Neck dissection in cases of clinical N0 neck
The individual treatment concept of the lymphatic drainage depends on the therapy of the primary tumors as well as the presence or absence of suspect lymph nodes in the imaging diagnosis. While in the context of surgical intervention in cases of clinical suspicion of present lymph node metastases of squamous cell caricnomas of the head and neck a modified radical neck dissection with removal of all five cervical lymph node levels is performed, especially the management of occult metastases in clinical N0 neck is the topic of controversial discussions. The conception of conservative procedure with careful follow-up examination in the sense of wait-and-see policy is opposed to the performance of elective neck dissection.
The problem of clinical N0 neck results from the partly insufficient sensitivity and specificity of non-invasive examination techniques. If neither clinically nor after performance of imaging diagnosis no hint for the presence of lymphogenic metastasis can be found, occult metastases must nonetheless be expected in 12–50% of the cases, depending o the location of the primary tumor [76]. A conservative procedure in the sense of wait-and-see policy bears the risk to overlook those subclinical metastases.
The diagnostically most reliable procedure for definitive assessment of the lymph node status is the operative exploration of the neck in the sense of elective neck dissection including histological examination of the tissue. The dilemma is now that the increased sensitivity of a histological assurance of the dignity in the context of an operative intervention is associated with an increased postoperative morbidity for the patients. Opponents of elective neck dissection indicate in this context that about 70% of the patients with clinical N0 neck and without metastasis undergo too excessive surgical treatment with all the related risks.
In clinical oncology, increasingly the sentinel lymphadenectomy is promoted. This minimally invasive procedure that may be considered as reliable and established method for determination of the lymph node status in cases of breast cancer and malignant melanomas, also gained attention for the therapy of head and neck cancer [77], [78]. The aim of sentinel lymphadenectomy is hereby to exclude intraoperatively occult lymph node metastasis and thus to reduce the extent of elective neck dissection to a minimum. Supporters of sentinel lymphadenectomy indicate the assumedly lower morbidity as well as better functional and cosmetic results in comparison to selective neck dissection [79].
The problem of the sentinel node procedure in the head and neck results from the high density of lymph nodes of about 300 lymph nodes and the close neighbourhood of primary tumor and the first draining lymph node stations in this area. So the exact determination of the sentinel lymph node by means of gamma probes may be difficult because of interferences of the radioactively marked tracer. Further the exact location of the searched lymph node may only be possible after turning the neck dissection specimen from the operation site. Also the injection of the tracer in the head and neck represents a high challenge for the examiner. Based on the close relationship of different lymphatic drainage regions there is the risk to inject in a drainage region neighbouring the main drainage. Finally the application of a too high volume may lead to an inadequate interstitial pressure increase of the surrounding tissue which leads to an accumulation in the neighbouring drainage regions as well as multiple, no longer representative lymph nodes [80], [81]. Additionally cancers may possibly drain to different lymph nodes at the same time because of the complex lymphatic architecture according to the location and the tumor stage. This might lead to the presence of several sentinel lymph nodes. Some authors think that the assessment of one lymph node is not significant and so they recommend the identification of two to maximal three lymph nodes (SN1, SN2, SN3) to exclude false-negative results [82]. Another disadvantage of sentinel lymphadenectomy in the head and neck is the often difficult operative description of single lymph nodes in rather inaccessible areas. In this context especially the only small operative access with an insufficient overview of the surgery site is considered as risk factor for the intraoperative damage of non-lymphatic structures.
In an evalauation performed at the Department of Otolaryngology, Marburg, Germany, it could be revealed that the distance of only one lymph node in the sense of a sentinel node is associated with a false-negative rate of nearly 40% [83]. Further the last mentioned article could show that at least two or three lymph nodes must be removed in order to exclude the presence of occult metastases with certain reliability. The mentioned results do not seem to support the intensive efforts on the performance of the sentinel node procedure in the head and neck region and so selective neck dissection in patients with N0 necks seems to be the oncologically most appropriate procedure. Additionally, the functional results after selective neck dissection are acceptable (see chapter 11). So it is recommended that based on the oncological appropriateness and the acceptable functional and aesthetic results selective neck dissection should be performed in patients with head and neck cancer, independently from the clinical evidence of locoregional metastasis. If the primary tumor is treated initially by surgery regardless its location, the definition of the treatment concepts must always consider a possible neck dissection.
The extent of selective neck dissection depends directly on the location and extent of the tumor. In cases of T1 oral cancery, the oropharynx, or the supraglottis, the follow-up may include the sonographic control of the neck or selective neck dissection according to the compliance of the patient and the sonographic experience of the treating physician. The important risk of wait-and-see is that the initially occult metastases reach a stage of inoperability because of a sometimes very rapid growth. If the treating physician has any objections the questions must be asked if it is more appropriate to initially perform a surgical treatment concept that may at least be applied as staging procedure. The possible identification of occult lymph node metastases plays a major role in the decision process of the further therapeutic options, especially with regard to radiotherapy.
Selective neck dissection of levels I-III should be performed in case of cancers of the upper lip, the lower lip, and the oral cavity. For cancers of the upper and lower lip the parotid lymph nodes must be examined. For cancers of the body of the tongue level IV should be included in the neck dissection (see chapter 5.2). The incidence of lymphogenic metastasis of a T1 cancer of the lower lip amounts to about 4–15%. With this background of the related low probability of occult metastatic spread in assumed N0 necks a wait-and-see strategy after removal of the primary tumor can be justified. For T2 cancers of the lower lip the metastatic probability increases to values of 16–35%, a fact that recommends selective neck dissection. The dissection of level II, however, may still be discussed controversially. It is considered necessary to dissect the levels I and II. For cancers of the oropharynx, selective neck dissection of levels II and III is recommended.
For cancers of the larynx the levels II–IV should be selectively dissected. In a prospective and randomised evaluation of T2-4N0 supraglottic and transglottic cancers no significant difference could be observed in the prognosis, locoregional recurrence rate, and complications with regard to modified radical neck dissection and selective neck dissection of level II–IV [84]. In more recent prospective investigations only selective neck dissection of level IIA and III is recommended for clinical N0 necks because of the rarely affected lymph nodes of levels IIB and IV, without impairing the oncologic result [85]. Prospective evaluations of laryngeal cancers show a rare affection of the lymph nodes in level IIB (less than 1%) in cases of clinical N0 neck [86], [87], [88], [89]. The metastatic spread in level IV is similar. According to an evaluation of 58 patients with supraglottic cancers no isolated metastases were detected in level IV after elective neck dissection [90]. Ferlito summarized the data of 175 patients with laryngeal cancer and clinical N0 neck from three prospective studies [91]. Only in 6 patients (3.4%) metastases in level IV could be detected histologically and partly molecular genetically.
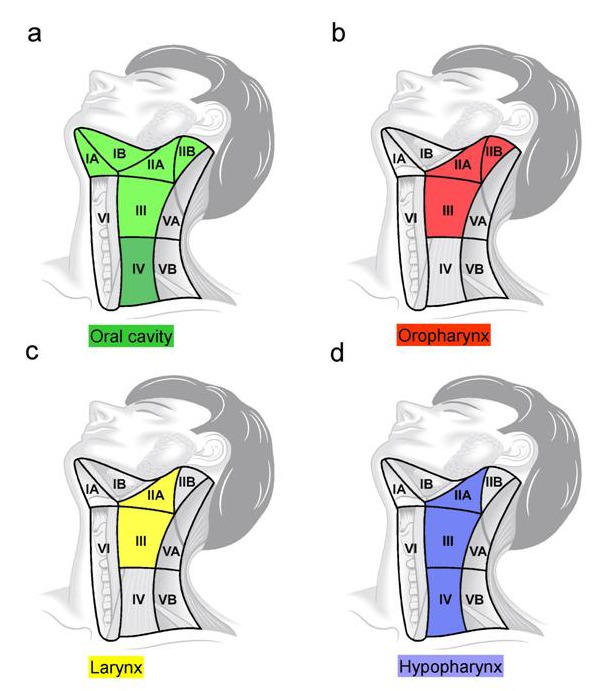
Comparably, also the dissection of the sublevel IIB is refused in hypopharyngeal cancer in order to avoid a dysfunction of the accessory nerve [92], [93], [94]. For cancers of the hypopharynx, selective neck dissection of the levels IIA–IV is recommended. A summary of the recommended extent of selective neck dissection in cases of clinical N0 neck is described in Figure 5 (Fig. 5).
Figure 5. Extent of selective neck dissection in clinical N0 neck. a) For cancers of the oral cavity it is level I–III that has to be dissection, while for cancers of the tongue levels I–IV must be considered, b) For oropharyngeal cancers it is level II–III, c) For glottic and supraglottic cancers of the larynx it is level IIA–III, d) For hypopharyngeal cancers it is level IIA–IV.
Finally it must be emphasized that the planning of selective neck dissection with different extent is only an appropriate therapy with adequate possibilities for follow-up in combination with ultrasound examinations.
8 Extirpation of the submandibular gland in neck dissections
The extirpation of the submandibular gland during neck dissection is of clinical relevance for two reasons. The submandibular gland with secretion of about 70% of the basal salivary volume is the main source of the mainly mucous saliva. A prospective evaluation of the salivary glands by means of scintigraphy after extirpation of the submandibular gland in the context of neck dissection showed that the extirpation of the gland leads to a clear reduction of the salivary volume without the possibility of compensation by other salivary glands [95]. Another aspect is the incidence of occult lymph node metastases of cancers of the oral cavity in early stages that are given with a rate of about 20–45%, however often without differentiation between the levels IA and IB [96]. Especially lymph nodes between the gland and the mylohyoid muscle are important that cannot be accessed easily without extirpation of the gland.
In contrast to the parotid gland, the submandibular gland does not contain intraglandular lymph nodes due to developmental reasons so that no lymphatic metastases are expected in the submandibular gland. Further the submandibular gland is located within a fibrous capsula that is often a barrier against tumor infiltration. The extirpation of the submandibular gland is indicated to be included in the dissection of level I for observation of the oncologic safety if an infiltration of level I by the cancer of the floor of the mouth or a lymph node metastasis in level I is present. The question now is if the extirpation of the submandibular gland is indicated for the safe dissection of occult lymph node metastases in level IB, especially for T1/2N0 cancers of the oral cavity.
Some retrospective investigations consider the preservation of the submandibular gland in the context of those cancers as appropriate [97], [98]. In a prospective evaluation of the group around Robbins about 33 neck dissection specimens of cancers of the oral cavity of different stages the dissection of leve lIB was performed in three successive steps [99]. First the dissection of the periglandular soft part tissue and the lymph nodes was performed, followed by the extirpation of the submandibular gland and finally the residual tissue in dorsal direction of the gland. All lymph nodes were already removed in the first step without detection of the lymph nodes in the residual tissue. In this article the technical possibility of dissecting all lymph nodes of leve lIB without the necessity of extirpating the gland was discussed.
9 Late cervical lymph node metastases
After selective or modified radical neck dissection some patients with or without radiochemotherapy may develop locoregional metastases, especially within the first two years after primary therapy. In the literature, however, the problem of late cervical lymph node metastases is only rarely discussed. Up to now the exact incidence of late cervical lymph node metastases is unclear because the studies performed up to now generally evaluated relatively inhomogeneous patient groups with and without radiotherapy, cervical lymph node metastases (pN+), and local recurrences. Further the extent of neck dissection varied.
In the case of multiple lymph node metastases or the presence of extracapsular growth, especially without adjuvant radiochemotherapy, locoregional metastatic spread can be expected. The incidence of late metastatic development seems to depend from the location and the extension of the primary tumor. In a retrospective analysis of 2550 patients suffering from hypopharyngeal and laryngeal cancer, the total incidence of late cervical lymph node metastases amounted to 12.4% [100]. In the mentioned study, the highest incidence for late locoregional metastases was found in cancers of the piriform sinus (31.1%) and the aryepiglottic folds (21.9%). In this study it could be confirmed that late metastases are significantly associated with an advanced tumor stage, lymph node metastases, and locoregional tumor recurrences. In a multicenter retrospective investigation of 826 patients with cancers of the oral cavity late lymph node metastases were found in 4% of the patients without tumor recurrence and initial pN0 neck while 12% of the patients with late metastatis were initially staged N+ [101]. In this evaluation, a relatively inhomogeneous group of patients with local recurrence and adjuvant radiotherapy were included. An investigation of the MD Anderson Cancer Center about patients with head and neck cancer who underwent selective neck dissection, found a late metastatic spread in 1.9% of the patients with pN0 neck without radiotherapy [102].
A retrospective analysis of the patients of the ENT Department of Marburg who developed late lymph node metastasis is presented. The clinical data of 61 patients suffering from head and neck cancer and who had undergone elective neck dissection with pN0 neck were analysed. Only patients without local recurrence, secondary primary, or radiochemotherapy were considered. Late lymph node metastases were found in 4 (6.5%) cases for all primary tumor locations that were located at the margins or outside the initially removed lymph node levels. In those patients the primary tumor was located in the area of the oral cavity (n=3) or the oropharynx (n=1) and was staged T1 or T2 in all cases. Lymph node metastases were identified in level I (n=2), II (n=1) and IV (n=1). Late lymph node metastases were detected in 4 of 29 patients (13.8%) with cancers of the oral cavity and the oropharynx after elective neck dissection. None of the patients of the present study with hypopharyngeal or laryngeal cancer developed late lymph node metastases (data not yet published).
For better understanding of the late lymph node metastases it must be considered that a negative histopathological examination of the cervical lymph nodes does not mean the safe exclusion of occult metastases so that the presence of subpathological metastases must be observed in this context (see chapter 4). Further it is clear that the borders between the different neck levels must not be understood very strictly and overlapping between the levels is possible. Late lymph nodes may be located in the levels that were not included in the selective neck dissection. The low incidence of this small group of patients with late lymph node metastases after selective neck dissection, however, does not justify the performance of extended types of neck surgery in patients with clinical N0 neck.
10 Neck dissection after primary radiochemotherapy
The primary radiochemotherapy is increasingly applied in the treatment concept of advanced cancers of the upper aerodigestive tract, especially oropharynx, hypopharynx, and larynx, with lymphogenic metastasis. Despite partly good response rates in the area of the primary tumor the cervical lymph nodes show comparably low response rates after primary radiochemotherapy. In an investigation a tumor-free stage in the area of the primary tumor could be achieved in 86% of the patients whereas the cervical lymph nodes showed a complete response in 69% of the patients; the response of the cervical lymph nodes depended on the their size (N stage) [103].
Patients with a complete response rate of the cervical lymph nodes after primary radiochemotherapy show a cervical recurrence rate of less than 5% in the follow-up time [104] so that neck dissection does not seem to be required in this patient group. Additionally, similar cervical control rates are reported about patients without hint to cervical tumor residues after performed salvage neck dissection [105]. In this patient group a narrow sonographic follow-up should be performed. There is no clear recommendation for patients with initial N3 neck and without indication for tumor residues because the number of patients in this subgroup is relatively low in most of the investigation.
However, salvage neck dissection is required in cases of residual tumor in the neck area. It could be confirmed that the lymph nodes in the levels I and V (except from cancers of the oral cavity) show only rarely tumor residues while metastatic tumor residues occur nearly exclusively in the levels II–IV. With this background and based on the fact that the (modified) radical neck dissection with condition after radiochemotherapy may show a relatively higher morbidity, selective neck dissection of level II–IV is recommended in cases of suspected cervical tumor residues [106], [107]. The choice of selective neck dissection after radiochemotherapy is emphasized by the fact that no correlation between selective and modified radical neck dissection and the detection of residual tumors, locaregional tumor control rate, and survival rate of those patients could be revealed [108].
The key for the possible planning of neck dissection after primary radiochemotherapy is the detection of cervical residual tissue. In this context we indicate chapter 4. PET-CT with a high negative predictive value seems to be a valuable examination method for determination of the residual tumor in the neck area. The time for PET-CT staging for possible planning of neck dissection amounts to 8–12 weeks after radiotherapy.
The complete cervical response is reduced from about 80% for N1 necks to about 40% for N3 necks [109]. Thus the response rate of lymph node metastases depends, as already mentioned, from their size. Additionally, patients with extended cervical metastases may reveal a high rate of distant metastases after primary radiochemotherapy especially in cases of N3 necks [110]. Those metastases may be observed about 6 months on the average after radiochemotherapy especially in the area of the lung. With this background PET-CT of the whole body is recommended beside the identification of cervical metastases, also in order to verify possible distant metastases in cases of advanced cervical metastatic spread.
11 Morbidity after neck dissection
As every type of invasive therapy, also neck dissection bears the risk of postoperative complications. The mortality and morbidity depend on the extent of surgical intervention as well as the specific conditions of the patients such as relevant previous diseases, e.g., heart or lung diseases, immunosuppression, or preoperative radiotherapy.
Radical neck dissection leads to a significant functional and cosmetic morbidity of the affected patients. It could be confirmed that a modification of the radical neck dissection and postoperative rehabilitations measures may improve the postoperative quality of life of the patients [111], [112].
The reliability and safety of neck dissection can only be assured if ENT specialists are aware of possible complications and they know about the topographiy anatomy of the neck as well as they aim at minimizing the morbidities [113]. Some important complications after neck dissection are presented here.
Apart from the intraoperative opening of the internal jugular vein, postoperative thrombosis is one possible complication after neck dissection. The change of the flow in the direct postoperative phase could be shown in ultrasound examinations and contrast-enhanced CT scan, however the long-term closure of the internal jugular vein after neck dissection can be considered as very rare event with neglectable morbidity [114]. Different evaluations show that wound healing disturbances lead to an increased risk of thrombosis. At the same time hypercoagulability caused by the tumor as well as the reduced blood flow during intubation anaesthesia are possible origins for the development of vascular thrombosis. There are several additional factors, apart from neck dissection, that influence the risk of closure of the internal jugular vein. Among those is the application of flaps for defect coverage as well as pre- or postoperative radiotherapy. Apart from the reduced surgery time, the selective neck dissection has the advantage of a comparably lower exposition of the blood vessels. Thus vascular dehydration and trauma are avoided.
Based on the high variability in the anatomy of the thoracic duct in the neck region the dissection of level IV may be associated with an iatrogenic lesion of those structures. The risk of developing a chylus fistula after radical neck dissection amounts to average 1–2.5% with a majority (75–90%) on the left side [115]. Regarding this aspect, a preoperative radiotherapy may significantly increase the incidence of postoperative chylus fistulas.
In about 1% of the patients an impairment of the function of the facial nerve can be observed after neck dissection [116]. It could be shown that the incidence to functional disturbance of the marginal facial nerve in cases of neck dissection can be compared to the incidence after extirpation of the submandibular gland in the context of benign diseases.
With relation to the functional result after neck dissection a functional disturbance of the accessory nerve may lead to complex clinical symptoms, the so-called shoulder-arm syndrome. Those are described as pains, weakness, and atrophy of the shoulder girdle. It leads to a restriction of the arm abduction and the frontal flexion. Also the scapula is only insufficiently stabilized during shoulder movement which leads to a mechanical overstress of the different shoulder structures.
This is one of the possible reasons for the development of chronic pain symptoms. Clinical evaluations showed that patients who had undergone selective neck dissection had less functional disturbances of the accessory nerve and less shoulder dysfunctions than patients with other types of neck dissection [117]. In opposition to those results, some authors did not find a significant correlation between the type of neck dissection and the degree of shoulder function loss [118]. Electrophysical investigations revealed that a surgical manipulation of the accessory nerve in the context of selective neck dissection may lead to an impairment of forwarding impulses especially when the dissection had been performed along the posterior triangle of the neck (level V). Subclinical or nearly asymptomatic nerve dysfunctions could be observed when level IIB was dissected [119].
In comparison to sentinel node biopsy the surgical extent of the different types of selective neck dissection seems to be closely related to a higher postoperative morbidity although no significant differences could be detected in the health related quality of life of both procedures [120]. In a retrospective evaluation of 52 patients, the functional and aesthetic results after selective neck dissection of different extent and regions were analysed [121]. The postoperative long-term course amounted to at least six months. The evaluation of the functional results showed that in 82% of the patients no difference in the side of the arm abduction was observed. A difference of the side regarding mobility of the arm of up to 20 degrees could be found in the remaining patients. Regarding the turning of the head, 84% did not state differences of the side. A limited turning of the head of up to 30 degrees to the non-operated neck side could be observed in 13% of the cases. In this context it must be considered that 80% of those patients had undergone previous irradiation. With regard to bending of the head to the side there was no difference in the side in 79%. Patients who had impairment to the non-operated side were irradiated in 75% of the cases. Questions on the subjective sensation of the patients showed that 96% of the patients had subjectively no impairment of the head and neck mobility. Regarding the shoulder-arm mobility, 86% of the patients reported about the absence of impairments. The evaluation of the aesthetic results showed that 98% of the patients were satisfied with the aesthetic result while 85% considered the treatment result as good or very good.
A comparative investigation of the quality of life of patients after exclusive radiochemotherapy and neck dissection after radiochemotherapy revealed that patients with additionally performed neck dissection had not significant difference in the quality of life [122]. Only in the domain of pains significant differences could be revealed.
12 Endoscopic neck dissection
In the fields of visceral surgery and orthopaedics endoscopic procedures are increasingly applied with great success. Due to the minimally invasive approach and the application of endoscopes in already existing cavities patients can be treated more carefully and gently and released after shorter inpatient durations.
The idea of endoscopic neck dissection originates from a time when the clinical relevance of sentinel lymphadenectomy for squamous cell carcinomas of the head and neck was still discussed. There are more and more studies on endoscopic procedures especially for therapy of thyroid diseases. The studies published up to now on endoscopic neck dissection for squamous cell carcinomas of the upper aerodigestive tract are often limited to the preparation of cadavers or animal models [123], [124]. So this procedure has currently an experimental significance and no clinical relevance [125]. First reports on endoscopic lymphadenectomy and the possible enlargement of the intervention to selective neck dissection have already been published [126]. It could be revealed that all relevant structures in level II and III can be reliably exposed via a skin incision of approximately 2 cm by means of a special spreader instrument [127].
In the neck, there is no preformed cavity and the lymph nodes are in anatomically complex areas in close neighbourhood to clinically relevant structures so that a reliable endoscopic removal of several neck regions can only be realised with technical limitations. Further, the oncologic and surgical safety cannot be assured with the techniques that are available today. The better cosmetic result by multiple small skin incisions has no particular clinical relevance for this surgery. Skin incisions performed in the context of selective neck dissection in the area of the tension lines of the skin lead to cosmetically satisfactory results so that those scars do not represent a relevant impairment of the affected cancer patients.
The definitive clinical importance of endoscopic neck dissection can currently not be answered. In this context further technical development of this procedure has to be made. Some authors, however, critically discuss the sense of this kind of intervention even after technical establishment in the future [128].
13 Prognostic relevance of neck dissection
Lymphogenic metastasis is a complex process of several steps. The tumor cells with metastatic potential disseminate from the area of the primary tumor and a low number of those cells start to metastasize. An even smaller subpopulation of those cells has the capacity to develop micrometastases. These micrometastases can be inactive for a long time and then a small fraction can develop after a latency to become clinical metastatic triggered by an unknown mechanism. The sequence of the events is described in chapter 3 and the selection of the tumor cells is only one dimension in the metastatic process. Further the kinetic of the metastatic progression and the affection of the metastatic organs where those steps take place are important. Some of these processes are determined by different genetic classes for initiation, progression, and virulence of metastatic spread. The function of the virulence genes is the determination of the organ specificity of the metastases [129].
It could be shown that the disseminated tumor cells in patients with breast cancer in M0 stage had different genetic changes with significantly lower chromosomal aberration than their primary tumor and metastatic cells of patients with M1 stage. This revelation lets expect that the dissemination of tumor cells is an early event in the tumor development and that disseminated tumor cells may further develop independently from the primary tumor [130]. This statement is emphasized by the clinical observation that patients with initial M0 stage after R0 tumor resection without detection of locoregional recurrences may have distant metastases in the further course. Also the CUP syndrome represents a situation where early disseminated tumor cells acquire favourable mutations and develop faster than primary tumor cells.
If metastases do not metastasize and develop independently from their primary tumor, the question must be asked if neck dissection in head and neck cancer may influence the risk of hematogenous metastasis and the prognosis of the patients. Up to now there is no meta-analysis consistent randomized study for any tumor (LoE 1A study) that confirms the prognostic benefit of elective lymph node dissection [131]. An extensive review article analyses the significance of lymphadenectomy in solid tumors and revealed that lymph node dissection does not lead to a significant improvement of the total survival [132]. So the exact value of neck dissection in N0 or N+ necks cannot be defined exactly at the moment.
There are however some well-known clinical aspects that must be considered in the discussion of the prognostic relevance of neck dissection. The presence of extracapsular spread of the cervical metastases as most important prognostic factor is related to a significantly higher locoregional recurrence rate [133]. In this context the question is asked if a metastasis penetrating the lymph node capsula after wait-and-see is as “dangerous” as the early detection of extracapsular spread at the time of diagnosis regarding the risk of distant metastasis. Another aspect concerns the therapy of lately detected clinical manifest lymph node metastases in patients with initial cN0 neck that primarily did not undergo neck dissection. Single articles on these metastases that were not treated in the occult stage give a hint on the hereby poorer prognosis of the affected patients [134]. Further there is the discussion of metastases of level IV. It is well-known that the detection of isolated lymph node metastases in level IV is associated with a fatal prognosis. In this context the question arises why the prognosis of this location is so poor if metastases in level IV do not play a role in the process of distant metastasis.
These and further questions show the necessity of further investigations for exact prognostic evaluation of neck dissection. It is confirmed that neck dissection can significantly improve the regional tumor control rate. Additionally, neck dissection is currently the gold standard as staging procedure.
14 Neck dissection for cancers of the major salivary glands
While most of the head and neck cancers develop in squamous cells the tumors of the salivary glands are characterized by a high heterogeneity of the histomorphologic appearance. This is the main reason for the often not predictable clinical behaviour and the controversies in the treatment concept of different salivary gland tumors.
Cervical lymph node metastases occur in patients with salivary gland tumors with an incidence of about 15–25% at the time of first diagnosis. Regional metastases can influence the prognosis of those patients in a decisive way. The 5-year survival rate of parotid cancers with cervical lymph node metastasis at the time of first diagnosis amounts to 9%. The survival rate increases to 17% when the lymph node metastasis develops after initial treatment and amounts to 74% after five years in patients without affection of the regional lymph nodes [135].
The treatment of the clinically positive neck consists often in a modified radical neck dissection completed by postoperative radiotherapy in cases of multiple metastasis or extranodal growth. There are however controversial discussions in the few publications regarding the surgical treatment of the ipsilateral cervical lymph nodes and the extent in patients with probably occult lymph node metastasis. While some authors are rather reluctant concerning neck dissection in the treatment concept of N0 necks, others recommend neck dissection for certain tumor entities or after histological diagnosis of positive lymph nodes [136].
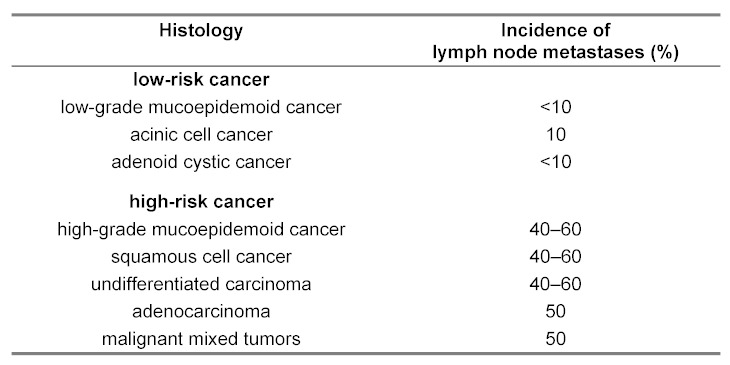
The different frequencies of cervical lymph node metastases for certain tumor entities are summarized in Table 2 (Tab. 2). An especially high risk of cervical lymph node metastases is described for adenocancers, undifferentiated cancers, high-grade mucoepidermoid cancers, squamous cell carcinomas, and cancers of the salivary ducts [137]. Lymph node metastases of adenoid cystic cancers occur more often than of poorly differentiated cancers with solid growth patterns. Another histomorphological risk factor is the extraparotid extent and lymphangiosis cancertosa, in particular in patients over 54 years. Those patients have a 95% probability of occult lymph node metastases in comparison to 1% in patients without the mentioned risk factors [138].
Table 2. Incidence of locoregional metastasis of different cancers of the parotid gland.
The tumor size correlates with the incidence of lymph node metastases. According to a multivariate analysis of risks for occult lymph node metastases they amount to 20% for tumors larger than 4 cm, in comparison to 4% for smaller tumors [139]. Facial paresis further contributes to the high risk of developing lymphogenous metastases of parotid cancer. Lymph node metastases may occur in about 65–75% of the patients with facial paresis [140], [141]. Finally there are references on an accumulation of lymph node metastases that seem to be related to different molecular parameters. The analysis of p53 expression in parotid tumors shows a significantly higher metastatic spread for tumors with a high expression of this onco-protein [142].
For assessment of the metastatic behaviour of parotid cancers, the knowledge of the incidence of regional occult metastases is essential as for other cancers of the head and neck. So-called high-grade cancers have a frequency of about 50% for occult lymph node metastases in comparison to maximal 10% for low-grade cancers. The incidence of occult lymph node metastases for parotid cancers after elective neck dissection in clinical N0 necks amounts to 1.1–15.9% according to a literature review [143]. Based on the high frequency of lymph node metastases of high-grade cancers an elective neck dissection is recommended in this patient group. Regarding the extent of neck dissection there are different opinions [144].
The decision for neck dissection must be made individually, depending on the characteristics of the primary tumor. It is justified to indicate selective neck dissection for high-grade cancers. Regarding additional parameters (>T2, lymphangiosis cancertosa) neck dissection is also recommended for low-grade cancers. An elective neck dissection should include the levels I, II, III, and VA which can be performed without further relevant morbidity parallel to parotidectomy [143].
The explanations up to now were related to more frequently occurring cancers of the parotid gland whereas the cancers of the submandibular gland represent only 5–10% of the salivary gland cancers. About half of all tumors of the submandibular gland are malignant while adenoid cystic cancers, followed by mucoepidermoid cancers, form the most frequent group. In case of presence of lymph node metastases the procedure is comparable to the one of parotid cancers. For high-grade cancers and cancers with an extraglandular growth (>T1) an elective neck dissection is more frequently indicated for the levels I–III [145], [146].
15 Neck dissection for cutaneous malignancies of the head and neck
15.1 Squamous cell carcinoma
Lymph nodes in the area of the parotid gland mainly drain to the skin areas of the ipsilateral front, temple, eyelids, cheeks, and auricle and thus represent the potential region for metastases of patients with squamous cell carcinoma of the skin from the mentioned head areas. About 40% of all metastases in the area of the parotid gland are caused by squamous cell carcinomas of the skin. A long-term study reports on a metastatic rate of up to 5% for squamous cell carcinomas of the scalp while auricular cancers have a higher metastatic rate of up to 10%. The mentioned metastatic rates are especially true for immuno-competent patients with previously untreated cancers that are generally smaller than 1.5 cm in diameter at the time of first diagnosis. This relative low incidence for locoregional metastases is opposed to a clearly higher metastatic incidence of so-called high-risk cancers. In particular patients over 70 years and immuno-suppressed patients with squamous cell carcinomas of the scalp of more than 1.5 cm in diameter are the main risk group for developing parotid metastases. The incidence of metastases of this small group of patients with skin cancer is significantly underestimated [147].
The parotid metastases are in close neighbourhood to the cervical lymph nodes and are often associated with cervical lymph node metastases such as the primary high-grade cancers of the parotid gland. Cervical metastases can be expected in more than 50% of the cases of present parotid metastasis. The metastases in the area of the parotid lymph nodes and their close lymphogenous contact to cervical lymph nodes have a high prognostic importance for patients with squamous cell carcinomas of the scalp. The 5-year survival rate of patients with locoregionally metastasized squamous cell carcinomas of the skin amounts to about 50% according to the literature. Those patients are primarily curable, however, with a high risk of about 20-25% for development of local recurrences despite aggressive multimodal therapy. Those local recurrences are basically incurable and are associated with a high risk of distant metastasis especially in the area of the lung [148].
The local tumor control rate as well as the survival rate of patient with parotid metastasis of a sqamous cell cancer of the skin dependy mainly on the size of the parotid metastasis, the infiltration of the facial nerve and the skull base. The extent of the cervical metastatic spread with existing parotid metastasis is of special prognostic relevance. O’Brien et al. [149] could show that patients with parotid metastases without cervical metastases or with an isolated cervical metastasis of up to 3 cm have a 5-year survival rate of 65–70% while the 5-year survival of patients with multiple cervical metastases or metastases of more than 3 cm amounts to 30%. This and other studies confirm that the extent of parotid and cervical metastatic spread significantly influence the survival rate of the affected patients.
The vast majority of the parotid metastases develop within the first two years after diagnosis of the skin cancer. The sonographic examination in narrow intervals of the parotid and cervical lymph nodes is required for patients with so-called high-risk cancers, especially within the first two years after diagnosis of the skin cancer in order to early diagnose locoregional metastases. Currently no solid data for elective parotidectomy are present.
The therapy of those metastases contains a partial or total parotidectomy in combination with neck dissection with subsequent radiotherapy. In case of suspect cervical lymph nodes modified radical neck dissection for treatment of the neck should be performed. In the case of clinical N0 neck and the presence of parotid metastases a selective neck dissection of the levels I, II, III, and VA is indicated (see also chapter 14) [143].
15.2 Merkel cell cancer
The Merkel cell carcinoma is a rare and very aggressive cutaneous neuroendocrine cancer with a high rate of local recurrences, regional lymph node metastases and distant metastases occurring in the further course of the disease. More than a third of the patients have regional lymph node metastases at the time of first presentation and develop lymph node metastases in up to 75%. The 5-year survival rate amounts without and with locoregional metastasis 75% or 60% respectively. The best result for local and regional control of the disease is achieved by surgical excision of the primary tumor with postoperative radiotherapy [150].
Although the clinical suspicion of lymph node metastaes justifies modified radical neck dissection, if needed completed by parotidectomy, there are different opinions regarding elective therapy of the locoregional lymph nodes for merkel cell cancers. The high incidence of lymph node metastases and micrometastases as most important prognostic factor justifies the recommendation of elective neck dissection and possibly parotidectomy for clinically negative regional lymph nodes for Merkel cell carcinoma of the skalp [151]. The significance of sentinel node biopsy for merkel cell cancers of the head and neck is still unclear.
15.3 Melanoma
Cutaneous malignant melanomas are only 5% of the all skin cancer but they are responsible for more than 65% of the deaths caused by skin cancer. In 20% of the cases, cutaneous malignant melanomas are located in the head and neck. Melanomas metastasize via lymphatic and hematogenous structures. About two third of the metastases occur first in the drainage region of the regional lymph nodes. A regional metastasis may appear as micrometastasis, identified by means of sentinel node biopsy, as satellite metastasis, in transit metastasis, or clinically visible regional lymph node metastasis. the 10-year survival rate amounts to 30–70% of the patients with micrometastases, 30–50% of the patients with satellite or in transit metastases, and 20–40% of the patients with clinically present lymph node metastases [152].
If locoregional lymph node metastases are detected clinically by means of imaging diagnosis, neck dissection sometimes completed by parotidectomy is recommended as standard therapy. For clinically negative lymph node findings the sentinel lymphadenectomy is considered as adequate procedure.
Adjuvant therapeutic procedures are applied in patients who did not show metastasis, but who have a high risk of further tumor development. This concerns mainly patients with tumors with a thickness of more than 1.5 mm, according to the AJCC staging this corresponds to a melanoma in stage II or III. The application of adjuvant radiotherapy for the treatment of cervical lymph node metastases of cutaneous melanomas of the head and neck is still controversially discussed. Data from randomized studies that confirm the benefit of adjuvant radiotherapy for patients with lymph node affection with high risk of recurrences (extranodal spread of the melanoma, more than two positive lymph nodes, significant lymph node enlargement or recurrence of previously extirpated lymph node area) are not present. Up to now, chemotherapy, immuno-stimulants or vaccinations have only contributed to a minimal success. Interferon (IFN) showed an effect on the recurrence-free survical in some clinical studies, however, without a clinically significant effect on the total survival [153].
16 Neck dissection for cancers of the thyroid gland
In contrast to the classification of the cervical lymph nodes into 6 levels, the neck regions for thyroid cancers are divided into compartments. The central compartment includes the lymph node groups medially on both sides of the carotid artery from the lingual bone to the brachiocephalic artery [154]. The most frequent lymph node groups that are affected by thyroid cancers are the prelaryngeal (Delphian), pretracheal, and paratracheal lymph nodes while the major part of the metastases is located in caudal direction of the larynx. The area caudally to the central compartment is called mediastinal compartment. The centrolateral compartment contains the area laterally to the vessel nerve sheath on both sides up to the anterior edge of the trapezius muscle.
For the lymphogenic metastatic process of thyroid cancers it is important to know that the lymphatic drainage of both thyroid lobes is not strictly divided. There is a wide-spread network of lymphatic relationships about which the prelaryngeal and pretracheal lymph nodes communicate. Further there are connections to the retropharyngeal lymph nodes and to lymph nodes located in the area of the upper mediastinum. Thyroid cancers show different metastatic behaviour according to the tumor entity.
The most frequent thyroid cancer with an incidence of about 60–80% is the papillary cancer. Papillary cancers metastasize preferably in lymphogenic way while the metastatic rate amounts to about 50%. On a microscopic level, lymph node metastases do not seem to have a prognostic relevance regarding those cancers [155], however, prospective randomized studies on the significance of central neck dissection are missing. The follicular cancers show a hematogenous metastatic tendancy with a lower lymphogenic metastatic rate of about 5–15%. For medullary cancers, the lymph node metastases are of decisive prognostic relevance and occur in about 50–80%. The anaplastic cancers show a rapidly progressing and aggressive growth accompanied by hematogenous metastases. Those cancers can have lymph node metastases in about 30% of the cases.
The therapy of these cancers includes generally a total thyroidectomy with central neck dissection in combination with radio-iodine therapy. Often further lymph node removal of the lateral and mediastinal compartments is required after detection of metastases. Beside ablation of probably still present thyroid tissue, the objective of radio-iodine-therapy the identification or exclusion of storing lymph node and distant metastases. An indication for radio-iodine-therapy is not given for papillary microcancers (pT1) and medullar and anaplastic cancers.
17 Perspectives
The reliable diagnosis of cervical lymph node metastases is a significant step for an optimized therapy and prognosis of patients with head and neck cancer. Clinical, radiological, and routinely performed histopathologic examinations are currently not in a position to detect locoregional metastases in head and neck cancer with certainty. Molecular procedures may contribute on the detection of occult subpathologic metastases and define the risk of lymphogenic metastasis based on the characteristics of the primary tumor. Current evaluations on the angiogenesis and measurements of the microvascular density of head and neck cancers as well as the analysis of expression of extra-cellular molecules such as MMP-1, -2, and integrin-3 are efforts to establish a marker for prediction of the lymphogenic metastasis. Further investigations show that DNA micro-array gene expression profiles may be useful for the prediction of the presence or development of cervical lymph node metastases of head and neck cancer. In the future, investigations especially on the prognostic biomarkers are required to define an individual risk profile and to determine the indication for neck dissection in N0 necks.
Notes
Acknowledgements
The authors want to thank Dr. S. Hoch, physician at the Department of Otolaryngology, Head & Neck Surgery, of Marburg, Germany, for his support in the creation of the figures.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Werner JA. Aktueller Stand der Versorgung des Lymphabflusses maligner Kopf-Halstumoren. Eur Arch Otorhinolaryngol. 1997;Suppl I:47–85. doi: 10.1055/s-2007-997495. [DOI] [PubMed] [Google Scholar]
- 2.Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer. 2009 Mar;115(5):922–935. doi: 10.1002/cncr.24123. Available from: http://dx.doi.org/10.1002/cncr.24123. [DOI] [PubMed] [Google Scholar]
- 3.Richard JM, Sancho-Garnier H, Micheau C, Saravane D, Cachin Y. Prognostic factors in cervical lymph node metastasis in upper respiratory and digestive tract carcinomas: study of 1,713 cases during a 15-year period. Laryngoscope. 1987 Jan;97(1):97–101. doi: 10.1288/00005537-198701000-00019. Available from: http://dx.doi.org/10.1288/00005537-198701000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Jones AS, Roland NJ, Field JK, Phillips DE. The level of cervical lymph node metastases: their prognostic relevance and relationship with head and neck squamous carcinoma primary sites. Clin Otolaryngol Allied Sci. 1994 Feb;19(1):63–69. doi: 10.1111/j.1365-2273.1994.tb01150.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 5.Puri SK, Fan CY, Hanna E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003 Apr;11(2):119–123. doi: 10.1097/00020840-200304000-00010. Available from: http://dx.doi.org/10.1097/00020840-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Crile GW. On the surgical treatment of cancer of the head and neck. With a summary of one hundred and twenty-one operations performed upon one hundred and five patients. Trans South Surg Gynecol Assoc. 1905;18:108–127. [Google Scholar]
- 7.Robbins KT, Denys D . Committee for Neck dissection Classification; American Head and Neck society. The american head and neck society’s revised classification for neck dissection. In: Johnson JT, Shaha AR, editors. Proceeding of the 5th International Conference in Head and Neck Cancer. Madson: Omnipress; 2000. pp. 365–371. [Google Scholar]
- 8.Hasegawa Y, Saikawa M, Hayasaki K. A new classification and nomenclature system for neck dissections: a proposal by the Japan Neck dissection Study Group. Jpn J Head Neck Cancer. 2005;31:71–78. [Google Scholar]
- 9.Hasegawa Y, Saikawa M. Update on the classification and nomenclature system for neck dissection: revisions proposed by the Japan Neck Dissection Study Group. Int J Clin Oncol. 2010 Feb;15(1):5–12. doi: 10.1007/s10147-009-0019-z. Available from: http://dx.doi.org/10.1007/s10147-009-0019-z. [DOI] [PubMed] [Google Scholar]
- 10.Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, Som PM, Day TA. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008 May;134(5):536–538. doi: 10.1001/archotol.134.5.536. Available from: http://dx.doi.org/10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 11.Ferlito A, Robbins KT, Shah JP, Medina JE, Silver CE, Al-Tamimi S, Fagan JJ, Paleri V, Takes RP, Bradford CR, Devaney KO, Stoeckli SJ, Weber RS, Bradley PJ, Suárez C, Leemans CR, Coskun HH, Pitman KT, Shaha AR, de Bree R, Hartl DM, Haigentz Mr, Jr, Rodrigo JP, Hamoir M, Khafif A, Langendijk JA, Owen RP, Sanabria A, Strojan P, Vander Poorten V, Werner JA, Bień S, Woolgar JA, Zbären P, Betka J, Folz BJ, Genden EM, Talmi YP, Strome M, González Botas JH, Olofsson J, Kowalski LP, Holmes JD, Hisa Y, Rinaldo A. Proposal for a rational classification of neck dissections. Head Neck. 2011 Mar;33(3):445–450. doi: 10.1002/hed.21614. Available from: http://dx.doi.org/10.1002/hed.21614. [DOI] [PubMed] [Google Scholar]
- 12.Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 2004 Jan;40(1):13–20. doi: 10.1016/S1368-8375(03)00127-1. Available from: http://dx.doi.org/10.1016/S1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, Shirasuna K. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol. 2009 Mar;34(3):673–680. doi: 10.3892/ijo_00000193. Available from: http://dx.doi.org/10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 14.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer. 2004 Sep;101(5):973–978. doi: 10.1002/cncr.20454. Available from: http://dx.doi.org/10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang Z, Jian P, Longjiang L, Bo H, Hongwei Z. Identification of oral cancer cell-induced changes in gene expression profile of lymphatic endothelial cell. Cancer Invest. 2008 Dec;26(10):1002–1007. doi: 10.1080/07357900802087234. Available from: http://dx.doi.org/10.1080/07357900802087234. [DOI] [PubMed] [Google Scholar]
- 16.Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007 Sep;110(6):1287–1294. doi: 10.1002/cncr.22900. Available from: http://dx.doi.org/10.1002/cncr.22900. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Carracedo D, Rodrigo JP, Astudillo A, Nieto CS, Gonzalez MV. Prognostic significance of lymphangiogenesis in pharyngolaryngeal carcinoma patients. BMC Cancer. 2010;10:416. doi: 10.1186/1471-2407-10-416. Available from: http://dx.doi.org/10.1186/1471-2407-10-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang Z, Jian P, Longjiang L, Bo H, Wenlin X. Altered phenotype of lymphatic endothelial cells induced by highly metastatic OTSCC cells contributed to the lymphatic metastasis of OTSCC cells. Cancer Sci. 2010 Mar;101(3):686–692. doi: 10.1111/j.1349-7006.2009.01444.x. Available from: http://dx.doi.org/10.1111/j.1349-7006.2009.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer--a review of mechanisms. Int J Oral Sci. 2010 Mar;2(1):5–14. doi: 10.4248/IJOS10006. Available from: http://dx.doi.org/10.4248/IJOS10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterz CM, Kulle C, Dakic B, Makarova G, Böttcher MC, Bette M, Werner JA, Mandic R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010 Feb;46(2):116–122. doi: 10.1016/j.oraloncology.2009.11.011. Available from: http://dx.doi.org/10.1016/j.oraloncology.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa MK, Furukawa M. Diagnosis of lymph node metastases of head and neck cancer and evaluation of effects of chemoradiotherapy using ultrasonography. Int J Clin Oncol. 2010 Feb;15(1):23–32. doi: 10.1007/s10147-009-0017-1. Available from: http://dx.doi.org/10.1007/s10147-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 22.Carter RL, Barr LC, O'Brien CJ, Soo KC, Shaw HJ. Transcapsular spread of metastatic squamous cell carcinoma from cervical lymph nodes. Am J Surg. 1985 Oct;150(4):495–499. doi: 10.1016/0002-9610(85)90162-X. Available from: http://dx.doi.org/10.1016/0002-9610(85)90162-X. [DOI] [PubMed] [Google Scholar]
- 23.Coatesworth AP, MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: the prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. 2002 Mar;24(3):258–261. doi: 10.1002/hed.10020. Available from: http://dx.doi.org/10.1002/hed.10020. [DOI] [PubMed] [Google Scholar]
- 24.Stern WB, Silver CE, Zeifer BA, Persky MS, Heller KS. Computed tomography of the clinically negative neck. Head Neck. 1990 Mar-Apr;12(2):109–113. doi: 10.1002/hed.2880120203. Available from: http://dx.doi.org/10.1002/hed.2880120203. [DOI] [PubMed] [Google Scholar]
- 25.van den Brekel MW, Castelijns JA, Stel HV, Golding RP, Meyer CJ, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol. 1993;250(1):11–17. doi: 10.1007/BF00176941. [DOI] [PubMed] [Google Scholar]
- 26.de Bondt RB, Nelemans PJ, Hofman PA, Casselman JW, Kremer B, van Engelshoven JM, Beets-Tan RG. Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol. 2007 Nov;64(2):266–272. doi: 10.1016/j.ejrad.2007.02.037. Available from: http://dx.doi.org/10.1016/j.ejrad.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Nahmias C, Carlson ER, Duncan LD, Blodgett TM, Kennedy J, Long MJ, Carr C, Hubner KF, Townsend DW. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg. 2007 Dec;65(12):2524–2535. doi: 10.1016/j.joms.2007.03.010. Available from: http://dx.doi.org/10.1016/j.joms.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008 May;100(10):712–720. doi: 10.1093/jnci/djn125. Available from: http://dx.doi.org/10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 29.van der Putten L, van den Broek GB, de Bree R, van den Brekel MW, Balm AJ, Hoebers FJ, Doornaert P, Leemans CR, Rasch CR. Effectiveness of salvage selective and modified radical neck dissection for regional pathologic lymphadenopathy after chemoradiation. Head Neck. 2009 May;31(5):593–603. doi: 10.1002/hed.20987. Available from: http://dx.doi.org/10.1002/hed.20987. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Kim JS, Yi JS, Lee JH, Choi SH, Nam SY, Cho KJ, Lee SW, Kim SB, Roh JL. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing salvage surgery for head and neck squamous cell carcinoma. Ann Surg Oncol. 2011 Sep;18(9):2579–2584. doi: 10.1245/s10434-011-1655-x. Available from: http://dx.doi.org/10.1245/s10434-011-1655-x. [DOI] [PubMed] [Google Scholar]
- 31.Yao M, Graham MM, Hoffman HT, Smith RB, Funk GF, Graham SM, Dornfeld KJ, Skwarchuk M, Menda Y, Buatti JM. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001–1010. doi: 10.1016/j.ijrobp.2004.01.040. Available from: http://dx.doi.org/10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Ong SC, Schöder H, Lee NY, Patel SG, Carlson D, Fury M, Pfister DG, Shah JP, Larson SM, Kraus DH. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med. 2008 Apr;49(4):532–540. doi: 10.2967/jnumed.107.044792. Available from: http://dx.doi.org/10.2967/jnumed.107.044792. [DOI] [PubMed] [Google Scholar]
- 33.Connell CA, Corry J, Milner AD, Hogg A, Hicks RJ, Rischin D, Peters LJ. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007 Nov;29(11):986–995. doi: 10.1002/hed.20629. Available from: http://dx.doi.org/10.1002/hed.20629. [DOI] [PubMed] [Google Scholar]
- 34.Sher DJ, Tishler RB, Annino D, Punglia RS. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol. 2010 May;21(5):1072–1077. doi: 10.1093/annonc/mdp405. Available from: http://dx.doi.org/10.1093/annonc/mdp405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corry J, Peters L, Fisher R, Macann A, Jackson M, McClure B, Rischin D. N2-N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders-results of Trans Tasman Radiation Oncology Group Study 98.02. Head Neck. 2008 Jun;30(6):737–742. doi: 10.1002/hed.20769. Available from: http://dx.doi.org/10.1002/hed.20769. [DOI] [PubMed] [Google Scholar]
- 36.Devaney SL, Ferlito A, Rinaldo A, Devaney KO. The pathology of neck dissection in cancer of the larynx. ORL J Otorhinolaryngol Relat Spec. 2000 Jul-Aug;62(4):204–211. doi: 10.1159/000027747. Available from: http://dx.doi.org/27747. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldo A, Devaney KO, Ferlito A. Immunohistochemical studies in the identification of lymph node micrometastases in patients with squamous cell carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2004;66(1):38–41. doi: 10.1159/000077232. Available from: http://dx.doi.org/10.1159/000077232. [DOI] [PubMed] [Google Scholar]
- 38.Becker MT, Shores CG, Yu KK, Yarbrough WG. Molecular assay to detect metastatic head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004 Jan;130(1):21–27. doi: 10.1001/archotol.130.1.21. Available from: http://dx.doi.org/10.1001/archotol.130.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki Y, Chiba I, Hirai A, Satoh C, Sakakibara N, Notani K, Iizuka T, Totsuka Y. Clinical value of genetically diagnosed lymph node micrometastasis for patients with oral squamous cell carcinoma. Head Neck. 2005 Aug;27(8):676–681. doi: 10.1002/hed.20200. Available from: http://dx.doi.org/10.1002/hed.20200. [DOI] [PubMed] [Google Scholar]
- 40.Werner JA, Dünne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003 Apr;25(4):322–332. doi: 10.1002/hed.10257. Available from: http://dx.doi.org/10.1002/hed.10257. [DOI] [PubMed] [Google Scholar]
- 41.Cantù G, Bimbi G, Miceli R, Mariani L, Colombo S, Riccio S, Squadrelli M, Battisti A, Pompilio M, Rossi M. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008 Feb;134(2):170–177. doi: 10.1001/archoto.2007.30. Available from: http://dx.doi.org/10.1001/archoto.2007.30. [DOI] [PubMed] [Google Scholar]
- 42.Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of "skip metastases" in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997 Jan;19(1):14–19. doi: 10.1002/(SICI)1097-0347(199701)19:1<14::AID-HED3>3.0.CO;2-Y. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199701)19:1<14::AID-HED3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, Ord RA, Wei WI, Zhao J. Sublingual lymph node metastasis of early tongue cancer: report of two cases and review of the literature. Int J Oral Maxillofac Surg. 2011 Jun;40(6):597–600. doi: 10.1016/j.ijom.2010.12.009. Available from: http://dx.doi.org/10.1016/j.ijom.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Asakage T, Yokose T, Mukai K, Tsugane S, Tsubono Y, Asai M, Ebihara S. Tumor thickness predicts cervical metastasis in patients with stage I/II carcinoma of the tongue. Cancer. 1998 Apr;82(8):1443–1448. doi: 10.1002/(SICI)1097-0142(19980415)82:8<1443::AID-CNCR2>3.0.CO;2-A. Available from: http://dx.doi.org/10.1002/(SICI)1097-0142(19980415)82:8<1443::AID-CNCR2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 45.Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009 Apr;115(7):1489–1497. doi: 10.1002/cncr.24161. Available from: http://dx.doi.org/10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 46.Preda L, Chiesa F, Calabrese L, Latronico A, Bruschini R, Leon ME, Renne G, Bellomi M. Relationship between histologic thickness of tongue carcinoma and thickness estimated from preoperative MRI. Eur Radiol. 2006 Oct;16(10):2242–2248. doi: 10.1007/s00330-006-0263-9. Available from: http://dx.doi.org/10.1007/s00330-006-0263-9. [DOI] [PubMed] [Google Scholar]
- 47.Okura M, Iida S, Aikawa T, Adachi T, Yoshimura N, Yamada T, Kogo M. Tumor thickness and paralingual distance of coronal MR imaging predicts cervical node metastases in oral tongue carcinoma. AJNR Am J Neuroradiol. 2008 Jan;29(1):45–50. doi: 10.3174/ajnr.A0749. Available from: http://dx.doi.org/10.3174/ajnr.A0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King AD, Ahuja AT, Leung SF, Lam WW, Teo P, Chan YL, Metreweli C. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck. 2000 May;22(3):275–281. doi: 10.1002/(SICI)1097-0347(200005)22:3<275::AID-HED10>3.0.CO;2-N. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(200005)22:3<275::AID-HED10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun Y, Liao X, Lin A, Liu M, Li L, Ma J. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer. 2009 Feb;115(3):680–688. doi: 10.1002/cncr.24049. Available from: http://dx.doi.org/10.1002/cncr.24049. [DOI] [PubMed] [Google Scholar]
- 50.Ng SH, Chang JT, Chan SC, Ko SF, Wang HM, Liao CT, Chang YC, Yen TC. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging. 2004 Aug;31(8):1073–1080. doi: 10.1007/s00259-004-1498-9. Available from: http://dx.doi.org/10.1007/s00259-004-1498-9. [DOI] [PubMed] [Google Scholar]
- 51.Liu LZ, Zhang GY, Xie CM, Liu XW, Cui CY, Li L. Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys. 2006 Nov;66(3):721–730. doi: 10.1016/j.ijrobp.2006.05.054. Available from: http://dx.doi.org/10.1016/j.ijrobp.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 52.Lee AW, Foo W, Law SC, Poon YF, Sze WM, O SK, Tung SY, Lau WH. Nasopharyngeal carcinoma: presenting symptoms and duration before diagnosis. Hong Kong Med J. 1997 Dec;3(4):355–361. [PubMed] [Google Scholar]
- 53.Shimizu K, Inoue H, Saitoh M, Ohtsuki N, Ishida H, Makino K, Amatsu M, Nibu K. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol. 2006 Aug;126(8):872–877. doi: 10.1080/00016480500504259. Available from: http://dx.doi.org/10.1080/00016480500504259. [DOI] [PubMed] [Google Scholar]
- 54.Gross ND, Ellingson TW, Wax MK, Cohen JI, Andersen PE. Impact of retropharyngeal lymph node metastasis in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004 Feb;130(2):169–173. doi: 10.1001/archotol.130.2.169. Available from: http://dx.doi.org/10.1001/archotol.130.2.169. [DOI] [PubMed] [Google Scholar]
- 55.Tauzin M, Rabalais A, Hagan JL, Wood CG, Ferris RL, Walvekar RR. PET-CT staging of the neck in cancers of the oropharynx: patterns of regional and retropharyngeal nodal metastasis. World J Surg Oncol. 2010;8:70. doi: 10.1186/1477-7819-8-70. Available from: http://dx.doi.org/10.1186/1477-7819-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dirix P, Nuyts S, Bussels B, Hermans R, Van den Bogaert W. Prognostic influence of retropharyngeal lymph node metastasis in squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2006 Jul;65(3):739–744. doi: 10.1016/j.ijrobp.2006.02.027. Available from: http://dx.doi.org/10.1016/j.ijrobp.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 57.Mendelsohn AH, Lai CK, Shintaku IP, Elashoff DA, Dubinett SM, Abemayor E, St John MA. Histopathologic findings of HPV and p16 positive HNSCC. Laryngoscope. 2010 Sep;120(9):1788–1794. doi: 10.1002/lary.21044. Available from: http://dx.doi.org/10.1002/lary.21044. [DOI] [PubMed] [Google Scholar]
- 58.Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, Kim MS. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012 Jan;34(1):10–14. doi: 10.1002/hed.21697. Available from: http://dx.doi.org/10.1002/hed.21697. [DOI] [PubMed] [Google Scholar]
- 59.Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPV+ and HPV- HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009 Jul;31(7):911–918. doi: 10.1002/hed.21040. Available from: http://dx.doi.org/10.1002/hed.21040. [DOI] [PubMed] [Google Scholar]
- 60.Iyer NG, Shaha AR, Ferlito A, Thomas Robbins K, Medina JE, Silver CE, Rinaldo A, Takes RP, Suárez C, Rodrigo JP, Bradley PJ, Werner JA. Delphian node metastasis in head and neck cancers--oracle or myth? J Surg Oncol. 2010 Sep;102(4):354–358. doi: 10.1002/jso.21640. Available from: http://dx.doi.org/10.1002/jso.21640. [DOI] [PubMed] [Google Scholar]
- 61.Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990 May-Jun;12(3):197–203. doi: 10.1002/hed.2880120302. Available from: http://dx.doi.org/10.1002/hed.2880120302. [DOI] [PubMed] [Google Scholar]
- 62.Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck. 2000 Jul;22(4):380–385. doi: 10.1002/1097-0347(200007)22:4<380::AID-HED11>3.0.CO;2-E. Available from: http://dx.doi.org/10.1002/1097-0347(200007)22:4<380::AID-HED11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 63.Kamiyama R, Saikawa M, Kishimoto S. Significance of retropharyngeal lymph node dissection in hypopharyngeal cancer. Jpn J Clin Oncol. 2009 Oct;39(10):632–637. doi: 10.1093/jjco/hyp080. Available from: http://dx.doi.org/10.1093/jjco/hyp080. [DOI] [PubMed] [Google Scholar]
- 64.Chan SC, Lin CY, Ng SH, Chang JT, Wang HM, Liao CT, Lo CW, Yen TC. 18F-FDG PET for retropharyngeal lymph node metastasis in oropharyngeal and hypopharyngeal cancers: impact on diagnosis and prediction analysis. Nucl Med Commun. 2010 Mar;31(3):260–265. doi: 10.1097/MNM.0b013e3283360133. Available from: http://dx.doi.org/10.1097/MNM.0b013e3283360133. [DOI] [PubMed] [Google Scholar]
- 65.Amatsu M, Mohri M, Kinishi M. Significance of retropharyngeal node dissection at radical surgery for carcinoma of the hypopharynx and cervical esophagus. Laryngoscope. 2001 Jun;111(6):1099–1103. doi: 10.1097/00005537-200106000-00031. Available from: http://dx.doi.org/10.1097/00005537-200106000-00031. [DOI] [PubMed] [Google Scholar]
- 66.Rudmik L, Lau HY, Matthews TW, Bosch JD, Kloiber R, Molnar CP, Dort JC. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011 Jul;33(7):935–940. doi: 10.1002/hed.21566. Available from: http://dx.doi.org/10.1002/hed.21566. [DOI] [PubMed] [Google Scholar]
- 67.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009 Mar;19(3):731–744. doi: 10.1007/s00330-008-1194-4. Available from: http://dx.doi.org/10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham MM, Badawi RD, Wahl RL. Variations in PET/CT methodology for oncologic imaging at U.S. academic medical centers: an imaging response assessment team survey. J Nucl Med. 2011 Feb;52(2):311–317. doi: 10.2967/jnumed.109.074104. Available from: http://dx.doi.org/10.2967/jnumed.109.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strojan P, Ferlito A, Langendijk JA, Corry J, Woolgar JA, Rinaldo A, Silver CE, Paleri V, Fagan JJ, Pellitteri PK, Haigentz M, Jr, Suárez C, Robbins KT, Rodrigo JP, Olsen KD, Hinni ML, Werner JA, Mondin V, Kowalski LP, Devaney KO, de Bree R, Takes RP, Wolf GT, Shaha AR, Genden EM, Barnes L. Contemporary management of lymph node metastases from an unknown primary to the neck: II. A review of therapeutic options. Head Neck. 2011 Oct; doi: 10.1002/hed.21899. Available from: http://dx.doi.org/10.1002/hed.21899. [DOI] [PubMed] [Google Scholar]
- 70.Reddy SP, Marks JE. Metastatic carcinoma in the cervical lymph nodes from an unknown primary site: results of bilateral neck plus mucosal irradiation vs. ipsilateral neck irradiation. Int J Radiat Oncol Biol Phys. 1997 Mar;37(4):797–802. doi: 10.1016/S0360-3016(97)00025-4. Available from: http://dx.doi.org/10.1016/S0360-3016(97)00025-4. [DOI] [PubMed] [Google Scholar]
- 71.Aslani M, Sultanem K, Voung T, Hier M, Niazi T, Shenouda G. Metastatic carcinoma to the cervical nodes from an unknown head and neck primary site: Is there a need for neck dissection? Head Neck. 2007 Jun;29(6):585–590. doi: 10.1002/hed.20581. Available from: http://dx.doi.org/10.1002/hed.20581. [DOI] [PubMed] [Google Scholar]
- 72.Nieder C, Gregoire V, Ang KK. Cervical lymph node metastases from occult squamous cell carcinoma: cut down a tree to get an apple? Int J Radiat Oncol Biol Phys. 2001;50:727–733. doi: 10.1016/s0360-3016(01)01462-6. [DOI] [PubMed] [Google Scholar]
- 73.Jereczek-Fossa BA, Jassem J, Orecchia R. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat Rev. 2004 Apr;30(2):153–164. doi: 10.1016/j.ctrv.2003.10.001. Available from: http://dx.doi.org/10.1016/j.ctrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Villeneuve H, Després P, Fortin B, Filion E, Donath D, Soulières D, Guertin L, Ayad T, Christopoulos A, Nguyen-Tan PF. Cervical lymph node metastases from unknown primary cancer: a single-institution experience with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012 Apr;82(5):1866–1871. doi: 10.1016/j.ijrobp.2011.02.031. Available from: http://dx.doi.org/10.1016/j.ijrobp.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 75.Shoushtari A, Saylor D, Kerr KL, Sheng K, Thomas C, Jameson M, Reibel J, Shonka D, Levine P, Read P. Outcomes of patients with head-and-neck cancer of unknown primary origin treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2011 Nov;81(3):e83–e91. doi: 10.1016/j.ijrobp.2011.01.014. Available from: http://dx.doi.org/10.1016/j.ijrobp.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 76.van den Brekel MW, van der Waal I, Meijer CJ, Freeman JL, Castelijns JA, Snow GB. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope. 1996 Aug;106(8):987–991. doi: 10.1097/00005537-199608000-00014. Available from: http://dx.doi.org/10.1097/00005537-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Werner JA, Dünne AA, Ramaswamy A, Folz BJ, Lippert BM, Moll R, Behr T. Sentinel node detection in N0 cancer of the pharynx and larynx. Br J Cancer. 2002 Sep;87(7):711–715. doi: 10.1038/sj.bjc.6600445. Available from: http://dx.doi.org/10.1038/sj.bjc.6600445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Werner JA. Selective sentinel lymphadenectomy for head and neck squamous cell carcinoma. Cancer Treat Res. 2005;127:187–206. doi: 10.1007/0-387-23604-X_10. Available from: http://dx.doi.org/10.1007/0-387-23604-X_10. [DOI] [PubMed] [Google Scholar]
- 79.Santaolalla F, Sanchez JM, Ereno C, Sanchez A, Martinez A. Comparative study of patients with and without sentinel lymph node biopsy (SLNB) in oral and oropharyngeal cancer: is SLNB an accurate and useful procedure? Acta Otolaryngol. 2009 Feb;129(2):199–204. doi: 10.1080/00016480802032827. Available from: http://dx.doi.org/10.1080/00016480802032827. [DOI] [PubMed] [Google Scholar]
- 80.Werner JA, Dünne AA, Ramaswamy A, Brandt D, Külkens C, Folz BJ, Moll R, Lippert BM. Das Sentinel Node Konzept bei Plattenepithelkarzinomen der oberen Luft- und Speisewege - eine kritische Analyse an 100 Patienten. [The sentinel node concept in head and neck squamous cell carcinoma--a critical analysis in 100 patients]. Laryngorhinootologie. 2002 Jan;81(1):31–39. doi: 10.1055/s-2002-20114. (Ger). Available from: http://dx.doi.org/10.1055/s-2002-20114. [DOI] [PubMed] [Google Scholar]
- 81.Werner JA, Dünne AA, Folz BJ, Moll R, Behr T. Value of sentinel lymphadenectomy in head and neck cancer. Ann Surg Oncol. 2004 Mar;11(3 Suppl):267S–270S. doi: 10.1007/BF02523643. [DOI] [PubMed] [Google Scholar]
- 82.Werner JA, Dünne AA, Brandt D, Ramaswamy A, Külkens C, Lippert BM, Folz BJ, Joseph K, Moll R. Untersuchungen zum Stellenwert der Sentinel Lymphonodektomie bei Karzinomen des Pharynx und Larynx. [Studies on significance of sentinel lymphadenectomy in pharyngeal and laryngeal carcinoma]. Laryngorhinootologie. 1999 Dec;78(12):663–670. doi: 10.1055/s-1999-8768. (Ger). Available from: http://dx.doi.org/10.1055/s-1999-8768. [DOI] [PubMed] [Google Scholar]
- 83.Werner JA, Dünne AA, Ramaswamy A, Dalchow C, Behr T, Moll R, Folz BJ, Davis RK. The sentinel node concept in head and neck cancer: solution for the controversies in the N0 neck? Head Neck. 2004 Jul;26(7):603–611. doi: 10.1002/hed.20062. Available from: http://dx.doi.org/10.1002/hed.20062. [DOI] [PubMed] [Google Scholar]
- 84.End results of a prospective trial on elective lateral neck dissection vs type III modified radical neck dissection in the management of supraglottic and transglottic carcinomas. Head Neck. 1999;21:694–702. doi: 10.1002/(SICI)1097-0347(199912)21:8<694::AID-HED3>3.0.CO;2-B. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199912)21:8<694::AID-HED3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 85.Ferlito A, Silver CE, Rinaldo A. Selective neck dissection (IIA, III): a rational replacement for complete functional neck dissection in patients with N0 supraglottic and glottic squamous carcinoma. Laryngoscope. 2008 Apr;118(4):676–679. doi: 10.1097/MLG.0b013e31815f6f25. Available from: http://dx.doi.org/10.1097/MLG.0b013e31815f6f25. [DOI] [PubMed] [Google Scholar]
- 86.Coskun HH, Erisen L, Basut O. Selective neck dissection for clinically N0 neck in laryngeal cancer: is dissection of level IIb necessary? Otolaryngol Head Neck Surg. 2004 Nov;131(5):655–659. doi: 10.1016/j.otohns.2004.04.014. Available from: http://dx.doi.org/10.1016/j.otohns.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 87.Elsheikh MN, Mahfouz ME, Salim EI, Elsheikh EA. Molecular assessment of neck dissections supports preserving level IIB lymph nodes in selective neck dissection for laryngeal squamous cell carcinoma with a clinically negative neck. ORL J Otorhinolaryngol Relat Spec. 2006;68(3):177–184. doi: 10.1159/000091396. Available from: http://dx.doi.org/10.1159/000091396. [DOI] [PubMed] [Google Scholar]
- 88.Sezen OS, Kubilay U, Haytoglu S, Unver S. Frequency of metastases at the area of the supraretrospinal (level IIB) lymph node in laryngeal cancer. Head Neck. 2007 Dec;29(12):1111–1114. doi: 10.1002/hed.20646. Available from: http://dx.doi.org/10.1002/hed.20646. [DOI] [PubMed] [Google Scholar]
- 89.Paleri V, Kumar Subramaniam S, Oozeer N, Rees G, Krishnan S. Dissection of the submuscular recess (sublevel IIb) in squamous cell cancer of the upper aerodigestive tract: prospective study and systematic review of the literature. Head Neck. 2008 Feb;30(2):194–200. doi: 10.1002/hed.20682. Available from: http://dx.doi.org/10.1002/hed.20682. [DOI] [PubMed] [Google Scholar]
- 90.Cağli S, Yüce I, Güney E. Is routine inclusion of level IV necessary in neck dissection for clinically N0 supraglottic carcinoma? Otolaryngol Head Neck Surg. 2007 Feb;136(2):287–290. doi: 10.1016/j.otohns.2006.08.009. Available from: http://dx.doi.org/10.1016/j.otohns.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 91.Ferlito A, Silver CE, Suárez C, Rinaldo A. Preliminary multi-institutional prospective pathologic and molecular studies support preservation of sublevel IIB and level IV for laryngeal squamous carcinoma with clinically negative neck. Eur Arch Otorhinolaryngol. 2007 Feb;264(2):111–114. doi: 10.1007/s00405-006-0209-5. Available from: http://dx.doi.org/10.1007/s00405-006-0209-5. [DOI] [PubMed] [Google Scholar]
- 92.Kim YH, Koo BS, Lim YC, Lee JS, Kim SH, Choi EC. Lymphatic metastases to level IIb in hypopharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006 Oct;132(10):1060–1064. doi: 10.1001/archotol.132.10.1060. Available from: http://dx.doi.org/10.1001/archotol.132.10.1060. [DOI] [PubMed] [Google Scholar]
- 93.Celik B, Coskun H, Kumas FF, Irdesel J, Zarifoglu M, Erisen L, Onart S. Accessory nerve function after level 2b-preserving selective neck dissection. Head Neck. 2009 Nov;31(11):1496–1501. doi: 10.1002/hed.21112. Available from: http://dx.doi.org/10.1002/hed.21112. [DOI] [PubMed] [Google Scholar]
- 94.Wiegand S, Esters J, Müller HH, Jäcker T, Roessler M, Werner JA, Sesterhenn AM. Is it necessary to dissect levels I and IIB in hypopharyngeal cancer? Acta Otolaryngol. 2010 Jun;130(6):747–752. doi: 10.3109/00016480903384168. Available from: http://dx.doi.org/10.3109/00016480903384168. [DOI] [PubMed] [Google Scholar]
- 95.Jaguar GC, Lima EN, Kowalski LP, Pellizon AC, Carvalho AL, Alves FA. Impact of submandibular gland excision on salivary gland function in head and neck cancer patients. Oral Oncol. 2010 May;46(5):349–354. doi: 10.1016/j.oraloncology.2009.11.018. Available from: http://dx.doi.org/10.1016/j.oraloncology.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 96.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005 Dec;27(12):1080–1091. doi: 10.1002/hed.20275. Available from: http://dx.doi.org/10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- 97.Razfar A, Walvekar RR, Melkane A, Johnson JT, Myers EN. Incidence and patterns of regional metastasis in early oral squamous cell cancers: feasibility of submandibular gland preservation. Head Neck. 2009 Dec;31(12):1619–1623. doi: 10.1002/hed.21129. Available from: http://dx.doi.org/10.1002/hed.21129. [DOI] [PubMed] [Google Scholar]
- 98.Takes RP, Robbins KT, Woolgar JA, Rinaldo A, Silver CE, Olofsson J, Ferlito A. Questionable necessity to remove the submandibular gland in neck dissection. Head Neck. 2011 May;33(5):743–745. doi: 10.1002/hed.21451. Available from: http://dx.doi.org/10.1002/hed.21451. [DOI] [PubMed] [Google Scholar]
- 99.Dhiwakar M, Ronen O, Malone J, Rao K, Bell S, Phillips R, Shevlin B, Robbins KT. Feasibility of submandibular gland preservation in neck dissection: A prospective anatomic-pathologic study. Head Neck. 2011 May;33(5):603–609. doi: 10.1002/hed.21499. Available from: http://dx.doi.org/10.1002/hed.21499. [DOI] [PubMed] [Google Scholar]
- 100.Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, Perez CA. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001 Jun;111(6):1079–1087. doi: 10.1097/00005537-200106000-00028. Available from: http://dx.doi.org/10.1097/00005537-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 101.Brugère JM, Mosseri VF, Mamelle G, David JM, Buisset E, Vallicioni J, de Raucourt D, Szpirglas HJ, Asselain BJ. Nodal failures in patients with NO N+ oral squamous cell carcinoma without capsular rupture. Head Neck. 1996 Mar-Apr;18(2):133–137. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<133::AID-HED4>3.0.CO;2-2. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199603/04)18:2<133::AID-HED4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 102.Byers RM, Clayman GL, McGill D, Andrews T, Kare RP, Roberts DB, Goepfert H. Selective neck dissections for squamous carcinoma of the upper aerodigestive tract: patterns of regional failure. Head Neck. 1999 Sep;21(6):499–505. doi: 10.1002/(SICI)1097-0347(199909)21:6<499::AID-HED1>3.0.CO;2-A. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199909)21:6<499::AID-HED1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 103.Hanna E, Alexiou M, Morgan J, Badley J, Maddox AM, Penagaricano J, Fan CY, Breau R, Suen J. Intensive chemoradiotherapy as a primary treatment for organ preservation in patients with advanced cancer of the head and neck: efficacy, toxic effects, and limitations. Arch Otolaryngol Head Neck Surg. 2004 Jul;130(7):861–867. doi: 10.1001/archotol.130.7.861. Available from: http://dx.doi.org/10.1001/archotol.130.7.861. [DOI] [PubMed] [Google Scholar]
- 104.Wee JT, Anderson BO, Corry J, D'Cruz A, Soo KC, Qian CN, Chua DT, Hicks RJ, Goh CH, Khoo JB, Ong SC, Forastiere AA, Chan AT. Management of the neck after chemoradiotherapy for head and neck cancers in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009 Nov;10(11):1086–1092. doi: 10.1016/S1470-2045(09)70266-9. Available from: http://dx.doi.org/10.1016/S1470-2045(09)70266-9. [DOI] [PubMed] [Google Scholar]
- 105.Johnson CR, Silverman LN, Clay LB, Schmidt-Ullrich R. Radiotherapeutic management of bulky cervical lymphadenopathy in squamous cell carcinoma of the head and neck: is postradiotherapy neck dissection necessary? Radiat Oncol Investig. 1998;6(1):52–57. doi: 10.1002/(SICI)1520-6823(1998)6:1<52::AID-ROI6>3.0.CO;2-H. Available from: http://dx.doi.org/10.1002/(SICI)1520-6823(1998)6:1<52::AID-ROI6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 106.Mukhija V, Gupta S, Jacobson AS, Eloy JA, Genden EM. Selective neck dissection following adjuvant therapy for advanced head and neck cancer. Head Neck. 2009 Feb;31(2):183–188. doi: 10.1002/hed.20944. Available from: http://dx.doi.org/10.1002/hed.20944. [DOI] [PubMed] [Google Scholar]
- 107.Dhiwakar M, Robbins KT, Vieira F, Rao K, Malone J. Selective neck dissection as an early salvage intervention for clinically persistent nodal disease following chemoradiation. Head Neck. 2012 Feb;34(2):188–193. doi: 10.1002/hed.21707. Available from: http://dx.doi.org/10.1002/hed.21707. [DOI] [PubMed] [Google Scholar]
- 108.Sewall GK, Palazzi-Churas KL, Richards GM, Hartig GK, Harari PM. Planned postradiotherapy neck dissection: Rationale and clinical outcomes. Laryngoscope. 2007 Jan;117(1):121–128. doi: 10.1097/01.mlg.0000246709.93530.72. Available from: http://dx.doi.org/10.1097/01.mlg.0000246709.93530.72. [DOI] [PubMed] [Google Scholar]
- 109.Forest VI, Nguyen-Tan PF, Tabet JC, Olivier MJ, Larochelle D, Fortin B, Gélinas M, Soulières D, Charpentier D, Guertin L. Role of neck dissection following concurrent chemoradiation for advanced head and neck carcinoma. Head Neck. 2006 Dec;28(12):1099–1105. doi: 10.1002/hed.20479. Available from: http://dx.doi.org/10.1002/hed.20479. [DOI] [PubMed] [Google Scholar]
- 110.Ballonoff A, Raben D, Rusthoven KE, Bassetti M, Kane M, Song JI, Chen C. Outcomes of patients with n3 neck nodes treated with chemoradiation. Laryngoscope. 2008 Jun;118(6):995–998. doi: 10.1097/MLG.0b013e31816a7120. Available from: http://dx.doi.org/10.1097/MLG.0b013e31816a7120. [DOI] [PubMed] [Google Scholar]
- 111.Inoue H, Nibu K, Saito M, Otsuki N, Ishida H, Onitsuka T, Fujii T, Kawabata K, Saikawa M. Quality of life after neck dissection. Arch Otolaryngol Head Neck Surg. 2006 Jun;132(6):662–666. doi: 10.1001/archotol.132.6.662. Available from: http://dx.doi.org/10.1001/archotol.132.6.662. [DOI] [PubMed] [Google Scholar]
- 112.Nibu K, Ebihara Y, Ebihara M, Kawabata K, Onitsuka T, Fujii T, Saikawa M. Quality of life after neck dissection: a multicenter longitudinal study by the Japanese Clinical Study Group on Standardization of Treatment for Lymph Node Metastasis of Head and Neck Cancer. Int J Clin Oncol. 2010 Feb;15(1):33–38. doi: 10.1007/s10147-009-0020-6. Available from: http://dx.doi.org/10.1007/s10147-009-0020-6. [DOI] [PubMed] [Google Scholar]
- 113.Kerawala CJ, Heliotos M. Prevention of complications in neck dissection. Head Neck Oncol. 2009;1:35. doi: 10.1186/1758-3284-1-35. Available from: http://dx.doi.org/10.1186/1758-3284-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cappiello J, Piazza C, Berlucchi M, Peretti G, De Zinis LO, Maroldi R, Nicolai P. Internal jugular vein patency after lateral neck dissection: a prospective study. Eur Arch Otorhinolaryngol. 2002 Sep;259(8):409–412. doi: 10.1007/s00405-002-0479-5. Available from: http://dx.doi.org/10.1007/s00405-002-0479-5. [DOI] [PubMed] [Google Scholar]
- 115.de Gier HH, Balm AJ, Bruning PF, Gregor RT, Hilgers FJ. Systematic approach to the treatment of chylous leakage after neck dissection. Head Neck. 1996 Jul-Aug;18(4):347–351. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<347::AID-HED6>3.0.CO;2-Y. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199607/08)18:4<347::AID-HED6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 116.Prim MP, De Diego JI, Verdaguer JM, Sastre N, Rabanal I. Neurological complications following functional neck dissection. Eur Arch Otorhinolaryngol. 2006 May;263(5):473–476. doi: 10.1007/s00405-005-1028-9. Available from: http://dx.doi.org/10.1007/s00405-005-1028-9. [DOI] [PubMed] [Google Scholar]
- 117.Cheng PT, Hao SP, Lin YH, Yeh AR. Objective comparison of shoulder dysfunction after three neck dissection techniques. Ann Otol Rhinol Laryngol. 2000 Aug;109(8 Pt 1):761–766. doi: 10.1177/000348940010900811. [DOI] [PubMed] [Google Scholar]
- 118.Köybasioglu A, Tokcaer AB, Uslu S, Ileri F, Beder L, Ozbilen S. Accessory nerve function after modified radical and lateral neck dissections. Laryngoscope. 2000 Jan;110(1):73–77. doi: 10.1097/00005537-200001000-00014. Available from: http://dx.doi.org/10.1097/00005537-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 119.Cappiello J, Piazza C, Giudice M, De Maria G, Nicolai P. Shoulder disability after different selective neck dissections (levels II-IV versus levels II-V): a comparative study. Laryngoscope. 2005 Feb;115(2):259–263. doi: 10.1097/01.mlg.0000154729.31281.da. Available from: http://dx.doi.org/10.1097/01.mlg.0000154729.31281.da. [DOI] [PubMed] [Google Scholar]
- 120.Schiefke F, Akdemir M, Weber A, Akdemir D, Singer S, Frerich B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck. 2009 Apr;31(4):503–512. doi: 10.1002/hed.21001. Available from: http://dx.doi.org/10.1002/hed.21001. [DOI] [PubMed] [Google Scholar]
- 121.Teymoortash A, Hoch S, Eivazi B, Werner JA. Postoperative morbidity after different types of selective neck dissection. Laryngoscope. 2010 May;120(5):924–929. doi: 10.1002/lary.20894. Available from: http://dx.doi.org/10.1002/lary.20894. [DOI] [PubMed] [Google Scholar]
- 122.Donatelli-Lassig AA, Duffy SA, Fowler KE, Ronis DL, Chepeha DB, Terrell JE. The effect of neck dissection on quality of life after chemoradiation. Otolaryngol Head Neck Surg. 2008 Oct;139(4):511–518. doi: 10.1016/j.otohns.2008.07.007. Available from: http://dx.doi.org/10.1016/j.otohns.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dulguerov P, Leuchter I, Szalay-Quinodoz I, Allal AS, Marchal F, Lehmann W, Fasel JH. Endoscopic neck dissection in human cadavers. Laryngoscope. 2001 Dec;111(12):2135–2139. doi: 10.1097/00005537-200112000-00010. Available from: http://dx.doi.org/10.1097/00005537-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Terris DJ, Monfared A, Thomas A, Kambham N, Sáenz Y. Endoscopic selective neck dissection in a porcine model. Arch Otolaryngol Head Neck Surg. 2003 Jun;129(6):613–617. doi: 10.1001/archotol.129.6.613. Available from: http://dx.doi.org/10.1001/archotol.129.6.613. [DOI] [PubMed] [Google Scholar]
- 125.Hartl DM, Ferlito A, Silver CE, Takes RP, Stoeckli SJ, Suárez C, Rodrigo JP, Sesterhenn AM, Snyderman CH, Terris DJ, Genden EM, Rinaldo A. Minimally invasive techniques for head and neck malignancies: current indications, outcomes and future directions. Eur Arch Otorhinolaryngol. 2011 Sep;268(9):1249–1257. doi: 10.1007/s00405-011-1620-0. Available from: http://dx.doi.org/10.1007/s00405-011-1620-0. [DOI] [PubMed] [Google Scholar]
- 126.Werner JA, Sapundzhiev NR, Teymoortash A, Dünne AA, Behr T, Folz BJ. Endoscopic sentinel lymphadenectomy as a new diagnostic approach in the N0 neck. Eur Arch Otorhinolaryngol. 2004 Oct;261(9):463–468. doi: 10.1007/s00405-003-0706-8. Available from: http://dx.doi.org/10.1007/s00405-003-0706-8. [DOI] [PubMed] [Google Scholar]
- 127.Sesterhenn AM, Folz BJ, Werner JA. Surgical technique of endoscopic sentinel lymphadenectomy in the N0 neck. Oper Tech Otolaryngol Head Neck Surg. 2008;19:26–32. doi: 10.1016/j.otot.2008.03.010. Available from: http://dx.doi.org/10.1016/j.otot.2008.03.010. [DOI] [Google Scholar]
- 128.Richtsmeier WJ. Dissecting the "endoscopic neck". Arch Otolaryngol Head Neck Surg. 2003 Jun;129(6):612. doi: 10.1001/archotol.129.6.612. Available from: http://dx.doi.org/10.1001/archotol.129.6.612. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009 Apr;9(4):274–284. doi: 10.1038/nrc2622. Available from: http://dx.doi.org/10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 130.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003 Jun;100(13):7737–7742. doi: 10.1073/pnas.1331931100. Available from: http://dx.doi.org/10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hölzel D, Engel J, Löhrs U. Sind elektive Lymphknotendissektionen in der Karzinomchirurgie noch zeitgemäss? [Elective lymph node dissections--still a standard in cancer surgery?]. Zentralbl Chir. 2008 Dec;133(6):582–589. doi: 10.1055/s-0028-1098738. (Ger). Available from: http://dx.doi.org/10.1055/s-0028-1098738. [DOI] [PubMed] [Google Scholar]
- 132.Gervasoni JE, Jr, Sbayi S, Cady B. Role of lymphadenectomy in surgical treatment of solid tumors: an update on the clinical data. Ann Surg Oncol. 2007 Sep;14(9):2443–2462. doi: 10.1245/s10434-007-9360-5. Available from: http://dx.doi.org/10.1245/s10434-007-9360-5. [DOI] [PubMed] [Google Scholar]
- 133.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001 Dec;92(12):3030–3036. doi: 10.1002/1097-0142(20011215)92:12<3030::AID-CNCR10148>3.0.CO;2-P. Available from: http://dx.doi.org/10.1002/1097-0142(20011215)92:12<3030::AID-CNCR10148>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 134.Haddadin KJ, Soutar DS, Oliver RJ, Webster MH, Robertson AG, MacDonald DG. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999 Sep;21(6):517–525. doi: 10.1002/(SICI)1097-0347(199909)21:6<517::AID-HED4>3.0.CO;2-C. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199909)21:6<517::AID-HED4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 135.Spiro RH, Huvos AG, Strong EW. Cancer of the parotid gland. A clinicopathologic study of 288 primary cases. Am J Surg. 1975 Oct;130(4):452–459. doi: 10.1016/0002-9610(75)90483-3. Available from: http://dx.doi.org/10.1016/0002-9610(75)90483-3. [DOI] [PubMed] [Google Scholar]
- 136.Medina JE. Neck dissection in the treatment of cancer of major salivary glands. Otolaryngol Clin North Am. 1998 Oct;31(5):815–822. doi: 10.1016/S0030-6665(05)70089-X. Available from: http://dx.doi.org/10.1016/S0030-6665(05)70089-X. [DOI] [PubMed] [Google Scholar]
- 137.Régis De Brito Santos I, Kowalski LP, Cavalcante De Araujo V, Flávia Logullo A, Magrin J. Multivariate analysis of risk factors for neck metastases in surgically treated parotid carcinomas. Arch Otolaryngol Head Neck Surg. 2001 Jan;127(1):56–60. doi: 10.1001/archotol.127.1.56. [DOI] [PubMed] [Google Scholar]
- 138.Frankenthaler RA, Byers RM, Luna MA, Callender DL, Wolf P, Goepfert H. Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg. 1993 May;119(5):517–520. doi: 10.1001/archotol.1993.01880170041008. Available from: http://dx.doi.org/10.1001/archotol.1993.01880170041008. [DOI] [PubMed] [Google Scholar]
- 139.Armstrong JG, Harrison LB, Thaler HT, Friedlander-Klar H, Fass DE, Zelefsky MJ, Shah JP, Strong EW, Spiro RH. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992 Feb;69(3):615–619. doi: 10.1002/1097-0142(19920201)69:3<615::AID-CNCR2820690303>3.0.CO;2-9. Available from: http://dx.doi.org/10.1002/1097-0142(19920201)69:3<615::AID-CNCR2820690303>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 140.Eneroth CM. Facial nerve paralysis. A criterion of malignancy in parotid tumors. Arch Otolaryngol. 1972 Apr;95(4):300–304. doi: 10.1001/archotol.1972.00770080494002. Available from: http://dx.doi.org/10.1001/archotol.1972.00770080494002. [DOI] [PubMed] [Google Scholar]
- 141.Conley J, Hamaker RC. Prognosis of malignant tumors of the parotid gland with facial paralysis. Arch Otolaryngol. 1975 Jan;101(1):39–41. doi: 10.1001/archotol.1975.00780300043012. Available from: http://dx.doi.org/10.1001/archotol.1975.00780300043012. [DOI] [PubMed] [Google Scholar]
- 142.Gallo O, Franchi A, Bianchi S, Boddi V, Giannelli E, Alajmo E. p53 oncoprotein expression in parotid gland carcinoma is associated with clinical outcome. Cancer. 1995 Apr;75(8):2037–2044. doi: 10.1002/1097-0142(19950415)75:8<2037::AID-CNCR2820750802>3.0.CO;2-V. Available from: http://dx.doi.org/10.1002/1097-0142(19950415)75:8<2037::AID-CNCR2820750802>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 143.Teymoortash A, Werner JA. Value of neck dissection in patients with cancer of the parotid gland and a clinical NO neck. Onkologie. 2002 Apr;25(2):122–126. doi: 10.1159/000055221. Available from: http://dx.doi.org/10.1159/000055221. [DOI] [PubMed] [Google Scholar]
- 144.Ferlito A, Shaha AR, Rinaldo A, Mondin V. Management of clinically negative cervical lymph nodes in patients with malignant neoplasms of the parotid gland. ORL J Otorhinolaryngol Relat Spec. 2001 May-Jun;63(3):123–126. doi: 10.1159/000055726. Available from: http://dx.doi.org/10.1159/000055726. [DOI] [PubMed] [Google Scholar]
- 145.Bhattacharyya N. Survival and prognosis for cancer of the submandibular gland. J Oral Maxillofac Surg. 2004 Apr;62(4):427–430. doi: 10.1016/j.joms.2003.06.012. Available from: http://dx.doi.org/10.1016/j.joms.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 146.Pohar S, Venkatesan V, Stitt LW, Hall SF, Hammond JA, Read N, Yoo J, Fung K, Pavamani S. Results in the management of malignant submandibular tumours and guidelines for elective neck treatment. J Otolaryngol Head Neck Surg. 2011 Jun;40(3):191–195. [PubMed] [Google Scholar]
- 147.Teymoortash A. Glandula parotis als Hauptmetastasierungsort der Plattenepithelkarzinome der Kopfhaut. [Parotid gland as the main site of metastasis of the cutaneous squamous cell carcinoma of the head]. Laryngorhinootologie. 2007 Oct;86(10):699–704. doi: 10.1055/s-2007-966813. (Ger). Available from: http://dx.doi.org/10.1055/s-2007-966813. [DOI] [PubMed] [Google Scholar]
- 148.Teymoortash A, Schultz ES, Werner JA. Klinische Bedeutung parotidealer Metastasen von Plattenepithelkarzinomen der Kopfhaut. [Significance of parotid metastases of squamous cell carcinoma of scalp]. Hautarzt. 2007 Apr;58(4):323–327. doi: 10.1007/s00105-006-1275-8. (Ger). Available from: http://dx.doi.org/10.1007/s00105-006-1275-8. [DOI] [PubMed] [Google Scholar]
- 149.O'Brien CJ, McNeil EB, McMahon JD, Pathak I, Lauer CS, Jackson MA. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002 May;24(5):417–422. doi: 10.1002/hed.10063. Available from: http://dx.doi.org/10.1002/hed.10063. [DOI] [PubMed] [Google Scholar]
- 150.Pellitteri PK, Takes RP, Lewis JS, Jr, Devaney KO, Harlor EJ, Strojan P, Rodrigo JP, Suárez C, Rinaldo A, Medina JE, Woolgar JA, Ferlito A. Merkel cell carcinoma of the head and neck. Head Neck. 2012 Sep;34(9):1346–1354. doi: 10.1002/hed.21787. Available from: http://dx.doi.org/10.1002/hed.21787. [DOI] [PubMed] [Google Scholar]
- 151.Kokoska ER, Kokoska MS, Collins BT, Stapleton DR, Wade TP. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997 Dec;174(6):688–693. doi: 10.1016/S0002-9610(97)00193-1. Available from: http://dx.doi.org/10.1016/S0002-9610(97)00193-1. [DOI] [PubMed] [Google Scholar]
- 152.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001 Aug;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 153.Guadagnolo BA, Zagars GK. Adjuvant radiation therapy for high-risk nodal metastases from cutaneous melanoma. Lancet Oncol. 2009 Apr;10(4):409–416. doi: 10.1016/S1470-2045(09)70043-9. Available from: http://dx.doi.org/10.1016/S1470-2045(09)70043-9. [DOI] [PubMed] [Google Scholar]
- 154.Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, Randolph GW, Stack BC, Jr, Steward DL, Terris DJ, Thompson GB, Tufano RP, Tuttle RM, Udelsman R. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009 Nov;19(11):1153–1158. doi: 10.1089/thy.2009.0159. Available from: http://dx.doi.org/10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 155.Shaha AR. Prophylactic central compartment dissection in thyroid cancer: a new avenue of debate. Surgery. 2009 Dec;146(6):1224–1227. doi: 10.1016/j.surg.2009.10.020. Available from: http://dx.doi.org/10.1016/j.surg.2009.10.020. [DOI] [PubMed] [Google Scholar]