Abstract
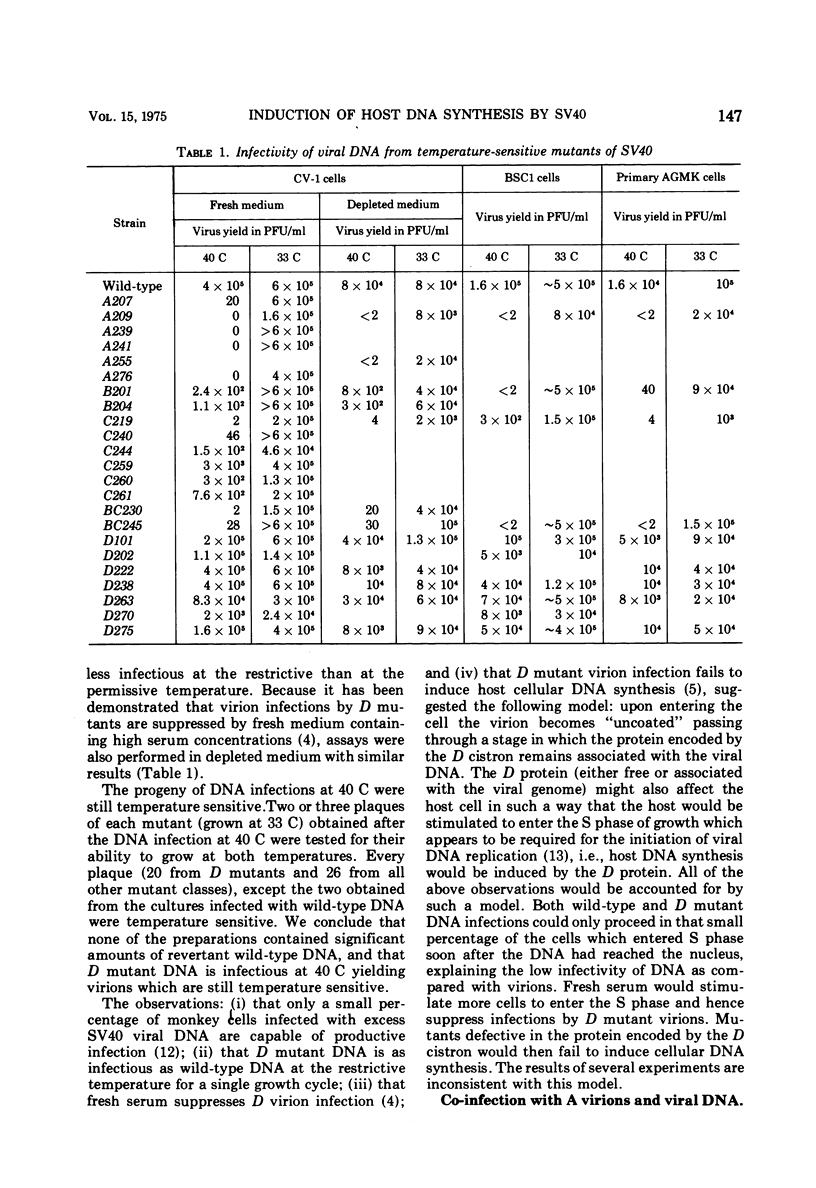
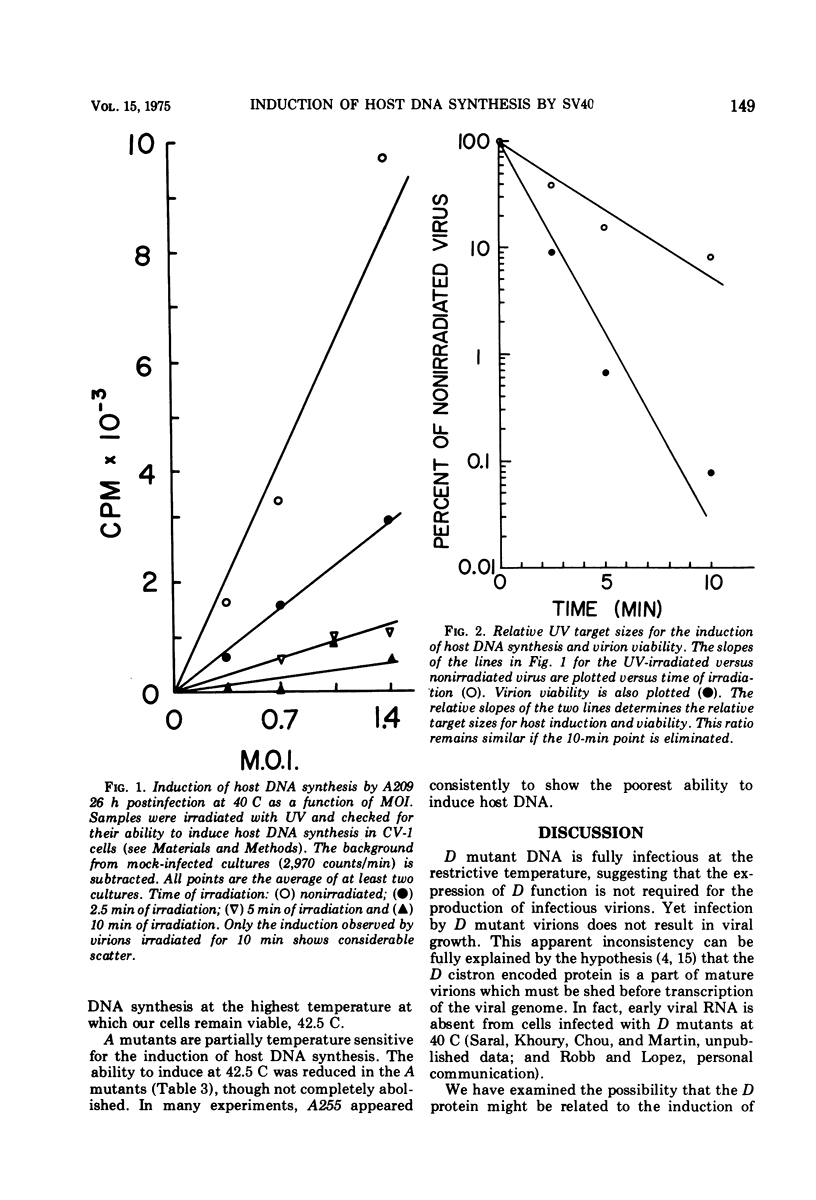
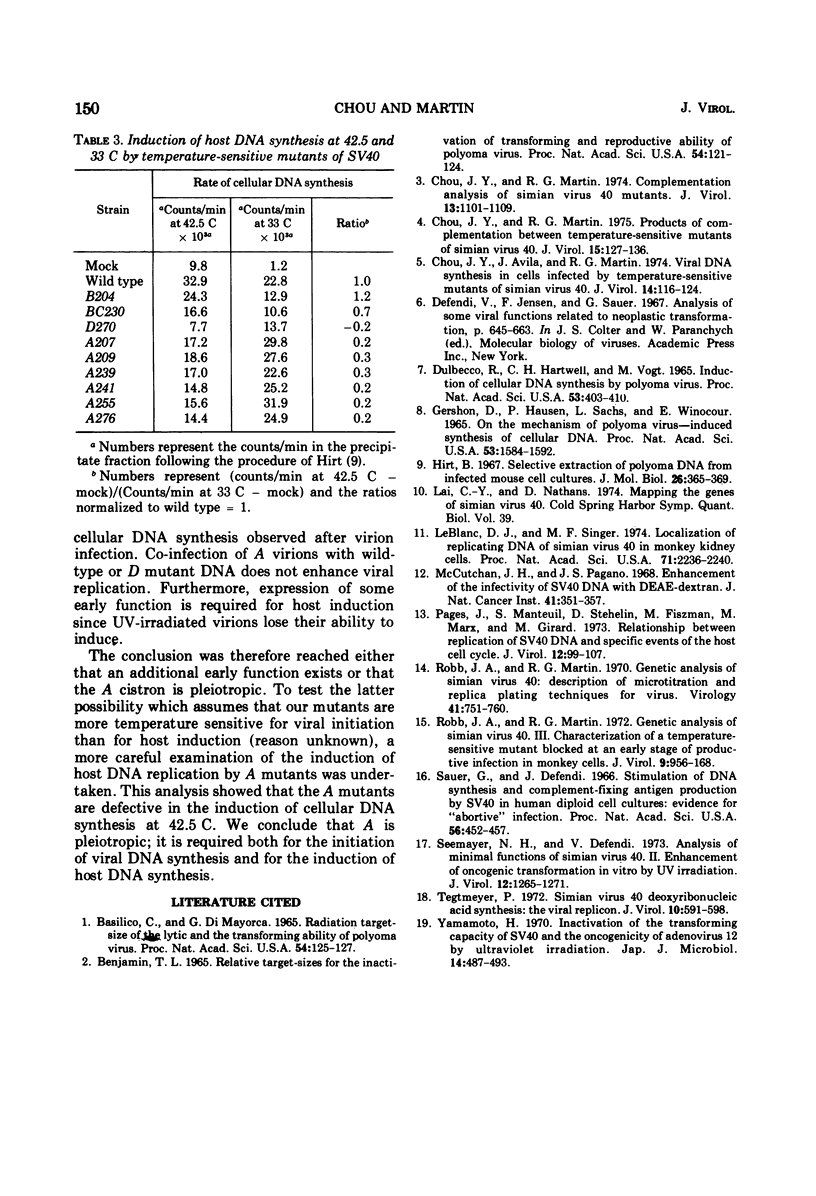
Host DNA synthesis is induced when CV-1 (monkey kidney) cell cultures are infected at 40 C with wild-type virions or with temperature-sensitive Simian virus 40 mutants of the "early" complementation group A. Host DNA synthesis is not induced when cultures are infected with mutants of the late complementation group D. The simplest explanation for these observations, that induction depends not upon the expression of some early gene function but rather on the presence of an active D protein in the infecting virion, has been examined. Indirect experiments suggest that this explanation is not correct. Moreover, the induction of host DNA synthesis is impaired when cultures are infected with mutants of the A group at 42.5 C rather than 40 C, suggesting that the A function may be responsible for host induction. The inability of D virions to induce host DNA synthesis may reflect their inability to "uncoat" at 40C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Products of complementation between temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):127–136. doi: 10.1128/jvi.15.1.127-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Hausen P., Sachs L., Winocour E. On the mechanism of polyoma virus-induced synthesis of cellular DNA. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1584–1592. doi: 10.1073/pnas.54.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Singer M. F. Localization of replicating DNA of simian virus 40 in monkey kidney cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2236–2240. doi: 10.1073/pnas.71.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Pages J., Manteuil S., Stehelin D., Fiszman M., Marx M., Girard M. Relationship between replication of simian virus 40 DNA and specific events of the host cell cycle. J Virol. 1973 Jul;12(1):99–107. doi: 10.1128/jvi.12.1.99-107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. 3. Characterization of a temperature-sensitive mutant blocked at an early stage of productive infection in monkey cells. J Virol. 1972 Jun;9(6):956–968. doi: 10.1128/jvi.9.6.956-968.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Sauer G., Defendi V. Stimulation of DNA synthesis and complement-fixing antigen production by SV40 in human diploid cell cultures: evidence for "abortive" infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):452–457. doi: 10.1073/pnas.56.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer N. H., Defendi V. Analysis of minimal functions of simian virus 40. II. Enhancement of oncogenic transformation in vitro by UV irradiation. J Virol. 1973 Dec;12(6):1265–1271. doi: 10.1128/jvi.12.6.1265-1271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H. Inactivation of the transforming capacity of SV40 and the oncogenicity of adenovirus 12 by ultraviolet irradiation. Jpn J Microbiol. 1970 Nov;14(6):487–493. [PubMed] [Google Scholar]



