Abstract
The concentration of antigen or mitogenic stimuli is known to play an important role in controlling the differentiation of naïve CD4+ T cells into different effector phenotypes. In particular, whereas TCR engagement at low antigen doses in the presence of TGF-β and IL-2 can promote differentiation of Foxp3-expressing induced regulatory T cells (iTregs), high levels of antigen have been shown in vitro and in vivo to prevent Foxp3 upregulation. This tight control of iTreg differentiation dictated by antigen dose likely determines the quality and duration of an immune response. However, the molecular mechanism by which this high dose-inhibition of Foxp3 induction occurs is not well understood. In this study, we demonstrate that when cells are in the presence of CD28 costimulation, TCR-dependent NF-κB signaling is essential for Foxp3 inhibition at high doses of TCR engagement in mouse T cells. Prevention of Foxp3 induction depends on the production of NF-κB-dependent cytokines by the T cells themselves. Moreover, T cells that fail to upregulate Foxp3 under iTreg-differentiating conditions and high TCR stimulation acquire the capacity to make TNF and IFN-γ, as well as IL-17 and IL-9, especially if IFN-γ signaling is antagonized. Thus, NF-κB helps T cells control their differentiation fate in a cell-intrinsic manner and prevents peripheral iTreg development under conditions of high antigen load that may require more vigorous effector T cell responses.
Introduction
Upon antigen encounter, T cells undergo proliferation and differentiation into functionally polarized effector cells. While the specific cytokines present during this differentiation are essential to determine the phenotype and function that T cells will acquire, the dose of antigen that T cells encounter also plays an important role (1). Stimulation of CD4+ naïve T cells in the presence of TGF-β and IL-2 promotes upregulation of the transcription factor Foxp3 and differentiation into iTregs (2, 3). Conversion of naïve T cells into iTregs can also occur in vivo, for instance upon oral administration of antigen, systemic injection of soluble antigen in the absence of adjuvant, or solid organ transplantation under cover of anti-CD154 immunosuppressive therapy (4–8). Interestingly, the dose of antigen encountered by T cells is essential in determining whether naïve T cells fail or succeed in differentiating to iTregs both in vitro and in vivo. Whereas low doses of peptide or of polyclonal TCR stimuli could induce Foxp3 expression in vitro, high doses of these mitogens prevented Foxp3 upregulation (9). Similarly, the greatest percentage of CD4+CD25+ cells in vivo (before Foxp3 was routinely used to identify Tregs) followed systemic injection of limiting doses of antigen whereas induction of these cells was abrogated upon administration of high doses of antigen (6). However, the molecular mechanism by which high TCR stimulation prevents iTreg induction is not well understood. A correlation between antigen dose and activation of the Akt/mTOR pathways has been reported (9, 10) and this signaling pathway is known to antagonize thymic nTreg development and iTreg differentiation (11–13), but whether signaling via this axis is the cause by which high TCR stimulation prevents Foxp3 induction is not completely clear.
NF-κB is a transcription factor activated upon TCR/CD28 engagement that plays a critical role in the thymic development of natural Tregs (nTregs) (14). Following T cell activation via TCR ligation, the scaffolding molecules CARMA1, Bcl-10 and Malt-1 recruit and induce the activity of the IKK complex, resulting in phosphorylation and degradation of the NF-κB inhibitor IκB that normally binds to and retains dimers of NF-κB subunits in the cytoplasm. Release from IκB reveals nuclear localization sequences in the NF-κB subunits that drive their nuclear translocation, allowing their transcriptional activity (15). The NF-κB subunit c-Rel has been shown to bind to enhancer sequences located in the promoter and third intron of the Foxp3 gene and plays a direct role in Foxp3 expression during thymic nTreg development (16–19). In contrast, the role of c-Rel in driving Foxp3 transcription during iTreg differentiation is more controversial ( 17, 19, 20). Furthermore, mice lacking CARMA1 or Bcl-10, adaptors that couple the TCR to NF-κB have been recently shown to lack nTregs but retain differentiation of naïve T cells into iTregs (21–24), suggesting that TCR-driven NF-κB activity is not required for iTreg differentiation, at least if sufficient exogenous IL-2 is present. Surprisingly, our results demonstrate that at high doses of TCR stimulation, NF-κB activity is, at least in part, responsible for the inhibition of TGF-β/IL-2-mediated iTreg differentiation. Therefore, NF-κB is not only dispensable for Foxp3 expression in iTregs, but can in fact antagonize it at greater levels of TCR engagement, via T cell-intrinsic production of effector cytokines that oppose iTreg differentiation. These data shed light on the T cell-intrinsic molecular mechanisms that control conversion of naïve T cells into iTregs and operate in parallel to APC-derived cytokine-mediated signaling to ultimately control the differentiation phenotype of stimulated T cells.
Materials & Methods
Mice
C57Bl/6 and BALB/c mice were purchased from Harlan (Indianapolis, IN). IκBαΔN-Tg mice (25), expressing a super-repressor form of IκBα directed by the Lck promoter and the CD2 enhancer were bred in house and backcrossed more than 20 generations to C57Bl/6. CARMA1−/− mice (26) were originally generated in the 129 background but were backcrossed for at least six generations to C57Bl/6 animals. DO11.10 transgenic (−Tg) (27) mice were kindly provided by Dr Anne Sperling (The University of Chicago). CAR-Tg mice (28), which express the coxsackie and adenovirus receptor under the Lck promoter, were kindly provided by Dr Thomas Gajewski (The University of Chicago). Bcl-2-Tg mice (29), which over-express the anti-apoptotic Bcl2 molecule under the control of the Vav promoter, were kindly provided by Dr Fotini Gounari (The University of Chicago). CD4-Cre x IKKβ-CA x Foxp3-mRFP1 were obtained by crossing CD4-Cre (kindly provided by Dr Fotini Gounari) to Rosa-IKKβ-constitutively active (CA)FL and Foxp3-mRFP1 (Jackson Laboratories). The Rosa26-StopFLIKKβ-CA (30) strain possesses a loxP-flanked STOP cassette that prevents transcription of the downstream constitutively active form of IKKβ. When bred to mice that express Cre recombinase under the CD4 promoter, the resulting offspring have the STOP cassette deleted in T cells, resulting in expression of the IKKβCA, that leads to constitutive activation of canonical NF-κB signaling. All the experiments were performed in agreement with the University of Chicago Institutional Animal Care and Use Committee and according to the National Institutes of Health guidelines for animal use.
T cell differentiation in vitro: naïve CD4+ T cells were first enriched from spleen and peripheral lymph nodes with a CD4+ T cell enrichment kit (Stem Cells) and then sorted with a FACSAria (BD Biosciences) as CD4+CD25− (Foxp3-mRFP1−) CD44lo cells. Naive CD4+ T cells were stimulated with plate-bound anti-CD3 mAb (0–10 μg/ml, Bio-XCell) and anti-CD28 mAb (1 μg/ml, Bio-XCell) for 3 days in the presence of rhIL-2 and rhTGF-β1 (10 U/ml and 2.5 ng/ml, respectively, R&D Systems). In certain experiments, anti-TNF (10 μg/ml, generously provided by Hideo Yagita, Juntendo University, Tokyo, Japan), anti-IL-6 (10 μg/ml, clone MP5-20F3, Bioxpress), anti-IL-4 (10 μg/ml, eBioscience), anti-IFN-γ (10 μg/ml, clone XMG1.2, eBioscience), anti-IL-17A (10 μg/ml, R&D Systems), IL-6 (10 ng/ml, Peprotech), IL-17A (10 ng/ml, eBioscience), IFN-γ (10 ng/ml, Peprotech), TNF (10 ng/ml, R & D Systems) or IL-4 (10 ng/ml, Peprotech) were added. BALB/c splenic dendritic cells were purified by digestion of spleens with collagenase IV (400 U/ml, Sigma-Aldrich) for 30 min and then purified with CD11c+ microbeads (Miltenyi Biotec) and AutoMacs, following the manufacturer’s instructions. Dendritic cells were co-cultured in vitro with naïve T cells in the presence of ovalbumin323-339 peptide (OVA, 0–5 μg/ml), rhTGF-β1 (2.5 ng/ml, R&D Systems) and IL-2 (10 U/ml) for 3 days. In some experiments, purified CD4+CD25−CD44lo cells were stained with carboxyfluorescein-succinimidyl ester (CFSE, 2.5 μM, Molecular Probes). Alternatively, cells were treated with PD98059 (50 μM, ERK inhibitor, Calbiochem), cyclosporine A (100 ng/ml, calcineurin inhibitor, Sigma-Aldrich), Akt1/2 inhibitor (1 μM, Calbiochem), SB203580 (10 μM, p38 MAPK inhibitor, Calbiochem) or SP600125 (10 μM, JNK inhibitor, Calbiochem).
Adenoviral vectors: an adenoviral vector containing IKKβ–CA (IKKβEE) and an adenoviral vector without a coding cDNA (EV) were generated in HEK 293 cells as described by Marks et al (31).
Adenoviral transduction of CAR-Tg T cells
CAR Tg CD4+CD25−CD44lo naïve T cells were FACS-cell sorted in the FACSAria (BD Biosciences) and resuspended at 2 × 107 cells/ml in DMEM supplemented with 2% FCS. The cells were then mixed with an equal volume of DMEM containing 2% FCS and the indicated multiplicity of infection (MOI) of adenoviral particles and incubated at 37°C for 1 h. The cell/virus mixture was then transferred to a 10-cm tissue cell culture dish, incubated overnight at 37°C in an 8% CO2 atmosphere, then washed and used for experiments.
Flow Cytometry: toassess intracellular cytokine expression, the cells were restimulated with PMA (150 ng/ml) and ionomycin (500 ng/ml), in the presence of brefeldin A (10 μg/ml, Biolegend) for 5h. Cells were fixed and permeabilized with 1% paraformaldehyde in FACS buffer (0.1% bovine serum albumin, 0.1 NaN3 in PBS) with 0.1% saponin (IC buffer) and then stained in IC buffer with fluorochrome-conjugated antibodies to Foxp3, IL-17A, IL-17F, IFN-γ and IL-9 or their respective isotype controls. For the assessment of phospho-proteins, cells were stimulated for 18h, fixed with 1% formaldehyde at 37°C for 10 min, permeabilized with ice-cold methanol for 30 min on ice, washed in FACS buffer and stained with fluorochrome-conjugated antibodies to CD4, phospho-Smad2 (Ser465/467)/Smad3 (Ser423/425) or phopsho-RelA(Ser536) in FACS buffer. All experiments were analyzed in BD LSR II flow cytometers (BD Biosciences). Antibodies were purchased from eBioscience except for anti-IL-9 (Biolegend) and all phospho-antibodies (Cell Signaling Technology).
Western Blot: whole-cell extracts (10 μg of proteins) were analyzed by SDS–PAGE, transferred to PVDF membranes and probed with rabbit polyclonal Ab against phospho-Smad2/3, phospho-Akt, phospho-S6 and mouse cyclophilin B (Cell Signaling Technologies), or mouse anti-β-actin (Millipore/Upstate). Bound antibodies were detectedusing peroxidase-labeled anti-rabbit or anti-mouse IgG (Bio-Rad) and ECL Plus chemiluminescent reagent (GE Healthcare) on Kodak BioMax films (Rochester).
Reverse transcription and Quantitative real-time PCR: total RNA was prepared from T cells with the use of RNEasy Plus Mini Kit (QIAGEN). cDNA was synthesized with iScript cDNA Synthesis Kit (Bio-Rad) and the samples diluted in water (1:10). A total volume of 25 μl containing 5 μl cDNA template, 0.3 μM of each primer and SYBR Green PCRMaster Mix (Applied Biosystems) was analyzed in triplicate. Gene expression was analyzed with an ABI PRISM 7300 Sequence Detector and ABI Prism Sequence Detection Software version 1.9.1 (Applied Biosystems). Results were normalized by division of the value for the tested gene by that obtained for β-actin. The primers used were: Foxp3-F 5′-TCTTCGAGGAGCCAGAAGAG-3′, Foxp3-R 5′-TACTGGTGGCTACGATGCAG-3′; β-Actin-F 5′-TGGAATCCTGTGGCATCCATGAAAG-3′; β-Actin-R 5′-TAAAACGCA GCCTCAGTAACAGTCCG-3′.
Chromatin Immunoprecipitation (ChIP) assay: a ChIP assay kit was used according to the manufacturer’s instructions (Upstate). Acetylated histone-3 (AcH3) and NF-κB/RelA were immunoprecipitated with anti-acetyl-H3 (Millipore/Upstate) and anti-RelA antibodies (Santa Cruz Biotechnologies), respectively. Purified DNA was then analyzed by quantitative real-time PCR and normalized to input DNA. The primers spanned Foxp3 sequence +6144 to +6280 from transcription start site (TSS): Foxp3-F 5′-CAACTTCTCCTGACTCTGCCTTCA-3′; Foxp3-R 5′-GGAACTGTGCTAGTGGGAAGTGTACT-3′, as previously described (11). The primers for the amplification of the NF-κB-containing site in the IL-2 promoter (−266 to −100 bp to TSS) were: IL-2NFkB-F 5′-ATATGGGGGTGTCACGATGT-3′ and IL-2NFkB-R 5′-GCCACCTAAGTGTGGGCTAA-3′.
Cytokine ELISAs
IFNγ and TNF ELISAs (kits from eBioscience) were performed according to the instructions of the manufacturer using purified mAbs as capture Abs and biotinylated mAbs as developing Abs, followed by incubation with streptavidin-alkaline phosphatase and substrate. Plates were read in a 96-well spectrophotometer (Spectra Max 250, Molecular Devices) and data were analyzed using Softmax software (Molecular Devices) by comparison against a standard curve generated using recombinant cytokines at known concentrations.
Electrophoretic mobility shift assays (EMSA)
CD4+CD25−CD44lo wildtype cells were purified by negative selection using a magnetic bead separation system, according to the manufacturer’s instructions (Stem Cell Technologies). In all cases, cell purity was greater than 95%, as analyzed by flow cytometry. Nuclear proteins were extracted from T cells that had been stimulated with immobilized anti-CD3 (0, 0.05, 0.5 or 5 μg/ml) mAb and anti-CD28 mAb (1 μg/mL each) for 24 h. Nuclear extracts were quantified by Bradford and 0.5 μg were mixed with a 5′-biotin IL-2-NF-κB consensus oligonucleotide, [ACC AAG AGG GAT TTC ACC TAA ATC]. The EMSA was performed using the LightShift Chemiluminescent EMSA Kit (Pierce/Thermo Scientific), as recommended by the manufacturer.
Immunofluorescence detection and analysis of RelA nuclear translocation
CD4-Cre and CD4-Cre x IKKβ-CA freshly isolated lymphocytes were fixed with 1% formaldehyde for 10 min, permeabilized with 90% methanol for 30 min on ice, washed in FACS buffer, and stained with goat anti-RelA (sc372G, Santa Cruz), Alexa Fluor 647-labeled anti-goat IgG (Invitrogen), APC-Alexa750-conjugated anti-CD4 (eBioscience), and DAPI (Invitrogen). Intracellular expression of RelA and its nuclear translocation were assessed by image-based fluorescence using the ImageStream® 100 multispectral imaging flow cytometer (Amnis Corp.), as described previously (32). Briefly, a minimum of 740 CD4+ T cells were collected and analyzed. RelA translocation was assessed by the similarity of pixel intensities between the RelA image in channel 11 (Alexa Fluor 647) and the nuclear image in channel 7 based on the image mask of DAPI on a pixel-by-pixel basis for each cell. The similarity score for each cell was calculated using a log transformation of Pearson’s correlation coefficient. Positive translocation events were assessed by comparison with the similarity score for the wild type negative control (correlation of darkfield scatter image with the nuclear image) Data were analyzed using the IDEAS software package (Amnis Corp.).
Statistical analysis: statistical significance was evaluated using the two-tailed unpaired t test. Values of p < 0.05 were considered significant
Results
High dose TCR stimulation prevents Foxp3 transcription and locus accessibility
It has been shown previously that high dose of TCR stimulation prevents iTreg differentiation in peripheral naïve CD4+ T cells (9), although the molecular mechanism of this inhibition remains to be characterized. In order to study this phenomenon, OVA-specific DO11.10-Tg CD4+CD25−CD44lo sorted naïve T cells were stimulated for 72h with increasing concentrations of OVA323-339 peptide in the presence of dendritic cells, TGF-β and IL-2. The highest proportion and numbers of cells upregulating Foxp3, as assessed by intracellular flow cytometry, was observed when T cells were stimulated with 0.01 to 0.5 μg/ml of OVA peptide (Figure 1A and 1B). In contrast, higher concentrations of antigen inhibited iTreg differentiation. To determine whether this inhibition was T cell-intrinsic or depended on the presence of APCs, polyclonal purified CD4+ naïve T cells were stimulated on plate-bound anti-CD28 mAb with increasing doses of plate-bound anti-CD3 mAb in the presence of TGF-β and IL-2. As observed with OVA peptide, stimulation with anti-CD3 mAb concentrations higher than 0.5–1 μg/ml resulted in a reduced percentage and total number of Foxp3+ cells (Figure 1C), suggesting that high doses of TCR stimulation decrease the number of Foxp3-expressing cells in a T cell-intrinsic manner. Unless otherwise stated, 0.5 μg/ml of anti-CD3 mAb was chosen as the prototypic low dose and 5 μg/ml as the prototypic high dose of TCR stimulation. The inhibitory effect of high dose TCR stimulation on the percentage of cells upregulating Foxp3 expression was observed at all time points following TCR stimulation (Figure 1D).
Figure 1. High dose of TCR stimulation reduces iTreg differentiation.
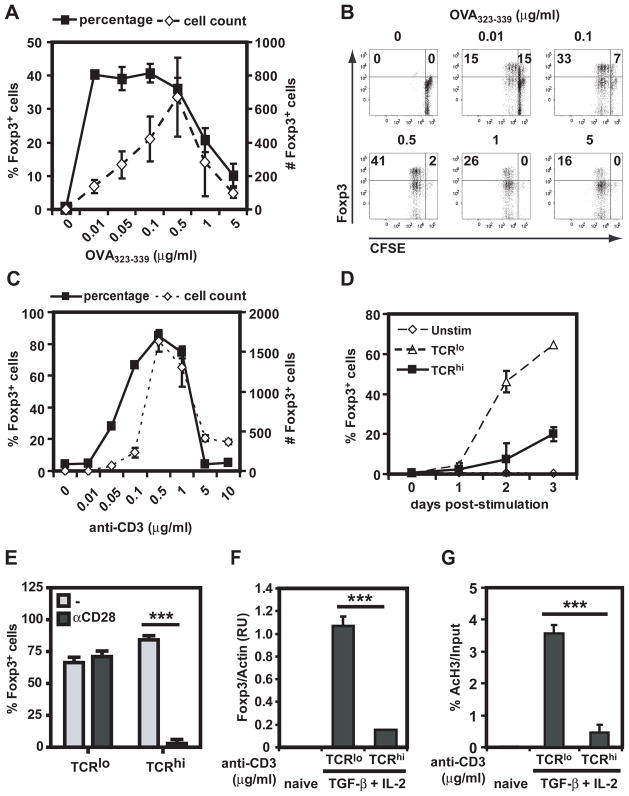
A. DO11.10-Tg CD4+CD25−CD44lo cells were stimulated with BALB/c splenic dendritic cells, TGF-β, IL-2 and increasing concentrations of OVA323-339 peptide. Percentage and numbers of Foxp3-expressing cells were assessed 72 h later by intracellular staining. Results show mean ± standard deviation (SD) of triplicates. B. CFSE-labeled CD4+CD25−CD44lo cells were stimulated as in A and proliferation was assessed by flow cytometry. C. Wild type C57BL/6 CD4+CD25−CD44lo cells were stimulated with plate-bound anti-CD28, TGF-β, IL-2 and increasing doses of anti-CD3 mAb. Percentage and numbers of Foxp3-expressing cells were assessed at 72h as in A. D. Wild type C57BL/6 CD4+CD25−CD44lo cells were stimulated with plate-bound anti-CD28, TGF-β, IL-2 and anti-CD3 mAb (0, 0.5, or 5 μg/ml). Percentage of cells expressing Foxp3 was assessed at the indicated times as in C. E. Cells were stimulated as in D in the absence (−) or presence (αCD28) of anti-CD28. Percentage of Foxp3-expressing cells was assessed as in C. E. C57BL/6 CD4+CD25−CD44lo cells were untreated or stimulated with anti-CD28, TGFβ + IL-2 in the presence of either 0.5 or 5 μg/ml of anti-CD3 mAb. Three days later, cells were processed and Foxp3 mRNA was assessed by RT-qPCR and normalized to levels of β-actin gene expresion. F. T cells were stimulated as in E and processed for chromatin immunoprecipitation using anti-AcH3 Ab, with subsequent amplification of the Foxp3 locus by qPCR. Results shown are representative of 2 independent experiments. All experimental points represent average values and SD from three replicates of representative experiments. ns: non-significant, **: p <0. 01, ***: p <0. 001.
CD28 costimulation augments TCR engagement-mediated T cell activation (33). To determine the role of CD28 costimulation in the prevention of iTreg differentiation by high dose of TCR stimulation, sorted CD4 naïve cells were activated for 3 days in the presence or absence of agonistic anti-CD28 mAb. Inhibition of iTreg differentiation upon high anti-CD3 stimulation was dependent on the presence of costimulation as the percentage (Figure 1E) and the total number (data not shown) of Foxp3+ cells remained high in the absence of CD28 mAb. For the remainder of the experiments the dose of anti-CD28 mAb was kept constant to examine the mechanism by which a high dose of TCR stimulus prevents iTreg differentiation in the presence of costimulation. To investigate if high TCR stimulus-mediated inhibition of iTreg differentiation occurred at the transcriptional level, Foxp3 mRNA was analyzed by RT-qPCR. mRNA expression of FoxP3 was much lower in T cells cultured under high than low dose of anti-CD3 mAb (Figure 1F). Foxp3 locus accessibility was probed by determining the presence of acetylated histone 3 (AcH3) by ChIP assay. AcH3 levels in the Foxp3 locus were significantly higher when cells were stimulated with low, but not high dose of anti-CD3 mAb (Figure 1G). These results suggest that high doses of TCR stimuli prevent accessibility of the Foxp3 locus and subsequent gene transcription.
The reduced percentage of Foxp3-expressing cells at high dose of TCR stimulation is not due to cell death
TCR stimulation results in T cell activation but also cell death (34). To determine if the reduced conversion of naïve T cells into iTregs at high doses of TCR stimuli was due to cell death, iTreg differentiation experiments were performed using T cells transgenic for the anti-apoptotic molecule Bcl-2. Although apoptosis of T cells induced by high concentration of anti-CD3 mAb was largely prevented by overexpression of Bcl-2, increased cell survival did not restore iTreg differentiation at high doses of anti-CD3 mAb (Figure 2), indicating that the reduced percentage of Foxp3-expressing cells observed upon high TCR stimulation is not due to cell death.
Figure 2. Inhibition of iTreg differentiation at high dose of TCR stimulation is not due to cell death.
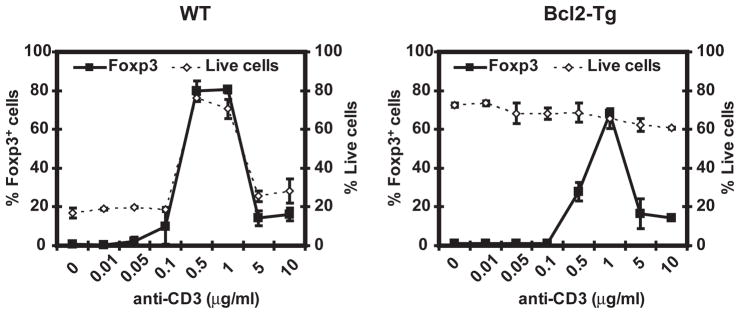
C57BL/6 (A) or Bcl-2-Tg (B) CD4+CD25−CD44lo cells were stimulated and analyzed as in Figure 1C, in the presence of increasing doses of anti-CD3. Results shown are representative of two independent experiments. All experimental points represent average values and SD from three replicates.
Reduced iTreg differentiation at high dose of TCR stimulation is not due to impaired TGF-β or IL-2 signaling
De novo expression of Foxp3 in peripheral CD4+ naïve T cells requires signaling by the cytokines TGF-β and IL-2, through phosphorylation of Smad2 and Smad3 and STAT5, respectively (35, 36). To investigate if high doses of TCR stimulation impaired TGF-β signaling, the expression of phospho-Smad2/3 was assessed by western blot (Figure 3A) and flow cytometry (Figure 3B). Increasing amounts of TGF-β resulted in a dose-dependent augmentation phospho-Smad 2/3 at both low and high concentrations of anti-CD3 mAb (Figure 3B), but even high concentrations of TGF-β could not restore iTreg differentiation in cells stimulated with high dose of anti-CD3 mAb (Figure 3C). Levels of phospho-STAT5 were also equivalent in cells stimulated with low and high doses of anti-CD3 mAb (Figure 3D). Hence, TGF-β and IL-2 signaling do not appear affected by the concentration of anti-CD3 mAb. To exclude that TGF-β and IL-2 were consumed in cells stimulated with high doses of anti-CD3 mAb, therefore preventing iTreg differentiation, daily addition of TGF-β and IL-2 was provided. These culture conditions did not increase the percentage of Foxp3-expressing cells upon stimulation with high doses of anti-CD3 mAb (Figure 3E). Taken together, our results suggest that inhibition of iTreg differentiation under conditions of high dose TCR stimulation is not due to impaired IL-2 or TGF-β signaling.
Figure 3. TGF-β and IL-2 signaling is not impaired at high dose of anti-CD3 stimulation.
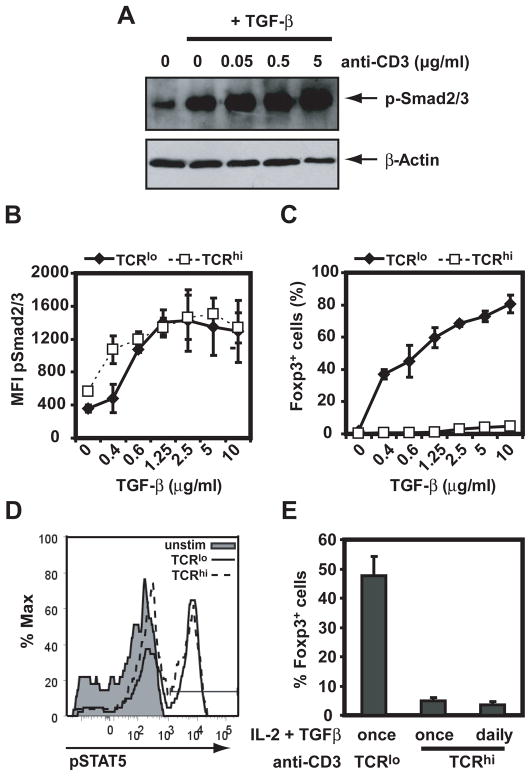
A. C57BL/6 CD4+CD25−CD44lo cells were stimulated for 30 min with plate-bound anti-CD28 in the presence or absence of TGF-β and increasing doses of plate-bound anti-CD3 mAb. Smad2/3 phosphorylation was assessed by immunoblot and normalized to β-actin. B and C. C57BL/6 CD4+CD25−CD44lo cells were stimulated with IL-2 and plate-bound anti-CD28 in either 0.5 (solid line) or 5 μg/ml (dashed line) of plate-bound anti-CD3 mAb, in the presence of increasing doses of TGF-β. Smad2/3 phosphorylation at 18h (B) or Foxp3 expression at 72h (C) were assessed by flow cytometry. D. C57BL/6 CD4+CD25−CD44lo cells were stimulated with IL-2, TGF-β, plate bound anti-CD28 and either 0 (filled histogram), 0.5 (solid line) or 5 μg/ml (dashed line) of anti-CD3 mAb. STAT5 phosphorylation was assessed by flow cytometry at 18h. E. C57BL/6 CD4+CD25−CD44lo cells were stimulated with TGF-β and IL-2, and 0.5 μg/ml or 5 μg/ml of anti-CD3 mAb. TGF-β and IL-2 were added either at the beginning of the culture (once) or every 24h (daily). Results shown are representative of two independent experiments. All experimental points represent average values and SD from three replicates.
Akt/mTOR activity may not explain high TCR stimuli-mediated inhibition of iTreg differentiation
It has been previously shown that Akt and mTOR negatively regulate Foxp3 expression (11, 12). Therefore, it was conceivable that higher concentrations of TCR stimuli resulted in increased Akt/mTOR activity, in turn preventing Foxp3 induction. However, phosphorylation of S6 and Akt, as analyzed by western blot, was equivalent in cells stimulated with either 0.5 or 5 μg/ml of anti-CD3 (Figure 4A), conditions that respectively promote and prevent Foxp3 upregulation. Similar results were obtained when analyzing mean fluorescence intensity of cells expressing phospho-S6 and phospho-Akt by flow cytometry (data not shown). Furthermore, inhibition of Akt resulted in an increased percentage of Foxp3-expressing cells at both low and high doses of anti-CD3 stimulation (Figure 4B), suggesting that Akt/mTOR signaling has a negative effect on iTreg differentiation regardless of TCR stimulation dose. Thus, Akt/mTOR signaling does not seem to explain the selective inhibitory effects of high TCR stimulation.
Figure 4. Activation of Akt may not explain inhibition of Foxp3 expression at high doses of TCR stimulation.
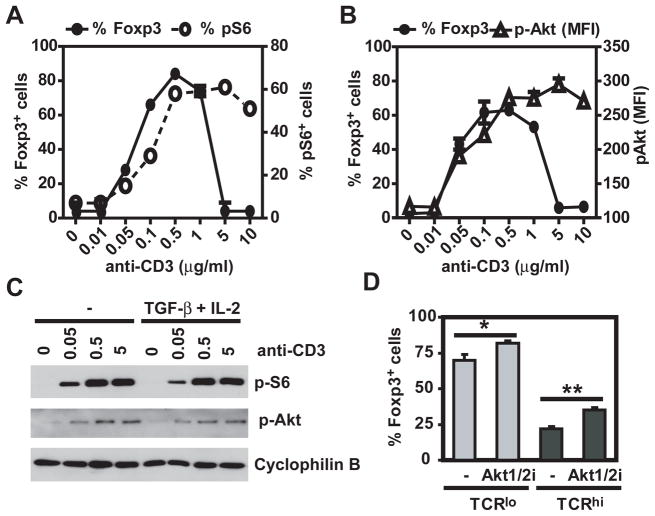
A. C57BL/6 CD4+CD25−CD44lo cells were stimulated for 30 min with plate bound anti-CD28 and increasing doses of anti-CD3 in the presence or absence of TGF-β and IL-2. Phosphorylation of S6 and Akt was assessed by immunoblot and levels normalized to cyclophilin B. B. C57BL/6 CD4+CD25−CD44lo cells were stimulated with TGF-β, IL-2, plate bound anti-CD28, 0.5 (TCRlo) or 5 μg/ml (TCRhi) of anti-CD3, in the presence or absence of an Akt1/2 inhibitor. Percentage of Foxp3-expressing cells was evaluated 72h post stimulation by flow cytometry. All experimental points represent average values and SD from three replicates. *: p <0.05, **: p <0.01.
High dose TCR-mediated inhibition of iTreg differentiation is NF-κB-dependent
TCR/CD28 stimuli are known to be potent inducers of NF-κB activity (33). High dose TCR triggering resulted in significantly increased recruitment of RelA/NF-κB to the NF-κB binding site of the IL-2 promoter compared to low dose anti-CD3 mAb (Figure 5A), demonstrating a correlation between the level of NF-κB activity and the inhibition of iTreg differentiation. To determine if NF-κB activity was necessary for high dose TCR-mediated inhibition of iTreg differentiation, IκBαΔN-Tg and CARMA1-deficient CD4+ T cells were used as genetic models of NF-κB-impaired T cells (Figure 5B). Increasing concentrations of anti-CD3 mAb resulted in a dose-dependent induction of NF-κB activity in wild type but not, IκBαΔN-Tg or CARMA1-deficient cells, as assessed by EMSA (Figure 5B). Both IκBαΔN-Tg and CARMA1-deficient CD4+ T cells were partially resistant to the high dose TCR-mediated inhibition of iTreg differentiation (Figure 5C and D). This was not due to reduced proliferation by NF-κB-impaired T cells, as the exogenous IL-2 present in iTreg culture conditions compensated for the reduced production of IL-2 by (23, 25) these T cells and allowed NF-κB-impaired T cells to proliferate as well as wild type T cells (Supplementary Figure 1). These results suggest that T cell-NF-κB is required for high TCR stimulation-mediated prevention of iTreg differentiation.
Figure 5. Inhibition of iTreg differentiation at high doses of TCR stimulation is NF-κB-dependent.
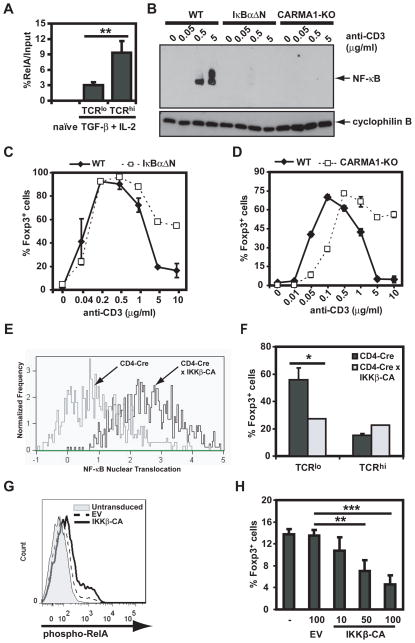
A. Wild type CD4+CD44loCD25− cells were left alone or stimulated for 20h with anti-CD28 and anti-CD3 (0.5 or 5 μg/ml) in the presence of TGF-β and IL-2. Chromatin was immunoprecipitated with anti-RelA antibody and qPCR was performed spanning the NF-κB site in the IL-2 promoter. B. Wild type (WT), IκBαΔN and CARMA1-deficient CD4+CD25−CD44lo cells were stimulated for 24h with TGF-β, IL-2, plate bound anti-CD28 and increasing doses of anti-CD3 mAb. Nuclear extracts were prepared and NF-κB activity was assessed by EMSA. Immunoblot for cyclophilin B was used for protein normalization. C. Expression of Foxp3 was assessed in wild type, and IκBαΔN-Tg CD4+CD25−CD44lo cells, stimulated and analyzed as in Figure 1C. D. CD4+CD25−CD44lo wild type and CARMA1-deficient cells were stimulated and analyzed as in Figure 1C. E. Freshly isolated CD4+ cells from CD4-Cre x Foxp3RFP and CD4-Cre x IKKβ-CA x Foxp3RFP were analyzed for RelA nuclear translocation using the ImageStream® 100 multispectral imaging flow cytometer, as described in Materials and Methods. F. CD4+CD25−CD44loFoxp3RFP− from CD4-Cre x Foxp3RFP and CD4-Cre x IKKβ-CA x Foxp3RFP were stimulated with TGF-β, IL-2, plate bound anti-CD28 and 0.5 (TCRlo) or 5 μg/ml (TCRhi) plate bound of anti-CD3. Percentage of Foxp3-expressing cells was evaluated 72h post-stimulation by intracellular flow cytometry. G and H. CAR-Tg CD4+CD25−CD44lo cells were left untreated, or infected with either control adenovirus (empty vector, EV), or increasing MOIs of adenovirus encoding for IKKβ-CA. Twenty hours later RelA phosphorylation was evaluated by intracellular flow cytometry (G) or Foxp3 expression was assessed in cells further stimulated for 72h with TGF-β and IL-2 in the presence of anti-CD3 and anti-CD28 mAbs. Results shown are representative of three independent experiments. All experimental points represent average values and SD from three replicates. **: p <0. 01, ***: p <0. 001.
To test the sufficiency of NF-κB in inhibiting Foxp3 induction, we used two complementary approaches. First, we analyzed T cells from CD4-Cre x Rosa26-StopFLIKKβCA (CD4-Cre x IKKβ-CA) mice that express constitutive NF-κB activity as demonstrated by the constitutive nuclear translocation of RelA assessed by flow-based immunofluorescence (Figure 5E). To enable purification of FoxP3-negative T cells, these mice were crossed to Foxp3-mRFP transgenic mice (37), in which cells expressing Foxp3 are co-marked with monomeric red fluorescent protein (mRFP). CD4+CD25−CD44loFoxp3-mRFP− cells sorted from CD4-Cre x Foxp3-mRFP and CD4-CrexIKKβ-CAxFoxp3-mRFP mice were stimulated in the presence of IL-2, TGF-β, anti-CD28 and either low or high doses of anti-CD3 mAb. Constitutive activity of NF-κB reduced the percentage of iTregs induced by low dose anti-CD3 stimulation (Figure 5F). As an alternative approach, CAR-Tg CD4+ naïve T cells were transduced with adenovirus encoding IKKβ-CA resulting in a subset of cells expressing phospho-RelA (Figure 5G). T cells transduced with IKKβ-CA at increasing MOIs, but not empty vector-transduced T cells, displayed a dose-dependent reduction in the percentage of FoxP3+ cells (Figure 5H), further indicating that increased activation of NF-κB is sufficient to reduce iTreg differentiation. Taken together, these results show correlation, necessity and sufficiency and therefore strongly implicate the TCR-CARMA1-NF-κB axis in the inhibition of iTreg differentiation by high doses of TCR stimulation.
NF-κB-dependent cytokines induced by high doses of anti-CD3 stimulation prevent iTreg differentiation
At high doses of TCR stimulation, NF-κB may either directly inhibit gene expression of Foxp3 or may induce the transcription of NF-κB-target genes capable of suppressing Foxp3 expression. Target genes of NF-κB include a number of cytokines. To determine whether cytokines produced by T cells stimulated with high doses of anti-CD3 mAb could reduce iTreg differentiation, congenic wild type and CARMA1-deficient naïve CD4+ T cells were cultured in the presence of TGF-β, IL-2 and high doses of anti-CD3 mAb. Whereas CARMA1-deficient T cells were resistant to the inhibitory effect of high doses of TCR stimulation on iTreg differentiation when cultured alone, co-culture with wild type T cells resulted in a significantly reduced percentage of CARMA1-deficient T cells expressing Foxp3 (Figure 6A). Similar results were obtained whether the co-culture was carried out in the same well or if wild type and CARMA1-deficient T cells were separated in transwells (Figure 6B), suggesting that soluble factors produced by wild type T cells were suppressing iTreg differentiation by CARMA1-deficient and wild type T cells. Whereas individual neutralization of IFN-γ, IL-6, TNF, IL-17 and IL-4 did not restore iTreg differentiation of wild type T cells upon high TCR stimulation, combined blockade of these cytokines almost abrogated the ability of high doses of anti-CD3 mAb to suppress Foxp3 induction (Figure 6C). In order to address which cytokines were sufficient to inhibit Foxp3 expression, wild type naïve T cells were subjected to iTreg differentiation under low concentration of anti-CD3 mAb. The only combination of cytokines that reduced the percentage of FoxP3+ cells was TNF and IFN-γ (Figure 6D). Consistent with this result, secretion of both cytokines was maximal when wild type cells were stimulated with high doses of anti-CD3 mAb that simultaneously prevented iTreg differentiation (Figure 6E). Thus, NF-κB-dependent inhibition of Foxp3 expression upon high TCR stimulation is secondary to the production of TNF and IFN-γ by the activated T cells.
Figure 6. High doses of anti-CD3 stimulation induce NF-κB-dependent cytokines that prevent Foxp3 expression.
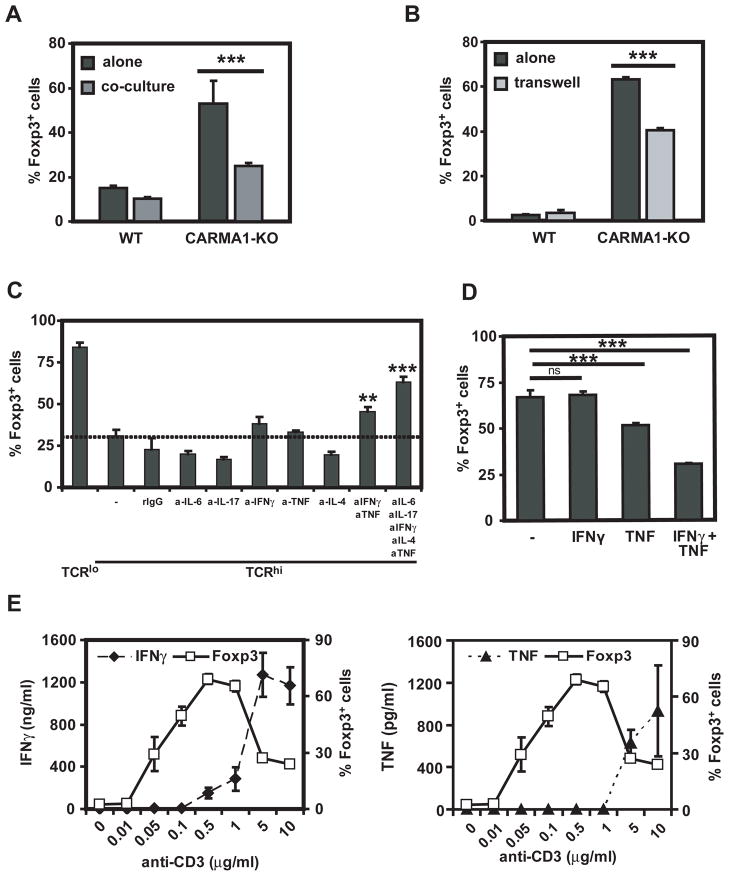
A. Wild type (CD45.1+) and CARMA1-KO (CD45.2+) CD4+CD25−CD44lo congenic cells were stimulated with TGF-β, IL-2, anti-CD28 and 5 g/ml (, TCRhi) of anti-CD3 mAb. Cells were cultured apart or together. Three days later expression of Foxp3 was assessed by intracellular staining in WT (CD45.1+) or CARMA1-KO (CD45.2+) cells. B. Wild type and CARMA1-KO CD4+CD25−CD44lo cells were stimulated with TGF-β, IL-2, anti-CD28 and 5 μg/ml of anti-CD3 mAb. Cells were cultured apart or separated by transwell. Three days later expression of intracellular Foxp3 was assessed by flow cytometry. C. Expression of Foxp3 was assessed in wild type cells stimulated as in 6A, in the presence of neutralizing antibodies to IL-6, IL-17A, IFN-γ, TNF, IL-4, alone or in combination. D. Wild type cells were stimulated with 0.5 μg/ml of anti-CD3 with TGF-β and IL-2 in the presence or absence of IFNγ and TNF. Foxp3 expression was assessed 72h later by intracellular staining. E. Wild type CD4+CD25−CD44lo cells were stimulated for three days as in Figure 1C. Foxp3 expression was assessed by intracellular flow and IFNγ and TNF secretion was evaluated by ELISA. Results shown are representative of three independent experiments. All experimental points represent average values and SD from three replicates. **: p <0.01, ***: p <0.001
Upon high doses of TCR stimulation, differentiating iTregs acquire effector phenotypes
The high levels of TNF and IFNγ produced by cells stimulated with high doses of TCR triggering suggested that cell differentiation had switched from iTreg to Th1. In addition, TGF-β can promote not only the generation of suppressive iTregs, but also differentiation of naïve T cells into cells producing IL-17A/F or IL-9 (38). In order to assess whether high doses of TCR stimulation also diverted iTreg differentiation into Th17 or Th9 development, expression of IL-17A, IL-17F and IL-9 was analyzed. High concentrations of anti-CD3 mAb induced the expression of IL-17A, IL-17F and IL-9, both by intracellular flow cytometry (Figure 7A) and ELISA (data not shown). However, the percentage of cells expressing these pro-inflammatory cytokines was low unless IFN-γ was blocked (Figure 7B), as it has been shown that IFN-γ inhibits differentiation of non-Th1 subsets (38). These results suggest that despite a cytokine milieu that promotes the differentiation of iTregs (TGF-β and IL-2) the strength of TCR stimulation can determine in a T cell-intrinsic manner whether differentiation proceeds towards iTreg or towards pro-inflammatory T cell subsets.
Figure 7. High doses of TCR stimulation switch differentiating iTregs to IL-17 or IL-9 production.
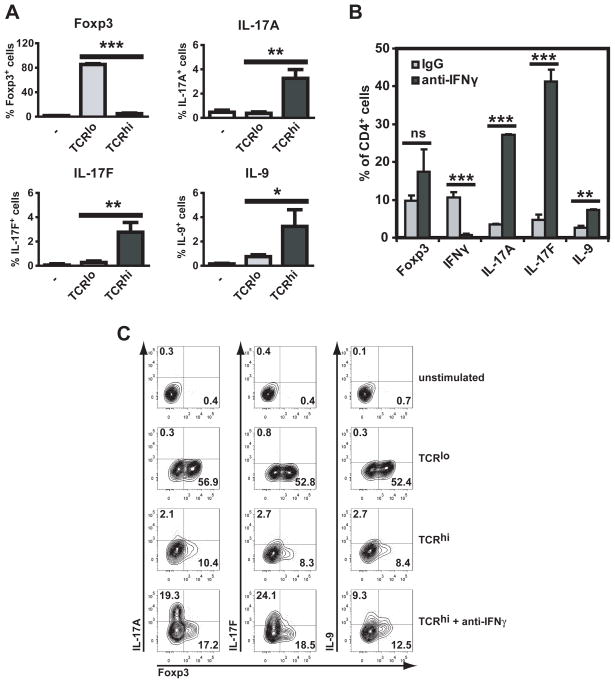
A. Wild type CD4+CD25−CD44lo cells were stimulated with TGF-β, IL-2 and anti-CD28 in the absence (−) or presence of 0.5 μg/ml (TCRlo) or 5 μg/ml (TCRhi) of anti-CD3 mAb. Three days later cells were restimulated with PMA and ionomycin and expression of Foxp3, IL-17A, IL-17F and IL-9 was assessed by intracellular flow cytometry. B. Wild type CD4+CD25−CD62L+ cells were stimulated with TGF-β and IL-2 with 5 μg/ml of anti-CD3 mAb, in the presence of an irrelevant IgG or an IFN-γ-neutralizing antibody. Three days later cells were restimulated with PMA and ionomycin and expression of Foxp3, IFN-γ, IL-17A, IL-17F and IL-9 was assessed by intracellular flow cytometry. Results shown are representative of three independent experiments. All experimental points represent average values and SD from three replicates. ns, not significant, *, p <0.05, **: p <0.01, ***: p <0.001.
Discussion
High doses of TCR stimuli have been shown to prevent conversion of naïve T cells into iTregs and overrule the ability of TGF-β/IL-2 to drive Foxp3 expression, although the molecular mechanism by which expression of Foxp3 is inhibited was not well understood. Our results indicate that TCR-driven NF-κB activity is, at least in part, responsible for this high dose-inhibition of Foxp3 expression, and operates via the induction of NF-κB-dependent, T cell-intrinsic effector cytokines that antagonize iTreg differentiation. These data highlight the potential of T cells to undergo a fate differentiation distinct to that specified by the APC-produced cytokine milieu surrounding them, and identify a signaling pathway that enables such fate changes in a T cell-intrinsic manner.
Our data, as well as those previously published using CARMA1- or Bcl10-deficient T cells suggest that TCR-mediated NF-κB activity is not required for expression of Foxp3 in peripheral T cells stimulated in the presence of exogenous TGF-β and IL-2 (21, 23). This is in contrast to the high dependency on NF-κB activity for the thymic development of nTreg and of their precursors (22–24, 39), and the demonstration of direct transcriptional control of Foxp3 at least by c-Rel in nTregs (16, 17). These results suggest independence from TCR-driven NF-κB for Foxp3 transcription in iTregs but not nTregs. However, an alternative explanation is that TCR-mediated NF-κB activity is only required in the context of iTreg differentiation for the induction of IL-2 production. Because exogenous IL-2 is provided for iTreg differentiation in vitro, developing iTregs may be able to upregulate Foxp3 in an NF-κB-independent manner. This hypothesis is supported by our unpublished results showing that CARMA1-KO, but not wild type T cells, fail to upregulate Foxp3 if IL-2 is omitted from the tissue culture, as only wild type T cells can produce their own IL-2 upon TCR stimulation. This hypothesis is also consistent with results on iTreg differentiation using c-Rel-deficient T cells. Indeed, it has been suggested that c-Rel is essential for the differentiation of iTregs (17) either via its regulation of IL-2 production (20) or of peripheral homeostatic proliferation of Tregs (19). Regardless of the requirement for NF-κB-mediated Foxp3 expression at low dose of TCR stimulation, our results demonstrate that NF-κB upon high TCR stimulation abrogates Foxp3 induction.
Our data support the notion that inhibition of iTreg differentiation at high dose of TCR stimulation depends on CD28 costimulation. The role of CD28 in iTreg differentiation has been controversial, whereby some authors claim that CD28 requirement is due to its ability to promote IL-2 production (40), whereas others show that costimulation can be detrimental (41). However, the dose of TCR stimulation was not taken into account in these studies and may explain the opposite results. Our results indicate that CD28 costimulation inhibits iTreg differentiation at high doses of TCR stimulation, whereas it helps differentiation at very low doses of TCR triggering (≤0.1 μg/ml, data not shown). Consistent with an impact of costimulation on iTreg differentiation, Francisco and collaborators (42) have recently shown that triggering of the inhibitory receptor PD-1 resulted in increased percentage of Foxp3-expressing cells.
While NF-κB is activated by combined CD3 and CD28 engagement, other signaling pathways such as NFAT, ERK and JNK are also induced upon CD3/CD28 ligation (43, 44). Thus, one hypothesis is that any TCR-driven signal may be detrimental for Foxp3 induction at high level of TCR ligation. However, pharmacological inhibition of the calcineurin/NFAT pathway reduced iTreg differentiation at both low or high doses of anti-CD3 (Supplementary Figure 2), confirming the absolute requirement for this pathway during de novo expression of Foxp3 (45) rather than a selective detrimental effect at high antigen dose. In contrast, pharmacological inhibition of JNK and ERK signaling promoted iTreg differentiation upon high TCR triggering, but had minimal or no effect upon stimulation with low doses of anti-CD3 mAb (Supplementary Figure 2). However, because TGF-β signaling also induces activation of JNK and ERK (46), we cannot distinguish whether the increase in iTreg differentiation upon inhibition of ERK and JNK is due to reducing those signaling pathways downstream of TGF-β or TCR engagement. Taken together, our data suggest that NF-κB, along with other signaling pathways, is required for high dose TCR-mediated inhibition of iTreg differentiation.
Our results indicate that effector cytokines expressed by the differentiating naïve T cells following activation with high doses of TCR stimuli can antagonize their differentiation into iTregs despite the presence of TGF-β and IL-2. Furthermore, our data reveal a mechanism for this effect showing its dependence on NF-κB activity. In fact, the two cytokines that were sufficient to prevent Foxp3 induction, TNF and IFN-γ, are direct target genes of NF-κB (47–49). These data complement those by Hill and colleagues who recently described the ability of memory T cells capable of producing the effector cytokines IFN-γ, IL-4 and IL-21 to prevent Foxp3 induction in naïve T cells (50). Interestingly, retinoic acid was able to curtail production of these cytokines and restore iTreg differentiation. Remarkably, retinoic acid has been shown to prevent CD28-mediated inhibition of de novo Foxp3 expression (41). It remains to be established whether retinoic acid or other pro-regulatory factors can also antagonize the inhibitory effects of high TCR stimuli on iTreg differentiation.
Although we show that NF-κB-dependent, T cell-derived, effector cytokines antagonize Foxp3 induction in differentiating iTregs, NF-κB may also prevent Foxp3 upregulation by other means. Our data demonstrate that high TCR stimulation is associated with reduced AcH3 at specific sites in the Foxp3 locus when compared with low TCR stimulation. NF-κB activity induced at high TCR stimulation may directly or indirectly maintain a closed conformation of the Foxp3 locus, although NF-κB family members have generally been associated with opening rather than closing of chromatin structures in T cells (51, 52).
The Akt/mTOR signaling pathway is known to prevent iTreg differentiation (11, 12, 53). For instance, premature termination of TCR signaling, inhibition of PI3K, Akt or mTOR have all been shown to facilitate Foxp3 induction, whereas maintenance of TCR signaling or constitutive PI3K/Akt/mTOR activity antagonized its expression (11). The level of Akt activity has recently been correlated with antigen dose in T cells stimulated with pulsed dendritic cells and has therefore been hypothesized to drive the inhibition of Foxp3 expression at high doses of TCR stimuli (9). However, differences in phospho-S6 mean fluorescence intensity were more modest when T cells were stimulated with anti-CD3 mAb alone in the absence of APCs (9). Interestingly, Gottschalk and collaborators have recently shown that increasing doses of TCR peptide ligands prevent the upregulation of Foxp3 in vivo, and this phenomenon was not associated with enhanced Akt phosphorylation (54). Our data confirm a slight increase in the proportion of T cells expressing phospho-Akt or phospho-S6 at high versus low TCR stimulation by flow cytometry (data not shown), although this difference is unlikely to account for the dramatic decrease in cells expressing Foxp3 at high versus low TCR activation. Our data indicate that inhibition of Akt (Figure 4B) and PI3K (data not shown) can promote iTreg differentiation at any dose of TCR stimulation (data not shown), suggesting that this pathway may act in parallel to, rather than downstream of, the TCR signaling cascade.
Our data also demonstrate that a proportion of naïve T cells activated with high TCR stimuli under iTreg conditions that fail to upregulate Foxp3 instead express IL-17A, IL-17F, or IL-9, cytokines whose acquisition depends on TGF-β-mediated signaling. It has been recently shown that the concentration of anti-CD3 mAb positively correlates with the percentage of cells induced to express IL-17A upon Th17 differentiation, which itself depends on NFAT activity (55). Conversely, it is known that Foxp3 associates with NFAT upon iTreg differentiation preventing it from binding AP-1 (56), and can also bind the Th17 lineage-determining transcription factor RORγt (57), inhibiting the latter from driving Th17 differentiation. When Foxp3 induction is inhibited at high TCR stimulation during iTreg differentiation, these transcription factors may therefore be available for promoting effector rather than iTreg differentiation, which is reflected by the emergence of IL-17- and IL-9-expressing cells in our cultures. This tendency was exacerbated when IFN-γ production was blocked, as IFN-γ is known to prevent differentiation of Th17 cells (58). These results suggest that specific subsets of effector Th subsets may be preferentially generated upon high antigen exposure even in pro-regulatory environments.
Overall, our data prompt the speculation that inhibition of NF-κB in T cells, or of NF-κB-dependent T cell effector cytokines, may restore regulation under circumstances of high antigen exposure, such as in autoimmunity settings.
Acknowledgments
We are grateful to Hideo Yagita for providing anti-TNF antibody as well as to Fotini Gounari, Anne Sperling, Daniel Littman and Thomas Gajewski for providing Bcl-2-Tg, DO11.10-Tg, CARMA1-deficient and CAR-Tg mice, respectively. We thank the University of Chicago Flow Cytometry Core Facility for expert technical help.
Footnotes
This work was supported by NIH RO1 AI1052352 to MLA and an AHA fellowship to LLM.
References
- 1.Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.04.010. in press. [DOI] [PubMed] [Google Scholar]
- 2.Zheng S, Sun G2CM, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 7.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Ahmed EM, Wang T, Ochando JC, Chong AS, Alegre ML. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 11.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 15.Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol Rev. 2009;228:199–211. doi: 10.1111/j.1600-065X.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, Gerondakis S. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visekruna A, Huber M, Hellhund A, Bothur E, Reinhard K, Bollig N, Schmidt N, Joeris T, Lohoff M, Steinhoff U. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. Eur J Immunol. 2010;40:671–676. doi: 10.1002/eji.200940260. [DOI] [PubMed] [Google Scholar]
- 21.Jana S, Jailwala P, Haribhai D, Waukau J, Glisic S, Grossman W, Mishra M, Wen R, Wang D, Williams CB, Ghosh S. The role of NF-kappaB and Smad3 in TGF-beta-mediated Foxp3 expression. Eur J Immunol. 2009;39:2571–2583. doi: 10.1002/eji.200939201. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-{kappa}B activation. Proc Natl Acad Sci U S A. 2004;16:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, Beutler B, Hoebe K. Commitment to the Regulatory T Cell Lineage Requires CARMA1 in the Thymus but Not in the Periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol. 2009;182:6736–6743. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O’Brien W, Thome M, Littman DR. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc Natl Acad Sci U S A. 2000;97:13784–13789. doi: 10.1073/pnas.250356297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks RE, Ho AW, Rivas F, Marshall E, Janardhan S, Gajewski TF. Differential Ras signaling via the antigen receptor and IL-2 receptor in primary T lymphocytes. Biochem Biophys Res Commun. 2003;312:691–696. doi: 10.1016/j.bbrc.2003.10.168. [DOI] [PubMed] [Google Scholar]
- 32.George TC, Fanning SL, Fitzgerald-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, Morrissey PJ, Lynch DH. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 34.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 35.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 36.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vang KB, Yang J, Pagan AJ, Li LX, Wang J, Green JM, Beg AA, Farrar MA. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol. 2010;184:4074–4077. doi: 10.4049/jimmunol.0903933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 Is Essential for TGF-beta-Mediated Induction of Foxp3+ T Regulatory Cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 41.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francisco LM, V, Salinas H, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 45.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 46.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 50.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao S, Gerondakis S, Woltring D, Shannon MF. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J Immunol. 2003;170:3724–3731. doi: 10.4049/jimmunol.170.7.3724. [DOI] [PubMed] [Google Scholar]
- 52.Holloway AF, Rao S, Chen X, Shannon MF. Changes in chromatin accessibility across the GM-CSF promoter upon T cell activation are dependent on nuclear factor kappaB proteins. J Exp Med. 2003;197:413–423. doi: 10.1084/jem.20021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183:6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- 54.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 57.Zhou L, Lopes JE, Chong MM, Ivanov, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]


