Abstract
Background
Hyperkalemia is a potential threat to patient safety in chronic kidney disease (CKD). This study determined the incidence of hyperkalemia in CKD and whether it is associated with excess mortality.
Methods
This retrospective analysis of a national cohort comprised of 2,103,422 records from 245,808 veterans with at least one hospitalization and at least one inpatient or outpatient serum potassium record during fiscal year 2005. CKD and treatment with ACE-I and/or ARBs (RAAS blockers) were the key predictors of hyperkalemia. Death within one day of a hyperkalemic event was the principal outcome.
Results
Of the 66,529 hyperkalemic events (3.2% of records), more occurred inpatient (34937 (52.7%)) versus outpatient (31322 (47.3%)). The adjusted rate of hyperkalemia was higher in patients with CKD than without CKD among individuals treated with RAAS blockers (7.67 vs. 2.30 per 100 patient months, p<0.0001) and those without RAAS blocker treatment (8.22 vs. 1.77 per 100 patient months, p<0.0001). The adjusted odds (OR) of death with a moderate (K+≥ 5.5 and < 6.0mg/dl) and severe (K+≥ 6.0 mg/dl) hyperkalemic event was highest with no CKD (OR: 10.32, 31.64, respectively), versus Stage 3 (5.35, 19.52), Stage 4 (OR: 5.73, 11.56), or Stage 5 CKD (OR: 2.31, 8.02) with all p<0.0001 versus normokalemia and no CKD.
Conclusion
The risk of hyperkalemia is increased with CKD, and its occurrence increases the odds of mortality within one day of the event. These findings underscore the importance of this metabolic disturbance as a threat to patient safety in CKD.
Keywords: chronic kidney disease, hyperkalemia, patient safety
Introduction
Chronic kidney disease (CKD) is a common disease1, affecting a growing number of Americans, and it may be associated with a variety of electrolyte disturbances2. One such disturbance, hyperkalemia, is of great concern to providers treating patients with CKD because of its possible implications for patient safety related to the potential for associated adverse cardiac outcomes3–6. Patients with CKD may be predisposed to hyperkalemia for a variety of reasons. Principal causes include their impaired glomerular filtration rate (GFR) combined with a frequently high dietary potassium intake relative to residual renal function, a commonly observed extracellular shift of potassium caused by the metabolic acidosis of renal failure2,3, and most importantly, recommended treatment with renin-angiotensin aldosterone system (RAAS) blockers that inhibit renal potassium excretion7–9.
Few studies have determined the frequency of hyperkalemia in a large CKD population and the extent that this metabolic disturbance is associated with adverse outcomes. Such information is needed to determine the significance of hyperkalemia as a disease specific patient safety measure. In this study, we examined a national cohort of patients cared for over a single year in the Veterans Health Administration (VHA) to determine the incidence of hyperkalemia in CKD relative to non-CKD patients, and in the presence or absence of treatment with a RAAS blocker. We determined the significance of hyperkalemia by examining its association with subsequent short-term mortality in this population.
Methods
Study Design
This was a retrospective observational study of a national cohort of veterans cared for in the Veterans Health Administration (VHA).
Setting and Data Sources
The setting and data sources used for this study have been previously reported and described10. Briefly, the VHA national health care system provides care to veterans at numerous inpatient and outpatient facilities across the country and is considered the largest integrated national health care system in the United States11. The study data set was derived from fiscal year 2005 and linked acute inpatient data to outpatient laboratory data and vital status data for each veteran. The source data files included the Patient Treatment File (PTF), the Outpatient Care File (OPC), and the Decision Support System (DSS) Laboratory Result Files11;12. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore and the Research & Development Committee of the Maryland VA Healthcare System.
Participants
The study population consisted of veterans with at least one hospitalization from 10/1/2004–9/30/2005, an outpatient measure of serum creatinine prior to hospitalization (index CKD date) and complete demographic data (race, gender, age) to determine estimated glomerular filtration rate (eGFR) and CKD classification. The index GFR was calculated from the serum creatinine obtained between one week and one year and most proximate to the admission date of the index hospitalization. For inclusion in the study, patients also needed at least one potassium value measured during the study year, and this potassium value could be recorded during either an inpatient or outpatient period. If there was more than one record per subject per day, we selected only the record with the highest potassium value of that day for inclusion in the data set.
Variables
The primary exposure was the presence of CKD, defined by an eGFR < 60 131 ml/min/1.73m2 using the abbreviated MDRD equation13. We elected to use one measure of renal function to classify subjects with or without CKD because of the unavailability of repeated measures among a substantial portion of the study cohort. The use of a single measure of renal function to identify patients with CKD is consistent with other large cohort studies1;14–16. If patients met the requirements for CKD, they were further classified by stage of CKD based on their eGFR17.
Incidence of hyperkalemia was the primary outcome. Using previously defined thresholds for hyperkalemia, we set moderate hyperkalemia to be a serum potassium level greater than or equal to 5.5 mg/dl but less than 6.0 mg/dl18, with more severe hyperkalemia set at greater than or equal to 6.0 mg/dl19. To determine the significance of hyperkalemia after its occurrence, mortality on the day of or the day following (1-day mortality) the hyperkalemic event was chosen as the key adverse outcome. If there were multiple potassium records associated with a 1-day period, the record with the highest potassium value was included in the analysis, regardless of when it occurred in the 1-day period. We chose this short time interval to ensure a feasible link between the hyperkalemic event and mortality and to minimize the effect of potential case-mix confounders to this association.
Covariates
Standard demographic characteristics, comorbidities, and use of RAAS blockers were included in the analysis. Demographic data included gender, age at date of hospital admission (18–56, 57–64, 65–75, and 76+), and race, which was characterized as Caucasian, African-American or other. Comorbidities used in the analysis included cancer (excluding non-melanomatous skin cancer), cardiovascular disease (a composite of cerebrovascular disease, myocardial infarction and/or congestive heart failure), and diabetes. The Charlson comorbidity index (CCI) was computed for each patient for descriptive purposes and excluded cancer, diabetes and cardiovascular disease20. All comorbidities were determined using International Classification of Diseases, 9th revision (ICD-9CM) diagnosis codes from inpatient and outpatient VHA records from October 1, 1999 to the date of the index date. A variable indicating whether the patient had a prescription for Angiotensin II Receptor Blockers (ARB) and/or Angiotensin Converting Enzyme Inhibitors (ACE-I) within 30 days of a potassium record was included in the analysis.
Measurements
To determine incidence of hyperkalemia, we expressed the cumulative frequency of events per 100 patient-months of the contributed time of veterans to each sub-group during the study period. To determine the association between hyperkalemia and mortality, survival status within 1-day was linked with each record. We also conducted sub-analyses of inpatient and outpatient events.
Statistical Methods
For descriptive analyses, we reported continuous variables with their mean values and standard errors and N (%) for categorical variables. We used a Poisson regression model, allowing for multivariate adjustments and variable follow-up times, to determine the incidence rates of hyperkalemia in individuals with and without CKD. Results were reported as adjusted rates (events per 100 patient months) within key strata including with and without CKD, and with or without exposure to a RAAS blocker, and the average for each adjustment variable.
To determine the adjusted risk of mortality associated with a potassium event, we calculated an odds ratio of 1-day mortality using generalized estimating equations (GEE). This allowed for repeated assessments of multiple potassium records and repeated vital status assessment within each subject and controlling for multiple covariates.
Results
Participants
The base study cohort included 247,176 patients; however, 1368 of these were excluded because of missing index GFR (n=16) or potassium values (n=1352). The final study population of 245,808 individuals had 2,764,658 potassium records; this set of records was further restricted by limiting multiple values on any given day, leaving the final data set including 2,103,422 records.
Descriptive Data
Table 1 lists the baseline characteristics of the study patients classified into groups based on CKD status and whether or not the patient had experienced any hyperkalemic event (≥ 5.5 mg/dl). Patients with CKD were older, more likely to be male, white, on ACE-I and/or ARBs, and have diabetes, CVD, cancer, and other comorbidities represented by the CCI. Patients who experienced hyperkalemia were more likely to be male, African American, older, and have diabetes, CVD, and CKD across all stages. Table 1 also includes the results of a logistic regression model identifying risk factors for hyperkalemia in the study population. The strongest risk factor for the development of hyperkalemia was the presence and severity (stage) of CKD. As renal function declined, the odds of developing hyperkalemia greatly increased. Use of ACE-I and/or ARBs was also a significant risk factor for development of hyperkalemia as was the presence of diabetes and two or more other comorbidities represented by the CCI.
Table 1.
Patient Characteristics by Chronic Kidney Disease and Hyperkalemia Status
| No CKD | CKD | K+ < 5.5 mg/dl | K+ ≥5.5 mg/dl* | Odds (OR) of K+ ≥5.5 mg/dl | ||
|---|---|---|---|---|---|---|
| Patients (row %) | 174935 (71.2) | 70873 (28.8) | 212171 (86.3) | 33637 (13.7) | 245808 (100.0) | |
| Sex (column %) | ||||||
| Male | 167202 (95.6) | 68572 (96.8) | 202867 (95.6) | 32907 (97.8) | 1.00 | |
| Female | 7733 (4.4) | 2301 (3.2) | 9304 (4.4) | 730 (2.2) | 0.61 (0.57, 0.66) | |
| Race | ||||||
| White | 139213 (79.6) | 58745 (82.9) | 172054 (81.1) | 25904 (77.0) | 1.00 | |
| Black | 34000 (19.4) | 11332 (16.0) | 37958 (17.9) | 7374 (21.9) | 1.29 (1.25, 1.32) | |
| Other | 1722 (1.0) | 796 (1.1) | 2159 (1.0) | 359 (1.1) | 1.03 (0.91, 1.15) | |
| Age (median) | 61.0±0.1 | 73.0±0.1 | 64.0±0.1 | 68.0±0.1 | 1.01 (1.00, 1.01) | |
| ACE-I/ARB | ||||||
| None | 89928 (51.4) | 24410 (34.4) | 102953 (48.5) | 11385 (33.8) | 1.00 | |
| Either | 82528 (47.2) | 44047 (62.1) | 105492 (49.7) | 21083 (62.7) | 1.41 (1.37, 1.44) | |
| Both | 2479 (1.4) | 2416 (3.4) | 3726 (1.8) | 1169 (3.5) | 1.67 (1.55, 1.80) | |
| CCI | ||||||
| 0 | 86402 (49.4) | 27977 (39.5) | 101850 (48.0) | 12529 (37.2) | 1.00 | |
| 1 | 59589 (34.1) | 26786 (37.8) | 73745 (34.8) | 12630 (37.5) | 1.24 (1.20, 1.27) | |
| 2+ | 28944 (16.5) | 16110 (22.7) | 36576 (17.2) | 8478 (25.2) | 1.57 (1.51, 1.62) | |
| Cancer | ||||||
| No | 135842 (77.7) | 51359 (72.5) | 162591 (76.6) | 24610 (73.2) | 1.00 | |
| Yes | 39093 (22.3) | 19514 (27.5) | 49580 (23.4) | 9027 (26.8) | 1.16 (1.13, 1.19) | |
| Diabetes | ||||||
| No | 117799 (67.3) | 35129 (49.6) | 137417 (64.8) | 15511 (46.1) | 1.00 | |
| Yes | 57136 (32.7) | 35744 (50.4) | 74754 (35.2) | 18126 (53.9) | 1.51 (1.47, 1.55) | |
| CVD | ||||||
| No | 124946 (71.4) | 33156 (46.8) | 140886 (66.4) | 17216 (51.2) | 1.00 | |
| Yes | 49989 (28.6) | 37717 (53.2) | 71285 (33.6) | 16421 (48.8) | 1.14 (1.12, 1.17) | |
| CKD Stage | ||||||
| No CKD | 174935 (100) | 0 (0) | 159450 (75.2) | 15485 (46.0) | 1.00 | |
| Stage 31 | 0 (0) | 57798 (81.6) | 45840 (21.6) | 11958 (35.6) | 2.24 (2.17, 2.30) | |
| Stage 42 | 0 (0) | 8351 (11.8) | 4835 (2.3) | 3516 (10.5) | 5.91 (5.63, 6.20) | |
| Stage 53 | 0 (0) | 4724 (6.7) | 2046 (1.0) | 2678 (8.0) | 11.00 (10.34, 11.69) | |
inclusive of all hyperkalemic events
Stage 3: 60 ml/min/1.73 m2>GFR≥30 ml/min/1.73 m2
Stage 4: 30 ml/min/1.73 m2>GFR≥15 ml/min/1.73 m2
Stage 5: GFR<15 ml/min/1.73 m2
Of the 33637 individuals who experienced hyperkalemia, 17822 (53.0%) had only one hyperkalemic event ≥ 5.5 mg/dl during the study period and the remainder had more than one event. There were 70 individuals (0.21%) who had more than twenty events, and a greater proportion of these patients had CKD, ACE-I and/or ARBs use, and diabetes than the general population with hyperkalemia.
Incidence of Hyperkalemia
Table 2 categorizes the subjects into four groups based on CKD and treatment status with ACE-I and/or ARBs. For each of these groups, the cumulative number of hyperkalemic events, based on severity, was summed within the cumulative 100 patient-months of that group. A total of 66259 hyperkalemic events (3.2% of total laboratory values) were recorded, with a decreasing incidence of more 9 severe events. The adjusted rates show that, regardless of treatment status, individuals with CKD were significantly more likely to have a hyperkalemic event than those without CKD. However, the highest number of hyperkalemic events per 100 patient months occurred in patients who had CKD and no treatment with an ACE-I and/or ARB within the prior 30 days.
Table 2.
Incidence of Hyperkalemia (per 100 patient-months) among patients with and without CKD and/or Renin Angiotensin AldosteroneSystem (RAAS) blocker treatment
| Potassium ≥ 5.5 mg/dl (inclusive) |
Potassium ≥5.5 and <6.0 mg/dl |
Potassium ≥ 6.0 mg/dl |
||
|---|---|---|---|---|
| Total Number of Potassium Events | 66259 | 44907 | 21352 | |
| Total Number of Patient Months | 1581299 | 1581299 | 1581299 | |
| RAAS Blocker Treatment | ||||
| CKD | ||||
| Number of events | 29023 | 19371 | 9652 | |
| Number of Patient Months | 317701 | 317701 | 317701 | |
| Adjusted Rate1,2 | 7.67 (7.57, 7.78) | 5.06 (4.97, 5.14) | 2.60 (2.54, 2.66) | |
| NO CKD | ||||
| Number of events | 15302 | 10714 | 4588 | |
| Number of Patient Months | 577747 | 577747 | 577747 | |
| Adjusted Rate1,2 | 2.30 (2.26, 2.33) | 1.63 (1.59, 1.66) | 0.67 (0.65, 0.69) | |
| No RAAS Blocker Treatment | ||||
| CKD | ||||
| Number of events | 12709 | 8506 | 4203 | |
| Number of Patient Months | 146884 | 146884 | 146884 | |
| Adjusted Rate1,2 | 8.22 (8.07, 8.37) | 5.43 (5.31, 5.56) | 2.76 (2.68, 2.85) | |
| NO CKD | ||||
| Number of events | 9225 | 6316 | 2909 | |
| Number of Patient Months | 538967 | 538967 | 538967 | |
| Adjusted Rate1,2 | 1.77 (1.73, 1.81) | 1.24 (1.21, 1.27) | 0.53 (0.51, 0.55) | |
Adjusted rates are per 100 patient months, all p-values<0.0001, (95%CI)
Adjusted for confounders: race, gender, age, CCI, cancer, diabetes, CVD, and RAAS blocker treatment within 30 days
Risk of mortality with hyperkalemia
There were 6996 patients (2.8%) that died within one day of a blood draw when potassium levels were recorded. Figure 1 shows the adjusted 1-day odds of death associated with each hyperkalemic event based on severity of hyperkalemia and stage of CKD. The reference group included those normokalemic records from individuals with neither CKD nor hyperkalemia. Of note, among normokalemic events, there was a tendency toward a higher risk of 1-day mortality when the events occurred in individuals with CKD, and the risk reached significance among patients with stage 5 CKD relative to their counterparts without CKD. For those events recorded within each stage of CKD, as the level of hyperkalemia increased, the odds of death also increased. In contradistinction to the trend seen with normokalemia, within each discrete level of hyperkalemia, there was an inverse relationship between stages of CKD and odds of death; as the stage of CKD becomes more severe, the odds of death decrease.
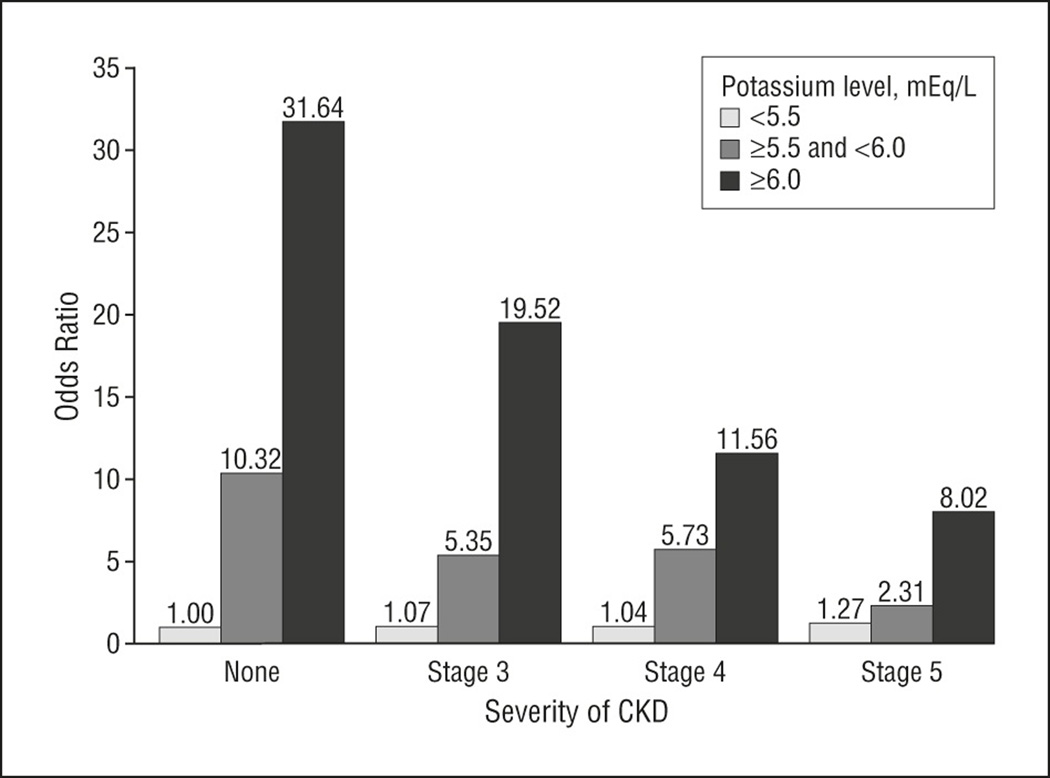
The odds ratio of death within 1 day of a moderate (K ≥ 5.5 and < 6.0 mg/dl) and severe (≥ 6.0 mg/dl) hyperkalemic event in the study population stratified by stage of CKD. Normokalemia (K < 5.5 mg/dl) and no CKD is the reference group. P < 0.001 for all moderate and severe hyperkalemia groups, and p < 0.05 for Stage 5 with normokalemia versus reference group.
Table 3 illustrates the odds of 1-day mortality associated with inpatient and outpatient records from patients with and without CKD. The reference group in both the inpatient and outpatient analyses was comprised of normokalemic records from patients without CKD. Of the 66529 hyperkalemic events, 34937 (52.7%) occurred as inpatient and 31322 (47.3%) occurred as outpatient events. In both the inpatient and outpatient analyses, the trends were the same. The odds of death increased with severity of hyperkalemia; however, the risk of death was greater in the absence of CKD versus when CKD was present. The risk estimates in all categories tended to be higher with inpatient records than with outpatient records.
Table 3.
Odds of Death within 1 Day of a Hyperkalemic Event, by Potassium Category and CKD
| Potassium< 5.5 mg/dl (No hypokalemia) |
Potassium ≥ 5.5 and <6.0 mg/dl |
Potassium ≥ 6.0 mg/dl | ||
|---|---|---|---|---|
| INPATIENT | ||||
| Number of records | 1206165 | 24190 | 10747 | |
| No CKD | ||||
| OR (95% CI)1,2 | 1 (reference) | 11.38 (10.08, 12.84) | 33.36 (29.72, 37.43) | |
| Number of records | 762136 | 9611 | 4227 | |
| Number of death events3 | 2304 (0.3%) | 312 (3.2%) | 365 (8.6%) | |
| CKD | ||||
| OR (95% CI)1,2 | 1.12 (1.05, 1.19) | 5.40 (4.72, 6.18) | 15.82 (13.97, 17.93) | |
| Number of records | 444029 | 14579 | 6520 | |
| Number of death events3 | 1693 (0.4%) | 256 (1.8%) | 310 (4.8%) | |
| OUTPATIENT | ||||
| Number of events | 830998 | 20717 | 10605 | |
| No CKD | ||||
| OR (95% CI)1,2 | 1 (reference) | 6.17 (4.16, 9.16) | 27.74 (20.66, 37.25) | |
| Number of records | 554777 | 7419 | 3270 | |
| Number of death events3 | 336 (0.06%) | 27 (0.4%) | 54 (1.7%) | |
| CKD | ||||
| OR (95% CI)1,2 | 0.99 (0.82, 1.18) | 2.73 (1.80, 4.11) | 13.06 (9.85, 17.30) | |
| Number of records | 276221 | 13298 | 7335 | |
| Number of death events3 | 200 (0.07%) | 25 (0.2%) | 63 (0.9%) | |
Odds ratio (OR) estimated from logistic regression models accounting for repeated glucose measures within individuals.
Adjusted for confounders: race, gender, age, CCI, cancer, diabetes, CVD, treatment with RAAS blocker within 30 days
Number of 1-day death events by records in each subgroup (%)
Discussion
Over the past decade, research has increasingly focused on studying patient safety in the general population. Little attention, however, has been given to disease specific measures of patient safety that are relevant to populations with chronic illness like CKD. In this analysis, we focused on hyperkalemia as one measure of disease specific patient safety relevant to health care provides managing patients with CKD.
Our results demonstrate that patients with CKD were significantly more likely than patients without CKD to have a hyperkalemic event at all stages of renal disease. The results, however, indicate that patients with CKD treated with a RAAS blocker are slightly less likely to have both moderate and severe hyperkalemia than CKD patients who are not on this treatment. The 1-day odds of death were greater after a hyperkalemic event compared to a normokalemic event. However, hyperkalemic events in patients without CKD were associated with higher odds of 1-day mortality than hyperkalemic events in patients with CKD. There tended to be an inverse relationship between the severity (stage) of CKD and odds of 1-day mortality following a hyperkalemic event, and more severe hyperkalemia was associated with higher odds of death.
Prior studies7, 9, 21–23 have documented modest hyperkalemia associated with the use of ACE-I and/or ARB in patients with CKD in the setting of a clinical trial. In six separate clinical trials of over 1500 individuals with renal insufficiency, elevations in serum potassium levels between 0.3 and 0.6 mg/dl occurred in 3–5% of subjects randomized to an ACE inhibitor24. Other trials have shown similar increases among patients assigned to treatment with ARBs24. The observation in the present study that hyperkalemia is less common among patients with CKD treated with a RAAS blocker has several potential explanation. It is possible that the predisposition of CKD patients to developing hyperkalemia may cause treating physicians to screen out prior to treatment of patients who are prone to this metabolic disturbance. In those patients in whom they elect to treat with a RAAS blocker, physicians may increase their surveillance for hyperkalemia or spend more time determining means by which a patient can avoid this consequence of therapy. However, our ability to elucidate the mechanism underlying this finding is limited and beyond the scope of this study.
The observed lower 1-day mortality rate among patients with CKD versus those patients without CKD and hyperkalemia has some plausible explanations. First, as kidney function declines, there is likely to be an adaptive response which leads to a new increased steady state serum potassium level. In patients with more severe kidney disease, there is also an increased level of gut potassium excretion..
Potassium adaption is the response of the kidneys to a high dietary potassium intake25;26. In patients with chronic renal insufficiency, prior studies have described a compensatory response to chronic hyperkalemia in which the body eventually develops a new steady state potassium level which is often significantly higher than normal27–31. As GFR declines, serum potassium levels increase as potassium filtration and secretion fall. When aldosterone or other kaliuretic factors fail to maintain potassium homeostasis, extracellular [K+] may rise until it reaches a level sufficient to produce a sustained increase in renal potassium excretion32. Increased extrarenal potassium excretion through the gut is another adaptive mechanism which gradually develops in patients with chronic renal failure as kidney function declines33–35.
The lower mortality seen with hyperkalemia in CKD is consistent with evidence suggesting that these patients may be less susceptible to cardiac toxicity from this metabolic disturbance than patients without kidney disease. This may be attributable to the fact that patients with CKD are more likely to develop chronic hyperkalemia, which is reported to be better tolerated than an acute rise29;36;37. It has been noted that patients with CKD who develop chronic hyperkalemia can have serum potassium levels in excess of 6.0 mg/dl without apparent electrocardiographic or cardiovascular manifestations38–40. Acute hyperkalemia can cause a rapid reduction in resting membrane potential leading to increased cardiac depolarization, muscle excitability and possible ECG changes41;42. These cardiac changes may be altered in the renal population where the presence of other electrolyte disturbances could influence the cardiac membrane potential36.
The findings reported in this study have important clinical implications. They suggest that patients with CKD and those who are treated with RAAS blocker have an increased risk of hyperkalemia, but that risk may be somewhat lowered with proper patient selection for RAAS treatment. The increased risk of death associated with hyperkalemia underscores the importance of clinical monitoring and follow-up of patients with CKD or who are treated with RAAS blockers to avoid the adverse outcomes that are associated with this metabolic disturbance. Others have reported that a substantial portion of patients who have hyperkalemia detected in a clinical setting do not have timely follow-up of this abnormal lab value43;44 Even though this study revealed an attenuation in the morality risk associated with hyperkalemia and CKD consistent with the adaptive mechanisms described, the higher frequency of this metabolic disturbance in this disease population suggests that patients with CKD should be closely monitored to minimize adverse outcomes.
Retrospective analyses such as this one have several inherent limitations which should be considered when interpreting the findings. First, the incidence of hyperkalemia is only detected at the time of a clinically ordered blood test and does not account for the occurrence of this metabolic disturbance at unobserved times. Therefore, our estimation of the incidence of hyperkalemia is subject to the frequency of laboratory monitoring, which is non-random among the veterans in this cohort. Nevertheless, the demonstrated odds of death associated with hyperkalemia in both inpatient and outpatient labs, highlights the significance of the detected events in this study. Using 1-day mortality as the outcome, we were able to minimize the influence of confounding by severity of illness in the study. The results of this study only included RAAS blockers, which are known to increase serum potassium levels. However, it is quite possible that other agents may have influenced the incidence of hyperkalemia. The emphasis in this study, however, was on that class of agents which are a key element to disease management in CKD. Finally, patients with Stage 5 CKD may have included individuals with ESRD who are on dialysis and as a result may handle potassium differently than those subjects who were dialysis independent. Given the small number of patients with Stage 5 CKD in the cohort, the proportion on dialysis was likely to be small.
The findings of this study support the consideration of hyperkalemia as a patient safety measure in CKD. We have shown that this metabolic disturbance is more common in CKD than in patients without this condition. Moreover, hyperkalemia is linked to increased odds of mortality although the risk is lower in CKD versus non-CKD populations. These findings suggest that the role of disease management in the manifestation of this candidate safety measure is complex. However, given the association of hyperkalemia with CKD and the elevated odds of death associated with hyperkalemia, this metabolic disturbance should be considered a disease-specific safety event. More work is needed to see to what extent alterations in disease management will reduce the incidence of this patient safety indicator.
Acknowledgments
NIDDK 1R21DK075675, Short Term Research Training Program, Office of Student Research/Dean's Office, University of Maryland School of Medicine
Footnotes
Disclosures: None
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult 358 patients in the intensive care unit. Am J Health Syst Pharm. 2007;64(20 Suppl 13):S13–S20. doi: 10.2146/ajhp040300. [DOI] [PubMed] [Google Scholar]
- 3.Williams ME. Hyperkalemia. Crit Care Clin. 1991;7(1):155–174. [PubMed] [Google Scholar]
- 4.Esposito C, Bellotti N, Fasoli G, Plati A, Dal Canton A. Hyperkalemia-induced ECG abnormalities in patients with reduced renal function. Clin Nephrol. 2004;62(6):465–468. doi: 10.5414/cnp62465. [DOI] [PubMed] [Google Scholar]
- 5.Obialo CI, Ofili EO, Mirza T. Hyperkalemia in CHF patients aged 63 to 85 years with subclinical renal disease. Am J Cardiol. 2003;90(6):663–665. doi: 10.1016/s0002-9149(02)02581-x. [DOI] [PubMed] [Google Scholar]
- 6.Mandal AK. Hypokalemia and hyperkalemia. Med Clin North Am. 1997;81(3):611–539. doi: 10.1016/s0025-7125(05)70536-8. [DOI] [PubMed] [Google Scholar]
- 7.Weir M. Are drugs that block the renin-angiotensin system effective and safe in patients with renal insufficiency? American Journal of Hypertension. 1999;12(12 Pt 3):195S–203S. doi: 10.1016/s0895-7061(99)00104-1. [DOI] [PubMed] [Google Scholar]
- 8.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin aldosterone system. N Engl J Med. 2004;351(6):585–591. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 9.Reardon LC, Macpherson DS. Hyperkalemia in Outpatients Using Angiotensin Converting Enzyme Inhibitors. Arch Intern Med. 1998;158(1):26–32. doi: 10.1001/archinte.158.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC. Chronic kidney disease adversely affects patient safety. J Am Soc Nephrol. 2008;19(12):2414–2419. doi: 10.1681/ASN.2008010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowper DC, Hynes DM, Kubal JD, Murphy PA. Using administrative databases for outcomes research: select examples from VA Health Services Research and Development. J Med Syst. 1999;23(3):249–259. doi: 10.1023/a:1020579806511. [DOI] [PubMed] [Google Scholar]
- 12.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004;27(Suppl 2):B22–B26. doi: 10.2337/diacare.27.suppl_2.b22. [DOI] [PubMed] [Google Scholar]
- 13.Avorn J, Bohn RL, Levy E, Levin R, Owen WF. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med. 2002;162(17):2002–2006. doi: 10.1001/archinte.162.17.2002. [DOI] [PubMed] [Google Scholar]
- 14.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kid Dis. 2002;2(Suppl 1):S46–S75. [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 18.Kuperman GJ. How promptly are inpatients treated for critical laboratory results? J Am Med Inform Assoc. 1998;5(1):112–119. doi: 10.1136/jamia.1998.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoll GA. Renin-angiotensin system blockade and the risk of hyperkalemia in 395 chronic hemodialysis patients. Am J Med. 2002;112(2):110–114. doi: 10.1016/s0002-9343(01)01068-3. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9 CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Hannedouche T, Landais P, Goldfarb B, et al. Randomised controlled trial of enalapril and beta-blockers in non-diabetic chronic renal failure. BMJ. 1994;309(6958):833–837. doi: 10.1136/bmj.309.6958.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.August P, Cody RJ, Sealey JE, Laragh JH. Hemodynamic responses to converting enzyme inhibition in patients with renal disease. Am J Hypertens. 1989;2(8):599–603. doi: 10.1093/ajh/2.8.599. [DOI] [PubMed] [Google Scholar]
- 23.Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24(2):168–175. doi: 10.1016/j.semnephrol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Bakris GL, Siomos M, Richardson D, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. Kidney Int. 2000;58(5):2084–2092. doi: 10.1111/j.1523-1755.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 25.Malnic G, Klose R, Giebisch G. Micropuncture study of renal potassium excretion in the rat. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- 26.Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol Renal Physiol. 1999;277(6 Pt2):F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 27.Kunau RT, Stein JH. Disorders of hypo and hyperkalemia. Clin Nephrol. 1977;7(4):173–190. [PubMed] [Google Scholar]
- 28.Salem MM, Rosa RM, Batlle DC. Extrarenal potassium tolerance in chronic renal failure: implications for the treatment of acute hyperkalemia. Am J Kidney Dis. 1991;18(4):421–440. doi: 10.1016/s0272-6386(12)80110-7. [DOI] [PubMed] [Google Scholar]
- 29.Allon M. Treatment and Prevention of Hyperkalemia in End Stage Renal Disease. Kidney Int. 1993;43(6):1197–1209. doi: 10.1038/ki.1993.170. [DOI] [PubMed] [Google Scholar]
- 30.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol. 1971;221(2):437–448. doi: 10.1152/ajplegacy.1971.221.2.437. [DOI] [PubMed] [Google Scholar]
- 31.Silva P, Brown RS, Epstein FH. Adaptation to potassium. Kidney Int. 1977;11(6):466–475. doi: 10.1038/ki.1977.64. [DOI] [PubMed] [Google Scholar]
- 32.Gennari FJ, Segal AS. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int. 2002;62(1):1–9. doi: 10.1046/j.1523-1755.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed J, Weisberg L. Hyperkalemia in Dialysis Patients. Semin Dial. 2001;14(5):348–356. doi: 10.1046/j.1525-139x.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 34.Hayes CP, Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physician. 1967;80:207–216. [PubMed] [Google Scholar]
- 35.Sandle GI, Gaiger E, Tapster S, Goodship TH. Evidence for large intestinal control of potassium homeostasis in uraemic patients undergoing long-term dialysis. Clin Sci. 1987;73(3):247–252. doi: 10.1042/cs0730247. [DOI] [PubMed] [Google Scholar]
- 36.Allon M. Disorders of potassium metabolism. In: Greenburg A, editor. Primer on Kidney Diseases. 3rd ed. San Diego: Academic Press; 2001. pp. 98–107. [Google Scholar]
- 37.Surawicz B, Chlebus H, Mazzoleni A. Hemodynamic and electrocardiographic effects of hyperpotassemia. Differences in response to slow and rapid increases in concentration of plasma K. Am Heart J. 1967;73:647–664. doi: 10.1016/0002-8703(67)90174-3. [DOI] [PubMed] [Google Scholar]
- 38.Aslam S, Friedman EA, Ifudu O. Electrocardiography is unreliable in detecting potentially lethal hyperkalaemia in haemodialysis patients. Nephrol Dial Transplant. 2002;17:1639–1642. doi: 10.1093/ndt/17.9.1639. [DOI] [PubMed] [Google Scholar]
- 39.Szerlip HM, Weiss J, Singer I. Profound hyperkalemia without electrocardiographic manifestations. Am J Kidney Dis. 1986;7:461–465. doi: 10.1016/s0272-6386(86)80185-8. [DOI] [PubMed] [Google Scholar]
- 40.Wong KC. Management of Electrolyte Abnormalities. In: Stanley TH, Petty WC, editors. New Anesthetic Agents, Devices and Monitoring Techniques. Springer; 1983. pp. 10–19. [Google Scholar]
- 41.Lyons CJ, Burgess MJ, Abildskov JA. Effects of acute hyperkalemia on cardiac excitability. Am Heart J. 1977;94(6):755–763. doi: 10.1016/s0002-8703(77)80217-2. [DOI] [PubMed] [Google Scholar]
- 42.Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18(6):721–729. doi: 10.1053/ajem.2000.7344. [DOI] [PubMed] [Google Scholar]
- 43.Moore CR, Lin JJ, O'Connor N, Halm EA. Follow-up of markedly elevated serum potassium results in the ambulatory setting: implications for patient safety. Am J Med Qual. 2006;21:115–124. doi: 10.1177/1062860605285047. [DOI] [PubMed] [Google Scholar]
- 44.Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: Causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158:917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]


