Abstract
Background. Aging is a multifactorial process characterized by a progressive decline in physiological function. Decreased kidney function is associated with cardiovascular disease and mortality. Therefore, increasing our insight into kidney aging by understanding the anatomic, physiologic, and pathologic changes of aging in the kidney is important to prevent disastrous outcomes in elderly people. Methods. Male two-, 12-, and 24-month-old C57/BL6 mice were used in this study. We measured histological change, oxidative stress, and aging-related protein expression in the kidneys. Results. Twenty-four-month-old mice displayed increased albuminuria. Creatinine clearance decreased with aging, although this was not statistically significant. There were increases in mesangial volume and tubulointerstitial fibrosis in 24-month-old mice. There were also increases in F4/80 expression and in apoptosis detected by TUNEL assay. Urine isoprostane excretion increased with aging and SOD1 and SOD2 were decreased in 24-month-old mice. Oxidative stress may be mediated by a decrease in Sirt1, PGC-1α, ERR-1α, and PPARα expression. Klotho expression also decreased. Conclusions. Our results demonstrate that Sirt1 was decreased with aging and may relate to changed target molecules including PGC-1α/ERR-1α signaling and PPARα. Klotho can also induce oxidative stress. Pharmacologically targeting these signaling molecules may reduce the pathologic changes of aging in the kidney.
1. Introduction
Aging in most species is associated with impaired adaptive and homeostatic mechanisms, leading to susceptibility to environmental or internal stresses with increasing rates of disease [1, 2]. A number of different theories of primarily disease-independent renal aging, which can be categorized as evolutionary, molecular, cellular, and systemic, have been proposed in the past and recent studies have provided evidence for some of these theories [3].
Age-related changes lead to a functional decline of several organs, including the kidney [4]. Age-related changes in renal structure and function have been described [5], not just in humans but in a wide range of other species, including rats, mice, hamsters, dogs, and cats. Modifications of the kidneys related to aging include changes to glomerular structure. As the functional kidney becomes older, there is glomerular basement membrane thickening and wrinkling associated with loss of capillary loops, and mesangial matrix expansion that may be the outcome of an imbalance between formation and removal of extracellular matrix [6].
Mild changes in renal function with aging are often accompanied by tubular functional changes, which may not necessarily be recognized in humans unless they lead to disease or accentuation of a pathological condition. These changes include a reduction in sodium homeostasis associated with the loss of the ability to concentrate or dilute the urine to the degree seen in younger individuals [7], which can accentuate dehydration in the elderly. There is also impairment of transport of a range of other electrolytes and ions across the tubular epithelium, as well as reduced drug excretion. Direct and indirect hormonal functions of the kidney also change with age, including blunted renin responses [8].
It is well known that the aging process is associated with disturbances of normal intracellular physiological phenomena including telomere shortening, reduction in Klotho expression, inactivation of silent information regulator T1 (SIRT1), activation of the renin-angiotensin system, and oxidative stress. Diabetes and metabolic syndrome are considered to contribute to the same types of organ damage as the aging process, especially in the kidneys, heart, and nerves. We therefore examined the processes of renal change during the aging process in mice, focusing on the changes in SIRT1,peroxisome proliferator-activated receptor α (PPARα), Klotho, and oxidative stress-related enzymes [9–11].
2. Materials and Methods
2.1. Animal Model
The Animal Care Committee of The Catholic University approved the experimental protocol. Aging C57BL/6 mice were purchased from the Korea Research Institute of Bioscience and Biotechnology (Chungcheongbuk-do, Republic of Korea). Mice were housed in a controlled temperature and controlled light environment. Mice were divided into three groups as follows: 2-month-old group (2 M group, n = 5), 12-month-old group (12 M group, n = 5), and 24-month-old group (24 M group, n = 4).
2.2. Renal Function
Mice were placed in individual mouse metabolic cages (Tecniplast, Gazzada, Italy) with access to water and food for 24 h to collect urine for subsequent measurements of albumin and creatinine concentrations. Albuminuria (Albuwell M, Exocell, Philadelphia, PA) and urine creatinine (The Creatinine Companion, Exocell) were measured using ELISA kits. Measurement of serum creatinine concentrations performed at Samkwang Medical Laboratories (Seoul, Korea) using enzymatic colorimetric methods (Modular DPP system, Roche, Hamburg, Germany). Creatinine clearance was calculated using a standard formula (urine creatinine (mg/dL) × urine volume (mL/24 h)/serum creatinine (mg/dL) × 1440 (min/24 h)).
2.3. Histology
Kidney samples were fixed in 10% formalin. The tissues were embedded in low-temperature melting paraffin, and 4-μm sections were processed and stained with hematoxylin-eosin (HE), periodic acid-Schiff (PAS), and Masson's trichrome. The glomerular volume and mesangial area were determined by examining PAS sections using a color-image analyzer (TDI Scope Eye, Version 3.5 for Windows, Olympus, Tokyo, Japan). The relative mesangial area was expressed as mesangial/glomerular surface area. A finding of tubulointerstitial fibrosis was defined as a matrix-rich expansion of the interstitium with tubular dilatation, tubular atrophy, tubular cast formation and thickening of the tubular basement membrane. Ten fields per section were assessed.
2.4. Immunohistochemistry
Paraffin sections were deparaffinized in xylene and hydrated in ethanol before staining for markers of inflammation including macrophages (F4/80, AbD Serotec, Oxford, UK). Sections were treated with an antigen-unmasking solution consisting of 10 mM sodium citrate, pH 6.0 and then washed with phosphate-buffered saline (PBS). Sections were incubated with 3% H2O2 in methanol to block endogenous peroxidase activity. Nonspecific binding was blocked with 10% normal horse serum. After incubating with primary antibody (F4/80 1 : 50) at 4°C overnight, antibodies were visualized with a peroxidase-conjugated secondary antibody using the Vector Impress kit (Vector Laboratories, Burlingame, CA). Sections were then dehydrated in ethanol, cleared in xylene and mounted without counterstaining. All sections were assessed using a color-image analyzer (TDI Scope Eye, Version 3.5 for Windows, Olympus).
2.5. TUNEL Assay
Apoptosis was quantified using an in situ detection TUNEL assay kit (Chemicon International, Temecula, CA). The number of TUNEL-positive cells in the kidney was determined using 1,000× magnification.
2.6. Western Blot Analysis
Total protein was extracted from the renal cortical tissues with a Pro-Prep Protein Extraction Solution (Intron Biotechnology, Gyeonggi-Do, Republic of Korea) according to the manufacturer's instructions. Western blot analysis was performed using the following antibodies: SIRT 1 (1 : 2000, LifeSpan BioSciences, Seattle, WA), Klotho (1 : 5000, Kyowa Hakko Kogyo Co. Ltd, Shizuoka, Japan), PPARα (1 : 1000, Abcam, Cambridge, MA), PGC-1α (1 : 2000, Novus Biologicals, Littleton, CO), estrogen-related receptor-1α (ERR-1α) (1 : 2000, Millipore, Billerica, MA), superoxide dismutase 1 (SOD1) (1 : 5000, Assay Designs, Rochester, NY) and superoxide dismutase 2 (SOD2) (1 : 10000, Abcam), and β-actin (1 : 10000, Sigma, St Louis, MO).
2.7. Assessment of Renal Oxidative Stress
To evaluate oxidative stress we measured the 24-h urinary 8-epi-prostaglandin F2α (isoprostane) (OXIS Health Products Inc., Foster City, CA) concentration.
2.8. Statistical Analysis
Data are expressed as means ± standard error (SE). Differences between the groups were examined for statistical significance using ANOVA with Bonferroni correction (SPSS v. 11.5, IBM, Armonk, NY). A P value of less than 0.05 was considered significant.
3. Results
3.1. Albuminuria, Serum Creatinine, and Creatinine Clearance
The 24 M group displayed higher 24 hour albuminuria than the 2 M group (P < 0.05) (Figure 1(a)). Although the difference was not significant, serum creatinine was increased in the 24 M group compared with the other groups (Figure 1(b)) and creatinine clearance was also decreased in the 24 M group compared with the 12 M group (Figure 1(c)). Thus, these results demonstrated that aging is associated with deterioration of renal function.
Figure 1.
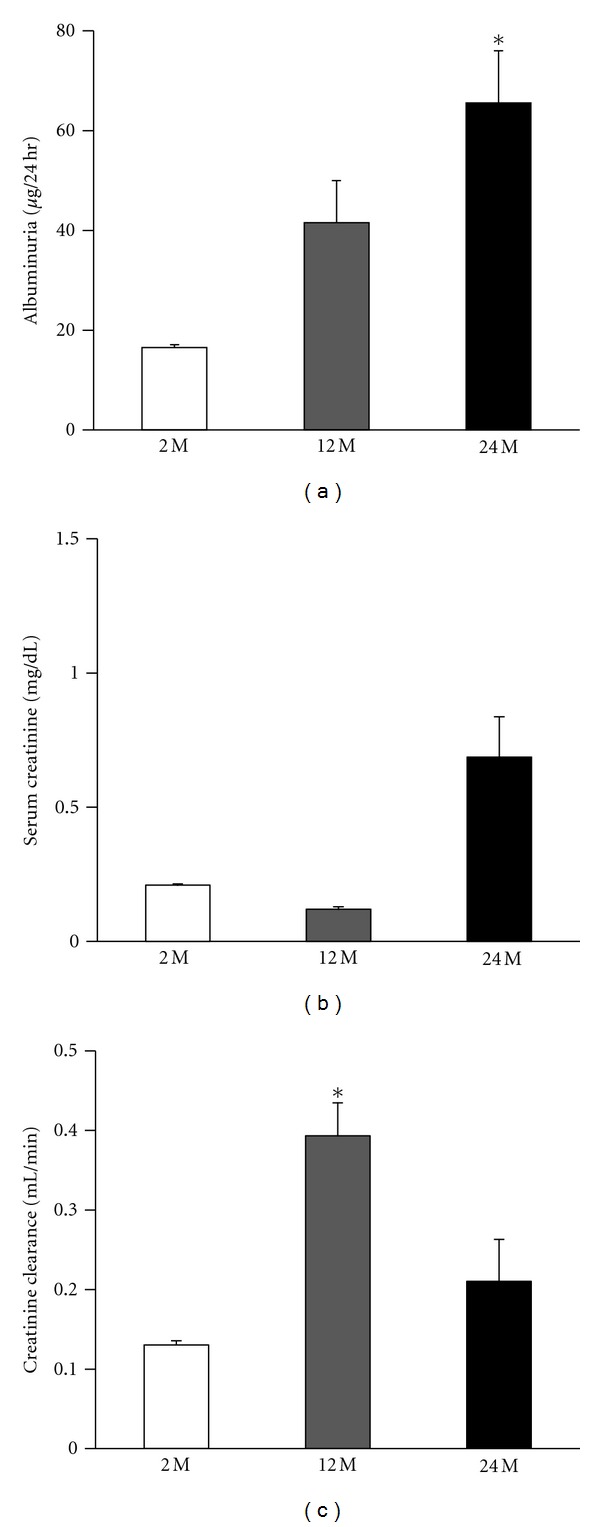
Increased renal damage in aged mice. The 24 M group displayed higher 24 h albuminuria than other groups ((a) 16.5 ± 1.1 versus 41.5 ± 8.4, 65.5 ± 10.4; *P < 0.05 versus 2 M). Serum creatinine was increased in the 24 M group compared with the other groups ((b) 0.21 ± 0.008 versus 0.12 ± 0.009, 0.68 ± 0.15). Creatinine clearance was decreased in the 24 M group compared with the 12 M group ((c) 0.13 ± 0.004 versus 0.39 ± 0.04, 0.21 ± 0.05; *P < 0.05 versus 2 M). Values are shown as mean ± SE.
3.2. Histologic Examination
Histological examination revealed that the mesangial area was expanded in the 24 M group by 3.5 and 2.1 times, compared with the 2 M group and 12 M group, respectively (P < 0.01 versus 2 M) (Figures 2(a), and 2(c)). There was also significantly increased tubulointerstitial fibrosis in the 24 M group, 19 and 18.2 times higher than in the 2 M group and 12 M group, respectively (P < 0.01 versus 2 M) (Figures 2(b), and 2(d)).
Figure 2.
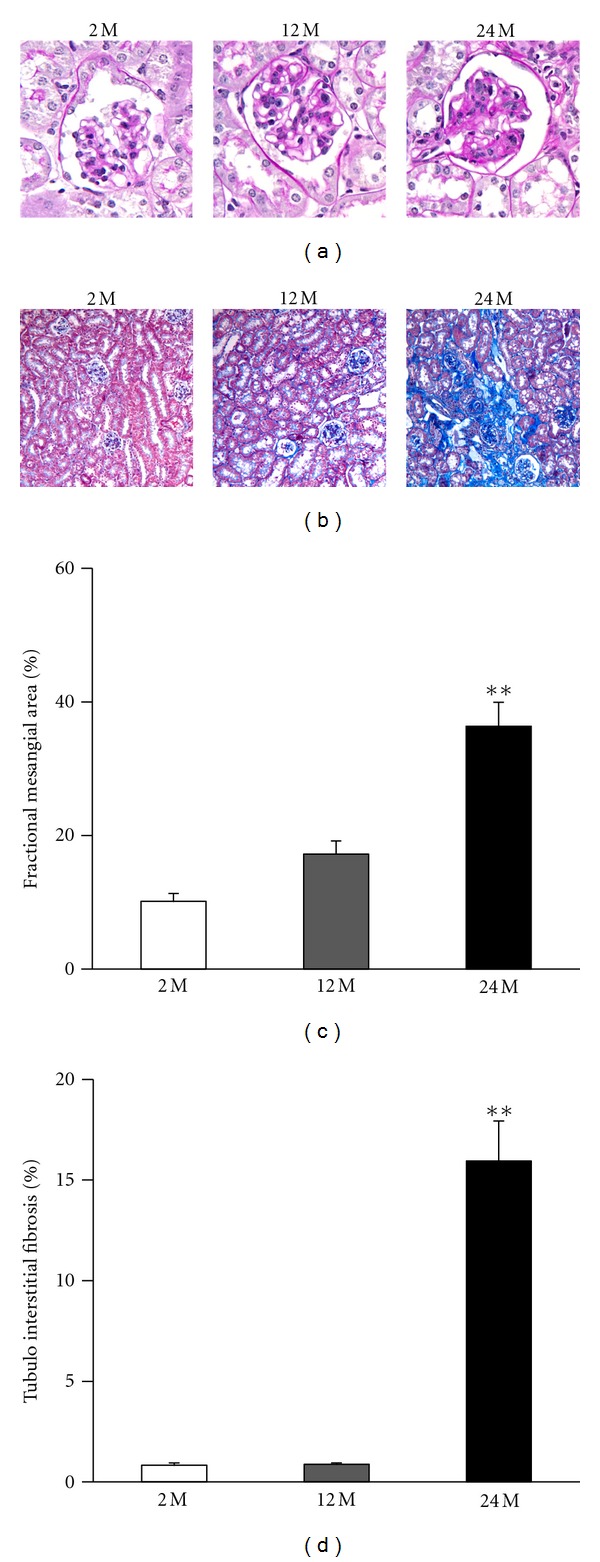
Renal histopathology of the glomerulus and tubulointerstitial areas. Representative images of PAS ((a) original magnification 400×) and Trichrome stain ((b) original magnification 200×) from renal tissue as indicated. Quantitative assessment of the areas of extracellular matrix in the glomerulus (c) and the tubulointerstitial fibrosis (d) from indicated groups. There was increased mesangial area expansion in the 24 M group compared with other groups (10.1 ± 1.19% versus 17.2 ± 2%, 36.3 ± 3.58%; **P < 0.01 versus 2 M), and significantly increased tubulointerstitial fibrosis in the 24 M group compared with other groups. (0.83 ± 0.11% versus 0.87 ± 0.07%, 15.9 ± 1.99%; **P < 0.01 versus 2 M). Values are shown as mean ± SE.
3.3. Immunochemistry for F4/80 and TUNEL Assay
Inflammatory cell infiltration, indicated by the presence of positive F4/80 cells, was markedly increased in the 24 M group compared with the 2 M group and 12 M group (P < 0.01 versus 2 M) (Figure 3). We also investigated TUNEL-positive cells in the kidneys from the three experimental groups. Compared with the 2 M group, the 24 M group had a significantly greater number of TUNEL-positive cells in the glomerulus (P < 0.01 versus 2 M) (Figures 4(a), and 4(b)) and cortical tubular areas (P < 0.01 versus 2 M) (Figures 4(a), and 4(c)). These results demonstrate that aging is associated with increased mesangial expansion and tubulointerstitial fibrosis, which could be related to inflammation and apoptosis.
Figure 3.
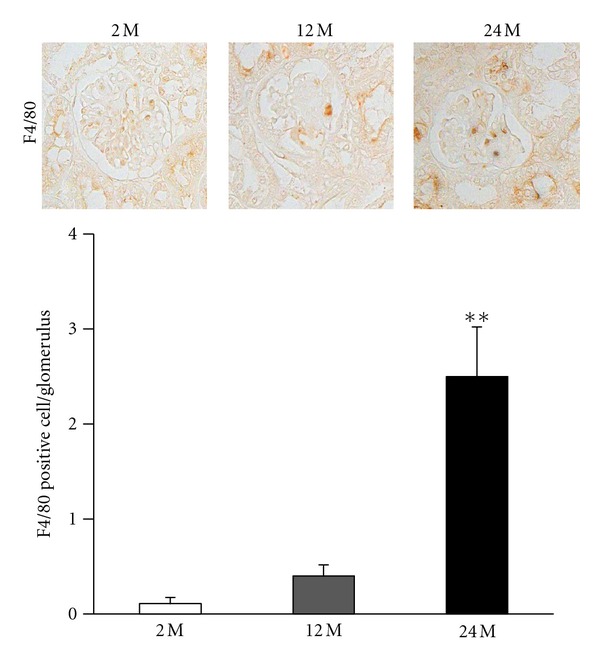
Immunohistochemistry for F4/80. Result of staining for F4/80-positive cells and quantitative analyses in the 2 M, 12 M, and 24 M groups ((a), original magnification 400×). There was a marked increase in the 24 M group compared with other groups (0.11 ± 0.06% versus 0.4 ± 0.11%, 2.5 ± 0.52%; **P < 0.01 versus 2 M). Values are shown as mean ± SE.
Figure 4.
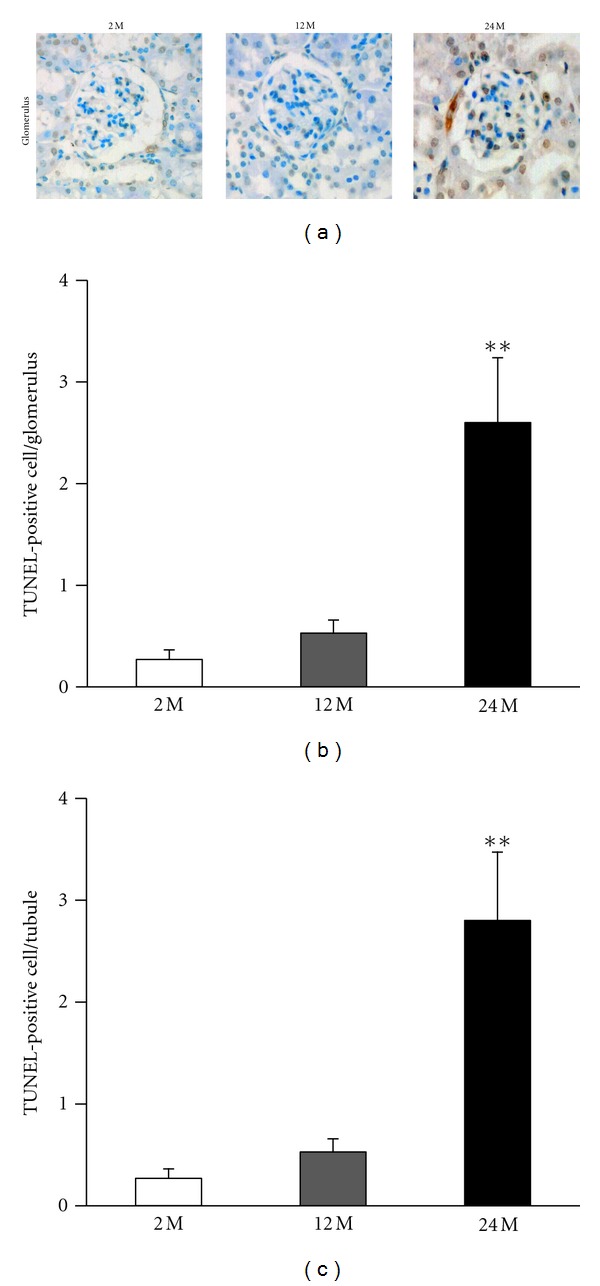
Increase in apoptotic cells in the glomerulus and tubules of the kidneys from the 24 M group. Representative pictures of TUNEL-positive mesangial cells ((a) original magnification 400×) in the glomerulus and cortical tubular areas for all the study groups. Quantitative analyses of the results are shown ((b) 0.27 ± 0.09% versus 0.53 ± 0.12%, 2.6 ± 0.63%; (c) 0.27 ± 0.09% versus 0.53 ± 0.12%, 2.8 ± 0.67%; **P < 0.01 versus 2 M). Values are shown as mean ± SE.
3.4. 24-h Urinary Levels of 8-epi-PGF2α
Isoprostane concentrations were increased in the 12 M group and 24 M group than in the 2 M group (P < 0.05 and P < 0.01, respectively, versus 2 M) (Figure 5). These findings indicate that oxidative damage was increased in renal cells from aged mice.
Figure 5.
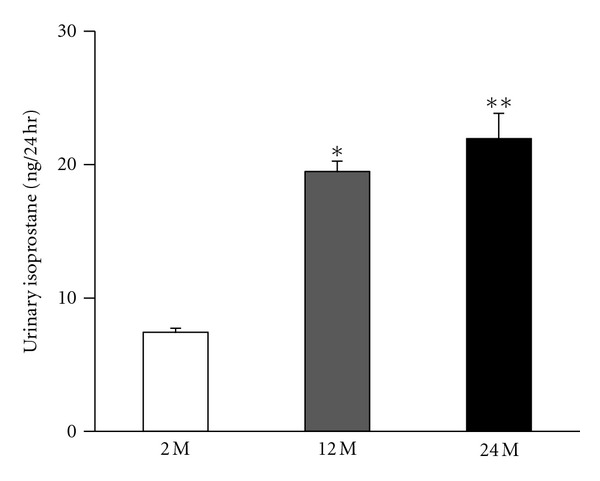
Intrarenal 24-h urinary 8-epi-prostaglandin F2α (isoprostane) concentrations in all the study groups. Quantitative analyses of the results are shown (7.4 ± 0.3% versus 19.4 ± 0.78%, 21.9 ± 1.9%; *P < 0.05 versus 2 M, **P < 0.01 versus 2 M). Values are shown as mean ± SE.
3.5. Expression of SOD1 and SOD2
By Western blot analysis, we examined the changes in the antioxidant proteins SOD1 and SOD2. The expression of SOD1 and SOD2 were decreased in the 24 M group compared with 2 M group and lower than 12 M group. (P < 0.05 and P < 0.01, resp., versus 2 M) (Figure 6).
Figure 6.
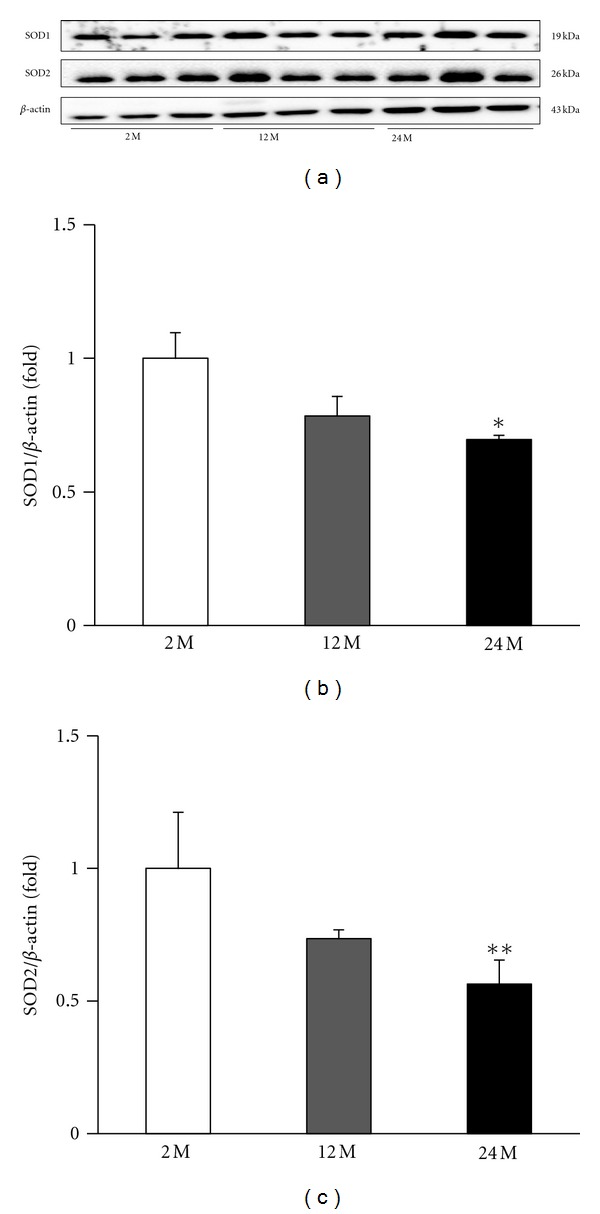
Representative Western blots of SOD1 and SOD2. Representative blots from the kidney are shown (a). The expression of SOD1 and SOD2 were decreased in the 24 M group compared with 2 M group and lower than 12 M group. Quantitative analyses of the results are shown (b, c). *P < 0.05 versus 2 M, **P < 0.01 versus 2 M.
3.6. Expression of SIRT1
SIRT1 activation can be influenced by several cellular conditions, such as oxidative stress. The relative content of SIRT1 protein was measured by Western blot analysis, which showed that the SIRT1 protein levels were markedly decreased in the 24 M group compared with the 2 M group and 12 M group (P < 0.01 versus 2 M) (Figures 7(a), and 7(b)).
Figure 7.
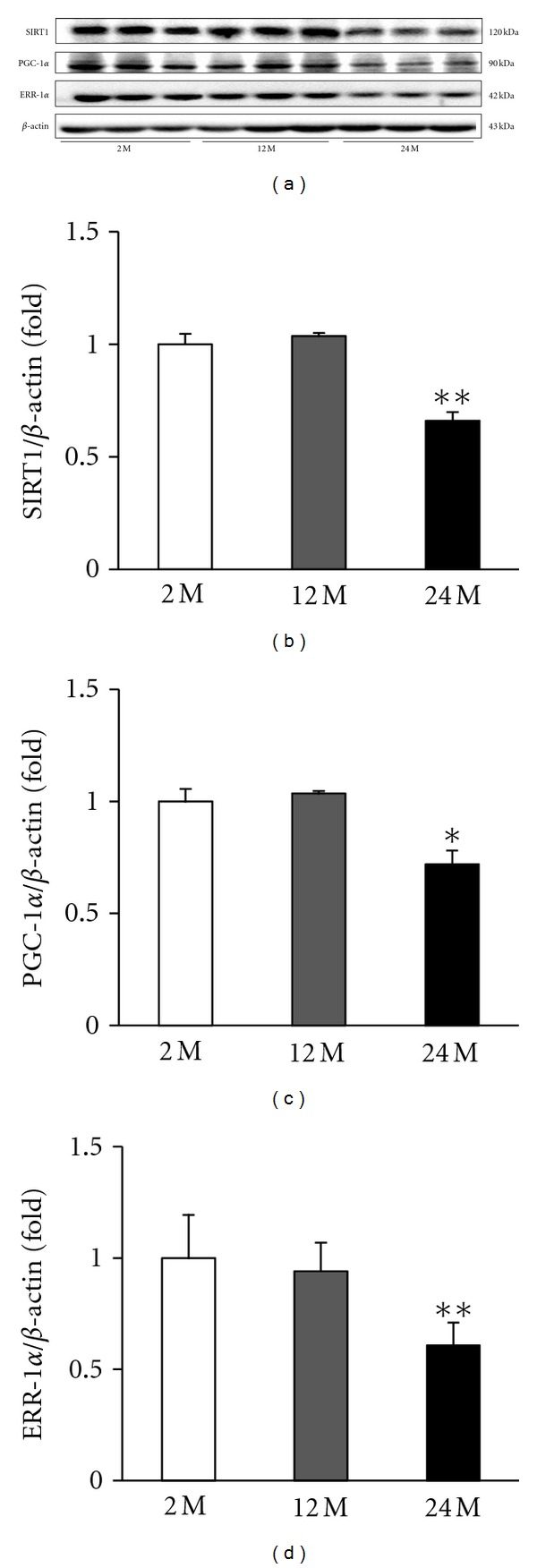
Representative Western blots of SIRT1, PGC-1α, and ERR-1α. Representative blots from the kidney are shown. (a) Western blot analysis showed that aging markedly decreased SIRT1, PGC-1α, and ERR-1α protein levels in the 24 M group compared with other groups. Quantitative analyses of the results are shown (b–d). *P < 0.05 versus 2 M, **P < 0.01 versus 2 M.
3.7. Expression of PGC-1α and ERR-1α
PGC-1α exerts a wide array of effects and directly coactivates multiple transcription factors, including nuclear receptors such as the PPARs and ERRs. In the aging kidney, decreased SIRT1 may regulate PGC-1α. Western blot analysis showed that PGC-1α was decreased in the 24 M group compared with the 2 M group and 12 M group (P < 0.05 versus 2 M) (Figures 7(a), and 7(c)). There was a gradual decrease in ERR-1α expression in the 12 M group and 24 M group compared with the 2 M group (P < 0.01 versus 2 M) (Figures 7(a), and 7(d)).
3.8. Expression of PPARα
PPARs play essential roles in the regulation of cellular differentiation, development, and metabolism and tumorigenesis in higher organisms. PPARα expression in the kidney was lower in the 24 M group compared with that in the 2 M group and 12 M group, as assessed by Western blot analysis (P < 0.01 versus 2 M) (Figure 8(a)).
Figure 8.
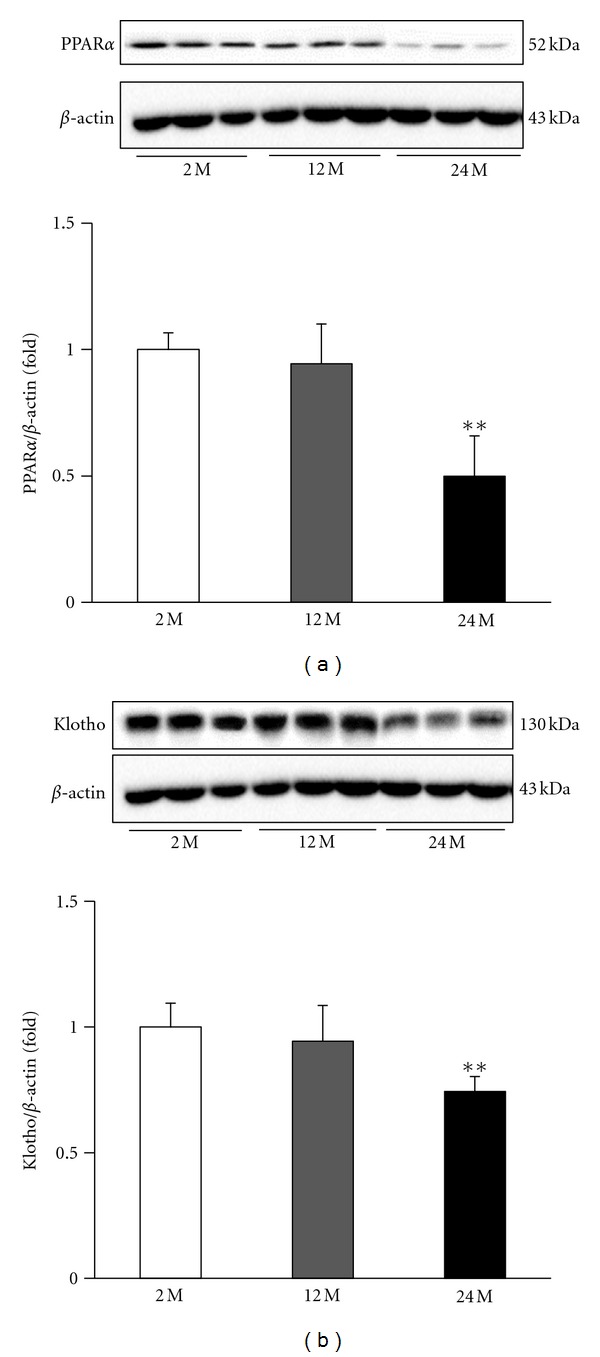
Representative Western blots of PPARα and Klotho. Representative blots from the kidney are shown. (a) Western blot analysis showed that aging decreased PPARα and Klotho expression in the 24 M group compared with other groups. Quantitative analyses of the results are shown (b, c). **P < 0.01 versus 2 M.
3.9. Expression of Klotho
Klotho is a transmembrane protein that, in addition to other effects, provides some control over the sensitivity of the organism to insulin and appears to be involved in aging. In the kidney, Klotho is reduced in aging. Our results showed decreased Klotho levels in the 24 M group compared with the 2 M group and the 12 M group (P < 0.01 versus 2 M) (Figure 8(b)).
4. Discussion
Aging is a multifactorial process characterized by a progressive decline in physiological function. In this study, we investigated the physiologic and pathologic changes related to aging in the kidney. Creatinine clearance decreased and albuminuria increased with aging. There were also increases in mesangial volume, tubulointerstitial fibrosis, inflammation (F4/80 positive cells) and apoptosis. The rate of pathologic change in aging may vary depending on the levels of angiotensin II, nitric oxide (NO), transforming growth factor β, advanced glycosylation end product (AGE), oxidative stress and lipid [12]. Our study showed that tissue injury in aging can occur from oxidative stress [13, 14] mediated by SIRT1, PGC-1α, PPARα, and Klotho [15].
Tissue injury in aging can occur from free radical production and/or antioxidant enzyme deficiency with subsequent lipid peroxidation and oxidative stress [13, 14]. Aged kidneys show increased levels of reactive oxygen species and thiobarbiturate acid-reactive substances, which are associated with lipid oxidative damage [16]. In addition, other markers of oxidative stress and lipid peroxidation, such as isoprostanes, AGE, and increased heme oxygenase, are also noted in aged rats [17]. The urine isoprostane excretion increased with age in this study and expression of SOD1 and SOD2 also decreased in 24-month-old mice. Many studies have been performed to assess methods of preventing the aging process with diets enriched in antioxidants such as vitamin E [17] or with angiotensin-converting enzyme inhibitor [18] or angiotensin-receptor blockers. The hemodynamic effect of angiotensin II enhances the aging process in kidney by inducing intraglomerular hypertension and subsequent glomerular damage. It also affects NO synthesis, oxidative stress, immunomodulation, growth factor induction, inflammation, cell migration and apoptosis [12, 19]. These finding suggest that renin-angiotensin-aldosterone blockade may be a potential therapeutic treatment to prevent the aging process.
In this study, we showed that SIRT1 expression was decreased in 24-month-old mice. SIRT1 is expressed throughout the body, has broad biological effects, and can significantly affect both cellular survival and longevity during acute and long-term injuries by mechanisms that involve both oxidative stress and cell metabolism [20]. In the cell, SIRT1 uses NAD+ as a substrate, but the level of NAD+ can also control the deacetylating activity of SIRT1. SIRT1 activation can be influenced by several cellular conditions, such as calorie restriction, exercise, and oxidative stress [21]. The hypothetical Sirt1 and AMP-activated protein kinase (AMPK) cycles regulate each other and share many common target molecules, such as PGC-1α, PPARs, Forkhead box (FoxO), and NF-κB [22]. It has been suggested that the activation of AMPK and SIRT1 allows for the concurrent deacetylation and phosphorylation of their target molecules and decreases the susceptibility to aging. In the aging kidney, decreased SIRT1 may regulate PGC-1α. We suggest that aging-induced suppression of SIRT1/PGC-1α signaling may increase oxidative stress in cooperation with AMPK. This signaling may also affect catabolism, mitochondrial function, angiogenesis, inflammation, and insulin resistance [22].
PGC-1α exerts a wide array of effects and directly coactivates multiple transcription factors, including nuclear receptors such as the PPARs [23, 24] or glucocorticoid receptors [25], estrogen receptors and ERRs [20, 26] and nonnuclear receptor transcription factors including myocyte enhancer factor-2 [27] and the FoxO family of transcription factors [28]. By simultaneously coactivating these transcription factors, PGC-1α can quickly and coordinately modulate a transcriptional program that governs energy metabolism. Although PGC-1α coactivation can change in response to different stimuli or in a tissue-specific manner, in the aging kidney PGC-1α exerts effects by enhancing the expression of PPARα and its target molecule ERR-1α. ERR-1α activation by PGC-1α and PPARα induces genes with roles in lipid transport [29], oxidative phosphorylation [30, 31], fatty acid oxidation [32, 33], the tricarboxylic acid cycle [23, 34, 35], mitochondrial biogenesis [30, 33], and oxidative stress defense [36]. In our study, expression of PGC-1α/ERR-1α also decreased with aging, which may induce oxidative stress.
PPARs play a critical physiological role in energy homeostasis and inflammation. PPARα stimulation has been shown to increase the NO system in cultured endothelial cells [37] and to induce overexpression both at protein and RNA level of enzymes such as SOD1 in cultured human umbilical vein endothelial cells [38]. PPARα suppression with aging could induce oxidative stress, leading to mesangial expansion and tubulointerstitial fibrosis. PPARα activation may improve renal function during the aging process [39].
The Klotho gene was originally identified as a putative age-suppressing gene in mice that extends lifespan when overexpressed [35]. There are two type of Klotho protein. Membrane Klotho functions as a receptor for a hormone that regulates excretion of phosphate and synthesis of active vitamin D in the kidney. Secreted Klotho functions as a humoral factor with pleiotropic activities, including suppression of growth factor signaling, suppression of oxidative stress, and regulation of ion channels and transporters [35]. Klotho is predominantly expressed in the renal distal tubular cells. Animal studies have showed that the nephroprotective effects of this protein are mostly attributable to the antioxidant properties of its soluble form [36]. Diabetes mellitus, hypertension, kidney diseases, and aging decrease expression of Klotho protein, which then increases sodium/phosphate cotransporter activity and oxidative stress, which in turn induces vascular calcification and endothelial dysfunction. There is emerging data from human studies that suggest that Klotho may be a modifiable factor involved in the pathogenesis of cardiovascular and renal disease in at-risk populations. Further data is required to confirm whether this novel protein has potential as a therapeutic tool that could be used to prevent or slow the progression of cardiorenal disease [36].
5. Conclusions
In conclusion, our results demonstrate that the aging process is associated with deterioration of renal function and increased mesangial expansion and tubulointerstitial fibrosis, which could be accompanied by inflammation, apoptosis and oxidative stress. SIRT1 expression is decreased with aging and may relate to change targets such as PGC-1α/ERR-1α signaling and PPARα. Reduction in Klotho can also induce oxidative stress. Pharmacologically targeting these signaling molecules may reduce the pathologic changes of aging in the kidney.
Authors' Contribution
C. W. Park and B. S. Choi contributed equally to this work.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0023126) and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant no. A111055).
References
- 1.Campbell KH, O’Hare AM. Kidney disease in the elderly: update on recent literature. Current Opinion in Nephrology and Hypertension. 2008;17(3):298–303. doi: 10.1097/MNH.0b013e3282f5dd90. [DOI] [PubMed] [Google Scholar]
- 2.O’Hare AM, Bertentha D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? Journal of the American Society of Nephrology. 2006;17(3):846–853. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 3.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies I, Fotheringham AP, Faragher BE. Age-associated changes in the kidney of the laboratory mouse. Age and Ageing. 1989;18(2):127–133. doi: 10.1093/ageing/18.2.127. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Corman B. The aging kidney: insights from experimental studies. Journal of the American Society of Nephrology. 1998;9(4):699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 6.Razzaque MS. Does renal ageing affect survival? Ageing Research Reviews. 2007;6(3):211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ. Vascular and glomerular changes in the ageing kidney. Journal of Pathology. 1977;121(2):65–78. doi: 10.1002/path.1711210202. [DOI] [PubMed] [Google Scholar]
- 8.Epsteina M, Hollenbeg NK. Age as a determinant of renal sodium conservation in normal man. Journal of Laboratory and Clinical Medicine. 1976;87(3):411–417. [PubMed] [Google Scholar]
- 9.Zhang H, Li Y, Fan Y, et al. Klotho is a target gene of PPAR-γ . Kidney International. 2008;74(6):732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 10.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes and Development. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim HA, Lee EK, Kim JM, et al. PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney. Biogerontology. 2012;13(2):133–145. doi: 10.1007/s10522-011-9361-4. [DOI] [PubMed] [Google Scholar]
- 12.Taal MW, Chertow GM, Brenner BM, et al. The Kidney. 9th edition. Philadelphia, Pa, USA: WB Saunders; 2011. Brenner & Rector's. [Google Scholar]
- 13.Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Molecular and Cellular Biochemistry. 1997;174(1-2):305–319. [PubMed] [Google Scholar]
- 14.Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 15.Chung HW, Lim JH, Kim MY, et al. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3α-PGC-1α pathway. Nephrology Dialysis Transplantation. 2012;27(6):2213–2225. doi: 10.1093/ndt/gfr613. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Torres P, Lucio J, Gonzalez-Rubio M, Rodríguez-Puyol M, Rodríguez-Puyol D. Oxidant/antioxidant balance in isolated glomeruli and cultured mesangial cells. Free Radical Biology & Medicine. 1997;22(1-2):49–56. doi: 10.1016/s0891-5849(96)00239-0. [DOI] [PubMed] [Google Scholar]
- 17.Reckelhoff JF, Kanji V, Racusen LC, et al. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. American Journal of Physiology. 1998;274(3):R767–R774. doi: 10.1152/ajpregu.1998.274.3.R767. [DOI] [PubMed] [Google Scholar]
- 18.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22(phox) is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. Journal of Biological Chemistry. 1996;271(38):23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 19.Wolf G, Ziyadeh FN, Schroeder R, Stahl RAK. Angiotensin II inhibits inducible nitric oxide synthase in tubular MCT cells by a posttranscriptional mechanism. Journal of the American Society of Nephrology. 1997;8(4):551–557. doi: 10.1681/ASN.V84551. [DOI] [PubMed] [Google Scholar]
- 20.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ: identification of novel Leucine-rich interaction motif within PGC-1α . Journal of Biological Chemistry. 2002;277(43):40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 21.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opinion on Therapeutic Targets. 2012;16(2):167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? American Journal of Physiology. 2010;298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Molecular and Cellular Biology. 2000;20(5):1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 25.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα) Journal of Biological Chemistry. 2003;278(11):9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 27.Michael LF, Wu Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puigserver P, Rhee J, Donocan J. Insulin-required hepatic gluconeogensis through FOXO1-PGC-1α interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Kume S, Araki S, et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney International. 2011;71(8):871–882. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber SN, Emter R, Hock MB, et al. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huss JM, Pineda Torra I, Staels B, Giguère V, Kelly DP. Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Molecular and Cellular Biology. 2004;24(20):9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrier JC, Deblois G, Champigny C, Levy E, Giguère V. Estrogen-related receptor α (ERRα) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. Journal of Biological Chemistry. 2004;279(50):52052–52058. doi: 10.1074/jbc.M410337200. [DOI] [PubMed] [Google Scholar]
- 33.Villena JA, Hock MB, Chang WY, Barcas JE, Giguère V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptive thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuro-o M. Klotho and the aging process. Korean Journal of Internal Medicine. 2011;26(2):113–122. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maltese G, Karalliedde J. The putative role of the antiageing protein klotho in cardiovascular and renal disease. International Journal of Hypertension. 2012;2012:5 pages. doi: 10.1155/2012/757469.757469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangwala SM, Li X, Lindsley L, et al. Estrogen-related receptor alpha is essential for the expression of antioxidant protection genes and mitochondrial function. Biochemical and Biophysical Research Communications. 2007;357(1):231–236. doi: 10.1016/j.bbrc.2007.03.126. [DOI] [PubMed] [Google Scholar]
- 37.Goya K, Sumitani S, Xu X, et al. Peroxisome proliferator-activated receptor α agonists increase nitric oxide synthase expression in vascular endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(4):658–663. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 38.Inoue I, Goto SI, Matsunaga T, et al. The ligands/activators for peroxisome proliferator-activated receptor α (PPARα) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50(1):3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 39.Chung S, Park CW. Role of peroxisome proliferator-activated receptor α in diabetic nephropathy. Diabetes & Metabolism. 2011;35(4):327–336. doi: 10.4093/dmj.2011.35.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


