Abstract
Adenovirus type 2 mRNA was translated in S30 extracts from Ehrlich ascites and wheat embryo cells. The in vitro products were identified by sodium dodecyl sulfate-gel electrophoresis after immunoprecipitation with specific antisera in the presence of urea. Seven virion polypeptides could be identified by immunoprecipitation. Three of these appear to be precursors to polypeptides of the virion. mRNA isolated late in adenovirus infection was separated into three size classes by zonal sedimentation. Material sedimenting at 26S was translated into polypeptides corresponding to the largest virion polypeptides II to IV, a 22S fraction corresponding to polypeptide V, and smaller polypeptides and a 15S fraction corresponding to polypeptide IX. A significant amount of polypeptide IX was also synthesized by the 26S and 22S RNA.
Full text
PDF



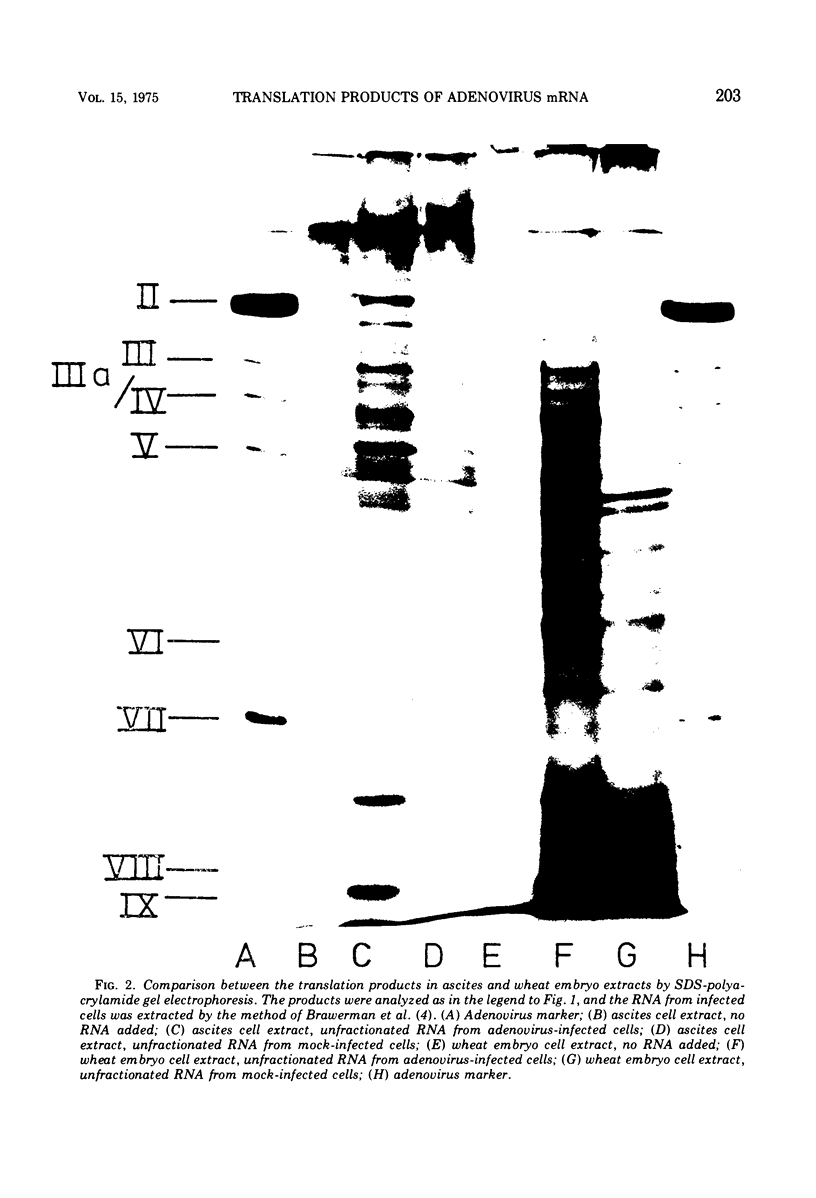

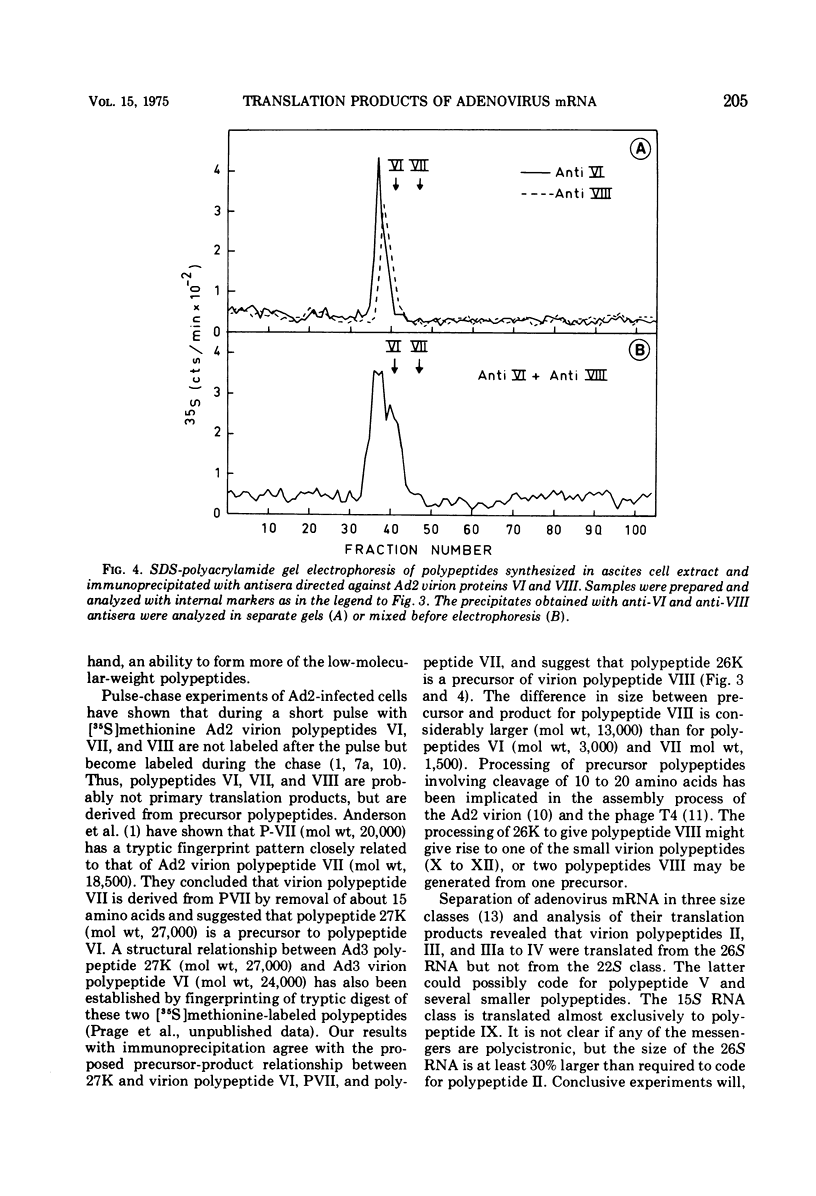


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Caffier H., Raskas H. J., Parsons T. J., Green M. Initiation of mammalian viral protein synthesis. Nat New Biol. 1971 Feb 24;229(8):239–241. doi: 10.1038/newbio229239a0. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Callahan R., Westphal H. Cell-free synthesis of adenovirus coat proteins. J Biol Chem. 1974 Oct 10;249(19):6331–6338. [PubMed] [Google Scholar]
- Eron L., Wesphal H., Callahan R. In vitro synthesis of adenovirus core proteins. J Virol. 1974 Aug;14(2):375–383. doi: 10.1128/jvi.14.2.375-383.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Philipson L. Structural proteins of adenoviruses. XI. Purification of three low molecular weight virion proteins of adenovirus type 2 and their synthesis during productive infection. Virology. 1974 Nov;62(1):253–269. doi: 10.1016/0042-6822(74)90320-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Jornvall H., Pettersson U., Philipson L. Structural studies of adenovirus type-2 hexon protein. Eur J Biochem. 1974 Oct 1;48(1):179–192. doi: 10.1111/j.1432-1033.1974.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Mammalian messenger RNA. Essays Biochem. 1973;9:59–102. [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Oberg B. F., Shatkin A. J. Initiation of picornavirus protein synthesis in ascites cell extracts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3589–3593. doi: 10.1073/pnas.69.12.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Replication of poliovirus RNA studied by gel filtration and electrophoresis. Eur J Biochem. 1969 Dec;11(2):305–315. doi: 10.1111/j.1432-1033.1969.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Perlman S., Hirsch M., Penman S. Utilization of messenger in adenovirus-2-infected cells at normal and elevated temperatures. Nat New Biol. 1972 Aug 2;238(83):143–144. doi: 10.1038/newbio238143a0. [DOI] [PubMed] [Google Scholar]
- Pettersson U. Structural proteins of adenoviruses. VI. On the antigenic determinants of the hexon. Virology. 1971 Jan;43(1):123–136. doi: 10.1016/0042-6822(71)90230-3. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U. Structure and function of virion proteins of adenoviruses. Prog Exp Tumor Res. 1973;18:1–55. doi: 10.1159/000393160. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrilo A., Beato M., Schutz G., Feigelson P., Allfrey V. G. Cell-free translation of the globin message within polydisperse high-molecular-weight ribonucleic acid of avian erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3641–3645. doi: 10.1073/pnas.70.12.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero C., Reibel L., Delaunay J., Schapira G. Translation of globulin mRNA among eukaryotes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1171–1177. doi: 10.1016/0006-291x(73)90815-2. [DOI] [PubMed] [Google Scholar]
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]





