Abstract
Broadly neutralizing Abs to HIV-1 are well described; however, identification of Ags that elicit these Abs has proven difficult. Persistent infection with GB virus type C (GBV-C) is associated with prolonged survival in HIV-1–infected individuals, and among those without HIV-1 viremia, the presence of Ab to GBV-C glycoprotein E2 is also associated with survival. GBV-C E2 protein inhibits HIV-1 entry, and an antigenic peptide within E2 interferes with gp41-induced membrane perturbations in vitro, suggesting the possibility of structural mimicry between GBV-C E2 protein and HIV-1 particles. Naturally occurring human and experimentally induced GBV-C E2 Abs were examined for their ability to neutralize infectious HIV-1 particles and HIV-1–enveloped pseudovirus particles. All GBV-C E2 Abs neutralized diverse isolates of HIV-1 with the exception of rabbit anti-peptide Abs raised against a synthetic GBV-C E2 peptide. Rabbit anti–GBV-C E2 Abs neutralized HIV-1–pseudotyped retrovirus particles but not HIV-1–pseudotyped vesicular stomatitis virus particles, and E2 Abs immune-precipitated HIV-1 gag particles containing the vesicular stomatitis virus type G envelope, HIV-1 envelope, GBV-C envelope, or no viral envelope. The Abs did not neutralize or immune-precipitate mumps or yellow fever viruses. Rabbit GBV-C E2 Abs inhibited HIV attachment to cells but did not inhibit entry following attachment. Taken together, these data indicate that the GBV-C E2 protein has a structural motif that elicits Abs that cross-react with a cellular Ag present on retrovirus particles, independent of HIV-1 envelope glycoproteins. The data provide evidence that a heterologous viral protein can induce HIV-1–neutralizing Abs.
Human immunodeficiency virus-1 vaccine development has relied primarily on the use of HIV-1 proteins as immunogens in an attempt to elicit either neutralizing Abs or cellular immune responses to prevent or modify HIV-related disease (reviewed in Refs. 1 and 2). Because of the high replicative rate of HIV-1 and the error-prone RNA-dependent DNA polymerase, neutralization and T cell escape mutants are generated on a daily if not hourly basis in infected individuals. Broadly neutralizing human HIV mAbs (hmAbs) have been isolated from HIV-infected individuals (3), including hmAbs directed against gp120 that interfere with CD4 binding (e.g., 2G12) or that react with the membrane proximal ectodomain region (MPER) of gp41 (e.g., 2F5 and 4E10) (3, 4). These Abs also react with a 36-residue peptide that overlaps with the MPER called T-20 (5). T-20 inhibits HIV replication by preventing virus envelope fusion with the cell membrane, and T-20 is an effective and licensed antiretroviral treatment (Fuzeon) (5, 6). 2F5 and 4E10 Abs are polyspecific and cross-react with cellular Ags including several lipids (7–12). Although Ags that interact with these Abs have been identified, active immunization with gp41, MPER, or T-20 does not elicit broadly neutralizing HIV Abs (13, 14). Clearly, new approaches to HIV-1 vaccines are needed (1, 2).
GB virus type C (GBV-C) is a common human infection that is not clearly associated with any disease. The virus replicates in B and T lymphocytes including CD4+ and CD8+ T cell subsets (15; reviewed in Ref. 16). Because of shared modes of transmission, the prevalence of GBV-C in HIV-infected people is high (17–42%) (17). Several studies and a meta-analysis of studies including 1294 HIV-infected individuals found that persistent GBV-C infection is associated with prolonged survival (18–22). GBV-C infection is also associated with decreased maternal-to-child HIV-1 transmission (23, 24). Abs to GBV-C are usually not detected during viremia; however, following clearance of GBV-C, Abs specific for the envelope glycoprotein (E2) are identified. Consequently, GBV-C E2 Ab serves as a marker of prior infection (reviewed in Ref. 16). Although persistent GBV-C viremia is associated with the best survival in epidemiological studies (25), one study found that subjects without viremia who have GBV-C E2 Abs survived longer than those without E2 Abs (20). Human GBV-C E2 Abs and all but one of characterized GBV-C E2 murine mAbs are conformation dependent (26). One mAb (M6) recognizes a linear epitope on E2 (27); however, the interaction is complex. M6 binds to six amino acids within E2 if there are four or eight amino acids added to the C or N termini, respectively, suggesting that there is a size and sequence requirement for interaction (26). A GBV-C E2 peptide encompassing this epitope has been proposed to be involved in GBV-C–cell membrane fusion, based on findings that it forms an amphipathic helix in the presence of lipids and model membranes (28, 29). In addition, another E2 peptide that overlaps the putative fusion peptide prevents oligomerization of the HIV-1 gp41 fusion peptide and membrane fusion in an in vitro model (30). Finally, incubation of PBMCs or CD4+ T cell lines with the GBV-C envelope glycoprotein E2 competitively inhibits HIV-1 entry in vitro (31, 32), raising the possibility that there is structural mimicry between GBV-C E2 and HIV-1 particles, or between E2 and a cell surface molecule carried on the HIV particle that is involved in HIV-1 entry. If this is true, Abs directed against GBV-C E2 may interfere with HIV attachment or fusion and potentially modify HIV-1 disease progression.
We examined naturally occurring human and experimentally induced murine and rabbit GBV-C E2 Abs and found that they cross-reacted with and neutralized diverse HIV-1 isolates. They also precipitated pseudotyped HIV-1 gag particles independent of the viral envelope used. In contrast, the Abs did not neutralize or precipitate mumps virus or yellow fever virus (YFV), and they did not neutralize vesicular stomatitis virus (VSV) particles, suggesting that the Ag is of cellular origin and that it is present on HIV-1 gag particles. Thus, immunization with GBV-C E2 protein elicited broadly neutralizing HIV Abs, a long sought-after goal in HIV-1 vaccine development.
Materials and Methods
Ags and Abs
GBV-C E2 protein truncated to remove the C-terminal transmembrane domain (nt 1167–2162 based on the infectious clone isolate; GenBank number AF121950) (33) was expressed in Chinese hamster ovary (CHO) cells as described previously (26). The CHO cells were adapted for serum-free growth, and supernatants containing GBV-C E2 protein were concentrated on an Amicon CP10 concentrator (Millipore, Billerica, MA). Three BALB/C mice were immunized with 25 μg rGBV-C E2 protein mixed with IFA, followed by two immunizations of E2 in IFA at 2-wk intervals. Rabbits were immunized with 50 μg E2 in IFA (Invitrogen, Carlsbad, CA) and boosted 6 and 12 wk later by the Iowa State University Hybridoma Facility (Ames, IA). Sera were collected before immunization (preimmune) and at various intervals after immunization for analysis and IgG purification as described previously (26). A synthetic peptide representing GBV-C E2 sequences (GGAGLTGGFYEPLVRRC) was provided by Dr. O. Sharma (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program [NARRRP], Rockville, MD). Rabbits were immunized with KLH-conjugated E2 peptide (100 μg) in IFA and boosted 2, 7, and 9 wk later.
HIV-, HCV- and HBsAg-negative sera were obtained from healthy blood donors. Anti-E2 Ab status was assessed by ELISA (μPlate anti-HGenv test; provided by Dr. G. Hess, Roche Diagnostics, Mannheim, Germany). Murine anti–GBV-C E2 mAb M1(virostat [VS]) was purchased (Virostat, Portland, ME), whereas M6 and M11 (Roche Laboratories, Penzberg, Germany) were provided by Dr. A. Engel (Roche Diagnostics). Isotype control Abs were purchased from Sigma-Aldrich (St. Louis, MO). M1(VS) and M11 recognize conformation-dependent epitopes, whereas M6 recognizes a linear epitope on GBV-C E2 (26). E2 immunoblot were performed as described previously (26). HIV-neutralizing hmAbs 2F5, 2G12, and 4E10 were obtained from NARRRP (catalog numbers 1475, 1476, and 10091, respectively).
Cells and viruses
PBMCs were isolated from blood obtained from healthy donors. All human subjects provided written informed consent, and the project was approved by the University of Iowa Institutional Review Board. CD4+ cell lines (MT-2 and human osteosarcoma [HOS] cells expressing human CD4, CCR5, and CXCR4) were maintained as described previously (34–36). The HOS cells were obtained from NARRRP and also contained GFP under the control of HIV-1 long terminal repeat (catalog numbers 3685 and 3944). Baby hamster kidney (BHK) 21 cells were maintained in DMEM containing 10% FCS and antibiotics. Table I summarizes the infectious HIV-1 isolates used in these studies. Briefly, using the nomenclature described in the data sheets for HIV-1 isolates obtained from NARRRP, we studied the following viruses: R5 isolates—HIV-1 92UGO31 (clade A; catalog number 1741), HIVJR-CSF (clade B; catalog number 394), and NFN-SX-heat stabile Ag (HSA) (clade B); and X4 isolates—HIV-1 92UGO29 (clade A; catalog number 1650), azidothymidine (AZT) intermediate (clade B; catalog number 1073), HIV-1IIIB/H9 (clade B; catalog number 398), HIVxxBru-JF (clade B; catalog number 2969), HIVELI (clade D; catalog number 2521), and NFN-HSA (pNL4.3 backbone; clade B). Infectious cDNA clones of two HIV-1 isolates were provided by Dr. B. Jamieson (University of California, Los Angeles, Los Angeles, CA): NFN-HSA (derived from pNL4.3 and expressing murine HSA [also known as CD24]) and NFN-SX-HSA (pNL4.3 env replaced with an R5-tropic env gene (37, 38). Two clinical isolates were obtained and characterized from the University of Iowa HIV/AIDS Clinic (Iowa City, IA). HIV stock viruses were prepared in MT-2 cells (X4 isolates), PBMCs (X4 and R5 isolates), or HOS cells as indicated in Results. YFV (vaccine strain, 17D; Sanofi-Pasteur, Swiftwater, PA) was propagated in BHK21 cells or MT-2 cells. The mumps virus was propagated in Vero Cells or MT-2 cells.
Table I.
Characteristics of HIV isolates used in neutralization assays
| Isolate | Isolate Name | Tropism | Derivationa | Clade | Catalog No.b | Cell Types Studied |
|---|---|---|---|---|---|---|
| 1 | HIV-1 92UGO31 | R5 | Clinical | A | 1741 | PBMC |
| 2 | AZT intermediatec | R5-X4 | Clinical | B | 1073 | PBMC |
| 3 | HIV-1 92UGO29 | X4 | Clinical | A | 1650 | MT-2; PBMC |
| 4 | HIV-1JR-CSF | R5 | Laboratory | B | 394 | PBMC |
| 5 | HIV-1IIIB/H9 | X4 | Laboratory | B | 398 | MT-2; PBMC |
| 6 | HIV-1xxBru-JF | X4 | Laboratory | B | 2969 | PBMC |
| 7 | HIV-1ELI | X4 | Laboratory | D | 2521 | MT-2; PBMC |
| 8 | NFN-HAS (pNL4-3) | X4 | Laboratory | B | NA | MT-2; PBMC |
| 9 | NFN-SX-HSA (pNL4-3) | R5 | Laboratory | B | NA | PBMC |
| 10 | UIVC | R5 | Clinical | B | NA | MT-2; PBMC |
| 11 | UIVC | R5/X4 | Clinical | B | NA | MT-2; PBMC |
Derivation refers to whether HIV isolate is a laboratory-adapted isolate or a clinical isolate.
NARRRP catalog number. Isolate name from NARRRP data sheet.
Isolate was initially mixed tropic (R5-X4), but following amplification in MT-2, cells reverted to CXCR4 tropic.
NA, not applicable (not obtained from NARRRP); R5, CCR5 tropic; UIVC, isolate obtained from University of Iowa Virology Clinic; X4, CXCR4 tropic.
Pseudotype virus production
293T cells were cotransfected with pNL4-3.Luc.R-E– (luciferase reporter inserted into pNL4-3 nef gene) and a plasmid that expresses the envelope glycoproteins of VSV (VSV-G), HIV (pHXB2-env), GBV-C E1-E2 (nt 555–2479), or no viral envelope protein using CaCl2 as described previously (39). Particles were collected 72 h posttransfection, filtered with a 0.45-μm filter, and concentrated by centrifugation at 12,000 rpm for 20 h at 4°C. Alternatively, 293T cells were transfected with pHXB2 env-expressing plasmid and tranduced with defective VSV particles with a GFP reporter gene provided by Dr. W. Maury (University of Iowa). Pseudotyped VSV particles were collected from supernatants and filtered with a 0.45-μm filter prior to use.
Viral infections and neutralization assays
The HIV-1 inocula (2 ng/ml p24 Ag per infection) or defective HIV or VSV particles (produced in 293T) were incubated with a range of concentrations of GBV-C E2 Ab-positive sera, IgG preparations or mAbs for 1 h at 37°C prior to adding to CD4+ T cell or HOS cell cultures. GBV-C E2 Ab-negative sera, E2 Ab-negative IgG preparations, and isotype control mAbs served as the negative control Abs. Inocula were removed, CD4+ cells (PBMCs or MT-2 as described in Results) were washed after 4 h, and culture supernatants were collected postinfection for measurement of HIV-1 replication by measuring HIV-1 p24 Ag (ELISA) or reporter gene expression 72 h posttransduction as described previously (19, 35). Pseudoparticle transduction was determined by measuring luciferase by the Bright-Glo Luciferase Assay System (Promega, Madison, WI) or GFP expression by flow cytometry. The concentration of each Ab preparation (micrograms per milliliter) required to reduce HIV-1 p24 Ag release into culture supernatants by 50% was calculated (IC50). YFV and mumps virus infectious titers (tissue culture infective dose 50%) were determined by terminal dilution in BHK21 cells or Vero cells, respectively (40, 41), and YFV and mumps viral RNA concentrations were measured by real-time PCR as described previously (42, 43). All neutralization experiments were performed in triplicate and independently repeated at least once with consistent results.
For some experiments, the standardized neutralization of HIV envelope-pseudotyped HIV particles was assessed after a single round of infection in TZM-bl cells as described previously (36). A total of 200 tissue culture infective dose 50% of virus was incubated with serial dilutions of test samples for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells were added to each well. No Ab control and cell only controls served as the background controls. Cells were lysed, and luminescence was measured 48 h after transduction using a Victor 2 luminometer. The serum concentration or IgG concentration that resulted in a 50% reduction in relative light units (RLUs) compared with virus control wells after subtraction of background RLUs was calculated (36).
HIV-1 and SIV envelopes used to pseudotype HIV-1 retroviral particles included SS1196.01, QH0692.42, PAVO.04, 6535.03, REJO.67, THRO.18, Du123.06, Du156.12, Du172.17, Du422.01, and control HIV, and SIV isolates included HIVMN (propagated in H9 cells) and SIVmac239/CS generated in PBMCs. In addition, HIV isolate SF162.LS was propagated in PBMCs and used in one TZM-bl experiment.
Immune precipitation
HIV-1, YFV, mumps virus, or defective HIV-1 pseudoparticles were mixed with Abs at various concentrations and incubated while mixing overnight at 4°C. All three viruses (HIV-1, YFV, and mumps) were prepared in PBMCs and pseudoparticles were prepared in 293T cells. Heat-killed, formalin-fixed Staphylococcus aureus cells with a coat of protein A (Pansorbin; Calbiochem, San Diego, CA) were added and incubated for 2 h at 4°C. Cells were pelleted, washed three times in TBS or PBS containing 0.02% Tween 20, and resuspended in PBS, and viral particle precipitation was measured (p24 Ag ELISA or real-time PCR for YFV and mumps RNA as above). For the E2 competition assay, GBV-C E2 or E2-negative cell supernatants were incubated with E2 Ab for 1 h at 37°C prior to incubating the Ab with virus particles.
ELISA
GBV-C E2 Ab reactivities with GBV-C E2 were assessed using a GBV-C mAb E2 capture assay. Plates were coated with E2-specific mAb (M5) in carbonate buffer (pH 9.6) overnight at 4°C, washed with PBS containing 0.02% Tween 20 (PBST), and blocked with PBS containing 1% BSA (PBSA) at 4°C overnight or 1 h at 37°C. E2 Ag was applied at 1 μg/ml in PBSA and allowed to adsorb for 1 h at 37°C. Plates were washed with PBST, and serial dilutions of Abs in PBSA were applied and incubated at 37°C for 1 h. Following washing, bound rabbit Abs were detected using alkaline phosphatase-labeled goat anti-rabbit Fc Ab (Sigma-Aldrich). Plates were washed, p-nitrophenylphosphate substrate was added, and the absorbance (405 nm) was measured on a Bio-Rad model 680 microplate reader. Previous studies found that human anti–GBV-C E2 Abs bound to E2 that was captured by M5 mAb (26, 27).
Flow cytometry
MT-2 cells and PBMCs were incubated with the anti–GBV-C M6 mAb or IgG2a isotype control Ab (BD Biosciences, Franklin Lakes, NJ) for 1 h at 4°C. Ab was added to cells that were untreated or following cell permeabilization using the BD Cytofix/Cytoperm kit as recommended by the manufacturer (BD Biosciences). Cells were washed, and PE-labeled anti-mouse secondary Ab (Southern Biotechnology Associates, Birmingham, AL) was applied for 30 min at 4°C. Ab binding was assessed by flow cytometry as described previously (44, 45).
Statistics
Statistics were performed using SigmaStat software V3.11 (Jandel Scientific, Chicago, IL). t tests were used for direct comparisons for individual data points, and results on experiments monitoring multiple days were analyzed by ANOVA.
Results
GBV-C E2 Abs neutralize HIV-1 infection
Heat-inactivated sera from healthy blood donors with and without GBV-C E2 Ab were tested for HIV-1–neutralizing activity. E2 Ab-positive, but not E2 Ab-negative, sera (diluted 1/100) neutralized a CCR5-tropic HIV isolate (R5, isolate 1) (Table I) and a CXCR4-tropic HIV isolate (X4, isolate 2) (Table I) in a PBMC-based neutralization assay. The percent inhibition compared with no Ab control is shown in Fig. 1A. Sera were maintained in culture media (1/100). None of these healthy blood donor sera reacted with denatured HIV-1 proteins in Western blot analyses (data not shown).
FIGURE 1.

Ab to the GBV-C envelope glycoprotein E2 neutralizes HIV-1 in vitro. Human serum (S) diluted 1/100 obtained from GBV-C E2 Ab-positive (P) subjects (n = 3; SP1, SP2, and SP3), IgG (I) purified from these sera (I-P1, I-P2, and I-P3; 10 μg/ml), murine mAbs [M1(VS) and M6], and human HIV-1 mAbs (2G12, 2F5, and 4E10) (5 μg/ml for all mAbs) neutralized CCR5- and CXCR4-tropic HIV-1 isolates in primary human PBMCs compared with no Ab controls, whereas E2 Ab-negative human sera (SN1 and SN2), IgG (I-N1 and I-N2), or murine isotype control (IC) Ab did not (A). HIV p24 Ag concentration was measured 5 d postinfection. The dose relationship between the human IgG preparations is shown (B).
To determine whether the effect was due to soluble factors in serum or whether it was due to serum Abs, IgGs purified from the serum samples were tested for HIV-1 neutralization activity in PBMCs (concentration 10 μg/ml) (Fig. 1A). The three human GBV-C E2 Ab-positive IgG preparations neutralized HIV-1 in MT-2 cells in a dose-dependent manner (Fig. 1B). Three murine GBV-C E2 mAbs M1(VS), M5, and M6 also inhibited HIV-1 isolates (concentration 5 μg/ml) in the PBMC-based assay (Fig. 1A). Three broadly neutralizing human HIV-1 mAbs (2G12, 2F5, and 4E10; 5 μg/ml) (Fig. 1A) served as positive control Abs.
GBV-C E2 immunogenicity
Mice and rabbits were immunized with rGBV-C E2 protein to determine whether it elicited HIV-1–neutralizing Abs. Murine serum obtained 10 d after the third E2 immunization demonstrated anti-E2 reactivity in a mAb capture ELISA, whereas the preimmune sera did not (data not shown). Sufficient sera were available to test two of the three mice by immunoblot assay, and both reacted with rE2 (Fig. 2A). All three murine sera neutralized the infectivity of HIV-1 (isolate 2) (Table I) in MT-2 cells compared with preimmune sera, as measured by HIV-1 p24 Ag release into culture supernatants 5 d post-HIV infection (Fig. 2B).
FIGURE 2.

GBV-C E2 protein elicits HIV-1–neutralizing Abs in mice. Mouse sera (diluted 1/5000) from two of three mice immunized with rGBV-C E2 protein reacted with CHO cell culture supernatant containing rE2 but not supernatant (Sup) from CHO cells not expressing E2. Pooled preimmune murine sera diluted 1/5000 did not react with E2 (A). There was insufficient serum from the third mouse to test by immunoblot. The concentrations of HIV p24 Ag produced in MT-2 cells 5 d following incubation with preimmune sera (1/1000) or postimmune (GBV-C E2 Ab positive) sera are shown in B. Data represent the average HIV p24 concentration released by cells following neutralization with the three mouse serum samples performed at the dilutions shown. The dashed line shows the amount of HIV-1 p24 Ag produced in the no Ab control cells.
GBV-C E2 protein and the GBV-C 17-aa E2 peptide were immunogenic in rabbits as well, as sera obtained from all rabbits reacted with E2 in a mAb capture ELISA (Fig. 3A) and by immunoblot analysis (Fig. 3B), whereas preimmune sera did not. Postimmune anti–GBV-C E2 IgG (n = 4 rabbits) significantly inhibited HIV-1 (isolate 2) (Table I) replication in MT-2 cells 3 and 6 d postinfection (Fig. 4A). In contrast, preimmune rabbit IgG, IgG purified from rabbit sera following immunization with a different GBV-C protein (NS5A) (46), or polyclonal goat IgG containing Ab to hepatitis B surface Ag did not inhibit HIV-1 infection compared with the no Ab control (Fig. 4A). All polyclonal Abs were used at a concentration of 10 μg/ml, and the Ab was maintained in the media following HIV-1 inoculation of cells.
FIGURE 3.

GBV-C E2 protein and antigenic peptide elicits E2 Abs in rabbits. Rabbit serum (n = 2) obtained following immunization with GBV-C E2 peptide (rPep-post) or GBV-C E2 protein (n = 4; R1, R2, R3, and R4) reacted with GBV-C E2 protein by ELISA (A), whereas the preimmune peptide (rPep-pre) and preimmune E2 sera (E2-pre) did not. All rabbit Abs reacted with E2 protein by immunoblot analysis (B). M6 is a control murine mAb that recognizes a linear epitope on GBV-C E2. M, the lane containing the m.w. marker; Pep, rabbit anti–GBV-C E2 peptide IgG.
FIGURE 4.
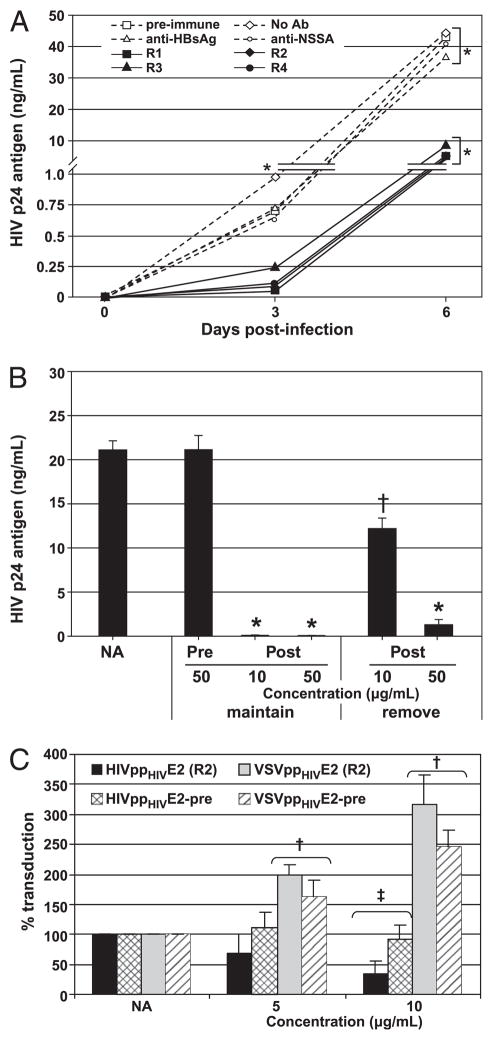
Rabbit anti–GBV-C E2 Abs neutralize infectious HIV-1 particles and HIV-1–pseudotyped HIV-1 particles but not HIV-1–pseudo-typed VSV particles. Rabbit anti–GBV-C E2 IgG from the four rabbits (R1, R2, R3, and R4) neutralized HIV replication in MT-2 cells compared with no Ab, rabbit preimmune IgG, and control Abs (anti-hepatitis B surface Ag and anti–GBV-C NS5A protein). *p < 0.001 for R1–R4 versus all controls (A). Postimmune rabbit IgG (Post) was maintained in the media or was removed from media following washing of cells postinfection. HIV-1 replication was monitored at day 5 postinfection (B). *p < 0.01; †p < 0.05, respectively, when to preimmune IgG. NA, no Ab control. Postimmune rabbit IgG (rabbit 2, R2) neutralized HIVenv-pseudotyped defective HIV-1 particles [HIVppHIV E2(R2)] but not HIVenv pseudotyped defective VSV particles (VSVppHIV), whereas preimmune rabbit IgG did not neutralize either (A). †p < 0.005 compared with no Ab control; ‡p < 0.001 compared with no Ab control and preimmune (C).
To ascertain whether the rabbit IgG inhibited the HIV-1 p24 Ag ELISA,HIV-1 was incubated with no Ab or pre- or postimmune rabbit IgG (10 μg/ml each), and the concentration was assessed. There was no difference between the no Ab control (11,107 pg/ml) and the pre-(11,219 pg/ml) and postimmune (11,106 pg/ml) (ANOVA; p = 0.99). In addition, incubation of MT-2 cells and PBMCs with rabbit pre-immune or anti-E2 IgG did not alter cell number or viability during 6 d in culture (data not shown). HIV-1 inhibition was diminished when Abs were not maintained in the cell culture media (Fig. 4B). Specifically, HIV replication was inhibited by >99% in MT-2 cells if E2 Ab was maintained in the media (p < 0.01); however, HIV-1 replication inhibition was diminished when Ab was removed after inoculation. Initial infection appears to be incompletely inhibited by the Abs, and HIV-1 cell-to-cell spread is suppressed in the presence of GBV-C E2 Abs.
Single cycle replication assays allow assessment of viral envelope specificity and provide information regarding which step(s) of HIV replication is inhibited. HIV-1 envelope protein (gp160) was used to pseudotype defective HIV-1 (gag) particles or defective VSV particles generated in 293T cells, and rabbit anti–GBV-C E2 IgG was assessed for neutralizing activity in a HOS cell-based assay. Anti–GBV-C E2 IgG preparation neutralized HIV-1–pseudotyped retrovirus particles in a dose-dependent fashion; however, this Ab did not neutralize HIV-1–pseudotyped VSV particles (Fig. 4C). Preimmune rabbit IgG did not neutralize either particle type. These data suggest that the anti–GBV-C E2 Abs neutralize HIV-1 particles independently from the HIV-1 envelope proteins.
HIV-1 neutralization by GBV-C E2 Abs is assay dependent
The breadth and potency of HIV-1–neutralizing activity present in GBV-C E2 Ab preparations was examined in virus replication assays using a diverse panel of HIV-1 isolates including clinical and laboratory derived R5 and ×4 isolates and representing clades A, B, and D (Table I). The Ab concentration (micrograms per milliliter) required to inhibit HIV replication by 50% (IC50) for human polyclonal anti-E2 Ab, rabbit anti-E2 Ab, rabbit anti-E2 peptide Ab, and murine mAbs M1(VS), M5, and M6 is shown in Table II. All anti-E2 Abs possessed neutralizing activity against all isolates tested except the rabbit anti-E2 peptide Ab (<50% reduction at 50 μg/ml Ab concentration). Three HIV-1–neutralizing hmAbs served as control Abs (2F5, 4E10, and 2G12). Because endotoxin contamination of Ab may lead to non–specific-neutralizing activity against HIV-1 (47), all of the rabbit IgG preparations were tested for endotoxin (assay; Cape Cod Associates, Woods Hole, MA) and were found to be endotoxin free (<0.25 EU/ml).
Table II.
Anti–GBV-C E2 Abs neutralize diverse HIV isolates
| Ab Description | Ab Identification | HIV Isolate Number (see Table I for characteristics)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Human anti-E2 polyclonal IgG | I-P1 | 8.1a | 3.4 | 2.9 | 1.6 | 2.2 | 2.3 | 4.1 | 3.5 | 2.8 | 3.7 | 5.1 |
| I-P2 | 2.6 | 8.3 | ||||||||||
| I-P3 | 5.8 | 1.7 | <10 | <10 | ||||||||
| Rabbit anti-E2 protein polyclonal IgG | R1b,c | 4.3 | 5.0 | |||||||||
| R2 | 1.5 | 2.1 | 1.1 | 3.1 | 1.6 | <10 | <10 | <10 | <10 | 1.5 | 1.2 | |
| R3 | 9.3 | 10 | ||||||||||
| R4 | 5.6 | 4.9 | <10 | <10 | <10 | <10 | <10 | 2.1 | 1.7 | |||
| Rabbit anti-E2 peptide polyclonal IgG | rPep-1c | >50 | >50 | >50 | >50 | >50 | >50 | >50 | ||||
| rPep-2 | >50 | >50 | >50 | >50 | ||||||||
| Mouse anti-E2 monoclonal IgG | M1 (VS) | 1.4 | 2.5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | ||
| M5 | <5 | <5 | ||||||||||
| M6 | 1.8 | 1.1 | ||||||||||
| Human anti-HIV monoclonal IgG | 2F5 | 1.7 | ||||||||||
| 4E10 | 1.3 | |||||||||||
| 2G12 | >5 | 0.2 | ||||||||||
IC50 results >5 μg/ml for mAbs or >30 μg/ml for polyclonal Abs are in bold.
Neutralization assays were performed in PBMCs for R5 isolates and MT-2 cells for X4 isolates.
Concentration (micrograms per milliliter) required to reduce HIV infectivity by 50% compared with control Ab (IC50).
Rabbits were immunized with either rGBV-C E2 protein (anti-E2) or the 17-aa peptide Ag recognized by the M6 murine mAb (anti-rPep) as described in Materials and Methods. Blank, not tested. The highest or lowest concentration tested is equal to or more than or less than, respectively.
Some HIV-neutralizing Abs perform differently in neutralization assays that use different cell types (48, 49). To see whether this were true for the GBV-C E2 Abs, a standardized TZM-bl assay was used to examine neutralization of a panel of HIV-enveloped pseudo-particles (36, 50, 51). Differences in neutralization in the TMZ-bl cells and the PBMC-, MT-2–, and HOS-based assays were observed (Table III). The murine mAb M5 did not neutralize any of the isolates tested in TZM-bl cells, and M6 mAb neutralized only one HIV envelopes (Du422.01) at concentrations of <5 μg/ml (Table III). In contrast, the murine M1(VS) mAb was broadly neutralizing and neutralized all viruses HIV-1 envelopes studied, except REJO.67 in the TZM-bl cell assay (Table III). The mAb M1 (VS) was also active against an SIV macaque envelope (SIVmac 239/CS), further suggesting that the interaction is independent of HIV-1 envelope glycoproteins. Although the rabbit anti-peptide IgG and E2-positive human IgG neutralized some HIV and SIV isolates at high concentrations (generally >20 μg/ml) (Table III), the specificity of this is unclear, because the E2-negative IgG also inhibited some isolates at high concentration (Table III). One of the HIV-negative blood donor human anti-E2 IgG preparations (I-P2) neutralized three HIV isolates at <10 μg/ml, indicating a more narrow neutralization effect on HIV-1 envelope-containing particles in the TZM-bl cell neutralization assay (Table III). Because these are polyclonal human Abs, the relative concentration of Ab that reacts with E2 is considerably lower than the HIV-neutralizing IC50 concentration.
Table III.
Interassay variation in GBV-C E2 Ab neutralization
| Ab | ID50 in TZM-bl Cellsa Virus Isolates
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MN (B) | SS1196.01 (B) | QH0692.42 (B) | PAVO 0.04 (B) | 6535 0.03 (B) | REJO 0.67 (B) | THRO 0.18 (B) | Du123 0.06 (C) | Du156 0.12 (C) | Du172 0.17 (C) | Du422.01 (C) | SIVmac 239/CS) | |
| M1 (VS) | 3.9 | 4.2 | 4.3 | 4.4 | 4 | >5 | 4 | 2.3 | 2.5 | 2.7 | 2.7 | 2.6 |
| M5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| M6 | > | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | 4.8 | >5 |
| rPep-1 | >50 | >50 | >50 | >50 | >50 | 45 | >50 | 41.7 | 30.3 | 43.5 | 41.7 | >50 |
| rPep-2 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | 27.8 | 21.3 | 38.5 | 27.8 | >50 |
| I-N2 | >50 | >50 | 45.5 | >50 | >50 | >50 | >50 | 34.5 | 25.6 | 38.5 | 29.4 | >50 |
| I-N1 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | 43.5 | 35.7 | >50 | >50 | >50 |
| I-P1 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | 27.8 | 18.9 | 37 | 40 | >50 |
| I-P2 | <1.9 | >50 | 8.3 | >50 | 38.5 | 6.8 | >50 | >50 | >50 | >50 | >50 | >50 |
| I-P3 | 21.7 | >50 | >50 | >50 | >50 | 37 | >50 | 26.3 | 23.3 | 40 | 29.4 | 34.5 |
IC50 results >5 μg/ml for mAbs or >30 μg/ml for polyclonal Abs are in bold.
Values represent concentration (micrograms per milliliter) at which RLUs were reduced 50% compared with virus control wells (no test sample). All HIV-1 isolates, except MN, are molecularly cloned pseudoviruses produced by transfection in 293T cells. M1(VS), M5, and M6 are murine anti–GBV-C E2 mAbs. rPep-1 and rPep-2 are rabbit anti-E2 peptide Abs (see Materials and Methods). I-N1 and I-N2 are two human IgG preparations from blood donors without E2 Ab, and I-P1, I-P2, and I-P3 are human IgG preparations from three blood donors with E2 Ab.
To determine whether the differences were related to the neutralization system used or to the viruses studied, human anti-E2 IgG and the three murine anti–GBV-C E2 mAbs were retested at Duke University (Durham, NC) in PBMC-based assays using HIV-1 isolates 1 and 2 (Table I). Two of the three human GBV-C E2 Ab-positive IgG preparations neutralized the R5 and ×4 isolate at <20 μg/ml (I-P2 and I-P3), and the three murine E2 mAbs neutralized HIV-1 in PBMC assays (Table IV). The TriMAb control (2G12, 4E10, and 2F5) did not neutralize the HIV-1 R5 isolate (Table I, isolate 1) in TZM-bl cells but did in PBMCs. Of the anti–GBV-C E2 mAbs, only M6 did not neutralize well in PBMCs, with a very high IC50 value for the isolate 1 (17 μg/ml) and no activity against the ×4 isolate number 2.
Table IV.
Comparison of HIV neutralization in TZM-bl cells and PBMCs
| Sample | ID50 in TZM-bl Cells (μg/ml)a
|
ID50 in PBMCs (μg/ml)b
|
|||
|---|---|---|---|---|---|
| SF162.LS Clade B | HIV-1 R5 Isolate 1 | HIV-1 X4 Isolate 2 | HIV-1 R5 Isolate 1 | HIV-1 X4 Isolate 2 | |
| I-N2 | >45 | >45 | >45 | >45 | >45 |
| I-P1 | >45 | >45 | >45 | >45 | 40 |
| I-P2 | >45 | >45 | >45 | 9 | 15 |
| I-P3 | >45 | >45 | >45 | 14 | 19 |
| M1 (VS) | 2.6 | 4.0 | 3.2 | 1 | 2 |
| M5 | >50 | >50 | >50 | 3 | 8 |
| M6 | >50 | >50 | >50 | 17 | >50 |
| TriMAbc | 0.04 | >25 | 0.58 | 2 | 1 |
IC50 results >5 μg/ml for mAbs or >30 μg/ml for polyclonal Abs are in bold. HIV isolates 1 (R5) and 2 (X4) refer to isolates described in Table I.
Values represent the Ab concentration (micrograms per milliliter) at which RLUs were reduced 50% compared with virus control wells (no test sample).
Values represent the Ab concentration (micrograms per milliliter) at which p24 Gag Ag synthesis was reduced by 50% relative to virus control wells (no test sample).
TriMAb is a positive control of three HIV-1 human neutralizing mAbs.
GBV-C E2 Abs precipitate HIV-1 particles
As noted above, naturally occurring human GBV-C E2 Abs are conformation dependent (26), although the rabbit anti-E2 Abs recognized denatured E2. The human GBV-C E2 Abs and the rabbit anti-E2 Abs did not react with denatured HIV-1 proteins (Refs. 52–54 and data not shown). In contrast, the rabbit anti-E2 IgG preparations precipitated infectious HIV-1 particles prepared in HOS cell lines (Fig. 5A), suggesting that the interaction is conformation dependent. Immune precipitation was inhibited by CHO cell culture supernatant fluid containing rE2 protein in a dose-dependent manner but not CHO supernatant without E2 (Fig. 5A).
FIGURE 5.

Rabbit anti–GBV-C E2 Abs precipitate HIV particles. HIV-1 particles were immune precipitated (IP) by the postimmune rabbit IgG (R1, R2, R3, and R4) but not preimmune (pre) rabbit anti-E2 IgG (A; *p < 0.01 compared with preimmune IgG). CHO cell culture supernatants containing E2 protein inhibited IP in a dose-dependent manner, whereas CHO cell culture supernatants without E2 did not (A). The protein concentration represents the total protein in culture supernatants and not E2-specific protein. Defective HIV gag particles displaying HIV, VSV, or GBV-C envelope glycoproteins, or HIV-1 particles with no virus envelope glycoproteins were precipitated by postimmune rabbit anti-E2 IgG and not by preimmune rabbit IgG (B; *p < 0.01 compared with preimmune IgG; †p < 0.05). YFV and mumps virus (MV) were not neutralized following incubation with pre- or postimmune (post) rabbit E2 IgG (left panel; C), and they were not IP by postimmune rabbit anti-E2 IgG (right panel; C). NA, no Ab control; IPV, the amount of input virus used in IP experiments.
Rabbit anti–GBV-C E2 IgG precipitated retrovirus particles displaying HIVenv, GBV-Cenv, or VSV envelope glycoprotein significantly more than preimmune IgG or no Ab control (Fig. 5B). In addition, defective HIV particles with no viral envelope protein were precipitated by the rabbit anti–GBV-C E2 Abs (Fig. 5B), indicating that precipitation did not involve the viral envelope proteins. GBV-C–enveloped HIV pseudoparticles (GBV-Cpp) were precipitated to a significantly greater extent than the other defective particles by anti-E2 Ab, suggesting that, in addition to the gag particle Ag recognized by these Abs, interactions with the GBV-C E2 protein contributed to the immune precipitation (Fig. 5B). The murine mAb M6 and the human anti-gp120 (2G12) mAb precipitated HIV-1–pseudotyped gag particles (HIVpp) more than the IgG2a and human polyclonal IgG control Abs (data not shown). However, the percentage of input of defective HIVpp and GBV-Cpp precipitated by all of these mAbs was very low (<2% of input virus), suggesting that their reactivity with gag particles is significantly weaker than the rabbit polyclonal anti–GBV-C E2 Ab.
The rabbit GBV-C E2 Abs did not precipitate or neutralize YFV or mumps virus prepared in MT-2 cells (Fig. 5C). Like HIV-1, the mumps virus has a class I envelope glycoprotein. YFV prepared in Vero cells, and mumps prepared in BHK cells were also not neutralized or precipitated by the E2 Abs (data not shown). Taken together, the data suggest that the anti-E2 Ab interactions are not dependent on HIV-1 or other viral envelope glycoproteins and that the antigenic site is not exposed on flavivirus or orthomyxovirus particles.
Anti–GBV-C E2 Abs interfere with HIV-1 particle binding
To determine whether GBV-C E2 Abs neutralized HIV-1 by binding to cell plasma membrane Ags and blocking either HIV-1 attachment or entry, MT-2 cells were incubated with no Ab, E2-negative rabbit IgG (preimmune; 10 μg/ml), or anti–GBV-C E2 rabbit IgG for 2 h at 4°C (postimmune; 10 μg/ml). Cells were washed, and HIV-1 was added prior to warming the cells to 37°C. HIV replication was monitored. Preincubation of cells with rabbit GBV-C E2 Ab did not inhibit HIV-1 replication compared with controls (Fig. 6A). Thus, if the Ag interacting with GBV-C E2 Abs is of cellular origin, it is either not exposed on the surface of MT-2 cells, or it is expressed in a different confirmation.
FIGURE 6.
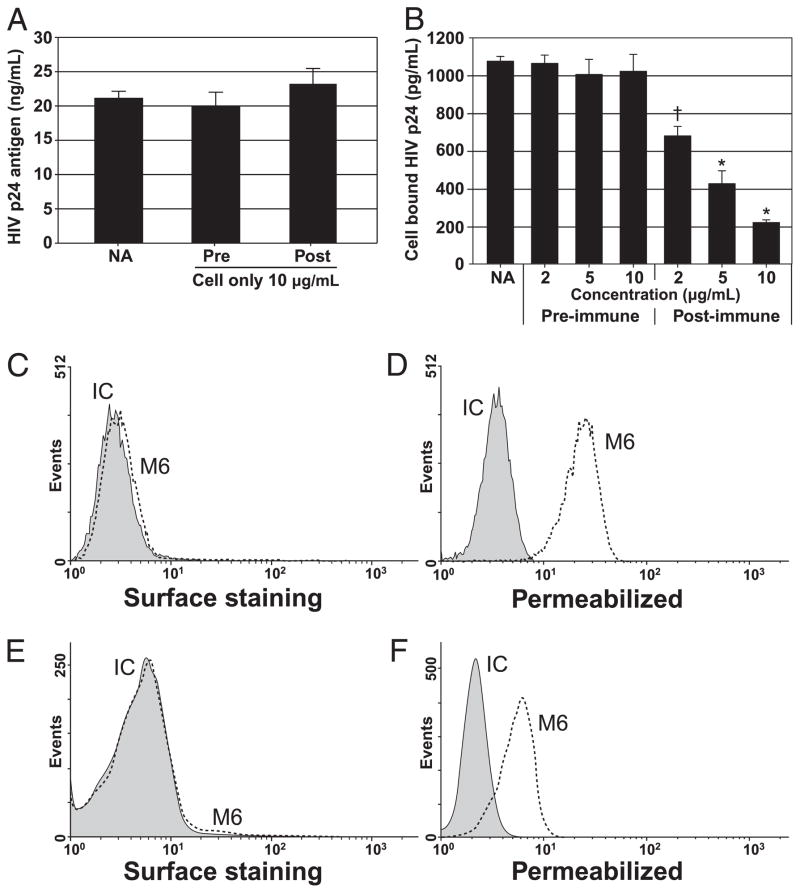
Anti–GBV-C E2 Abs do not inhibit HIV-1 by interacting with cell plasma membrane but interfere with HIV-1 cellular attachment. HIV replication was not altered when MT-2 cells were preincubated with no Ab (NA), pre- (Pre), or post-E2 (Post) rabbit IgG (10 μg/ml) (A). Incubation of HIV-1 particles with rabbit postimmune (anti–GBV-C E2 positive) IgG inhibited HIV-1 particle attachment to MT-2 cells compared with no Ab or preimmune IgG controls in a dose-dependent manner (B; see Materials and Methods) (*p < 0.01 compared with preimmune IgG; †p < 0.05). Surface staining of intact MT-2 cells and PBMCs by the murine anti-E2 M6 mAb is shown in C and E, respectively. M6 reactivity increased with both MT-2 cells and PBMCs following fixation and permeabilization (D and F, respectively). IC, IgG2a isotype control Ab.
To determine the step of HIV replication that is inhibited by GBV-C E2 Abs, HIV-1 was mixed with rabbit E2 Abs for 1 h prior to addition to PBMCs for 2 h at 4°C. Cells were washed to remove nonspecifically bound HIV-1 particles, and the amount of HIV-1 bound to the cell was measured by p24 Ag ELISA. Rabbit anti–GBV-C E2 Ab blocked HIV-1 binding in a dose-dependent manner, whereas preimmune IgG did not (Fig. 6B). The murine mAb 6 did not interact with intact MT-2 cells or PBMCs as measured by flow cytometry (Fig. 6C, 6E). However, following fixation and permeabilization, M6 reacted with MT-2 cells and PBMCs (Fig. 6D, 6F). Similarly, M6 did not react with intact CHO cells but did following fixation and permeabilization (data not shown). These data indicate that the E2 Abs react with cellular Ags that are not highly exposed on the cell surface.
To determine whether the anti–GBV-C E2 Abs inhibited HIV entry in addition to inhibiting HIV attachment, HIV-1 was incubated with cells for 2 h at 4°C to allow attachment. Following attachment, preimmune rabbit IgG, rabbit anti-E2, or rabbit anti-E2 Fab fragments were added to the cells at 4°C and cells were warmed to 37°C. One hour later, the medium was replaced with medium with or without the rabbit IgG or Fab fragments. In cells maintained in anti-E2 IgG or Fab fragments prepared from the polyclonal anti-E2 IgG preparation, HIV-1 replication was inhibited in a dose-dependent manner (Fig. 7A). HIV inhibition was not related to steric interactions between the Fc portion of the anti-E2 IgG. Similar to what was observed when anti-E2 IgG was removed from the media (Fig. 4B), inhibition was lost when anti-E2 Fab fragments were removed from the media. These data cannot distinguish between inhibition of entry during initial infection or subsequent inhibition of HIV-1 cell-to-cell spread.
FIGURE 7.

GBV-C Abs do not block HIV-1 entry following HIV-1 attachment to cells. Following attachment of HIV particles to MT-2 cells for 2 h at 4°C, rabbit anti–GBV-C E2 Ab or Fab fragments were added to cells for 1 h. Following washing, Abs were included in the media (maintain) or not (remove). Cells were warmed to 37°C, and HIV-1 p24 Ag release was measured 3 d later (A). HIV envelope pseudotyped defective HIV-1 particles were similarly analyzed in HOS cells. Following attachment at 4°C, pre- or postimmune rabbit GBV-C E2 IgG was added at the concentrations indicated (B). Cells were warmed to 37°C, and transduction (luciferase activity) was measured 72 h later and compared to the virus only control (B).
To assess this in a single cycle of infection, HIV-1 envelope pseudotyped gag particles generated in 293T cells were added to HOS cells expressing human CD4, CXCR4, and CCR5. Pre- or postimmune (anti-E2) rabbit IgG was added to the cells following viral attachment for 2 h at 4°C, and the cells were warmed to 37°C to allow viral entry. Luciferase activity was measured 72 h later, and HIV-1 transduction was not inhibited (Fig. 7B). Thus, once HIV particles bound to CD4, access to the cross-reacting Ag was blocked, and the Abs were not able to block subsequent entry steps.
Discussion
Naturally occurring GBV-C E2 Abs from HIV-negative blood donors and experimentally induced GBV-C E2 Abs neutralized HIV-1 infection in vitro. The Abs inhibited HIV-1 isolates obtained from patients from geographically diverse regions and representing three separate HIV-1 clades. Neutralization was independent of entry coreceptor usage (CCR5 or CXCR4), and similar to other characterized Abs, neutralization differed when neutralization assays used different cellular substrates. VSV particles displaying HIV-1 envelope glycoproteins, YFVs, and mumps viruses were not neutralized by the anti–GBV-C E2 Abs. Anti–GBV-C E2 Abs also precipitated infectious HIV-1 particles and defective HIV-1 particles but not YFV or mumps viruses. Immune precipitation was independent of the viral envelope, because retrovirus particles with HIV-1, VSV, GBV-C, or no envelope protein were precipitated.
Anti–GBV-C E2 Abs interacted with cellular Ag(s) following cell permeabilization, suggesting that anti-E2 Abs cross-react with an Ag(s) that is not exposed on the cell surface. Together with the neutralization and immune precipitation data, these data indicate that the anti-E2 Abs neutralize and precipitate HIV-1 particles via interactions with a cellular Ag that is present or enriched on HIV-1 gag particles. The Ab interactions appear to be similar to the well-characterized and broadly neutralizing HIV-1 Abs 2F5 and 4E10, which react with both HIV-1 gp41 peptides and permeabilized cells (10). These Abs also react with a variety of phosphatidylinositols and lipids (10). Studies are under way to further characterize the cellular Ag recognized by the anti–GBV-C E2 Abs (J.H. McLinden, J. Xiang, E.L. Mohr, T.M. Kaufman, Q. Chang, and J.T. Stapleton, manuscript in preparation).
HIV-1 neutralization required incubation of anti-E2 Abs with the viral particles before interaction with the cell surface, because the Abs did not bind to cells, and they did not block entry following viral attachment in single cycle infections. Preincubation of the E2 Abs with HIV-1 particles reduced virus-cell binding; thus, HIV-1 replication is inhibited at least in part by decreasing HIV-1 attachment (Fig. 6B). Of note, the GBV-C E2 peptide recognized by the M6 mAb did not elicit HIV-1–neutralizing Abs nor neutralize HIV-1 infectivity, whereas the rE2 protein did, illustrating that the peptide is insufficient to induce neutralizing HIV-1 Abs. Previous studies found that immunization with the MPER peptide sequence or T-20 is not immunogenic unless the peptide is presented as part of a structural particle (e.g., viral particles or liposomes) (9, 11, 55, 56).
GBV-C E2 protein inhibits HIV-1 entry in vitro through an unknown mechanism (31, 32). Thus, either viremia with GBV-C or the presence of E2 Ab may influence HIV-1 replication. Viremia appears to have the greatest impact on survival in HIV-infected people (20), perhaps because of a dose effect. GBV-C concentration in infected humans is typically >50 million genome equivalents/ ml plasma (57), and GBV-C is produced by B lymphocytes and both CD4+ and CD8+ T lymphocytes (15). Because each virus particle is predicted to have multiple copies of E2 protein on the surface, lymphocytes are constantly exposed to low concentrations of E2 protein. In contrast, the concentration of GBV-C E2 Abs in humans varies over time and may drop to levels below the limit of detection during longitudinal follow up (20, 53). It is likely that the E2 Ab concentration is an important variable in determining the magnitude of any potential clinical effect of GBV-C E2 Abs.
No significant amino acid sequence homology between GBV-C E2 and either HIV-1 or cellular proteins was identified in a protein-protein basic local alignment search tool search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Although several studies have identified HIV-1–neutralizing Abs in ~20% of humans with HIV-1 infection and a lower percentage in HIV-uninfected people (58–64), there are few data suggesting a beneficial clinical course in those with HIV-neutralizing Abs compared with those without. In contrast, a modest association between GBV-C E2 Ab and prolonged survival was observed by Tillmann et al. (20). There are clearly neutralizing Abs present in HIV-1–infected people that are not due to GBV-C E2 Abs; thus, measuring survival on the basis of the presence of HIV-1–neutralizing Abs independent of the presence of GBV-C E2 Ab will confound the analysis. It will be interesting to determine what proportion of HIV-1–neutralizing Abs in HIV-infected individuals are directed against the GBV-C E2 protein, and studies to address this question are under way.
One concern related to the use of non–HIV-1 Ags to invoke HIV-1–neutralizing Abs is that these Ags may induce autoantibodies. Because ~10% of healthy U.S. blood donors have Ab to GBV-C E2 protein (52, 65, 66), it appears that GBV-C Abs do not induce any ill effects in humans. Furthermore, the GBV-C E2 Abs appear to recognize cellular proteins carried on HIV-1 particles that are not displayed on the surface of cell plasma membranes.
Currently, no commercial GBV-C E2 mAbs or hybridoma cell lines producing GBV-C E2 mAbs are available (67). Consequently, the development of additional GBV-C E2 mAbs to allow further characterization of the Ag(s) that elicits the HIV-1–cross-reacting neutralizing Abs is needed. Characterization of the immunogenic domain(s) on the GBV-C E2 protein and its interactions with HIV particles may provide novel HIV-1 candidate vaccines.
Because a high proportion of HIV-1–infected people have GBV-C E2 Abs, it is unlikely that this Ab will, by itself, prove highly protective. Nevertheless, identification of immunogens that elicit HIV-1–neutralizing Abs against diverse isolates may contribute to protection induced by a multivalent HIV-1 vaccine, or they may delay or modify HIV-1 disease progression. Nevertheless, characterization of the E2 antigenic structure responsible for eliciting HIV-1–neutralizing activity may identify conserved targets for drug design. Our data suggest that testing of GBV-C E2 protein as a candidate HIV-1 vaccine Ag appears to be warranted.
Acknowledgments
We thank Dr. Beth Jamieson (University of California, Los Angeles) for providing NFN-HAS and NFN-SX-HSA HIV-1 isolates; Dr. Wendy Maury (University of Iowa) for providing defective VSV particles; and NARRRP, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, for the following HIV-1 isolates: HIV-192UG031 and HIV-192UG029 from the UNAIDS Network for HIV-1 Isolation and Characterization, AZT intermediate isolate from Dr. Douglas Richman, HIVJR-CSF from Dr. Irvin Chen, HTLV-IIIB/H9 from Dr. Robert Gallo, HIVxxBru-JF from Dr. John Mellors and Chaofu Shi, and HIVELI from Dr. Jean-Marie Bechet and Dr. Luc Montagnier. Catalog numbers are shown in Materials and Methods. We also thank Dr. Opendra Sharma (NARRRP) for providing the GBV-C synthetic peptides and Drs. Georg Hess and Alfred Engel (Roche Diagnostics and Laboratories) for providing GBV-C E2 ELISA kits and M6 mAb. We also thank Shawn Roach (University of Iowa) for assistance with the figures.
This work was supported in part by grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Merit Review to J.X. and J.T.S.), the National Institutes of Health (Grant R01 AI-58740 to J.T.S.), a Grand Challenges grant from the Bill and Melinda Gates Foundation (to J.X.), and the Biological Sciences Funding Program from the University of Iowa (to J.X.). E.L.M. received support from the University of Iowa Medical Scientist Training Program and from the Virology T-32 Training Grant.
Abbreviations used in this paper
- AZT
azidothymidine
- BHK
baby hamster kidney
- CHO
Chinese hamster ovary
- GBV-C
GB virus type C
- GBVpp
GBV-C enveloped retrovirus pseudoparticle
- HIVpp
HIV-enveloped retrovirus pseudoparticle
- HIVppHIV
HIV-enveloped retrovirus pseudoparticle
- hmAb
human mAb
- HOS
human osteocarcoma
- I
IgG
- IC
isotype control
- IP
immune precipitation
- IPV
the amount of input virus used in an immune-precipitated experiment
- M
the lane containing the m.w. marker
- MPER
membrane proximal ectodomain region
- MV
mumps virus
- NA
not applicable (not obtained from NARRRP)
- NARRRP
National Institutes of Health AIDS Research and Reference Reagent Program
- NS5A
GBV-C nonstructural protein 5A
- P
positive
- Pep
rabbit anti–GBV-C E2 peptide IgG
- Post
postimmune rabbit IgG
- R5
CCR5 tropic
- RLU
relative light unit
- S
serum
- Sup
supernatant
- UIVC
isolate obtained from University of Iowa Virology Clinic
- VS
virostat
- VSV
vesicular stomatitis virus
- VSVppHIV
VSV-enveloped retrovirus pseudoparticle
- X4
CXCR4 tropic
- YFV
yellow fever virus
Footnotes
Portions of this work were presented at the International Meeting of the Institute Human Virology, Baltimore, MD (October 31–November 4, 2004); the International AIDS Society Meeting (July 22–25, 2007) in Sydney, New South Wales, Australia; the Conference on Retroviruses and Opportunistic Infections, Montreal, Quebec, Canada (February 8–11, 2009); and the Keystone HIV Vaccines Conference, Banff, Alberta, Canada (March 21–26, 2010).
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, Hoxie JA, Martin M, Overbaugh J, Watkins DI, Mahmoud A, Greene WC. HIV vaccine research: the way forward. Science. 2008;321:530–532. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 2.Johnston MI, Fauci AS. An HIV vaccine—challenges and prospects. N Engl J Med. 2008;359:888–890. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 3.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 4.Dennison SM, Stewart SM, Stempel KC, Liao HX, Haynes BF, Alam SM. Stable docking of neutralizing human immunodeficiency virus type 1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J Virol. 2009;83:10211–10223. doi: 10.1128/JVI.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild C, Greenwell T, Matthews T. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retroviruses. 1993;9:1051–1053. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 6.Ferrantelli F, Hofmann-Lehmann R, Rasmussen RA, Wang T, Xu W, Li PL, Montefiori DC, Cavacini LA, Katinger H, Stiegler G, et al. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS. 2003;17:301–309. doi: 10.1097/00002030-200302140-00003. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Martínez S, Lorizate M, Hermann K, Kunert R, Basañez G, Nieva JL. Specific phospholipid recognition by human immunodeficiency virus type-1 neutralizing anti-gp41 2F5 antibody. FEBS Lett. 2006;580:2395–2399. doi: 10.1016/j.febslet.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF, Alam SM. HIV-1 hides an Achilles’ heel in virion lipids. Immunity. 2008;28:10–12. doi: 10.1016/j.immuni.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutant J, Yu H, Clément MJ, Alfsen A, Toma F, Curmi PA, Bomsel M. Both lipid environment and pH are critical for determining physiological solution structure of 3-D-conserved epitopes of the HIV-1 gp41-MPER peptide P1. FASEB J. 2008;22:4338–4351. doi: 10.1096/fj.08-113142. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 11.Matyas GR, Beck Z, Karasavvas N, Alving CR. Lipid binding properties of 4E10, 2F5, and WR304 monoclonal antibodies that neutralize HIV-1. Biochim Biophys Acta. 2009;1788:660–665. doi: 10.1016/j.bbamem.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Matyas GR, Wieczorek L, Beck Z, Ochsenbauer-Jambor C, Kappes JC, Michael NL, Polonis VR, Alving CR. Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS. 2009;23:2069–2077. doi: 10.1097/QAD.0b013e32832faea5. [DOI] [PubMed] [Google Scholar]
- 13.Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine. 2003;21:3370–3373. doi: 10.1016/s0264-410x(03)00335-9. [DOI] [PubMed] [Google Scholar]
- 14.Koff WC. Accelerating HIV vaccine development. Nature. 2010;464:161–162. doi: 10.1038/464161a. [DOI] [PubMed] [Google Scholar]
- 15.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23:137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton JT, Williams CF, Xiang J. GB virus type C: a beneficial infection? J Clin Microbiol. 2004;42:3915–3919. doi: 10.1128/JCM.42.9.3915-3919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrère JJ, Roudot-Thoraval F, Morand-Joubert L, Petit JC, Lerable J, Thauvin M, Mariotti M. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. J Infect Dis. 1999;179:783–789. doi: 10.1086/314671. [DOI] [PubMed] [Google Scholar]
- 19.Xiang J, Wünschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med. 2001;345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 20.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 21.Van der Bij AK, Kloosterboer N, Prins M, Boeser-Nunnink B, Geskus RB, Lange JMA, Coutinho RA, Schuitemaker H. GB virus C coinfection and HIV-1 disease progression: the Amsterdam Cohort Study. J Infect Dis. 2005;191:678–685. doi: 10.1086/427559. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med. 2006;7:173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 23.Supapol WB, Remis RS, Raboud J, Millson M, Tappero J, Kaul R, Kulkarni P, McConnell MS, Mock PA, Culnane M, et al. Reduced mother-to-child transmission of HIV associated with infant but not maternal GB virus C infection. J Infect Dis. 2008;197:1369–1377. doi: 10.1086/587488. [DOI] [PubMed] [Google Scholar]
- 24.Handelsman E, Cheng I, Thompson B, Hershow R, Mofenson LM, Hollinger FB, Chen KT, Burchett SK, Klinzman D, Stapleton JT, et al. Impact of GB virus type C infection on mother-to-child HIV transmission in the Women and Infants Transmission Study Cohort. [Published erratum in 2008 HIV Med. 9: 64.] HIV Med. 2007;8:561–567. doi: 10.1111/j.1468-1293.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med. 2004;350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 26.McLinden JH, Kaufman TM, Xiang J, Chang Q, Klinzman D, Engel AM, Hess G, Schmidt U, Houghton M, Stapleton JT. Characterization of an immunodominant antigenic site on GB virus C glycoprotein E2 that is involved in cell binding. J Virol. 2006;80:12131–12140. doi: 10.1128/JVI.01206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmolke S, Tacke M, Schmitt U, Engel AM, Ofenloch-Haehnle B. Identification of hepatitis G virus particles in human serum by E2-specific monoclonal antibodies generated by DNA immunization. J Virol. 1998;72:4541–4545. doi: 10.1128/jvi.72.5.4541-4545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larios C, Casas J, Alsina MA, Mestres C, Gómara MJ, Haro I. Characterization of a putative fusogenic sequence in the E2 hepatitis G virus protein. Arch Biochem Biophys. 2005;442:149–159. doi: 10.1016/j.abb.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Larios C, Casas J, Mestres C, Haro I, Alsina MA. Perturbations induced by synthetic peptides from hepatitis G virus structural proteins in lipid model membranes: a fluorescent approach. Luminescence. 2005;20:279–281. doi: 10.1002/bio.850. [DOI] [PubMed] [Google Scholar]
- 30.Herrera E, Gomara MJ, Mazzini S, Ragg E, Haro I. Synthetic peptides of hepatitis G virus (GBV-C/HGV) in the selection of putative peptide inhibitors of the HIV-1 fusion peptide. J Phys Chem B. 2009;113:7383–7391. doi: 10.1021/jp900707t. [DOI] [PubMed] [Google Scholar]
- 31.Jung S, Eichenmüller M, Donhauser N, Neipel F, Engel AM, Hess G, Fleckenstein B, Reil H. HIV entry inhibition by the envelope 2 glycoprotein of GB virus C. AIDS. 2007;21:645–647. doi: 10.1097/QAD.0b013e32803277c7. [DOI] [PubMed] [Google Scholar]
- 32.Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat. 2009;16:757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang J, Wünschmann S, Schmidt WN, Shao J, Stapleton JT. Full-length GB virus C (Hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J Virol. 2000;74:9125–9133. doi: 10.1128/jvi.74.19.9125-9133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wünschmann S, Stapleton JT. Fluorescence-based quantitative methods for detecting human immunodeficiency virus type 1-induced syncytia. J Clin Microbiol. 2000;38:3055–3060. doi: 10.1128/jcm.38.8.3055-3060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet. 2004;363:2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 36.Montefiori D. Evaluating neutralizing antibodies against HIV, SIV and SHIV in a luciferase reporter gene assay. In: Coligan JE, Kruisbeek AM, Margulies DH, Sevach EM, Strober W, Coico R, editors. Current Protocols in Immunololgy. John Wiley & Sons; New York, NY: 2004. pp. 11.1–11.5. [Google Scholar]
- 37.Jamieson BD, Zack JA. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J Virol. 1998;72:6520–6526. doi: 10.1128/jvi.72.8.6520-6526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and σ-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78:1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 39.Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, Sanders DA, McCray PB., Jr Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor α. J Virol. 2003;77:5902–5910. doi: 10.1128/JVI.77.10.5902-5910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Methods. 2003;110:185–191. doi: 10.1016/s0166-0934(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 41.Sakata H, Hishiyama M, Sugiura A. Enzyme-linked immunosorbent assay compared with neutralization tests for evaluation of live mumps vaccines. J Clin Microbiol. 1984;19:21–25. doi: 10.1128/jcm.19.1.21-25.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantel N, Aguirre M, Gulia S, Girerd-Chambaz Y, Colombani S, Moste C, Barban V. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever-dengue vaccines. J Virol Methods. 2008;151:40–46. doi: 10.1016/j.jviromet.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Akiyama M, Kimura H, Tsukagoshi H, Taira K, Mizuta K, Saitoh M, Nagano M, Sutoh A, Noda M, Morita Y, et al. Development of an assay for the detection and quantification of the measles virus nucleoprotein (N) gene using real-time reverse transcriptase PCR. J Med Microbiol. 2009;58:638–643. doi: 10.1099/jmm.0.005439-0. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman TM, McLinden JH, Xiang J, Engel AM, Stapleton JT. The GBV-C envelope glycoprotein E2 does not interact specifically with CD81. AIDS. 2007;21:1045–1048. doi: 10.1097/QAD.0b013e3280f77412. [DOI] [PubMed] [Google Scholar]
- 45.Wünschmann S, Medh JD, Klinzmann D, Schmidt WN, Stapleton JT, Stapleton JT. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol. 2000;74:10055–10062. doi: 10.1128/jvi.74.21.10055-10062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang J, Martinez-Smith C, Gale M, Jr, Chang Q, Labrecque DR, Schmidt WN, Stapleton JT. GB virus type C NS5A sequence polymorphisms: association with interferon susceptibility and inhibition of PKR-mediated eIF2α phosphorylation. J Interferon Cytokine Res. 2005;25:261–270. doi: 10.1089/jir.2005.25.261. [DOI] [PubMed] [Google Scholar]
- 47.Geonnotti AR, Bilska M, Yuan X, Ochsenbauer C, Edmonds TG, Kappes JC, Liao HX, Haynes BF, Montefiori DC. Differential inhibition of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and TZM-bl cells by endotoxin-mediated chemokine and gamma interferon production. AIDS Res Hum Retroviruses. 2010;26:279–291. doi: 10.1089/aid.2009.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polonis VR, Schuitemaker H, Bunnik EM, Brown BK, Scarlatti G. Impact of host cell variation on the neutralization of HIV-1 in vitro. Curr Opin HIV AIDS. 2009;4:400–407. doi: 10.1097/COH.0b013e32832edc50. [DOI] [PubMed] [Google Scholar]
- 49.Fenyö EM, Heath A, Dispinseri S, Holmes H, Lusso P, Zolla-Pazner S, Donners H, Heyndrickx L, Alcami J, Bongertz V, et al. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One. 2009;4:e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mascola JR, D’Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polonis VR, Brown BK, Rosa Borges A, Zolla-Pazner S, Dimitrov DS, Zhang M-Y, Barnett SW, Ruprecht RM, Scarlatti G, Fenyö E-M, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Tacke M, Schmolke S, Schlueter V, Sauleda S, Esteban JI, Tanaka E, Kiyosawa K, Alter HJ, Schmitt U, Hess G, et al. Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of hepatitis G virus exposure among healthy blood donors. Hepatology. 1997;26:1626–1633. doi: 10.1002/hep.510260635. [DOI] [PubMed] [Google Scholar]
- 53.Linnen J, Wages J, Jr, Zhang-Keck ZY, Fry KE, Krawczynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 54.Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 55.Menendez A, Chow KC, Pan OCC, Scott JK. Human immunodeficiency virus type 1-neutralizing monoclonal antibody 2F5 is multi-specific for sequences flanking the DKW core epitope. J Mol Biol. 2004;338:311–327. doi: 10.1016/j.jmb.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 56.Huarte N, Lorizate M, Kunert R, Nieva JL. Lipid modulation of membrane-bound epitope recognition and blocking by HIV-1 neutralizing antibodies. FEBS Lett. 2008;582:3798–3804. doi: 10.1016/j.febslet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Barnes A, Allen JB, Klinzman D, Wang Z, Chaloner K, Stapleton JT. GBV-C persistence does not require CD4+ T cell preservation, and GBV-C viral load (VL) is weakly inversely related to HIV VL. Fourth IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, New South Wales, Australia. July 22–27, 2007.2007. [Google Scholar]
- 58.Galéa P, le Contel C, Coutton C, Chermann JC. Rationale for a vaccine using cellular-derived epitope presented by HIV isolates. Vaccine. 1999;17:1700–1705. doi: 10.1016/s0264-410x(98)00432-0. [DOI] [PubMed] [Google Scholar]
- 59.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sei Y, Tsang PH, Roboz JP, Sarin PS, Wallace JI, Bekesi JG. Neutralizing antibodies as a prognostic indicator in the progression of acquired immune deficiency syndrome (AIDS)-related disorders: a double-blind study. J Clin Immunol. 1988;8:464–472. doi: 10.1007/BF00916952. [DOI] [PubMed] [Google Scholar]
- 61.Lopalco L, Barassi C, Pastori C, Longhi R, Burastero SE, Tambussi G, Mazzotta F, Lazzarin A, Clerici M, Siccardi AG. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 In vitro. J Immunol. 2000;164:3426–3433. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- 62.Hasselrot K, Bratt G, Hirbod T, Säberg P, Ehnlund M, Lopalco L, Sandström E, Broliden K. Orally exposed uninfected individuals have systemic anti-HIV responses associating with partners’ viral load. AIDS. 2010;24:35–43. doi: 10.1097/QAD.0b013e3283329853. [DOI] [PubMed] [Google Scholar]
- 63.Hasselrot K, Säberg P, Hirbod T, Söderlund J, Ehnlund M, Bratt G, Sandström E, Broliden K. Oral HIV-exposure elicits mucosal HIV-neutralizing antibodies in uninfected men who have sex with men. AIDS. 2009;23:329–333. doi: 10.1097/QAD.0b013e32831f924c. [DOI] [PubMed] [Google Scholar]
- 64.Mazzoli S, Lopalco L, Salvi A, Trabattoni D, Lo Caputo S, Semplici F, Biasin M, Bl C, Cosma A, Pastori C, et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999;180:871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 65.Heuft HG, Berg T, Schreier E, Künkel U, Tacke M, Schwella N, Hopf U, Salama A, Huhn D. Epidemiological and clinical aspects of hepatitis G virus infection in blood donors and immunocompromised recipients of HGV-contaminated blood. Vox Sang. 1998;74:161–167. [PubMed] [Google Scholar]
- 66.Pilot-Matias TJ, Carrick RJ, Coleman PF, Leary TP, Surowy TK, Simons JN, Muerhoff AS, Buijk SL, Chalmers ML, Dawson GJ, et al. Expression of the GB virus C E2 glycoprotein using the Semliki Forest virus vector system and its utility as a serologic marker. Virology. 1996;225:282–292. doi: 10.1006/viro.1996.0602. [DOI] [PubMed] [Google Scholar]
- 67.Blankson JN, Klinzman D, Astemborski J, Thomas DL, Kirk GD, Stapleton JT. Low frequency of GB virus C viremia in a cohort of HIV-1–infected elite suppressors. AIDS. 2008;22:2398–2400. doi: 10.1097/QAD.0b013e328316c3fb. [DOI] [PubMed] [Google Scholar]


