Abstract
Purpose.
Phagocytized melanosomes in ARPE-19 cells were previously shown to decrease susceptibility to oxidative stress induced by hydrogen peroxide treatment and increase stress due to light irradiation relative to cells containing control black latex beads. Here we asked whether differential expression of antioxidant enzymes in cells containing pigment granules could explain the outcomes.
Methods.
ARPE-19 cells were loaded by phagocytosis with porcine RPE melanosomes or black latex beads (control particles). Heme oxygenase-1 (HO-1), HO-2, glutathione peroxidase (GPx), and catalase were quantified by Western blot analysis before and after treatment with sublethal hydrogen peroxide or blue light (400–450 nm). The stress was confirmed as sublethal by cell survival analysis using real-time quantification of propidium iodide fluorescence.
Results.
Phagocytosis itself produced transient changes in protein levels of some antioxidant enzymes, but steady-state levels (7 days after phagocytosis) did not differ in cells containing melanosomes versus beads. Sublethal stress, induced by either hydrogen peroxide or light, had no effect on catalase or HO-2 in either particle-free or particle-loaded cells. In contrast, HO-1 protein was upregulated by treatment with both hydrogen peroxide and light. Particle content did not affect the HO-1 increase induced by hydrogen peroxide, but the increase induced by blue light irradiation was partially blocked in cells containing black beads and blocked even more in cells containing melanosomes.
Conclusions.
The results do not implicate differential antioxidant enzyme levels in stress protection by melanosomes against hydrogen peroxide, but they suggest a multifaceted role for melanosomes in regulating light stress susceptibility in RPE cells.
In this study, we compared ARPE-19 cells containing phagocytized melanosomes or control particles (latex beads) to address questions relating to hydrogen peroxide and light-induced stress and the effect of melanosomes on protein expression of antioxidant enzymes.
Introduction
Phagocytized porcine melanosomes were previously shown to protect ARPE-19 cells from oxidative stress induced by treatment with H2O2.1 Phagocytized latex beads, used as a phagocytosis control in the oxidative stress experiments, also conferred a detectable but smaller cytoprotection. To investigate the mechanism underlying the protective effect conferred by melanosomes, subsequent studies were performed to ask whether iron binding by melanin pigments could contribute.2 The rationale for this question came from observations on the pigment melanin made in model systems, which show that melanin is competent to bind divalent metal ions, including iron.3–8 Theoretically, therefore, pigment granules could reduce iron's availability to act as a cofactor in the Fenton reaction that generates the highly reactive hydroxyl radical from H2O2.9 To perform this function, pigment granules inside cells must retain the capacity to bind iron, a property that was recently demonstrated.2
Although melanosomes phagocytized by ARPE-19 cells are competent to bind iron, it is unclear whether this property plays a direct role in cytoprotection against H2O2-induced stress because granules loaded with different levels of bound iron produced similar outcomes in oxidative stress assays.2 Iron-loaded melanosomes nonetheless had an interesting secondary effect: they induced increased levels of the iron storage protein ferritin, which was used as a reporter for iron release into the cytosol.10–13 This observation not only implies a broader role for pigment granules in regulating cellular iron homeostasis, but it also raises the possibility that cells containing pigment granules may differ in expression levels of other iron-sensitive proteins aside from ferritin. Of possible importance to understanding how pigment granules may protect against H2O2-induced stress is the antioxidant enzyme heme oxygenase-1 (HO-1). Like the gene for ferritin,14–17 the HO-1 gene contains iron-responsive elements, making HO-1 expression iron sensitive.18–21 HO-1 expression is also sensitive to H2O2,22–27 and H2O2 may be generated during phagocytosis,28–32 raising the possibility that cells that had recently phagocytized particles may have higher levels of HO-1. Further, HO-1 can protect cells against H2O2-induced stress.25,33–36 Taken together, these observations suggest that ARPE-19 cells containing phagocytized melanosomes may differ in H2O2-induced stress susceptibility in part because of differences in expression levels of antioxidant enzymes, notably HO-1.
Here we compared ARPE-19 cells containing phagocytized melanosomes or control particles (latex beads) to address questions relating to hydrogen peroxide–induced stress and the effect of melanosomes on protein expression of antioxidant enzymes, focusing on HO-1. Also analyzed were catalase and glutathione peroxidase-1 (GPx-1), enzymes that are highly expressed in the RPE37–39 and known to be upregulated by38,40,41 or to protect against42,43 exposure to hydrogen peroxide or to sublethal blue light. Blue light was also used as a source of stress because light stress is highly relevant for the RPE44–46 and melanosomes are believed to play a role in determining susceptibility to photic damage. The role is complex, however, and could include exacerbating photo-damage due to melanin's ability to photo-generate potentially damaging species, including hydrogen peroxide.10,47,48 We previously observed a small increase, rather than the expected decrease, in light-induced cytotoxicity in ARPE-19 cells containing phagocytized melanosomes when compared with cells containing black latex beads, which have a similar ability to act as an optical screen.10 We attributed the higher cytotoxicity in cells containing melanosomes to the photoreactive properties of the melanin pigment. Here we asked whether differences in protective antioxidant enzymes in cells containing melanosomes versus beads could also contribute to the differential photic stress susceptibility.
The outcomes of our work show transient increases after particle phagocytosis in some enzyme proteins, notably HO-1, and the increases were particle specific, greater for melanosomes than for beads. Treatment with hydrogen peroxide also induced increases in HO-1 in cells containing particles; the increases were particle specific in that cells containing melanosomes exhibited greater survival than those with beads and were therefore competent to upregulate HO-1 at higher concentrations of the oxidant. The most provocative outcome, however, was for sublethal blue light stress. Light exposure increased HO-1 and GPx, but in cells containing particles, especially melanosomes, the increases were blocked. This observation suggests that the protection against light stress resulting from the ability of pigment granules to optically screen may have a competing negative effect: blocking the light-induced upregulation of other stress-protective mechanisms, such as antioxidant enzymes.
Materials and Methods
Cell Cultures
Cells from the human RPE line ARPE-19 (American Type Culture Collection, Rockville, MD) were maintained with twice-weekly feedings with minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) and antibiotics (penicillin 50 U/mL, streptomycin 50 U/mL, amphotericin B 2.5 μg/mL, and gentamicin 50 μg/mL; Gibco, Grand Island, NY). For experiments, cells were plated in 96-well plates at a density of 1 × 105 cells/cm2 and maintained for various intervals before phagocytosis and stress induction as described below.
Particle Phagocytosis
For phagocytosis, confluent ARPE-19 cultures at 7 days after plating were fed black latex beads (l μm, Molecular Probes, Eugene, OR) or porcine melanosomes isolated as previously described.41 Particles of both types were delivered to cultures in complete culture medium (MEM with FBS) using 7.5 × 107 particles per cm2 of culture substrate. Particle uptake proceeded for 24 hours, followed by re-feeding with fresh medium as previously described.1 Seven days after phagocytosis, cell counts using a Burker chamber (Danlab, Helsinki, Finland) were performed on triplicate culture wells lacking particles or containing either phagocytized latex beads or melanosomes. Data were expressed as the mean cell density per cm2 of culture substrate. Three independent experiments were performed.
Oxidative Stress Induction and Cell Survival Analysis
Oxidative stress was induced by either oxidant treatment or blue light irradiation using ARPE-19 cultures 7 days after particle uptake using previously described protocols.2 For oxidant-induced stress, cultures were treated with a range of concentrations of freshly prepared H2O2 (0–400 μM) in Hank's balanced salt solution with calcium and magnesium ions (Invitrogen, Carlsbad, CA) using the pulse delivery method that was previously described.1 For light-induced stress, cultures in complete culture medium were irradiated for intervals to 60 minutes by published methods46 using a ThermoOriel Solar Simulator (Pittsfield, MA) outfitted with a 1000-W xenon lamp (Newport Corporation, Stratford, CT), a 300- to 450-nm dichroic mirror (Newport), and an additional ultraviolet cutoff filter (390 nm; Newport).
To quantify cell survival after H2O2 treatment, a previously described real-time assay was used.1 Briefly, the fluorescent dye propidium iodide (PI; final concentration 100 μM) was added to culture medium at the time of oxidant addition, and PI fluorescence (555/617 nm excitation/emission) was measured at 10-minute intervals over 24 hours using a Biotek Synergy H4 plate reader (ThermoScientific, Palo Alto, CA). Triplicate culture wells were used for each oxidant concentration and all experimental groups were in the same culture plate. PI fluorescence intensity (in arbitrary units) was plotted versus time. Time of cell death onset and slopes of lines in the linear portions of the curves showing cell death rates were compared and analyzed for statistical significance using Graph Pad Prism 5 slope analysis (GraphPad Software, Inc., La Jolla, CA).
Cell survival following blue light treatment was quantified by the same assay as for H2O2 treatment by adding PI to the culture medium immediately after light irradiation followed by quantification of fluorescence over 24 hours. Additionally, light-treated cultures were examined by microscopy and paired fluorescence-phase images were captured of the living cultures at 24 hours using an inverted fluorescence microscope to visualize PI-positive nuclei.
Western Blotting for Antioxidant Enzyme Proteins
For Western blot analysis of antioxidant proteins, extracts of ARPE-19 cultures were collected directly in SDS-containing electrophoresis buffer supplemented with protease inhibitors by published protocols.3 Protein was quantified by the Bradford dye method (Bio-Rad Laboratories, Hercules, CA) and protein-equivalent samples from replicate cultures were loaded onto gels and separated by electrophoresis under reducing conditions using 15% SDS separating gels. Proteins were electroblotted and probed with the following primary antibodies: rabbit polyclonal antibodies to HO-1 (1:2000 dilution) or HO-2 (1:1000 dilution), both from Enzo Life Science (New York, NY); mouse polyclonal antibody to GPx (1:8000 dilution) or rabbit polyclonal antibody to catalase (1:10,000 dilution), both from Abcam (Cambridge, MA). Bands were visualized using LI-COR secondary antibodies and quantified by densitometry using the LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Results
Effects of Time in Culture and Phagocytosis on Antioxidant Enzymes in ARPE-19 Cells
The protective effect of phagocytized melanosomes in ARPE-19 cells subjected to H2O2 treatment was previously shown using cultures at several times after plating between 1 and 5 days.1 Here, in experiments to determine whether melanosome content and oxidative stress affected antioxidant enzyme protein expression, we sought to use consistent culture timing with the intent of maximizing our ability to detect small expression differences. Preliminary experiments were therefore conducted using quantitative densitometry of Western blots of samples from particle-free ARPE-19 cells to determine whether time postplating affected enzyme protein levels (Fig. 1). HO-1, HO-2, and catalase protein did not differ with time in culture; 1 and 7 days postplating are shown (Figs. 1A–C). However, GPx showed a time-dependent increase to 7 days (Fig. 1D) and plateauing thereafter (not shown). For subsequent experiments involving introduction of particles by phagocytosis, cultures at 7 days postplating were therefore used, a time when steady-state levels of enzyme proteins had been achieved.
Figure 1. .

Antioxidant enzyme proteins as a function of time postplating. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells prepared on days 1 or 7 postplating. For GPx, extracts were also prepared on day 3. Data are the mean band densities (error bars indicate SD), from triplicate cultures within a representative experiment, expressed in arbitrary units (a.u.) and shown as a percentage of the band density from day 1 cultures. For GPx, outcomes for days 3 and 7 differ from those for day 1 (asterisks; t-test analysis, P < 0.001).
To determine whether the process of particle phagocytosis affected antioxidant enzyme protein levels in ARPE-19 cells, cultures were given either control black latex beads or melanosomes for phagocytic uptake at 7 days postplating and extracts of cells without or with internalized particles were analyzed by Western blot analysis 24 hours later (Fig. 2). Phagocytosis did not affect protein levels of HO-2 (Fig. 2B) or catalase (Fig. 2C). However, phagocytosis induced increases in HO-1 (Fig. 2A) and GPx (Fig. 2D), and the increases differed with type of particle ingested: enzyme protein levels were consistently higher in cells that had internalized melanosomes as compared to latex beads. The differences in HO-1 and GPx protein following uptake of melanosomes versus beads were transient. By 7 days after phagocytosis, enzyme protein levels did not differ with particle type (Fig. 3), indicating that the presence of melanosomes within ARPE-19 cells did not produce a constitutively different antioxidant enzyme protein expression pattern as compared with particle-free cells or cells containing nonbiologic particles (latex beads).
Figure 2. .

Antioxidant enzyme proteins after phagocytosis. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells prepared 24 hours after the onset of phagocytosis of black latex beads (lx, hatched bars) or melanosomes (ms, black bars). Data are the mean band densities (error bars indicate SD) from triplicate cultures within a representative experiment, expressed in a.u. and shown as a percentage of the band density from paired control cultures that did not undergo phagocytosis (-, open bars). Single asterisks indicate significantly higher than control cultures; double asterisks indicate ms higher than lx (t-test analysis, P < 0.05).
Figure 3. .

Constitutive levels of antioxidant proteins in cells lacking particles or containing phagocytized latex beads or melanosomes. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells prepared 7 days after the onset of phagocytosis of black latex beads (lx, hatched bars) or melanosomes (ms, black bars), and cells that did not undergo phagocytosis and therefore contain no particles (-, open bars). Data are the mean band densities (error bars indicate SD), from triplicate cultures within a representative experiment, expressed in a.u. with the results for the lx and ms groups shown as a percentage of the control cultures that did not undergo phagocytosis (-). For all enzymes, results did not differ with type of ingested particle (t-test analysis).
Hydrogen Peroxide–Induced Stress and Antioxidant Enzyme Proteins in ARPE-19 Cells Containing Phagocytized Particles
Because phagocytosis produces a particle-specific but transient difference in levels of some antioxidant enzymes, the early post-phagocytosis interval was avoided in experiments to determine whether melanosomes have a granule-specific effect on antioxidant enzyme content in ARPE-19 cells subjected H2O2-induced stress. Experiments involving H2O2 treatment were therefore performed 7 days after particle uptake (14 days postplating) when baseline levels of enzymes were similar in cells regardless of particle content. Cultures of cells with no particles were not used as a control in these stress protocols because cell counts indicated that at 7 days post phagocytosis, cultures lacking phagocytized particles achieve a higher cell density (3.9 × 105 ± 0.37 cells/cm2) than cultures containing either latex beads (2.7 × 105 ± 0.27 cells/cm2) or melanosomes (2.6 × 105 ± 0.28 cells/cm2), which do not differ from one another (data are the mean ± SDs of triplicate culture wells per group in a representative experiment). Because RPE cell density is a known determinant of susceptibility to lethal stress,49–51 cells with no particles were not used in analyses involving H2O2-induced oxidative stress; cells containing melanosomes were compared with control cultures of cells containing latex beads.
The intent of the experiments involving H2O2-induced oxidative stress was to determine whether differential upregulation of antioxidant enzymes could be a contributing mechanism to the previously observed stress protection conferred by melanosomes.2 Experiments were therefore performed to confirm that stress protection persisted at the later time-in-culture interval (7 days post phagocytosis). Using kinetic analysis of PI fluorescence following H2O2 treatment, stress protection of ARPE-19 cells by phagocytized melanosomes relative to beads was confirmed (Fig. 4). Consistent with our previous observations,2 H2O2 concentrations at the borderline of lethality (0.2 mM H2O2 in the experiment shown [Fig. 4A]), produced a detectably greater rate of cell death for cells containing beads as compared to melanosomes. Higher concentrations (0.4 mM) produced very rapid cell death in bead-containing cells, whereas cells containing melanosomes were largely spared (Fig. 4B). At the higher oxidant dose (0.4 mM), the time of onset of cell death was earlier for cells containing beads (4.5 hours) than melanosomes (7.0 hours), and the slope of the lines thereafter describing the rate of cell death also differed with particle content. Time of cell death onset and slope of the line describing rate of death were previously shown to be sensitive measures of cytotoxicity using this real-time assay.1
Figure 4. .

Dynamic analysis of PI fluorescence in cells containing phagocytized particles and treated with hydrogen peroxide. ARPE-19 cells were exposed to a single dose of hydrogen peroxide at concentrations of (A) 0.2 mM or (B) 0.4 mM. PI fluorescence, expressed in a.u., was quantified at 10-minute intervals over 24 hours in triplicate wells of ARPE-19 cultures containing phagocytized black latex beads (lx, triangles) or melanosomes (ms, black circles). For the 0.2-mM treatment in (A), the inset shows an expanded y-axis scale bar to illustrate that the curves differ. Mean slopes of lines showing rates of cell death, determined as previously described,1 were 37.5 ± 1.6 (lx) and 16.6 ± 1.5 (ms) for 0.2 mM H2O2, or 566.0 ± 81.7 (lx) and 127.0 ± 6.1(ms) for 0.4 mM H2O2. Slopes differed significantly between particle groups at both oxidant concentrations (Graph Pad Prism 5 slope analysis, P < 0.0001).
Extracts were prepared for Western blot analysis of the oxidant-treated cultures shown in Figure 4 to determine whether there were differential affects on antioxidant enzyme levels in cells containing melanosomes versus beads (Fig. 5). H2O2 treatment of ARPE-19 cells did not affect levels of HO-2, catalase, or GPx, regardless of oxidant dose or content of internalized particles (Figs. 5B–D). Consistent with previous reports,22 H2O2 treatment did increase levels of HO-1 (Fig. 5A); however, there was no differential increase between cultures containing melanosomes as compared with beads in cultures treated with low oxidant doses. At a concentration of 0.2 mM H2O2, which produced little cell death (Fig. 4A), increases in HO-1 in treated cells containing beads and melanosomes were similar. At the higher oxidant dose (0.4 mM), HO-1 levels were sustained in the melanosome-containing cells but reduced in cells containing beads, likely due to the markedly reduced cell survival in the bead-loaded cells (Fig. 4B).
Figure 5. .

Antioxidant enzyme proteins in cells containing phagocytized particles and treated with hydrogen peroxide. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells preloaded by phagocytosis with control black latex beads (lx, hatched bars) or melanosomes (ms, black bars) and either untreated (0) or treated with 0.2 or 0.4 mM H2O2 as described for Figure 4. Extracts were prepared 24 hours after the onset of H2O2 treatment. Data are the mean band densities (error bars indicate SD) from triplicate cultures within a representative experiment, expressed in a.u., with the results for the ms group shown as a percentage of the lx group for each H2O2 treatment. The two particle groups differ significantly at the higher concentration of H2O2 for HO-1 and GPx (t-test analyses, P < 0.02). (E) Blot used for densitometry to illustrate that the same lanes were used for all enzyme proteins; the blot was cut to probe for individual proteins. The outer lanes show molecular mass markers.
Blue Light–Induced Stress and Antioxidant Enzyme Proteins in ARPE-19 Cells Containing Phagocytized Particles
As for experiments evaluating H2O2-induced stress, ARPE-19 cells at 7 days after particle phagocytosis were subjected to sublethal blue light irradiation to determine whether photic stress triggers changes in antioxidant enzyme levels and whether cells containing melanosomes differ from those containing beads. Kinetic analysis of PI fluorescence after irradiation confirmed that light treatment for 60 minutes was sublethal (Fig. 6); cell death over 24 hours postirradiation was minimal and did not differ for cells lacking particles (Fig. 6A), or containing phagocytized beads (Fig. 6B) or melanosomes (Fig. 6C).
Figure 6. .

Dynamic analysis of PI fluorescence in cells treated with sublethal blue light. ARPE-19 cells lacking particles (A; open circles) or containing phagocytized latex beads (B; triangles) or melanosomes (C; solid circles) were either untreated (black symbols) or blue light irradiated for 60 minutes (blue symbols) as described in the Methods section. PI fluorescence, expressed in a.u., was then quantified at 10-minute intervals over 24 hours in triplicate culture wells. Curves indicate no significant cell death on irradiation and no differences between no-light cultures and +light cultures for any particle group. The lower panels show images of cultures at the end of the experiment (24 hours) showing rare PI-positive nuclei (arrows). Scale bar: 20 μm.
Initial experiments were performed to probe the effects of sublethal light stress on antioxidant enzyme proteins in control, particle-free ARPE-19 cultures. Light stress produced no changes in HO-2 or catalase, but induced increases in both HO-1 and GPx (Fig. 7). For the latter two enzymes, increases in protein levels were shown to be light-dose dependent (Figs. 7A, 7D).
Figure 7. .

Antioxidant enzyme protein in cells treated with sublethal blue light. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells that were either untreated (no light, black bars) or blue light treated (+light, blue bars) for 60 minutes as for Figure 6. Blue light treatment for intermediate time points (15 and 30 minutes) was also performed to blot for HO-1 and GPx. Extracts were prepared at 24 hours after irradiation. Data are the means (error bars indicate SD) of three replicate cultures with the band density expressed in a.u. and shown as a percentage of the band density in extracts from nonirradiated controls. Single asterisks indicate significant differences for +light cultures relative to the paired no-light cultures (t-test analyses, P < 0.05).
ARPE-19 cultures preloaded by phagocytosis with melanosomes or black latex beads were then subjected to photic stress using 60 minutes of irradiation followed by Western blot analysis for antioxidant enzyme proteins (Fig. 8). As for particle-free cells (Fig. 7), cells containing particles of either type showed no irradiation-induced change in the amounts of HO-2 (Fig. 8B) or catalase (Fig. 8C). In contrast, the blue light–induced increase in HO-1 protein found in particle-free cells was suppressed in cells containing control black latex beads, and suppressed even further in cells containing phagocytized melanosomes (Fig. 8A). A similar particle-specific suppression effect was seen for the light-induced increase in GPx protein, and for this enzyme, the amounts after light treatment in cells containing melanosomes fell below levels in nonirradiated cells (Fig. 8D).
Figure 8. .
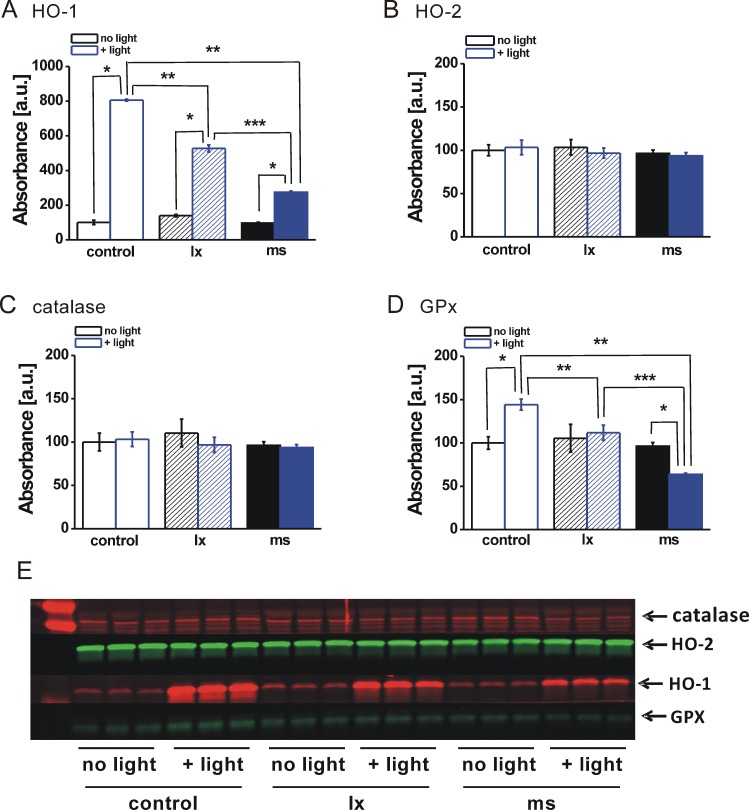
Antioxidant enzyme proteins in cells containing phagocytized particles and treated with sublethal blue light. Densitometric analysis of Western blots for (A) HO-1, (B) HO-2, (C) catalase, or (D) GPx in extracts of ARPE-19 cells lacking particles (control, open bars) or preloaded by phagocytosis with black latex beads (lx, hatched bars) or melanosomes (ms, solid bars) and either not light treated (no light, black bars) or light treated (+light, blue bars) for 60 minutes as for Figure 6. Single asterisks indicate significant differences for +light cultures relative to the paired no-light cultures within particle groups. Double asterisks indicate significant differences for +light groups in cultures containing either lx or ms relative to particle-free controls. Triple asterisks indicate significant difference for +light group cultures containing lx versus ms (t-test analyses, P < 0.05). (E) Blot used for densitometry to illustrate that the same lanes were used for all enzyme proteins; the blot was cut to probe for individual proteins. The left lane shows molecular mass markers.
Discussion
We previously showed that phagocytized melanosomes in ARPE-19 cells decrease the cytotoxic effects of oxidative stress induced by treatment with H2O21 and slightly increase the cytotoxic effects of stress induced by light treatment46 when compared with cells containing control phagocytized black latex beads. The mechanisms underlying these observations have not been established. Here we asked whether the presence of melanosomes within ARPE-19 cells differentially modulates levels of antioxidant enzymes that contribute to stress protection, especially HO-1. After controlling for the transient effects of phagocytosis itself on antioxidant enzyme protein expression, melanosomes were not shown to differentially modulate antioxidant enzymes in a way that could explain cytoprotection against oxidant-induced stress. However, melanosomes did modulate protein levels of HO-1 and GPx after photic stress in a direction that would predict greater toxicity; melanosomes suppressed the light-induced upregulation of the antioxidant enzymes to a greater extent than could be explained only by the pigment's ability to act as an optical screen. This observation illustrates the complexity of the role that melanosomes may play in regulating RPE susceptibility to oxidative stress, especially stress induced by light.
To generate an assay system that would detect what we predicted would be a small modulatory effect of phagocytized granules on antioxidant enzymes in ARPE-19 cells, we conducted multiple preliminary experiments to establish consistent baseline levels of the enzymes in the cultures. This led to the observation that time in culture affects protein expression of GPx, but not the other enzymes examined here. GPx has been studied in the past in ARPE-19 cells,37,42 but a dependence of enzyme levels on time postconfluency has not been reported. The observation here emphasizes the importance of controlling culture conditions when evaluating antioxidant enzymes and stress susceptibility in ARPE-19 cells, and perhaps in other RPE cell culture models as well.
Because melanosomes and control particles (latex beads) were introduced into ARPE-19 cells by phagocytosis, the effects of particle uptake on antioxidant enzyme proteins were also evaluated. Our intent was to control for the effects of phagocytosis, but the observation that particle uptake, both of melanosomes and of beads, induced a transient upregulation of HO-1and GPx may have biological relevance for the RPE in situ. A major ongoing function of the RPE within eyes is the phagocytosis of photoreceptor outer segments in the process of photoreceptor renewal.52,53 Given the high oxidative stress environment in which the RPE resides,2,54 including the potential for stress induced by internalization of peroxidized outer segment membranes,53,55 upregulation of antioxidant enzymes on phagocytosis could have protective benefits for the tissue. The mediators of the phagocytosis-induced upregulation observed here have not been established, but H2O2 is one possible candidate. H2O2 is reportedly produced during phagocytosis,28–30 and H2O2 is known to induce increased HO-122–27; this mechanism could therefore help explain increases in HO-1 following uptake of both types of particles. However, melanosome uptake induced greater increases in HO-1 than uptake of beads, which implies additional properties of the biological granule. One relevant property of melanosomes is iron binding and release. We previously observed that phagocytosis of melanosomes by ARPE-19 cells produces an upregulation of the protein ferritin that was in proportion to the iron content of the granules.2 We interpreted this outcome to indicate that release of iron from phagocytized granules into the cytosol could trigger upregulation of the iron-sensitive protein ferritin.2 Because HO-1 production is also iron sensitive,18 iron release from melanosomes could also underlie increases in this antioxidant enzyme after granule internalization.
Although phagocytosis could induce transient upregulation of some antioxidant enzymes in ARPE-19 cells, sustained higher levels of protective enzymes were not found in cells containing melanosomes as compared with beads that could explain the greater resistance of melanosome-containing cells to subsequent H2O2-induced stress.2 Similarly, changes in antioxidant enzyme levels following H2O2 treatment did not differ with particle type. Sublethal H2O2 treatment is known to induce increases in HO-122,56 and decreases in GPx,57 outcomes that were also obtained here for ARPE-19 cells containing phagocytized particles of both types. Only when doses of oxidant were high enough to produce differential cell death did melanosome-containing cells appear to have higher HO-1 than cells containing beads. However, the higher levels were likely a consequence of greater survival rather than a contributing cause of the stress protection conferred by melanosomes.
As for oxidant-induced stress, stress induced by light irradiation is also known to induce differential expression of antioxidant enzymes. Consistent with our observation, sublethal blue light treatment was shown to upregulate HO-1 in ARPE-19 cells,45 and more recently light damage in mouse eyes was shown to induce expression of the genes for HO-1 and GPx without affecting catalase transcript abundance.37 Similar outcomes were obtained here for antioxidant enzymes in light-irradiated ARPE-19 cells, supporting the validity of the culture model; after treatment of ARPE-19 cells with light doses that were confirmed to be sublethal, protein levels of HO-1 and GPx increased while catalase was unaffected. The increases in HO-1 and GPx were, however, blocked in cells containing black latex beads and blocked even more in cells containing phagocytized melanosomes. The blockage by black beads confirms a role for optical screening, but the consistently greater effect of melanosomes indicates that additional functions of pigment granules also contributed. One relevant function is the ability of melanin to act as an antioxidant by scavenging reactive oxygen species (ROS), including superoxide.3 It has been difficult to demonstrate that RPE melanin within cells explicitly performs an antioxidant function when light is the stressor, because light irradiation of melanin can have a competing pro-oxidizing effect resulting from the generation superoxide anions47 and additional ROS via interaction with mitochondrial cytochromes and flavin oxidases.58,59 A higher ROS environment resulting from irradiation of melanin would be expected to trigger greater HO-1 upregulation, which is a response to many forms of stress.18–21,60 However, there was apparently a reduced stimulus for HO-1 upregulation here, which argues for an ability of the melanosome to act as an antioxidant under conditions of light stress. The counterintuitive consequence for cells of this indirect effect of melanosomes would be a slightly diminished protection against light stress as compared with cells containing particles that can also absorb light (e.g., black beads) but that lack antioxidant properties. We have in fact previously observed this phenomenon of slightly greater phototoxicity for cells containing melanosomes when light absorbance was well controlled for by comparing to cells containing black beads.46
The potential biological ramifications of the observations made here indicating that melanosomes can modulate antioxidant enzyme proteins HO-1 and GPx under conditions of sublethal light stress are difficult to predict. Oxidative stress to the RPE, including photic stress, is believed to contribute to age-related macular degeneration.46,61 The role of the melanosomes in regulating RPE cell stress susceptibility may be significantly underappreciated and extend well beyond their accepted function of photoprotection by light absorbance. Further, age-related changes in melanosomes, including iron loading, photo-oxidation of melanin, and fusion with lipofuscin granules,62,63 may affect the complex biological functions of the granules, altering their ability to aid in stress protection.
Footnotes
Supported by research Grants R01EY019664 and P30EY01931 from the National Eye Institute (JMB), Grants 2011/03/N/NZ3/03854 (AP) and 2661/B/P01/2010/39 (TS) from the Poland National Science Center, an unrestricted grant from Research to Prevent Blindness, Inc., and donors from Milwaukee, Wisconsin, supporting macular degeneration research. This investigation was conducted in a facility constructed with support from National Center for Research Resources Grant C06 RR-RR016511 from the National Institutes of Health.
Disclosure: A. Pilat, None; A.M. Herrnreiter, None; C.M.B. Skumatz, None; T. Sarna, None; J.M. Burke, None
References
- 1. Burke JM, Kaczara P, Skumatz CMB, Zareba M, Raciti MW, Sarna T. Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol Vis. 2011; 17: 2864–2877 [PMC free article] [PubMed] [Google Scholar]
- 2. Kaczara P, Zaręba M, Herrnreiter A, et al. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012; 25: 804–814 [DOI] [PubMed] [Google Scholar]
- 3. Różanowski B, Burke JM, Boulton ME, Sarna T, Różanowska M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Invest Ophthalmol Vis Sci. 2008; 49: 2838–2847 [DOI] [PubMed] [Google Scholar]
- 4. Hong L, Simon JD. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J Phys Chem B. 2007; 111: 7938–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szpoganicz B, Gidanian S, Kong P, Farmer P. Metal binding by melanins: studies of colloidal dihydroxyindole-melanin, and its complexation by Cu(II) and Fe(II) ions. J Inorg Biochem. 2002; 89: 45–53 [DOI] [PubMed] [Google Scholar]
- 6. Enochs WS, Sarna T, Zecca L, Riley PA, Swartz HM. The roles of neuromelanin, binding of metal ions, and oxidative cytotoxicity in pathogenesis of Parkinson's disease: a hypothesis. J Neural Transm. 1994; 4: 83–100 [DOI] [PubMed] [Google Scholar]
- 7. Pilas B, Sarna T, Kalyanaraman B, Swartz HM. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic Biol Med. 1988; 4: 285–293 [DOI] [PubMed] [Google Scholar]
- 8. Sarna T, Hyde JS, Swartz HM. Ion-exchange in melanin: an electron spin resonance study with lanthanide probes. Science. 1976; 192: 1132–1134 [DOI] [PubMed] [Google Scholar]
- 9. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. New York: Oxford University Press; 1999. [Google Scholar]
- 10. Zareba M, Sarna T, Szewczyk G, Burke JM. Photobleaching of the melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress. Photochem Photobiol. 2007; 83: 925–930 [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2001; 434: 365–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood J. 2009; 114: 4546–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008; 320: 1207–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int J Biochem Cell Biol. 1999; 31: 1139–1152 [DOI] [PubMed] [Google Scholar]
- 15. Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelatation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol. 2005; 25: 2282–2288 [DOI] [PubMed] [Google Scholar]
- 16. Beaumont C, Leneuve P, Devaux I, et al. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995; 11: 444–446 [DOI] [PubMed] [Google Scholar]
- 17. Regan RF, Li Z, Chen M, Zhang X, Chen-Roetling J. Iron regulatory proteins increase neuronal vulnerability to hydrogen peroxide. Biochem Biophys Res Commun. 2008; 375: 6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzales S, Erario MA, Tomara ML. Heme oxygenase-1 induction and dependent increase in ferritin. Dev Neurosci. 2002; 24: 161–168 [DOI] [PubMed] [Google Scholar]
- 19. Schipper HM. Heme oxygenase-1: transducer of pathological brain iron sequestration under oxidative stress. Ann N Y Acad Sci. 2004; 1012: 84–93 [DOI] [PubMed] [Google Scholar]
- 20. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997; 94: 10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balla J, Vercellotti GM, Jeney V, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antiox Redox Signal. 2007; 9: 2119–2137 [DOI] [PubMed] [Google Scholar]
- 22. Alizadeh M, Wada M, Gelfman CM, Handa JT, Hjelmeland LM. Downregulation of differentiation specific gene expression by oxidative stress in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2001; 42: 2706–2713 [PubMed] [Google Scholar]
- 23. Chang SH, Garcia J, Melendez JA, Kilberg MS, Agarwal A. Haem oxygenase 1 gene induction by glucose deprivation is mediated by reactive oxygen species via the mitochondrial electron-transport chain. Biochem J. 2003; 371: 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cisowski J, Loboda A, Jozkowicz A, Chen S, Agarwal A, Dulak J. Role of heme oxygenase- 1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem Biophys Res Commun. 2005; 326: 670–676 [DOI] [PubMed] [Google Scholar]
- 25. Kim YS, Zhuang H, Koehler RC, Dore S. Distinct protective mechanisms of HO-1 and HO-2 against hydroperoxide-induced cytotoxicity. Free Radic Biol Med. 2005; 38: 85–92 [DOI] [PubMed] [Google Scholar]
- 26. Hori R, Kashiba M, Toma T, et al. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002; 277: 10712–10718 [DOI] [PubMed] [Google Scholar]
- 27. Otterbein LE, Choi AMK. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000; 279: L1029–L1037 [DOI] [PubMed] [Google Scholar]
- 28. Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994; 214: 242–249 [DOI] [PubMed] [Google Scholar]
- 29. Wu WC, Hu DN, Gao HX, et al. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Invest Mol Vis. 2010; 16: 1864–1873 [PMC free article] [PubMed] [Google Scholar]
- 30. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122: 598–614 [DOI] [PubMed] [Google Scholar]
- 31. Ballinger SW, van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999; 68: 765–772 [DOI] [PubMed] [Google Scholar]
- 32. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999; 5: 32 [PMC free article] [PubMed] [Google Scholar]
- 33. Ulyanova T, Szel A, Kutty RK, et al. Oxidative stress induces heme oxygenase-1 immunoreactivity in Muller cells of mouse retina in organ culture. Invest Ophthalmol Vis Sci. 2001; 42: 1370–1374 [PubMed] [Google Scholar]
- 34. Speit G, Dennog C, Eichhorn U, Rothfuss A, Kaina B. Induction of heme oxygenase-1 and adaptive protection the induction of DNA damage after hyperbaric oxygen treatment. Carcinogenesis. 2000; 21: 1795–1799 [DOI] [PubMed] [Google Scholar]
- 35. Lin Q, Weis S, Yang G, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007; 282: 20621–20663 [DOI] [PubMed] [Google Scholar]
- 36. Woo JM, Shin DY, Lee SJ, et al. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen species. Mol Vis. 2012; 18: 901–908 [PMC free article] [PubMed] [Google Scholar]
- 37. Hadziahmetovic M, Kumar U, Song Y, et al. Microarray analysis of murine retinal light damage reveals changes in iron regulatory, complement, and antioxidant genes in the neurosensory retina and isolated RPE. Invest Ophthalmol Vis Sci. 2012; 53: 5231–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng Y, Liu Y, Ge J, et al. Resveratrol protects human lens epithelial cell against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis. 2010; 16: 1467–1474 [PMC free article] [PubMed] [Google Scholar]
- 39. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999; 127: 694–709 [DOI] [PubMed] [Google Scholar]
- 40. Haque R, Chun E, Howell JC, Sengupta T, Chen D, Kim H. MicroRNA −30-b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS ONE. 2012; 7: e42542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tate DJ, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995; 36: 1271–1279 [PubMed] [Google Scholar]
- 42. Ohira A, Tanito M, Kaidzu S, Kondo T. Glutathione peroxidase induced in rat retinas to counteract photic injury. Invest Ophthalmol Vis Sci. 2003; 44: 1230–1236 [DOI] [PubMed] [Google Scholar]
- 43. Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VE. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007; 85: 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Różanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light induce-reactivity of retinal age pigment. J Biol Chem. 1995; 270: 18825–18830 [DOI] [PubMed] [Google Scholar]
- 45. Roehlecke C, Schaller A, Knels L, Funk RH. The influence of sublethal blue light exposure on human RPR cells. Mol Vis. 2009; 15: 1929–1938 [PMC free article] [PubMed] [Google Scholar]
- 46. Burke JM, Zareba M. Sublethal photic stress and the motility of RPE phagosomes and melanosomes. Invest Ophthalmol Vis Sci. 2009; 50: 1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Różanowska M, Korytowski W, Różanowski B, et al. Photoreactivity of aged human melanosomes: a comparison with lipofuscin. Invest Ophthalmol Vis Sci. 2002; 43: 2088–2096 [PubMed] [Google Scholar]
- 48. Zadlo A, Burke JM, Sarna T. Effect of untreated and photobleached bovine RPE melanosomes on the photoinduced peroxidation of lipids. Photochem Photobiol Sci. 2009; 8: 830–837 [DOI] [PubMed] [Google Scholar]
- 49. Zareba M, Raciti MW, Henry MM, Sarna T, Burke JM. Oxidative stress in ARPE-19 cultures: do melanosomes confer cytoprotection? Free Radic Biol Med. 2006; 40: 87–100 [DOI] [PubMed] [Google Scholar]
- 50. Wada M, Gelfman CM, Matsunaga H, et al. Density-dependent expression of FGF-2 in response to oxidative stress in RPE cells in vitro. Curr Eye Res. 2001; 23: 226–231 [DOI] [PubMed] [Google Scholar]
- 51. Garg TK, Chang JY. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ2. BMC Ophthalmol. 2003; 3: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiol. 2010; 25: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krohne TU, Holz FG, Kopitz J. Apical-to-basolateral transcytosis of photoreceptor outer segments induced by lipid peroxidation products in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 553–560 [DOI] [PubMed] [Google Scholar]
- 54. Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006; 51: 461–481 [DOI] [PubMed] [Google Scholar]
- 55. Sun M, Finnemann SC, Febbraio M, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium. J Biol Chem. 2005; 281: 4222–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45: 675–684 [DOI] [PubMed] [Google Scholar]
- 57. Espinoza SE, Guo H, Fedarko N, et al. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 2008; 63: 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005; 280: 21061–21066 [DOI] [PubMed] [Google Scholar]
- 59. le Bras M, Clement MV, Pervaiz S, Brenner C. Reactive oxygen species and mitochondrial signaling pathway of cell death. Histol Histopathol. 2005; 20: 205–220 [DOI] [PubMed] [Google Scholar]
- 60. Honda S, Hjelmeland LM, Handa JT. The use of hyperoxia to induce chronic mild oxidative stress in RPE cells in vitro. Mol Vis. 2000; 7: 63–70 [PubMed] [Google Scholar]
- 61. Beatty S, Koh HH, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115–134 [DOI] [PubMed] [Google Scholar]
- 62. Wihlmark U, Wrigstad A, Roberg K, Nilsson SE, Brunk UT. Lipofuscin accumulation in cultured retinal pigment epithelial cells causes enhanced sensitivity to blue light irradiation. Free Radic Biol Med. 1997; 22: 1229–1234 [DOI] [PubMed] [Google Scholar]
- 63. Dontsov AE, Glickman RD, Ostrovsky MA. Retinal pigment epithelium granules stimulate the photo-oxidation of unsaturated fatty acids. Free Radic Biol Med. 1999; 26: 1436–1446 [DOI] [PubMed] [Google Scholar]


