Abstract
It has long been recognized in breast cancer that the effect of hormone receptor (HR) status on recurrence rates varies over time and with the site of recurrence. However, there is relatively little in the literature on the effect of Human Epidermal Growth Factor Receptor 2 (HER2) on recurrence patterns. We wanted to assess whether the effect of HER2 status on the risk of distant recurrence changed over time and/or with HR status, and whether these relationships varied with site of recurrence.
We retrospectively studied 11,011 women diagnosed with stage I, II, or III breast cancer after 1997 who had data on HR status and HER2 status. 20% were HR negative and HER2 negative (so-called “triple-negatives”), 7% were HR negative and HER2 positive, 64% were HR positive and HER2 negative, and 10% were HR positive and HER2 positive.
The estimated overall cumulative incidence of developing distant metastases is 20% at 4 years, 30% at 8 years, and 36% at 12 years. The 12-year cumulative incidence was 23% for bone, 16% for liver, 14% for lung, 13% for distant lymph node, 10% for brain, and 8% for pleura. After adjusting for potential confounding factors, the nature of the effect of HER2 on recurrence rates was found to differ markedly across the sites of recurrence. For brain and pleura recurrences, the effect of HER2 depended on HR status in ways that significantly changed over time. For bone recurrences, the effect of HER2 did not depend on HR status, but did change significantly over time. For liver and distant lymph node recurrences, there was a significant effect of HER2 status that did not change with time or HR status. For lung recurrences, rates did not significantly vary with HER2 status.
Keywords: breast cancer, HER2 status, distant recurrence
Introduction
It has long been recognized in breast cancer that the effect of hormone receptor (HR) status on recurrence is transient in nature [1]. While HR negative patients initially have higher rates of recurrence, this difference diminishes over time. The nature of these changes in the effect of HR on recurrence rates over time varies with the site of recurrence. In addition to HR status, the status of a patient’s Human Epidermal Growth Factor Receptor 2 (HER2) is an important factor in making treatment decisions and assessing prognosis [2–4]. However, there is little in the published literature on the effect of HER2 status on recurrence patterns. We wanted to assess whether the effect of HER2 status in primary tumors on the risk of distant recurrence changed over time, whether the effect of HER2 status changed with HR status, and whether these relationships varied with site of recurrence.
The her2 gene is amplified in 20% of invasive breast cancers, and it is associated with decreased disease-free and overall survival rates [5, 6]. The introduction of trastuzumab monoclonal antibody therapy in the adjuvant setting changed the natural history of HER2-positive breast cancer [7–9]. Adjuvant trastuzumab-based chemotherapy has been shown to decrease the risk of distant metastasis by half and improve overall survival compared with chemotherapy alone in HER2-positive early-stage breast cancer [10–12]. However, 15% of patients will develop distant metastasis despite optimal local therapy and adjuvant trastuzumab-based therapy. Monitoring guidelines from the American Society of Clinical Oncology do not recommend intense monitoring after completion of curative therapy for any specific breast cancer subtype [13]. An improved understanding of patterns and sites of metastases may guide adjuvant sytemic therapy and stimulate investigation of novel surveillance approaches in HER2-positive breast cancer patients.
Material and Methods
Using an IRB-approved protocol, we obtained data on female breast cancer patients with invasive ductal carcinoma from the MDACC Breast Medical Oncology database. There were 12,315 patients diagnosed with stage I, II, or III breast cancer after 1997. Of these, 11011 (89%) had data on HR status and HER2 status and are included in this report. 2150 (20%) were HR negative and HER2 negative (so-called “triple-negatives”), 756 (7%) were HR negative and HER2 positive, 7037 (64%) were HR positive and HER2 negative, and 1068 (10%) were HR positive and HER2 positive.
Of the 11,011 patients studied 4404 (40%) were stage I, 4931 (45%) were stage II, and 1676 (15%) were stage III. 590 (5%) were nuclear grade I, 4256 (39%) were grade II, 5958 (54%)were grade III, and 207 (2%) were missing data on nuclear grade. 6754 (61%) were post-menopausal. The median age was 52 with range from 19 to 98. 7830 (71%) patients were white.
HER2 status was considered positive if positive by FISH or 3+ by IHC. HR status was considered positive if either estrogen receptor status or progesterone receptor status was positive by IHC.
For each of the distant recurrence sites of interest (brain, liver, lung, pleura, bone and distant lymph node), we computed for each patient the time to first development of a distant recurrence as that site and noted if they had not developed a distant recurrence at the site of interest whether they had died or were still alive at last follow-up. Using these data, we conducted for each site a competing risk analysis computing the cumulative incidence curves over time while treating death without recurrence as a competing risk [14].
To quantify the risk of developing distant recurrence, we use the cumulative incidence function because the usual Kaplan-Meier survival-based estimates are biased high due to patients dying without the distant recurrence of interest being counted as simple censoring events rather than as competing risks [14]. To compare cumulative incidence functions, we use Gray’s test [15]. To assess the effects of covariates on the cumulative incidence function, we use the proportional hazards model of Fine and Gray [16]. To assess the full complexity of the relationships between HER2 status, HR status, and time since diagnosis, we fit a version of the Fine-Gray model with a three-way interaction between HR status, HER2 status and a linear function of time. For each recurrence site, we successively removed higher order interaction terms until only significant terms remained. To adjust for potential confounding, we included terms in the models for Trastuzumab use, hormonal therapy, chemotherapy, stage, grade, age, menopausal status, and race.
All analyses were performed using R version 2.15.0 (R Foundation for Statistical Computing) including the cmprsk package.
Results
Of the 11,011 patients studied, 2298 had at least one distant recurrence, 475 died without a distant recurrence, and 8238 were alive and distant-recurrence-free at last follow-up. The estimated overall cumulative incidence of developing distant metastases is 11% at 2 years, 20% at 4 years, 26% at 6 years, 30% at 8 years, 33% at 10 years, and 36% at 12 years. The corresponding estimated risks of dying without distant recurrence are: 1%, 3%, 5%, 7%, 9% and 12%. There were 5046 patients at risk (i.e., alive and distant-recurrence-free) at 4 years, 1722 at 8 years, and 166 at 12 years.
For all patients combined, the general order of the sites of distant recurrence ordered by cumulative incidence is bone > liver > lung > distant lymph node > brain > pleura. The 12-year cumulative incidence was 23% for bone, 16% for liver, 14% for lung, 13% for distant lymph node, 10% for brain, and 8% for pleura. However, within the four subgroups formed by the joint values of HR status and HER2 status, the general ordering of sites by incidence is very different between groups. While lung recurrences are the most common for HR negative patients (regardless of HER2 status), bone recurrences are the most common for HR positive patients. As seen in Figure 1, of the four HR/HER2 subgroups, triple negative patients have the highest rates for lung, pleura, and distant lymph node recurrences for at least the first twelve years after diagnosis and for bone recurrences for the first six years after diagnosis. HR negative, HER2 positive patients have the highest incidence of brain recurrences for the first twelve years after diagnosis and for liver recurrences for the first six years after diagnosis. The cumulative incidence functions are statistically different between the four HR/HER2 subgroups for all six recurrence sites (p < 0.0001 for brain, liver, lung, pleura, and distant lymph nodes; p = 0.0011 for bone).
Figure 1.
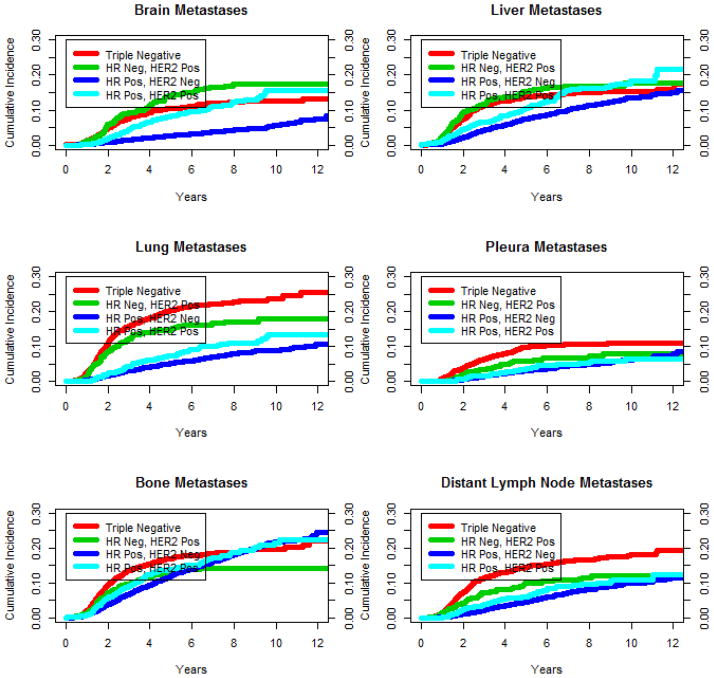
Cumulative incidence curve estimates by HR/HER2 subgroup and site of recurrence
We estimated the effect of HER2 status on recurrence rates separately for HR negative patients and HR positive patients and testing the significance of the differences for each site (Table 1). Technically, this is an assessment of the statistical interaction between HR status and HER2 status. For brain recurrences, the effect of HER2 status is significantly stronger in HR positive patients than in HR negative patients. For lung recurrences, among HR negative patients, HER2 positive patients have lower rates than HER2 negative patients; while among HR positive patients, HER2 positive patients have higher rates than HER2 negative patients. For pleura, bone and distant lymph node recurrences, among HR negative patients, HER2 positive patients have lower rates than HER2 negative patients; while among HR positive patients, recurrence rates did not vary significantly with HER2 status.
Table 1.
HER2 Hazard Ratios by HR Status and Site of Recurrence
| Site | HR Negative Patients | HR Positive Patients | p-value* |
|---|---|---|---|
| Brain | 1.40 (1.00, 1.70) | 2.88 (2.24, 3.71) | <0.0001 |
| Liver | 1.10 (0.89, 1.40) | 1.50 (1.22, 1.84) | 0.31 |
| Lung | 0.72 (0.58, 0.90) | 1.43 (1.12, 1.83) | <0.0001 |
| Pleura | 0.62 (0.43, 0.88) | 1.08 (0.76, 1.53) | 0.027 |
| Bone | 0.72 (0.56, 0.92) | 1.11 (0.92, 1.33) | 0.0056 |
| Dist LN** | 0.63 (0.48, 0.83) | 1.29 (1.00, 1.67) | 0.0002 |
p-value tests whether the effect of HER2 status varied significantly with HR status
Dist LN = distant lymph node
Numbers in parentheses are 95% confidence intervals for the hazard ratios.
The crossing of cumulative incidence curves for the four HR/HER2 subgroups indicates that relative differences in recurrence rates may change over time. To explore this phenomenon, we estimate competing risk hazard ratios for HER2 status by HR status separately for three time intervals: 0 to 4 years, 4 to 8 years, and 8 plus years (Table 2). These time intervals (0–4, 4–8, 8–12) were chosen because they were roughly equal in length, generally had a sufficient number of events for reliable analysis, and because three intervals was adequate to illustrate how effects change over time. The results indicate that the effect of HER2 status on rates of distant recurrence depends on (1) HR status, (2) site of recurrence, and (3) time since diagnosis. Generally speaking, among patients with HR positive tumors, HER2 positive patients have higher rates of recurrence than HER2 negative patients; while among patients with HR negative tumors, HER2 positive patients have lower rates of recurrence than HER2 negative patients among patients with HR negative tumors. Generally, among patients with HR positive tumors, the effect of HER2 status on recurrence rates declines over time, while among patients with HR negative tumors, the effect of HER2 status on recurrence rates increases over time. The effect of HER2 status on recurrence rates is most pronounced for recurrences in the brain and bone.
Table 2.
HER2 Hazard Ratios by HR Status, Years since Diagnosis, and Site of Recurrence
| Site | 0 – 4 years | 4 – 8 years | 8 – 12 years | |||
|---|---|---|---|---|---|---|
| HR− | HR+ | HR− | HR+ | HR− | HR+ | |
| Brain | 1.2 (0.9, 1.6) | 3.2 (2.3, 4.4) | 1.9 (1.1, 32) | 2.8 (1.8, 4.5) | 0.0 (0.0, 0.0) | 1.8 (0.8, 4.0) |
| Liver | 1.1 (0.9, 1.4) | 1.6 (1.2, 2.0) | 1.2 (0.6, 2.5) | 1.5 (1.0, 2.2) | 0.9 (0.1, 9.0) | 1.1 (0.5, 2.6) |
| Lung | 0.8 (0.6, 1.0) | 1.5 (1.1, 2.0) | 0.5 (0.3, 1.0) | 1.5 (0.9, 2.3) | 0.4 (0.0, 3.1) | 1.1 (0.4, 3.3) |
| Pleura | 0.6 (0.4, 0.9) | 1.1 (0.7, 1.8) | 0.6 (0.3, 1.3) | 1.2 (0.7, 2.2) | 5.1 (0.5, 57) | 0.7 (0.2, 2.3) |
| Bone | 0.7 (0.6, 1.0) | 1.4 (1.1, 1.7) | 0.7 (0.3, 1.4) | 0.7 (0.5, 1.1) | 0.0 (0.0, 0.0) | 0.8 (0.4, 1.7) |
| DLN* | 0.6 (0.4, 0.8) | 1.6 (1.2, 2.3) | 0.8 (0.4, 1.5) | 1.0 (0.6, 1.5) | 0.4 (0.0, 3.2) | 0.9 (0.3, 2.6) |
DLN = distant lymph node
Numbers in parentheses are 95% confidence intervals for the hazard ratios.
To further investigate how the effects of HER2 status on recurrence rates varies with respect to HR status and time since diagnosis, we constructed models with a three-way interaction between HR status, HER2 status and a linear function of time. Figure 2 shows estimates derived from these models of the adjusted log hazard ratio functions for HER2 status (comparing HER2 positive patients to HER2 negative patients) according to HR status and site of recurrence. Figure 2 is meant to complement and expand on the analysis presented in Table 2. They are two different ways of analyzing the changing hazard ratios over time. In Table 2, the change is modeled as a step-function of time with jumps at 4 and 8 years. In Figure 2, the change is modeled as a linear function of time. Furthermore, Table 2 is presents the results in tabular form, while Figure 2 presents the results in graphical form.
Figure 2.
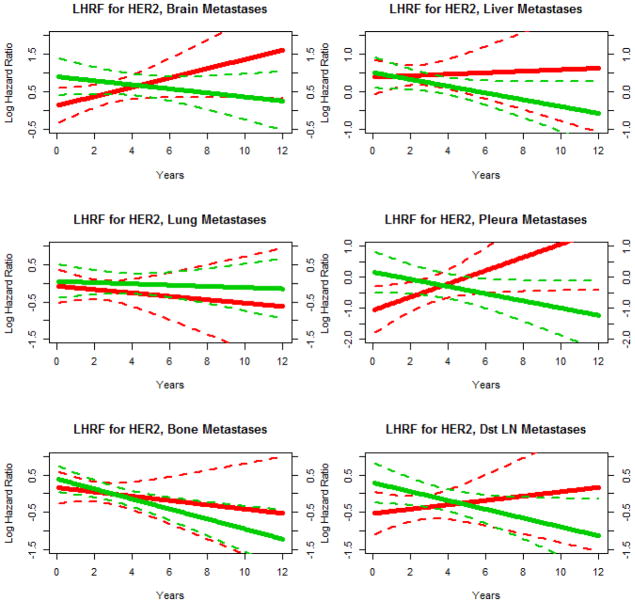
Adjusted competing risk model log hazard ratio function (LHRF) estimates for HER2 status (positive vs. negative) according to HR status, year since diagnosis, and site of recurrence. Red curves are for HR negative patients and green curves are for HR positive patients. Solid lines are point estimates and dashed lines are corresponding 95% confidence intervals.
For brain and pleura metastases, the dependence of the effect of HER2 status on HR status changes significantly over time. Among HR negative patients, HER2 positive patients initially have lower rates of recurrence than HER2 negative patients but this relationship switches such that later in time the reverse is true. Among HR positive patients, HER2 positive patients initially have somewhat lower rates of recurrence than HER2 negative patients and this protective effect becomes more pronounced over time. For bone metastases, the effect of HER2 status changed significantly over time but did not vary significantly with HR status. HER2 positivity became increasingly protective over time for both HR negative patients and HR positive patients. For liver and distant lymph node metastases, the effect of HER2 status did not vary significantly over time and did not vary significantly with HR status, but HER2 positive patients had significantly lower recurrence rates than HER2 negative patients. For lung metastases, the effect of HER2 status did not vary significantly over time and did not vary significantly with HR status, and the effect of HER2 itself was not significant.
Overall, 808 (44%) of the 1824 HER2 positive patients were treated with Trastuzumab, however this proportion increased over the study period: 15% in 2002, 35% in 2004, 61% in 2006, 73% in 2008 and 75% in 2010. In general, those HER2 positive patients treated with Trastuzumab had significantly lower cumulative incidence rates of recurrence than those HER2 positive patients treated without Trastuzumab. The competing risks hazard ratios, associated 95% CI, and p-values were 0.72 (0.51, 1.00) 0.053 for brain, 0.51 (0.36, 0.70) <0.0001 for liver, 0.77 (0.56, 1.00) 0.12 for lung, 1.0 (0.64, 1.70) 0.89 for pleura, 0.49 (0.36, 0.68) <0.0001 for bone, and 0.97 (0.68, 1.40) 0.87 for distant lymph nodes.
Overall, 6590 (81%) of the 8105 HR positive patients were treated with hormonal therapy and this proportion was stable throughout the study period. In general, those HR positive patients treated with hormonal therapy had significantly lower cumulative incidence rates of recurrence than those HR positive patients treated without hormonal therapy. The competing risks hazard ratios, associated 95% CI, and p-values for the difference in cumulative incidence curves for HR positive patients treated with vs. without hormone therapy were 0.48 (0.37, 0.63) <0.0001 for brain, 0.73 (0.60, 0.90) 0.0037 for liver, 0.56 (0.44, 0.70) <0.0001 for lung, 0.64 (0.47, 0.87) 0.0048 for pleura, 0.64 (0.54, 0.75) <0.0001 for bone, and 0.73 (0.57, 0.93) 0.012 for distant lymph nodes.
Discussion
In our retrospective study of over eleven thousand early-stage breast cancer patients we have shown that the nature of the effect of HER2 status on distant recurrence rates varied considerably from site to site. For brain and pleura recurrences, the effect of HER2 depended on HR status in ways that significantly changed over time. For bone recurrences, the effect of HER2 did not depend on HR status, but did change significantly over time. For liver and distant lymph node recurrences, there was a significant effect of HER2 status that did not change with time or HR status. For lung recurrences, rates did not significantly vary with HER2 status.
We are aware of only a few published studies assessing the effect of HER2 status on the patterns of risk of developing distant recurrences in breast cancer. Park et al [17] showed in a study of 886 women that distance recurrence patterns varied significantly according to HR status and HER2 status but did not assess how the risk or relative risk changed over time. Kennecke et al [18] showed in a study of 3,726 women that distance recurrence patterns varied significantly according to intrinsic molecular subtype (which are based in part on HR status and HER2 status) and found that HER2-enriched tumors had a higher rate of brain, liver and lung metastasis compared with luminal A tumors. One of the main limitations of these studies is the limited use of modern adjuvant systemic therapy, which may alter patterns of recurrence. For example, most patients did not receive adjuvant trastuzumab and aromatase inhibitors, which have become standard of care in the United States the last 5–7 years [10–12, 19].
Several studies have shown that HER2 positivity is a risk factor for subsequent development of central nervous system recurrence in patients with breast cancer [20–33]. However, central nervous system (CNS) involvement often follows seeding of lungs, liver and bones [34]. Arvold et al [21] reported an overall cumulative 5-year incidence of brain metastasis of 1.7% in 1,474 patients who had presented with early-stage breast cancer. Of the patients who developed brain metastasis (n=36), 25% (9 patients) had HER2-positive breast cancer at the time of initial diagnosis. Another retrospective study that included 9,524 women with early-stage breast cancer showed a 10-year rate of cumulative incidence of CNS metastasis of 6.8% in HER2-positive tumors, compared with a rate of 3.5% in HER2-negative tumors [29].
Musolino et al [24] evaluated the outcome of 214 patients with stage I–III HER2-positive breast cancer. Fifty-53 patients received adjuvant trastuzumab and 3 developed metastatic disease. Overall, 50 patients developed metastases; 3 patients had received adjuvant trastuzumab; 14 patients never received trastuzumab; and 33 patients did not receive adjuvant trastuzumab but were treated with trastuzumab for metastatic disease. As expected, patients treated with adjuvant trastuzumab had better overall survival rate than patients who did not receive adjuvant trastuzumab. The cumulative incidence of CNS metastases was 5%. Although patients with HER2-positive breast cancer who were not treated with trastuzumab fared worse than patients with HER2-negative disease, prior trastuzumab therapy was associated with diminished CNS metastases-free survival rate. A meta-analysis of randomized adjuvant trastuzumab trials revealed a higher incidence of CNS metastasis in the trastuzumab-containing arms compared with the non-trastuzumab containing arms [35]. The higher incidence of CNS metastasis in patients treated with trastuzumab probably reflects the inability of trastuzumab to cross the blood-brain barrier and the prolonged survival achieved by trastuzumab-based therapy in the metastatic setting [36–41].
Despite the elevated risk of CNS metastasis, the American Society of Clinical Oncology does not recommend any image-guided monitoring of HER2-positive breast cancer after curative locoregional and systemic therapy are administered, in the absence of signs or symptoms suggestive or recurrent disease [13]. Novel approaches are needed to identify HER2-positive patients at high risk for CNS metastasis before interventions to decrease recurrence can be implemented [42]. Whether prophylactic radiation therapy to the brain would be safe and effective in this setting is not known. In one study, HER2-positive early-stage breast cancer patients were monitored by magnetic resonance imaging of the brain after completion of adjuvant trastuzumab-based adjuvant therapy. Whole-brain irradiation in patients with occult CNS metastasis decreased mortality due to CNS disease but it didn’t prolong the overall survival rate [26]. Integration of novel systemic therapies that cross the blood-brain barrier would be of interest in this population [7]. Lapatinib may play a role in this setting, although the evidence available is limited [43–45]. In the pivotal phase III randomized trial of capecitabine/lapatinib versus capecitabine alone in patients with HER2-positive metastatic breast cancer after trastuzumab progression, the number of patients with CNS involvement was lower in the lapatinib arm, but the difference was not statistically significant [46]. The Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial will determine what sites of metastasis occur after lapatinib is added to standard trastuzumab-based therapy in the adjuvant setting.
HER2-positive breast cancer can be stratified in HR-positive and HR-negative [47, 48]. We found that the effect of HER2 status in the development of brain metastasis is stronger in HR positive patients than in HR negative patients. In contrast, Park et al [17] found no difference in the incidence of lung, liver and brain metastases between the HER2+/ER+ group and the HER2+/ER− group (n= 269; patients with HER2-positive metastatic breast cancer). These authors reported a higher rate of bone-only metastasis in the HER2+/ER+ group. These data suggest that HER2+/ER− tumors are more aggressive. Furthermore, these patients do not benefit from endocrine therapy.
Limitations of our study include its retrospective nature and it was completed at a single institution. Furthermore, we do not have detailed information regarding the HER2 and HR status of each metastatic tumor and heterogeneity is expected [49–51]. In conclusion, this is the largest study to date documenting the heterogeneity of recurrence patterns in HER2-positive breast cancer over time, taking into account the HR status of the primary tumor. Understanding tumor biology and optimization of HER2 targeted therapy and endocrine therapy will be required to improve the overall survival rate for patients with early-stage breast cancer.
Acknowledgments
This study was funded in part by the Breast Cancer Research Foundation. MDACC is supported by the National Institutes of Health through grant CA16672.
Footnotes
Potential Conflicts of Interest: KRH: None; FJE: None
References
- 1.Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 3.Peintinger F, Buzdar AU, Kuerer HM, et al. Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab. Ann Oncol. 2008;19:2020–2025. doi: 10.1093/annonc/mdn427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteva FJ, Hortobagyi GN, Sahin AA, et al. Expression of erbB/HER receptors, heregulin and P38 in primary breast cancer using quantitative immunohistochemistry. Pathol Oncol Res. 2001;7:171–177. doi: 10.1007/BF03032345. [DOI] [PubMed] [Google Scholar]
- 5.Esteva FJ, Hortobagi GN. Prognostic molecular markers in early breast cancer. Breast Cancer Res. 2004;6:109–118. doi: 10.1186/bcr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteva FJ, Sahin AA, Cristofanilli M, et al. Molecular prognostic factors for breast cancer metastasis and survival. Semin Radiat Oncol. 2002;12:319–328. doi: 10.1053/srao.2002.35251. [DOI] [PubMed] [Google Scholar]
- 7.Esteva FJ. Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. Oncologist. 2004;9:4–9. doi: 10.1634/theoncologist.9-suppl_3-4. [DOI] [PubMed] [Google Scholar]
- 8.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazouni C, Hall A, Broglio K, et al. Kinetics of serum HER-2/neu changes in patients with HER-2-positive primary breast cancer after initiation of primary chemotherapy. Cancer. 2007;109:496–501. doi: 10.1002/cncr.22418. [DOI] [PubMed] [Google Scholar]
- 10.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 14.Pintilie M. A Practical Perspective. Hoboken, NJ: John Wiley & Sons, Ltd; 2006. Competing Risks. [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;3:1141–1154. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazarda model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Park YH, Lee S, Cho EY, et al. Patterns of relapse and metastatic spread in HER2-overexpressing breast cancer according to estrogen receptor status. Cancer Chemother Pharmacol. 2010;66:507–516. doi: 10.1007/s00280-009-1190-7. [DOI] [PubMed] [Google Scholar]
- 18.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 19.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 20.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14:R129. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arvold ND, Oh KS, Niemierko A, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2243-x. [DOI] [PubMed] [Google Scholar]
- 22.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 23.Yap YS, Cornelio GH, Devi BC, et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Cancer. 2012;107:1075–1082. doi: 10.1038/bjc.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 25.Heitz F, Rochon J, Harter P, et al. Cerebral metastases in metastatic breast cancer: disease-specific risk factors and survival. Ann Oncol. 2011;22:1571–1581. doi: 10.1093/annonc/mdq625. [DOI] [PubMed] [Google Scholar]
- 26.Niwinska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77:1134–1139. doi: 10.1016/j.ijrobp.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Duchnowska R, Dziadziuszko R, Czartoryska-Arlukowicz B, et al. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat. 2009;117:297–303. doi: 10.1007/s10549-008-0275-z. [DOI] [PubMed] [Google Scholar]
- 28.Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 30.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 31.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 32.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 33.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 35.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Angulo AM, Cristofanilli M, Strom EA, et al. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004;101:1760–1766. doi: 10.1002/cncr.20530. [DOI] [PubMed] [Google Scholar]
- 37.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 38.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 39.Fornier M, Esteva FJ, Seidman AD. Trastuzumab in combination with chemotherapy for the treatment of metastatic breast cancer. Seminars in Oncology. 2000;27:38–45. [PubMed] [Google Scholar]
- 40.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow PK, Zambrana F, Esteva FJ. Recent advances in systemic therapy: Advances in systemic therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. 2009;11:207. doi: 10.1186/bcr2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999;13:457–472. doi: 10.1016/s0889-8588(05)70065-4. [DOI] [PubMed] [Google Scholar]
- 43.Dean-Colomb W, Esteva FJ. Emerging agents in the treatment of anthracycline- and taxane-refractory metastatic breast cancer. Semin Oncol. 2008;35:S31–38. doi: 10.1053/j.seminoncol.2008.02.008. quiz S40. [DOI] [PubMed] [Google Scholar]
- 44.Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 45.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 46.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 47.Koboldt DC, Fulton RS, McLellan MD, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012 doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwamoto T, Booser D, Valero V, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–734. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]
- 49.Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juul N, Szallasi Z, Eklund AC, et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: a retrospective analysis of five clinical trials. Lancet Oncol. 2010;11:358–365. doi: 10.1016/S1470-2045(10)70018-8. [DOI] [PubMed] [Google Scholar]
- 51.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]


