Abstract
Given the link between depression, anxiety, and cannabis abuse, a serotonin receptor (rs6311) and transporter polymorphism (rs2020936) were examined as moderators of neural response following a psychosocial treatment for cannabis use disorders (CUDs). While the proposed hypotheses wereunsupported, we found that the rs6311 C allelewas significantly related to brain activation (medial frontal gyrus, precuneus), indicating the role of this serotonin receptor in adolescent treatment response.
Keywords: cannabis, client language, genetics, fMRI, adolescents
1. Introduction
Adolescent cannabis use has been strongly correlated with a number of health risk behaviors (Chabrol et al., 2008; French and Dishion, 2003), protracted use (Swift et al., 2008), and poorer life outcomes during adulthood (Fergusson and Boden, 2008). These trends are even more striking for justice-involved adolescents (Aarons et al., 2001).Additionally, psychosocial treatments are the most frequently utilized approach for cannabis use disorders(CUDs; DHHS, 2009).However, only 26% successfully achieve abstinencefollowing treatment (Dutra et al., 2008).Notably, genetic factors may influence treatmentoutcomes (Anton et al., 2008; Bauer et al., 2007). For example, DRD4 was found tomoderate response toa motivational interviewing (MI) intervention targeting heavy drinking, with emerging adults with the protective allele evidencing better treatment response (Feldstein Ewing et al., 2009). Related, most of the research has focused on genesinvolved in craving, reward, and dependence (Filbey et al., 2010; Haughey et al., 2008; Schacht et al., 2009). Yet, the serotonergic system may be particularly salient to youths’ vulnerability to CUDs and subsequent treatment response (Lopez-Moreno et al., 2008; Otten and Engels, in press; Sadeh et al., 2010).
There are several reasons for this. First, serotonergic polymorphisms are involved in mood and anxiety symptoms. Risk alleles of single nucleotide polymorphisms (SNPs) on both the serotonin 2A receptor gene (5-HT-2A; rs6311 C allele; Dickel et al., 2007; Giegling et al., 2006; Unschuld et al., 2007) and the serotonin transporter gene (SLC6A4; rs2020936 A allele; Kramer et al., 2009; Wray et al., 2009) correspond with greater mood and anxiety disorders, aggression, and anger.Second, mood and anxiety symptoms are elevated among adolescent cannabis abusers (e.g., Diamond et al., 2006; Kaminer et al., 2008;Malmberg et al., 2010),with some arguing that youth use cannabis to cope with negative affect (Fox et al., 2011).Third, mood and anxiety symptoms have been found to influence treatment response (risk for relapse, e.g., Sinha and Li, 2007; Stein et al., 2011).
Despite these connections,we could find no study explicitly evaluating the relationship between serotonin polymorphisms and treatment response. Thus, wesought to investigate the association between two types of serotonin polymorphisms (transporter gene; receptor gene)and BOLD response during an active ingredient of a psychosocial intervention (change talk; CT). Based on prior researchindicating that genetic protective factors (DRD4) modulated psychosocial treatment response (Feldstein Ewing et al., 2009), recent research supporting the associations between depressive and anxiety symptoms and cue-induced BOLD response (Feldstein Ewing et al., 2010), and the clinical connection between adolescents’ cannabis use and mood and anxiety symptoms,we posited that the protective alleles of the serotonin polymorphisms (rs6311 T allele and rs2020936 G allele) would be correlated with greater activation of introspection/contemplative areas (medial frontal gyrus, insula, precuneus).
2. Materials and Methods
2.1 Participants
With institutional review board approval, 86 non-treatment seeking youth were recruited from southwest juvenile justice centers. All youth provided assent and parent/guardian consent. To participate, youth needed to be age 14-17, fluent in English, and a regular cannabis user (defined as use >7 of the past 30 days; Walker et al., 2006). Exclusion criteria included antipsychotics/anticonvulsants (serving as a proxy to detect psychotic symptoms),MRI contra-indications, and TBI with loss of consciousness >6 min.While not exclusion criteria, no participants within this study were taking other psychotropic substances, anxiolytics, or anti-depressants. Eligible participants could earn $105.
Of the 86 eligible youth, 7 did not completea scan(e.g., scanner problems, intoxication). Of these, 43 had motion within the selected threshold (<3 mm in any direction) and >1 run of data. Forty-one youth also had genetic data; these 41 were included in subsequent analyses. This sample was predominantly male (83%), age 16.09 (SD = 1.09), and Hispanic (53.5%), Caucasian (20.9%), Bi-/Multi-racial (14%), African-American (7%), and Native American (4.7%).
2.2Procedures
2.2.1 Baseline session
Participants completed a demographic questionnaire, as well as an assessment of their past month behavior, including problems related to their cannabis use(cannabis-related problems; “Have you had a persistent chest infection or cough?”, “Have you driven while stoned?”;CPQ-A; Martin et al., 2006), DSM-IV-TR criteria for cannabis dependence (cannabis dependence symptoms; “The need to smoke more marijuana to achieve the same high.”, “A failure to cut back or reduce your marijuana smoking habits.”; Stephens et al., 2000), frequency of cannabis use (cannabis use days; TLFB; Sobell & Sobell, 1992), depression (CDI-S; Kovacs, 2001), and anxiety (RCMAS; Reynolds & Richmond, 2008). Following other studies that have supported the use of a single motivational interviewing (MI) session to successfully catalyze change in substance use behaviors (Bertholet, et al., 2010; Miller & Wilbourne, 2002; Moyers et al., 2009), all participantsreceivedone60-minute session of MI targeting reducingcannabis use. During this session, all counselors elicited5 statements in favor of changing cannabis use (change talk; CT; e.g., I need to back off my marijuana use) and 5 statements in favor of sustaining cannabis use (sustain talk; ST; e.g., Marijuana isn’t a problem for me).
DNA was collected from saliva and extracted according to modified standard procedures (for details, see Walker et al., 1999).Samples were genotyped for the rs6311 and rs2020936 polymorphisms using Taqman PCR technology. Minor alleles were combined into one group. For rs6311, as halfof the sample was homozygous C/C (53.7%; n=22; C/T=36.6%, n=15; T/T=9.8%, n=4), C/T and T/T were combined into a second group (the T allele group). For rs2020936, as mostof the sample was homozygous A/A (65.9%; n=27), all G alleles were combined into a second group (the G allele group; A/G=24.4%, n=10; G/G=9.8%, n=4).HWE statistics were calculated for both SNPs with rs6311 falling within HWE assumptions (χ2=0.36) and rs2020936 deviating from HWE assumptions (χ2=3.41), due to an excess of dominant homozygotes compared to heterozygotes. Notably, no differences in allele distribution were observed by race/ethnicity, age, gender, frequency of cannabis use, or cannabis related problems.
2.2.2 Scan session
Consistent with studies indicating the ability to detect neural changes in response to treatment within a week of a single MI session (Feldstein Ewing et al., 2011; Houck, Moyers, & Tesche, in press), all participants returned within one week of their baseline session for a scan. All youth abstained from cannabis for 24 hours and from caffeine and cigarettes for the preceding hour, as verified by self-report. We utilized a 3T Siemens Trio with a 12-channel coil to collect MRI scansduring a task evaluating the effects of in-session-derived client statements on cue-elicited craving(for details, see Feldstein Ewing et al., in press).An anatomical MRI scan was collected with a T1-weighted multi-echo Magnetization Prepared Rapid Gradient Echo or MPRAGE (MEMPR) sequence (TR/TE/TI = 2300/2.74/900 ms, flip angle=8°, FOV=256×256 mm, Slab thickness=176 mm, Matrix=256×256×176, Voxel size=1×1×1 mm3, Number of echos=4, Pixel bandwidth=650 Hz). fMRI scans were collected using a gradient echo, echo-planar sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference [TR: 2.0 s, TE: 27ms (39ms for 1.5T), α: 70o, matrix size: 64×64, 32 slices, voxel size: 3×3×4 mm3] with a tilting acquisition to increase OFC signal-to-noise.
Briefly, following prior work (see Feldstein Ewing et al., in press), participants were visually and auditorily presented with 5 unique 16 s. CT statements and 5 unique 16 s. ST statements, followed by exposure toeither thetactile cannabis cue (marijuana pipe) or the control cue (pencil) for 20 s., ending with a 20 s. washout period to allow the hemodynamic response to return to baseline before the next trial. Youth were pseudorandomly presented with a single run of each of the 4statement/cue combinations[(1) CT/cannabis, (2) ST/cannabis, (3) CT/control, and (4) ST/control]. Each run consisted of 10×65 second trials (TR 2s/volume; 1 run=10 trialsx65 seconds/30 TRs per trial=325 TRs/10 min.).Each statement was presented to the participants twice yielding 10 CT and 10 ST statements per participant.
2.3 Analyses
Identical to our prior work (for details, see Feldstein Ewing et al, in press), the following SPM pre- and post-processing steps were conducted. To begin, exposure to tactile cues (cannabis/control) following CT and ST were modeled. Next, we contrasted: (a) exposure to (cannabis > control) cues following (ST > CT) and (b) exposure to (cannabis > control) cues following (CT >ST).To examine group differences in BOLD response for (cannabis > control) cues across different talk conditions, for the rs6311 SNP, atwo-sample t test was used to compare the T allele youth against C/C youth. Likewise, for rs2020936, the G allele group was compared with the A/A youth. Anatomical localization for the regions of activation was found using the Talairach Daemon software(Lancaster et al., 2000). For visualization and display, z-maps were overlaid on SPM’s T1 canonical MNI template.
3. Results
3.1 Cannabis use disorder, depression, and anxiety symptoms
This sample evidenced heavy cannabis use and related symptoms, as indicated by their past month frequency of cannabis use (M=17.78 days, SD=9.85), weekday cannabis use (97.6%), cannabis-related problems (M=16.46, SD=8.93), and cannabis dependence symptoms (M=3.22, SD=1.94). Youth reported low rates of depression (M=2.07; SD=2.21; note: total score >8 for girls and >10 for boys indicatesclinically-significantdepression; Kovacs et al., 2001) and anxiety (M t score = 50.00; SD=9.87; note: t score >70 indicates clinically-significant anxiety).
3.2 Genotype and BOLD response during (cannabis> control) cues following (change talk >sustain talk)
3.2.1 SNP rs6311
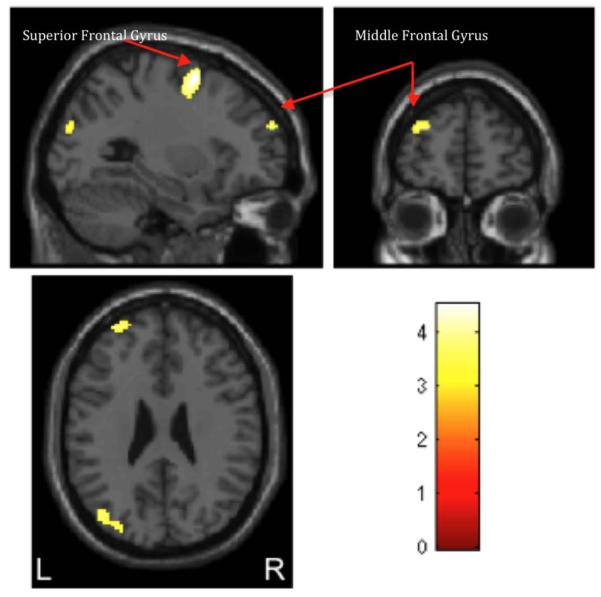
Compared with the T allele youth, the C/C youth had a greater cannabis-cue induced BOLD response following CT across the middle and superior frontal gyri, the temporo-occipital lobe, and parietal lobe (precuneus; uncorrected p<0.001, extent threshold>20 voxels; Figure 1). There were no regions where the T allele group had greater BOLD response than C/C youth.
Figure 1.
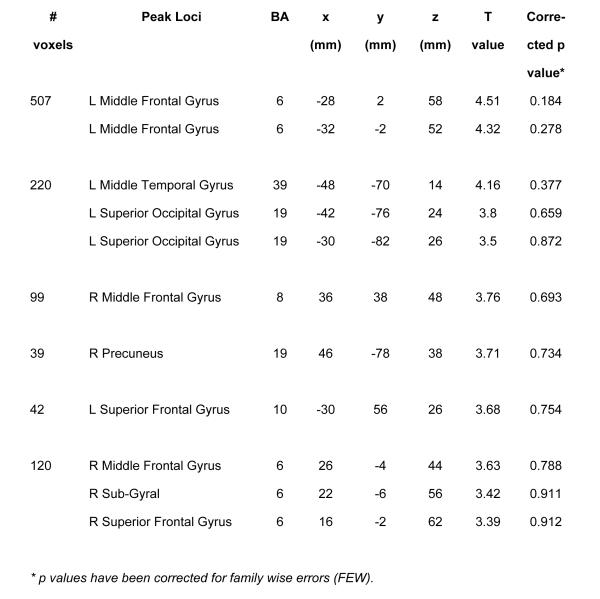
(a) Maximum loci of activation for the comparison of C/C vs. T allele groups (C/T and T/T) for SNP rs6311 during BOLD response to cannabis (> control) cues following change (> sustain) talk condition (uncorrected p < 0.001, extent threshold > 20 voxels). Total number of clusters: 6. L: Left; R: Right; BA: Brodmann Area.
(b) Activation maps obtained for the 2-sample t-test, where the C/C group showed significantly greater activation than the T allele groups (C/T and T/T) group for BOLD response for cannabis (> control) following change (> sustain) talk condition (uncorrected p< 0.001, extent threshold > 20 voxels). Activations are observed in the superior and middle frontal gyri along with middle temporal gyrus, superior occipital gyrus and precuneus. The color bar indicates t values with warmer colors denoting more significant activation. Left (L)/right (R) sides are marked in the bottom of the figure.
While there were no differences betweenthe C/C and T allele groups on self-reported depression (CDI-S) and anxiety (RCMAS), the C/C group had significant negative correlations between BOLD response and depression (inferior parietal lobule, supramarginal gyrus, superior temporal gyrus; uncorrected p<0.001, extent threshold>20 voxels) and anxiety (cuneus; uncorrected p<0.001, extent threshold>20 voxels).Similarly, the T allele group had significant negative correlations between BOLD response and depression (inferior parietal lobule, middle temporal gyrus, middle frontal gyrus; uncorrected p<0.001, extent threshold>20 voxels), as well as both significant positive (postcentral gyrus; uncorrected p<0.001, extent threshold>20 voxels) and negative correlations between BOLD response and anxiety (cingulate gyrus, middle temporal gyrus, precuneus, caudate, middle frontal gyrus; uncorrected p<0.001, extent threshold>20 voxels).
3.2.2 SNP rs2020936
There were no significant differences between the rs2020936 genotypes (A/A and G allele) on neural response to cues following CT and ST. There werealso no differences between allele groups on self-reported depression (CDI-S) and anxiety (RCMAS) scores.
4. Discussion
Based on prior research (Feldstein Ewing et al., 2009; 2010; 2011),we posited that protective alleles of the serotonin receptor (rs6311 T allele) and transporter genes (rs2020936 G allele) would modulate response to client change language. In contrast with expectations, neither the rs6311 T allele nor the rs2020936 G allele was correlated with greater levels of BOLD activation.Rather,the rs6311 C/C group demonstrated significantly greater activation in areas important totreatment response and self-awareness (medial frontal gyrus, precuneus; Falk et al., 2011; Gusnard et al., 2001).While more activation can reflect compensatory efforts to overcome dysfunction and/or inefficient use of neural resources, greater activation in these areas has been found to correspond with better treatment outcomesfor adolescent cannabis users (lower follow-up CUD; Feldstein Ewing et al., in press). Thus, we carefullyspeculate that the greater activation observed for rs6311 C/C in this studymightrepresent a protective role.
The C/C and T allele groups did not differ on self-reported depression and anxiety. However,consonant with prior studies with heavy drinking emerging adults, both the C/C and T allele groups showedsignificant relationships between BOLD activation and depression and anxiety symptoms, notably in regions that overlap with prior work (Feldstein Ewing et al., 2010).
There were also no areas in which the T allele evidenced greater BOLD activation than the C/C group. These findings diverge from prior studies, which have found the T allele to be protective against suicidal behavior (Giegling et al., 2006). Yet, theyare consistent with researchindicating the relationship between the T allele and risk behavior (Saiz et al., 2009).
For rs2020936, genotype groups did not significantly differ on neural activation patterns and self-reported depression and anxiety symptoms. These findings suggest that the observed relationships might not be important for serotonergic polymorphisms globally, but rather, may be specifically relevant to serotonin receptor genes. This is important in light of potential downstream clinical implications, such as considerations of which serotonin-based medications might be most effective for youth with CUDs (Cornelius et al., 2010; Findling et al., 2009).
4.1 Limitations and Future Directions
This study provides a first step towards understanding the basic biological substrates influencing treatment response with cannabis-using adolescents. However, examination of other genetic factors (e.g., CNR1, FAAH; Filbey et al., 2010), along with school-involved youth would be informative. Further, sample size and/or receipt of treatment may have influenced mood/anxiety symptoms observed within this sample.Additionally, small sample size and/or population stratification may have contributed to the rs2020936’s violation of HWE assumptions. Thus, replication of this paradigm with a larger and more clinically-severesample would be beneficial. Furthermore, some participants also consumed tobacco. Apost-hoc evaluationindicated a significant negative correlation between BOLD activation and frequency of nicotine use. While a full review of these findings is beyond the scope of this manuscript,this research teamis planning to directly evaluatethis relationship in future work. Similarly, apost-hoc evaluation of negative life events indicated that this sample had minimal levels of early life stress; however, as stress and early life events may have a moderating role for the serotonergic polymorphisms, greater attention to the relationship between risk groups and susceptibility to depression, anxiety, and motivation to use cannabis, particularly in the context of negative life events would strengthen future work.Moreover, it is possible the modulatory effect of genetic variation on brain activation following client statements could be due to an indirect effect. For example, mood and anxiety symptoms might have been lower for the risk (C/C) group following CT, which may have led to higher brain activation for this group; further deconstruction of this relationship in future studies is encouraged.Additionally,participants were predominantly male; as anxiety and depression are more prevalent among females, and different allele groups showed no differences on these scales, re-examination of this paradigm with females is important.Together, these data suggest the importance of crafting and empirically evaluating a developmentally-targeted translational model of adolescents’ response to psychosocial treatments.
Acknowledgement
The authors would like to thank Amber McEachern, Marilee Morgan, Sina Aslan, Dustin Truitt, Lindsay Chandler, Sara Blaine, Ben Ladd, Erin Tooley, Alisha Wray, and Shirley Smith for their project assistance.This research was supported by 1R03DA027892-01 (PI: first author). The authors declare that they have no competing financial or other conflicts of interest relating to the data included in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarons GA, Brown SA, Hough RL, Garland AF, Wood PA. Prevalence of adolescent substance use disorders across five sectors of care. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:419–426. doi: 10.1097/00004583-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in Project MATCH subjects. Alcoholism: Clinical and Experimental Research. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet N, Faouzi M, Gmel G, Gaume J, Daeppen JB. Change talk sequence during brief motivational intervention, towards or away from drinking. Addiction. 2010;105:2106–2012. doi: 10.1111/j.1360-0443.2010.03081.x. [DOI] [PubMed] [Google Scholar]
- Chabrol H, Chauchard E, Girabet J. Cannabis use and suicidal behaviors in high-school students. Addictive Behaviors. 2008;33:152–155. doi: 10.1016/j.addbeh.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addictive Behaviors. 2010;35:644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Panichelli-Mindel SM, Shera D, Dennis M, Tims F, Ungemack J. Psychiatric syndromes in adolescents with marijuana abuse and dependency in outpatient treatment. Journal of Child & Adolescent Substance Abuse. 2006;15:37–52. [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr., Hanna GL. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biological Psychaitry. 2007;61:322–329. doi: 10.1016/j.biopsych.2006.09.030. [DOI] [PubMed] [Google Scholar]
- DHHS . In: National survey on drug use and health. Studies O.o.A., editor. Ann Arbor, MI: 2009. [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcoholism: Clinical and Experimental Research. 2010;34:396–403. doi: 10.1111/j.1530-0277.2009.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How psychosocial alcohol interventions work: A preliminary look at what fMRI can tell us. Alcoholism: Clinical and Experimental Research. 2011;35:643–651. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, LaChance HA, Bryan AD, Hutchison KE. Do genetic and individual risk factors moderate the efficacy of motivational enhancement therapy? Drinking outcomes with an emerging adult sample. Addiction Biology. 2009;14:356–365. doi: 10.1111/j.1369-1600.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: Evaluating adolescents’ response to a cannabis intervention. Psychology of Addictive Behaviors. doi: 10.1037/a0029767. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht J, Myers U, Chavez R, Hutchison K. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Pagano ME, McNamara NK, Stansbrey RJ, Faber JE, Lingler J, Demeter CA, Bedoya D, Reed MD. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: A pilot randomized placebo-controlled trial. Child Adolescent Psychiatry Mental Health. 2009;3:11. doi: 10.1186/1753-2000-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CL, Towe SL, Stephens RS, Walker DD, Roffman RA. Motives for cannabis use in high-risk adolescent users. Psychology of Addictive Behaviors. 2011;25:492–500. doi: 10.1037/a0024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DC, Dishion TJ. Predictors of early initiation of sexual intercourse among high-risk adolescents. Journal of Early Adolescence. 2003;23:295–315. [Google Scholar]
- Giegling I, Hartmann AM, Moller HJ, Rejescu D. Anger- and agression-related traits are associated with polymorphisms in the 5-HT-2A gene. Journal of Affective Disorders. 2006;96:75–81. doi: 10.1016/j.jad.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck JM, Moyers TB, Tesche CD. Through a glass darkly: Some insights on change talk via magnetoencephalography. Psychology of Addictive Behaviors. doi: 10.1037/a0029896. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer Y, Conner DF, Curry JF. Treatment of comorbid adolescent cannabis use and major depressive disorder. Psychiatry. 2008;5:34–39. [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory: Technical manual. Multi-Health Systems, Inc.; New York: 2001. [Google Scholar]
- Kramer J, Wetherill L, Bucholz K, Chan G, Dick D, Bierut L, Hesselbrock V, Edenberg HJ, Foroud T, Kuperman S. Polymorphisms in the 5-HTT gene SLC6A4 are associated with anger and anxiety but not with DSM-IV alcohol dependence in the COGA sample. Alcoholism: Clinical & Experimental Research. 2009;33(s1) [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addiction Biology. 2008;13:160–187. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Malmberg M, Overbeek G, Monshouwer K, Lammers J, Vollebergh W, Engels R. Substance use risk profiles and associations with early substance use in adolescence. Journal of Behavioral Medicine. 2010;33:474–485. doi: 10.1007/s10865-010-9278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Copeland J, Gilmour S, Gates P, Swift W. The Adolescent Cannabis Problems Questionnaire (CPQ-A): Psychometric properties. Addictive Behaviors. 2006;31:2238–2248. doi: 10.1016/j.addbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL. Mesa Grande: A methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Otten R, Engels RC. Testing bidirectional effects between cannabis use and depressive symptoms: Moderation by the serotonin transporter gene. Addiction Biology. doi: 10.1111/j.1369-1600.2011.00380.x. in press. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Houck JM, Christopher PJ, Tonigan JS. From in-session behaviors to drinking outcomes: A causal chain for motivational interviewing. Journal of Consulting and Clinical Psychology. 2009;77:1113–1124. doi: 10.1037/a0017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children’s Manifest Anxiety Scale: Second Edition; RCMAS-2. Western Psychological Services; Torrance, CA: 2008. [Google Scholar]
- Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, Lejuez CW, Verona E. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology. 2010;119:604–609. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Florez G, Arango C, Corcoran P, Morales B, Bascaran MT, Alvarez C, San Narcisco G, Carreno E, Alvarez V, Coto E, Bobes J. Differential role of serotonergic polymorphisms in alcohol and heroin dependence. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33:695–700. doi: 10.1016/j.pnpbp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Schacht J, Selling R, Hutchison K. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology. 2009;203:511–517. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Time-line follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Humana Press; Totowa, N.J.: 1992. pp. 73–98. [Google Scholar]
- Stein LAR, Lebeau R, Colby SM, Barnett NP, Golembeske C, Monti PM. Motivational interviewing for incarcerated adolescents: Effects of depressive symptoms on reducing alcohol and marijuana use after release. Journal of Studies on Alcohol and Drugs. 2011;72:497–506. doi: 10.15288/jsad.2011.72.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Swift W, Coffey C, Carlin J, Dengenhardt L, Patton G. Adolescent cannabis users at 24 years: Trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103:1361–1370. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Erhardt A, Lucae S, Kloiber S, Kohli M, Salyankina D, Welt T, Kern N, Lieb R, Uhr M, Binder EB, Muller-Myhsok B, Holsboer F, Keck ME. Polymorphisms in the serotonin receptor gene HTR2A are associated with quantitative traits in panic disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:424–429. doi: 10.1002/ajmg.b.30412. [DOI] [PubMed] [Google Scholar]
- Walker AH, Najarian D, White DL, Jaffe JF, Kanetsky PA, Rebbeck TR. Collection of genomic DNA by buccal swabs for polymerase chain reaction-based biomarker assays. Environmental and Health Perspectives. 1999;107:517–520. doi: 10.1289/ehp.99107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DD, Roffman RA, Stephens RS, Wakana K, Berghuis J. Motivational Enhancement Therapy for adolescent marijuana users: A preliminary randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74:628–632. doi: 10.1037/0022-006X.74.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, Statham DJ, Pergadia ML, Madden PA, Heath AC, Montgomery GW, Martin NG. Accurate, large-scale genotyping of the 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biological Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


