Abstract
While general effects of glucocorticoids are well established, the specific cellular mechanisms by which these hormones exert tissue-dependent effects continue to be elaborated. Diseases that demonstrate altered glucocorticoid signaling have been associated with alterations in astrocytes, yet relatively little is known about the effects of glucocorticoids upon this cell type. We have analyzed mRNA expression patterns following glucocorticoid treatment of mouse primary astrocyte cultures. Microarray analysis of cortical astrocyte cultures treated with dexamethasone over an eight-point, 24 h time course identified 854 unique genes with ≥twofold change in mRNA expression at one or more time points. Clustering analysis associated subsets of these mRNA expression changes with gene ontology categories known to be impacted by glucocorticoids. Numerous mRNAs regulated by dexamethasone were also regulated by the natural ligand corticosterone; all of the mRNAs regulated ≥twofold by corticosterone were substantially attenuated by cotreatment with the glucocorticoid receptor antagonist RU486. Of the mRNAs demonstrating ≥twofold expression change in response to both glucocorticoids, 33 mRNAs were previously associated with glucocorticoid regulation, and 36 mRNAs were novel glucocorticoid targets. All genes tested by qPCR for glucocorticoid regulation in cortical astrocyte cultures were also regulated by glucocorticoids in hippocampal astrocyte cultures (18/18). Interestingly, a portion of glucocorticoid-regulated genes were astrocyte enriched; the percentage of astrocyte-enriched genes per total number of regulated genes was highest for the early time points and steadily decreased over the time course. These findings suggest that astrocytes in vitro may initially deploy cell type-specific patterns of mRNA regulatory responses to glucocorticoids and subsequently activate additional cell type-independent responses.
Keywords: transcription, corticosteroids, cells, cultured, neuroglia
the brain is composed of a number of different cell types, resulting in complex molecular processes that underlie both normal brain function and disease pathology. While studies have historically attributed numerous functions to neurons, glial cells (e.g., astrocytes, oligodendrocytes, microglia) are increasingly identified as active participants in a range of brain functions (e.g., Refs. 17, 30). For example, astrocytes are now known to be intricate contributors to many fundamental brain processes, including energy metabolism (5), metabolite transport (25), the blood brain barrier (1), synapse regulation (20), intercellular communication and gliotransmission (54), and injury response (6). In an effort to understand the transcriptional underpinnings of these intercellular distinctions, recent efforts in brain cell-specific transcriptome analyses have identified subsets of mRNAs that are enriched in a given cell type, including astrocytes (8). These results reinforce the notion that cell type context may be an important factor in understanding cellular mechanisms that contribute to defined neural circuits and related brain functions. How cell types defined by these differences in basal gene expression transcriptionally adapt to specific stimuli may inform our understanding of both normal and disease conditions.
The molecular distinctions between neurons and glial cell types are gaining interest as additional disease states are associated with glial pathology. Some single-gene diseases have been associated with specific glial alterations, e.g., Alexander's disease with astrocytes (44), myelination disorders with oligodendrocytes (23), but recent results implicate a glial component in many complex disorders as well, e.g., psychiatric disorders (reviewed in Ref. 13); schizophrenia (68, 72), bipolar disorder (55), major depression (12). Changes in astrocyte density and size in distinct brain regions of the postmortem brains of patients with depression imply that distinct subpopulations of astrocytes are disrupted in the disorder (46, 56). Postmortem brain transcriptional profiling studies have also identified astrocyte-associated mRNAs as being altered in specific brain regions in depression (2, 4), further suggesting the relevance of transcriptional control by cell type in this disorder.
Even with growing interest in glial biology in the face of increasing evidence of disease alteration, relatively little is known about effects of common transcriptional regulators in astrocytes. Given the differences in baseline gene expression between cell types, the effects of transcriptional regulators may also vary as a function of cell type. If cell-specific transcriptional alterations contribute to disease states associated with astrocytes, then discerning how known pathological alterations influence gene transcription in a cell type-dependent fashion may enhance our understanding of disease mechanisms. For example, in the case of major depression, a well-established physiological alteration is the clinical presentation of hypercortisolemia, i.e., elevated levels of cortisol/glucocorticoids in the blood (e.g., Ref. 28), reviewed in Refs. 26, 50.
Glucocorticoids are a class of steroid hormones that mediate the effects of the hypothalamic-pituitary-adrenal axis. This system is commonly associated with stress signaling and the “fight-or-flight” response (reviewed in Refs. 11, 16). Glucocorticoids act as powerful transcriptional regulators that signal through two types of receptors, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). MR is a high-affinity receptor associated with mechanisms involving lower glucocorticoid concentrations (e.g., circadian rhythmicity of cortisol). GR is a modestly lower-affinity receptor that responds to higher concentrations of glucocorticoids, such as levels associated with stressful experiences. Hypercortisolemia is thought to particularly impact GR-mediated regulation. Alterations in glucocorticoid signaling are thought to contribute to some disorders, e.g., major depression (41), Alzheimer's disease (27), and be causative for others, e.g., Cushing's syndrome (51). How stress hormones influence astrocyte gene expression at the transcriptome level and how these steroid-mediated transcription patterns change across time remains largely uncharacterized.
A small number of mRNAs have been identified individually as glucocorticoid-sensitive in astrocytes, e.g., glutamine synthetase (34), glial fibrillary acidic protein (GFAP) (60), suggesting unique impact of glucocorticoids by cell type. These mRNAs have been identified using two experimental strategies: 1) in vitro studies that use astrocyte cultures to examine glucocorticoid regulation of individual mRNAs (predominant strategy, e.g., Ref. 34) and 2) in vivo studies that manipulate stress steroid levels and identify known glial-enriched mRNAs as steroid-regulated (e.g., Ref. 52). However, neither of these approaches has addressed the temporal patterning of mRNA expression changes at the transcriptome level. These approaches were thus not able to investigate associations between multiple mRNAs regulated by glucocorticoids or previously uncharacterized mRNA targets of glucocorticoid regulation in astrocytes.
Here we have characterized global mRNA expression changes induced by glucocorticoid treatment in astrocytes in vitro. Primary mouse astrocyte cell cultures were treated with glucocorticoids in a series of experiments designed to determine GR-mediated mRNA regulation over a 24 h time course. We used the synthetic GR-selective agonist dexamethasone to identify temporally regulated mRNAs. Similarly, we demonstrate that all of the mRNAs regulated ≥twofold by the natural ligand corticosterone were attenuated with cotreatment of the GR-selective antagonist RU486. Understanding glucocorticoid transcriptional regulation in astrocytes may provide additional insights into cell-specific effects of glucocorticoids in the brain.
MATERIALS AND METHODS
Reagents.
Dulbecco's modified Eagle's medium (DMEM, 11960-044), fetal bovine serum (FBS, Cat. #16000-044), antibiotic-antimycotic (Cat. #15240-062), charcoal-stripped FBS (CS-FBS, Cat. #12676-029), trypsin (Cat. #15090-046), and Hanks' balanced salt solution (HBSS, Cat. #14170-112) were obtained from Invitrogen (Gibco). Dextrose (glucose, Cat. #BP350-1) and Trypan blue (Cat. #SV3008401) were obtained from Fisher Scientific. Dexamethasone (Cat. #D-2915), corticosterone (Cat. #C2505), and mifepristone (RU486, Cat. #M8046) were obtained from Sigma Aldrich. Standard cell culture medium was 10% FBS in DMEM, 1:100 dilution of antibiotic-antimycotic, and 33 mM glucose.
Astrocyte cell culture.
Adult C57B/6 mice were obtained from Charles River. All animal procedures at the University of Michigan were approved by the University Committee on Use and Care of Animals and monitored by the Unit for Laboratory Animal Medicine. Mice were bred to obtain postnatal brain tissue. Primary astrocyte cell culture was obtained by a protocol derived from previous studies (61). Brains were extracted from P0-P2 mice (average 6 mice/litter, meninges removed, placed in cold HBSS), and cortex and hippocampus were dissected using a dissecting microscope. Tissue dissections were pooled across animals and manually triturated. The tissue was then treated with a 0.05% trypsin solution in HBSS for ∼15 min to chemically release the cells from connective tissues. The trypsin was neutralized using 10% FBS/DMEM medium, and cells were pelleted via centrifugation. The supernatant was removed, and the cells were washed using 10% FBS/DMEM medium and centrifuged again. The resulting supernatant was then removed, and the cells were resuspended in 10% FBS/DMEM. Sample cell density was determined with a hemocytometer and Trypan blue dye. Cells were then plated on uncoated plastic six-well dishes (Costar 3516) at 250,000–500,000 cells/well in 2 ml 10% FBS/DMEM medium. Cells were grown in an incubator at 37°C/5% CO2. All media were removed 24 h after plating, and fresh media were then applied. For subsequent medium replacement, 50% of media was changed every 2–3 days. Cells were grown for 9–11 days in vitro until near confluence and then used for experiments. Cell culture derived by this protocol yielded 97% GFAP-positive cells (data not shown), implicating the majority of cells as GFAP+ astrocytes.
RNA sample generation: glucocorticoid and antagonist in vitro treatments.
On the day prior to glucocorticoid treatment, all media were removed and replaced with 10% CS-FBS/DMEM to limit residual serum steroid effects. For experiments, cells were treated with 50% fresh medium to a final concentration of 1) 100 nM glucocorticoids (corticosterone or dexamethasone), 2) 100 nM glucocorticoids + 500 nM antagonist (corticosterone + RU486), 3) 500 nM antagonist (RU486), or 4) vehicle (solution containing identical components to treatment group in a given experiment except for steroid/antagonist). For dexamethasone experiments, cells were exposed to a specific treatment for one of eight different lengths of time (0.25, 1, 2, 4, 6, 12, 18, 24 h). For corticosterone/RU486 experiments, cells were exposed to a specific treatment for 2, 4, or 6 h. For all treatment conditions examined, samples for the 2, 4, and 6 h treatments were generated as triplicate samples per group (i.e., 3 glucocorticoid treated, 3 vehicle treated); samples at other time points were generated as duplicate samples (i.e., 2 glucocorticoid treated, 2 vehicle treated). Total RNA samples were collected using TRIzol reagent per manufacturer's protocol (Invitrogen). RNA concentrations were obtained using a Nanodrop spectrophotometer (Thermo Scientific).
Microarray studies.
RNA samples were prepared using Illumina TotalPrep RNA Amplification kit (500 ng/sample, Ambion AMIL1791). Biotinylated RNA samples (1.5 μg/sample) were then applied to MouseWG-6 v2.0 Expression Beadchips (Illumina BD-201-0202) and processed per manufacturer's recommendations. Microarrays were scanned on a Illumina BeadArray Reader. Microarray data were analyzed using Illumina GenomeStudio software (version 1.1.1). GenomeStudio analyses were performed using quantile normalization with an Illumina custom error model and referenced to the corresponding vehicle group. Multiple testing corrections were applied using a Benjamini and Hochberg false discovery rate with differential expression defined as P < 0.05; P values were obtained through the GenomeStudio software. Subsequent calculations (e.g., fold-changes) were derived using Microsoft Excel. For the corticosterone studies, mRNAs were considered to be regulated by corticosterone and attenuated by RU486 by meeting one of the following statistical criteria: 1) corticosterone treatment P < 0.05 and corticosterone + RU486 cotreatment P > 0.05 or 2) corticosterone treatment P < 0.05 and corticosterone-RU486 cotreatment P < 0.05 but corticosterone-RU486 cotreatment resulted in ≥50% reduction in the magnitude of fold-change compared with corticosterone treatment. Regulated Illumina probes were manually analyzed using the National Center for Biotechnology Information (NCBI) gene database to update annotations and identify single genes associated with multiple regulated probes (http://www.ncbi.nlm.nih.gov/gene); for probes that targeted the same gene, the probe containing the highest magnitude of changes was used for downstream analyses. For Figure 1, if a cell type marker had multiple probes, the probe with the highest average signal across all samples was used and then averaged per group per time point for analysis (16 total values). All microarray data are deposited on the NCBI Gene Expression Omnibus database [accession numbers: GSE39270 (corticosterone), GSE39272 (dexamethasone)].
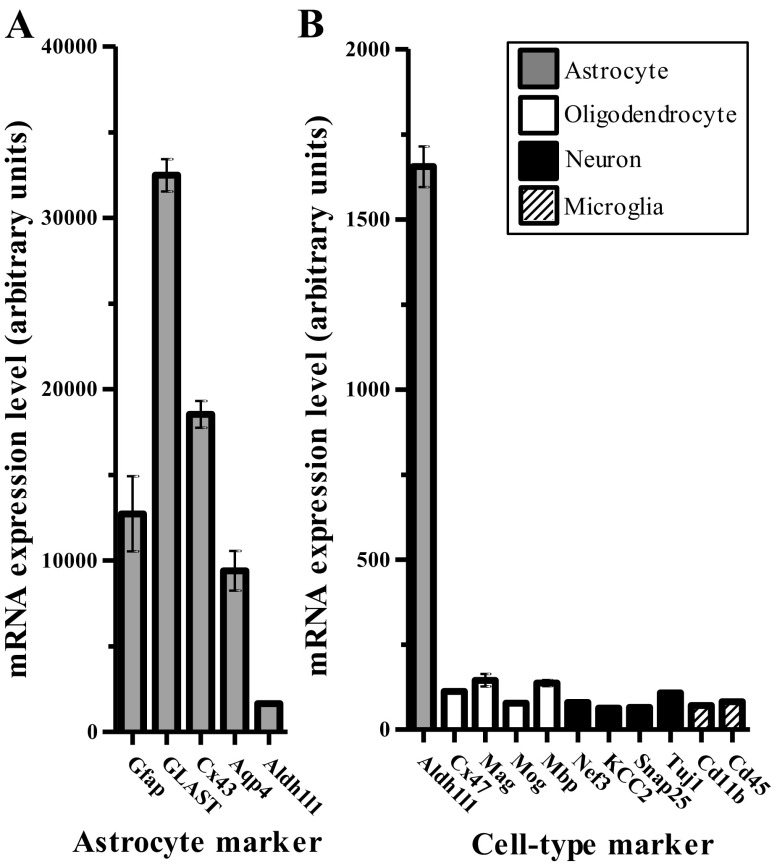
Fig. 1.
Cell culture system specifically expresses astrocyte mRNA cell markers. A: plot of average mRNA expression for subset of astrocyte cell markers among sample groups from 8 time points in Dexamethasone (Dex) time course. B: plot of average mRNA expression for subset of cell markers associated with additional brain cell types (neurons, oligodendrocytes, microglia). Bars for each mRNA are colored according to the cell type association. Error bars display SE.
Gene ontology analysis.
We conducted gene ontology (GO) analyses with the DAVID software database (15, 64) using Illumina probe ID notations for specified sets of mRNAs. In an effort to identify more specific GO terms (vs. broad), we performed analyses using default settings for terms of the GO biological processes (BP_FAT) and GO molecular function categories (MF_FAT).
Hierarchical clustering analysis.
Hierarchical clustering was performed using k-means clustering in R. Clustering analysis was performed on the set of all probes regulated ≥twofold at any time point by dexamethasone. Cluster number (8 clusters) was chosen based on analyzing the lowest number of clusters that produced a sum of squares statistically indifferent from immediately higher numbers of clusters.
qPCR validation studies.
The same RNA samples used for the microarray studies were also used for qPCR analyses. RNA samples (1 μg) were converted to cDNA using Superscript II via random hexamer priming (Invitrogen). We used ∼50% of each cDNA reaction for Applied Biosystems (ABI) Taqman mRNA qPCR assays in custom Low-Density Array (TLDA) format (ABI #4346799) with Taqman reagents (ABI #4440048). The Taqman arrays were processed on an Applied Biosystems Viia7 instrument. Genes measured were selected based on a series of factors (highest magnitude regulation, known/unknown glucocorticoid regulation, astrocyte enrichment, house-keeping gene expression controls). For validation experiments, genes were measured in technical triplicate per sample. Differential expression analysis was performed by the delta-delta-Ct method (Ref. 39, β-actin as control reference) using Statminer software (Integromics). An average of Ct values from technical replicates was taken as the Ct value for each gene measurement; individual Ct values identified as outliers via the Grubbs outlier test were excluded from downstream analyses. Specific Taqman mRNA assays used in this study are for the following targets: Actb (Mm01205647_g1), Adora2b (Mm00839292_m1), Aldh1l1 (Mm00550947_m1), Atp6v1b2 (Mm00431987_m1), Ch25h (Mm00515486_s1), Egr2 (Mm00456650_m1), Fkbp5 (Mm00487401_m1), Folh1 (Mm00489655_m1), Foxo1 (Mm00490672_m1), Gap43 (Mm00500404_m1), Gfap (Mm01253033_m1), Gja1 (Mm00439105_m1), Gjb6 (Mm01317508_m1), Glul (Mm00725701_s1), Klf9 (Mm00495172_m1), Mapk4 (Mm00554001_m1), Mertk (Mm00434920_m1), Pdk4 (Mm01166879_m1), Per1 (Mm00501813_m1), Phlda1 (Mm00456345_g1), Sgk1 (Mm00441380_m1), Slc1a2 (Mm00441457_m1), Slc1a3 (Mm00600697_m1), Sult1a1 (Mm01132072_m1), Syn2 (Mm00449780_m1), Txnip (Mm00452393_m1), Wnt7a (Mm00437354_m1).
Glucocorticoid regulation literature searching and astrocyte mRNA enrichment analyses.
Previously reported glucocorticoid mRNA regulation was assigned based on analysis of the literature for each gene (using PubMed and Google Scholar search tools, terms used: gene symbol and/or probe ID + “glucocorticoids, corticosteroids, corticosterone, dexamethasone, prednisone”). Astrocyte mRNA enrichment analyses were performed using in vivo astrocyte enrichment gene expression data from “Supplemental Table 4” from Cahoy and colleagues (8).
RESULTS
Astrocyte cell cultures robustly express astrocyte markers and do not express markers for other brain cell types.
Previous studies have utilized these cell culture protocols for studying astrocytes in vitro (61, 63). To confirm that our cell culture protocol successfully selected for astrocytes, we examined the mRNA expression of cell-type markers associated with different brain cell types (Fig. 1). Across all groups of the dexamethasone time course, the cell culture model expressed a number of astrocyte-specific cell markers [e.g., GFAP, connexin-43 (Gja1), GLAST (Slc1a3), aquaporin-4 (Aqp4), Aldh1l1], while exhibiting limited or no expression above background of cell markers associated with other brain cell types, i.e., connexin-47 (Cx47), myelin-associated glycoprotein (Mag), myelin oligodendrocyte glycoprotein (Mog), myelin basic protein (Mbp) (oligodendrocytes); neurofilament (Nef3), neuronal K-Cl cotransporter (KCC2/Slc12a5), synaptosomal-associated protein 25 (Snap25), neuron-specific β-tubulin (Tuj1/Tubb3) (neurons); Cd11b/Itgam, Cd45/Ptprc (microglia). These results suggest that the vast majority of cells in this culture system are indeed astrocytes. In addition, nearly identical gene expression patterns of these cell-type mRNA markers were detected in individual groups of samples (i.e., vehicle treated or glucocorticoid treated; data not shown), suggesting that glucocorticoid treatment did not affect the cell-type character of the culture system.
Dexamethasone dynamically regulates mRNAs in astrocytes over time in vitro.
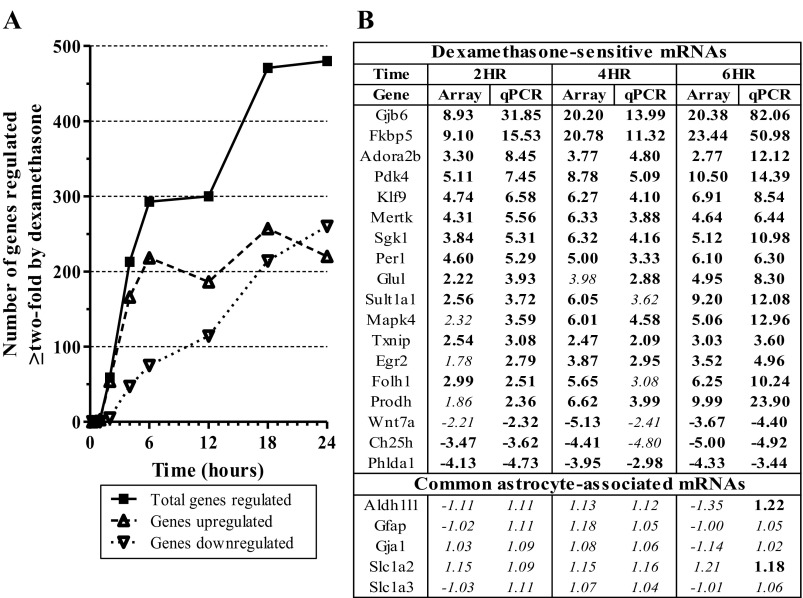
To establish the transcriptome profile of primary glucocorticoid receptor regulation in astrocytes in vitro, primary astrocyte cell cultures were treated with the GR-selective synthetic agonist dexamethasone (100 nM) at 8 different time intervals spanning a 24-h time course (i.e., 0.25, 1, 2, 4, 6, 12, 18, 24 h). Microarray analyses were then performed on the resulting RNA samples to identify glucocorticoid-sensitive mRNAs in this model system (Supplemental Table S1).1 Treatment of primary astrocyte cell cultures with the synthetic GR-agonist dexamethasone resulted in dynamic gene expression changes over a 24 h period of exposure (total 886 probes for 854 unique genes regulated ≥twofold, Fig. 2A, Supplemental Table S2a/b). The total number of mRNAs whose expression level changed ≥twofold increased with the duration of steroid exposure. In addition, the overall directionality of dexamethasone-mediated changes varied across the time course (e.g., majority of genes whose expression changed at early time points were upregulated by dexamethasone). These findings were also highly reproducible in a replicate microarray study of triplicate samples from the 2, 4, and 6 h time points based on the same statistical criteria for differential expression at similar magnitudes of regulation (≥97% overlap of mRNAs regulated ≥twofold in replicate data with mRNAs regulated ≥1.5-fold in time course data, data not shown). qPCR studies confirmed the observed microarray findings for all mRNAs tested for glucocorticoid regulation (18/18 mRNAs, Fig. 2B). The expression level of GR mRNA was similar in all data, suggesting that GR levels were consistent across astrocyte cultures (data not shown). Five astrocyte-associated genes (Slc1a3, Slc1a2, Gfap, Gja1, Aldh1l1) were not altered due to glucocorticoid treatment, indicating that observed mRNA changes were not generic to all astrocyte mRNAs (Fig. 2B).
Fig. 2.
Dex dynamically regulates specific mRNAs in astrocytes in vitro over 24 h time course. A: plot of number of genes that show >2-fold regulation by dexamethasone over time (P < 0.05). B: comparison of linear fold-change mRNA expression values of microarray data with qPCR data for select mRNAs regulated by Dex treatment (vs. vehicle). Data ordered based on magnitude of mRNA expression change in 2 h qPCR data. HR, hours; boldface = P < 0.05; italics = P > 0.05.
Hierarchical clustering analysis reveals subsets of dexamethasone-regulated genes associated with specific cellular functions.
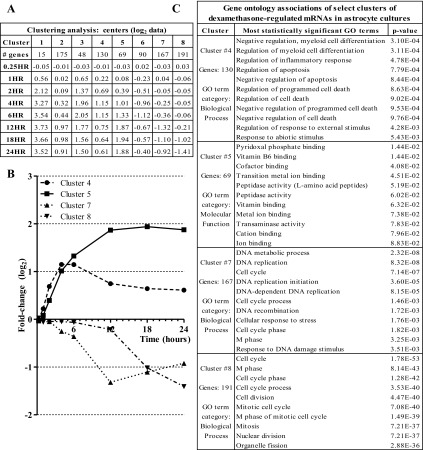
To determine temporal patterns of gene expression changes in astrocytes due to glucocorticoid treatment, we performed a clustering analysis on the set of all mRNAs regulated ≥twofold by dexamethasone using the k-means method. Based on sum of squares analysis, the k-value that best explained the temporal variation using the least amount of clusters was an eight-cluster analysis. Of the eight clusters, five of the cluster averages/centers were upregulated (clusters 1, 2, 3, 4, 5), while three were downregulated (clusters 6, 7, 8) by the 24 h time point (Fig. 3A). Some clusters exhibited average mRNA changes that peaked at earlier time points (e.g., 4 h, 6 h) and tailed off at later time points (clusters 3, 4, 6). Other clusters exhibited more delayed glucocorticoid-mediated changes (e.g., not changed until ≥12 h) (clusters 2, 7, 8). GO analyses were then performed on the genes in each cluster using the DAVID software. GO analysis of these clusters revealed distinct cellular targets of glucocorticoid regulation based on the duration of steroid exposure. Of the eight clusters examined, the most significantly associated biological processes in four of them were highly enriched for specific cellular functions; the other four clusters were not highly correlated with any one type of biological process. The four enriched clusters were associated with 1) apoptosis (cluster 4, increase of negative regulation), 2) cofactor and ion binding (cluster 5, positive correlation), 3) DNA replication (cluster 7, negative correlation), and 4) cell cycle (cluster 8, negative correlation) (Figs. 3, B and C; genes listed in Supplemental Table S3).
Fig. 3.
Clustering analysis of Dex gene expression time course data yields distinct sets of genes that contain similar gene ontology (GO) categories. A: table of number of genes per cluster and cluster centers (average log2 value) over the time course. B: graph of 4 GO term-associated cluster centers from k-means cluster analysis. C: table of graphed clusters and their corresponding most significant GO terms.
Dexamethasone-sensitive mRNAs also regulated by natural ligand corticosterone via the GR.
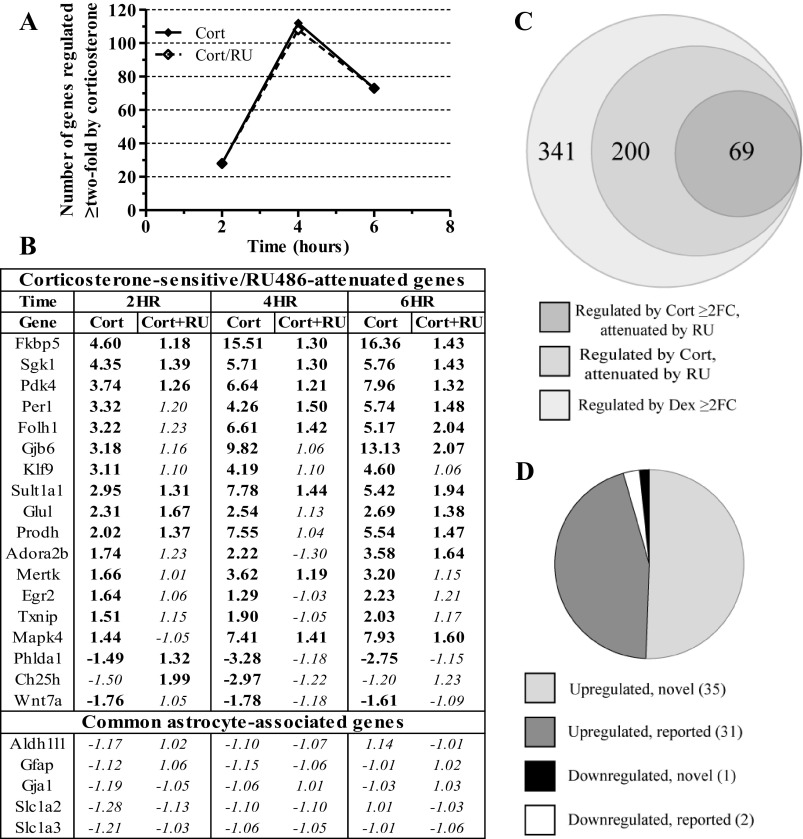
To determine if dexamethasone-regulated mRNAs were similarly regulated by the natural ligand of the GR, we treated primary astrocyte cultures with corticosterone (100 nM) for an abbreviated time course, targeting the time points of initial regulation in the dexamethasone time course (i.e., 2, 4, 6 h). Corticosterone treatment altered the expression level of numerous genes at these three time points (2 h, 28 mRNAs; 4 h, 112 mRNAs; 6 h, 73 mRNAs) (Fig. 4A). To determine if the observed mRNA expression differences were mediated through the GR, we treated parallel cultures with both corticosterone and RU486 (500 nM), a GR-selective antagonist. All 141 unique mRNAs with ≥twofold expression changes induced by corticosterone were attenuated by cotreatment with RU486 at one or more of the time points (Fig. 4A, Supplemental Table S4). qPCR data of a subset of corticosterone-regulated/RU486-attenuated mRNAs matched the directionality and magnitude of the microarray data (18/18 mRNAs, Fig. 4B). Comparison of corticosterone-regulated/RU486-attenuated mRNAs demonstrating ≥twofold expression change with mRNAs regulated ≥twofold by dexamethasone at similar time points revealed substantial but incomplete overlap (Fig. 4C), identifying more genes regulated ≥twofold by dexamethasone than corticosterone. The set of corticosterone-regulated/RU486-attenuated mRNAs as defined by statistical criterion but with the twofold change criterion removed (i.e., corticosterone P < 0.05, corticosterone + RU486 P > 0.05) contained a greater number of mRNAs regulated ≥twofold by dexamethasone (Fig. 4C). Based on this latter comparison, ∼60% of mRNAs regulated ≥twofold by dexamethasone were also regulated by corticosterone and attenuated by RU486. mRNAs that were regulated ≥twofold by corticosterone and dexamethasone and whose corticosterone regulation was attenuated by RU486 were primarily upregulated by glucocorticoid treatment (Fig. 4D). Of these 69 mRNAs regulated by both glucocorticoids, 33 have been previously associated with glucocorticoid regulation (Table 1), whereas 36 demonstrated novel glucocorticoid regulation (Table 2).
Fig. 4.
mRNAs regulated by synthetic glucocorticoid in primary astrocyte cultures are also regulated by natural ligand and the regulation is attenuated by GR antagonist. A: plot of number of genes that show >2-fold mRNA expression change due to corticosterone (Cort) that is attenuated by RU486 cotreatment (Cort/RU) (P < 0.05). B: table of qPCR validation data on select Cort-sensitive mRNAs. Genes ordered by magnitude of fold-change due to 2 h Cort exposure. C: proportional Venn diagram of overlap of genes regulated >2-fold by Dex at 2, 4, or 6 h vs. genes regulated by Cort (P < 0.05) and attenuated by RU486 (P > 0.05) at those same time points. D: graph characterizing genes regulated by both Dex and Cort ≥2-fold and also attenuated by RU486 in terms of previous literature reporting and direction of regulation. Regulated by Cort = P < 0.05, attenuated by RU = P > 0.05. FC, fold-change; boldface = P < 0.05, italics = P > 0.05.
Table 1.
Glucocorticoid-sensitive genes with reported GR-mediated regulation
| Gene Symbol | Definition | Direction | Neural Reported? |
|---|---|---|---|
| Ada | adenosine deaminase | upregulated | |
| Bcl6 | B-cell leukemia/lymphoma 6 | upregulated | |
| Cbs | cystathionine beta-synthase | upregulated | + |
| Ccnd2 | cyclin D2 | downregulated | |
| Errfi1 | ERBB receptor feedback inhibitor 1 | upregulated | + |
| Fam107a | family with sequence similarity 107 member A | upregulated | + |
| Fkbp5 | FK506 binding protein 5 | upregulated | + |
| Gadd45 g | growth arrest and DNA-damage-inducible 45 gamma | upregulated | + |
| Hdc | histidine decarboxylase | upregulated | + |
| Hspa1a | heat shock protein 1A | upregulated | |
| Ipas/Hif3a | inhibitory PAS domain AKA Hypoxia-inducible factor 3 | upregulated | + |
| Klf9 | Kruppel-like factor 9 | upregulated | + |
| Lcn2 | lipocalin 2 | upregulated | + |
| Lims2 | LIM and senescent cell antigen like domains 2 | upregulated | |
| Map 3k6 | mitogen-activated protein kinase kinase kinase 6 | upregulated | |
| Map k4 | mitogen-activated protein kinase 4 | upregulated | |
| Mertk | c-mer proto-oncogene tyrosine kinase | upregulated | + |
| M gll | monogly ceride lipase | upregulated | |
| Pdk4 | pyruvate dehy drogenase kinase, isozyme 4 | upregulated | + |
| Per1 | period homolog 1 | upregulated | + |
| Phlda1 | pleckstrin homology-like domain family A member 1 | downregulated | |
| Pim3 | proviral integration site 3 | upregulated | |
| Prodh | proline dehy drogenase | upregulated | |
| Rhou | Ras homolog gene family member U | upregulated | |
| S3-12 | plasma membrane associated protein S3–12 | upregulated | |
| Sesn1 | sestrin 1 | upregulated | |
| Sgk1 | serum/glucocorticoid regulated kinase 1 | upregulated | + |
| Sphk1 | sphingosine kinase 1 | upregulated | |
| Sult1a1 | sulfotransferase family 1A phenol-preferring member 1 | upregulated | + |
| Tsc22d3 | TSC22 domain family member 3 | upregulated | + |
| Txnip | thioredoxin interacting protein | upregulated | |
| Zbtb16 | zinc finger and BTB domain containing 16 | upregulated |
GR, glucocorticoid receptor.
Table 2.
Glucocorticoid-sensitive genes demonstrating novel GR-mediated regulation
| Gene Symbol | Definition | Direction |
|---|---|---|
| 1810011O10Rik | RIKEN cDNA 1810011O10 gene | upregulated |
| 9930004G02Rik | RIKEN cDNA 9930004G02 gene | upregulated |
| Accn1 | amiloride-sensitive cation channel 1 neuronal (degenerin) | upregulated |
| Acsl3 | acyl-CoA synthetase long-chain family member 3 | upregulated |
| Axud1 | AXIN1 up-regulated 1 | upregulated |
| Bcat1 | branched chain aminotransferase 1 cytosolic | upregulated |
| C030009J22Rik | RIKEN cDNA C030009J22 gene | upregulated |
| Chk | choline kinase alpha | upregulated |
| Cldn2 | claudin 2 | upregulated |
| E230024B12Rik | RIKEN cDNA E230024B12 gene | upregulated |
| Fam46b | family with sequence similarity 46, member B (Fam46b) | upregulated |
| Folh1 | folate hydrolase 1 | upregulated* |
| Galntl4 | UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 4 | upregulated |
| Gjb6 | gap junction protein beta 6 | upregulated |
| Gm22 | gene model 22 | upregulated |
| Got1l1 | glutamic-oxaloacetic transaminase 1-like 1 | upregulated |
| Hspb1 | heat shock protein 1 | upregulated |
| Kcnt1 | potassium channel subfamily T member 1 | upregulated |
| LOC385279 | LOC385279 | upregulated |
| Lpin3 | lipin3 | upregulated* |
| Lrrc8 | leucine-rich repeat containing 8A | upregulated |
| Mical2 | microtubule associated monoxygenase, calponin and LIM domain containing 2 | upregulated |
| Papss2 | 3′-phosphoadenosine 5′-phosphosulfate synthase 2 | upregulated* |
| Pex11a | peroxisomal biogenesis factor 11a | upregulated |
| Pgm5 | phosphoglucomutase 5 | upregulated |
| Rassf4 | Ras association (RalGDS/AF-6) domain family member 4 | upregulated |
| Rbpms | RNA binding protein gene with multiple splicing | upregulated |
| Scel | sciellin | upregulated |
| Slc10a6 | solute carrier family 10 (sodium/bile acid cotransporter family) member 6 | upregulated |
| Slc24a4 | solute carrier family 24 (sodium/potassium/calcium exchanger) member 4 | upregulated |
| Slc25a33 | solute carrier family 25 (pyrimidine nucleotide carrier) member 33 | upregulated |
| Smox | spermine oxidase | upregulated |
| Spsb1 | SplA/ryanodine receptor domain and SOCS box containing 1 | upregulated |
| Tcfcp2l1 | transcription factor CP2-like 1 | upregulated |
| Tilz3c | TSC22-related-inducible leucine zipper 3c | upregulated |
| Tmem200a | transmembrane protein 200A | downregulated |
Previously reported as glucocorticoid insensitive.
mRNAs regulated by glucocorticoids in cortical astrocyte cultures are also regulated in hippocampal astrocyte cultures.
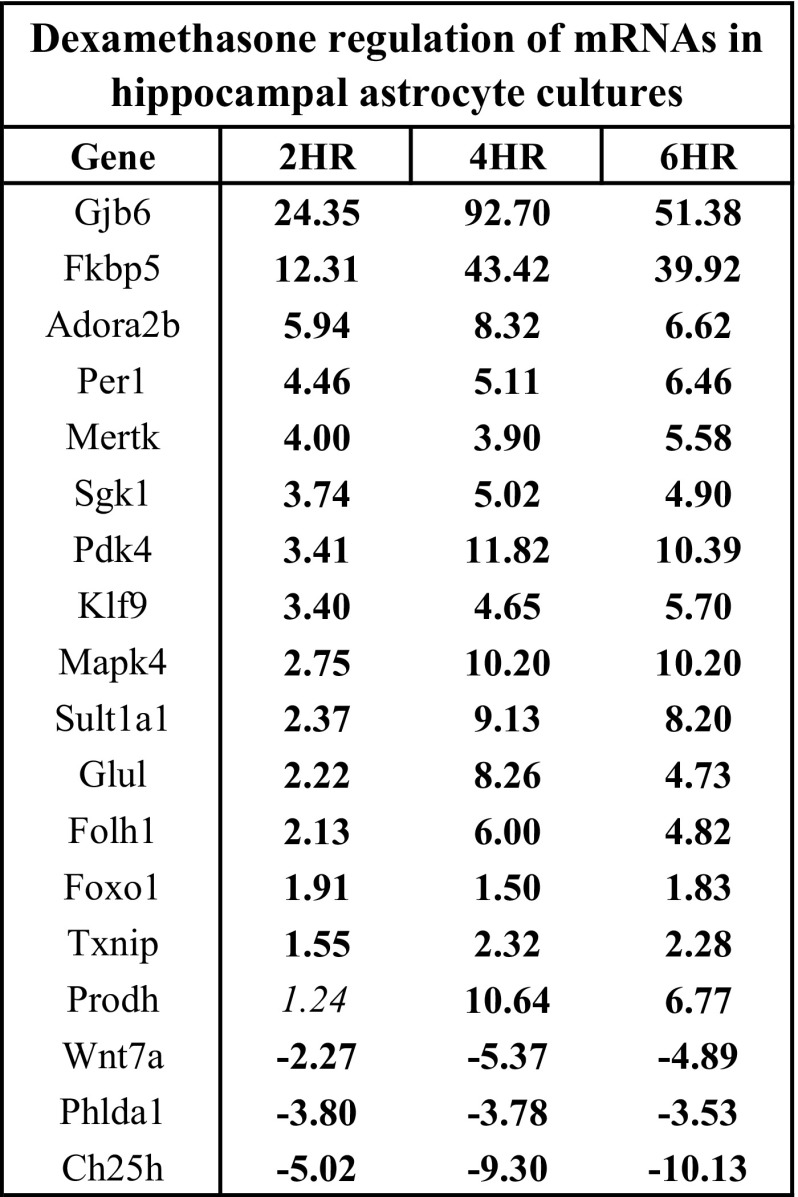
To determine if observed glucocorticoid-mediated mRNA changes were specific to astrocytes derived from cortical brain regions, additional qPCR experiments were conducted on RNA samples isolated from primary astrocyte cell cultures derived from hippocampus and treated with dexamethasone. All mRNAs tested for dexamethasone regulation by qPCR in cortical astrocyte cultures were also regulated by dexamethasone in hippocampal astrocyte cultures (18/18 mRNAs, P < 0.05) (Fig. 5).
Fig. 5.
A portion of mRNAs regulated by glucocorticoids in cortical astrocyte cultures is also regulated by glucocorticoids in hippocampal astrocyte cultures. qPCR data of select mRNAs regulated by Dex treatment in cortical astrocyte cultures that are also hippocampal astrocyte cultures. Genes ordered based on 2 h FC values. Boldface = P < 0.05, italics = P > 0.05.
Glucocorticoid-regulated mRNAs include sets of astrocyte-enriched genes.
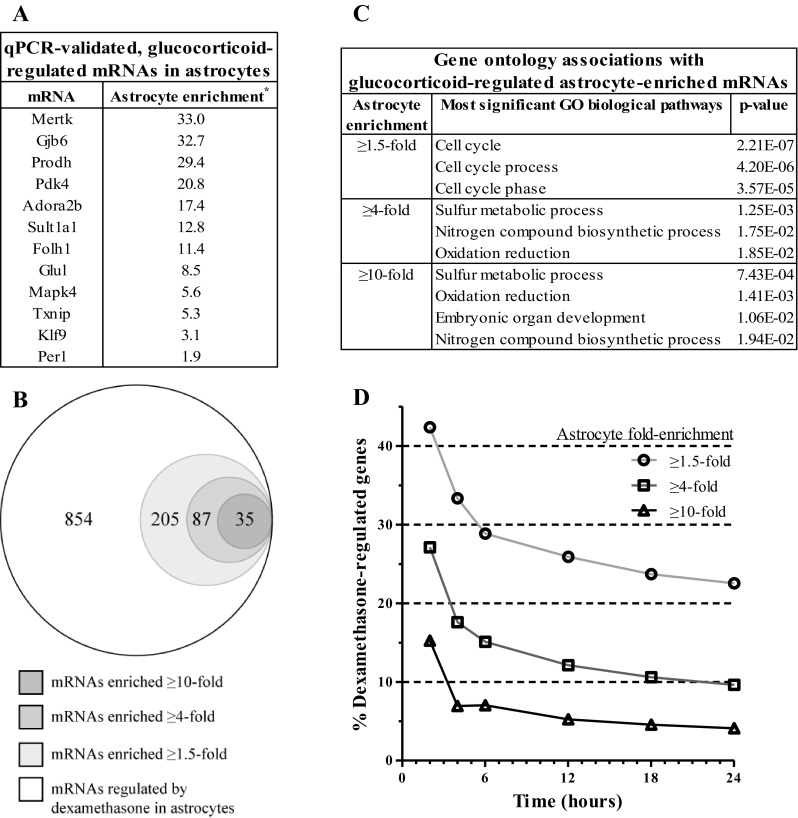
A portion of the mRNAs regulated by glucocorticoids in these data was known to be specifically expressed in astrocytes (e.g., Glul, Gjb6; Fig. 6A). In an effort to determine how glucocorticoids might modulate unique astrocyte cell functions, mRNAs regulated by glucocorticoids were compared with mRNAs reported to be enriched in astrocytes, thus described as “astrocyte enriched” (8). On average, the astrocyte cultures expressed ≥60–70% of astrocyte-enriched mRNAs (≥1.5-fold enrichment), which represented 13–14.5% of the detected mRNAs across samples (data not shown). Many genes identified as glucocorticoid sensitive in these data are reported to be astrocyte enriched (overall percentage 23.4%, Fig. 6B). To investigate the functional implications of these findings, glucocorticoid-regulated, we analyzed astrocyte-enriched genes for GO term association (Fig. 6C). The most significant “biological processes” term associated with the broadest enrichment category (≥1.5-fold enrichment) was related to cell cycle. The biological processes terms most associated with greater astrocyte-enrichment levels (≥4-fold, ≥10-fold) included 1) sulfur metabolic processes (genes upregulated), 2) nitrogen compound biosynthesis (genes upregulated), and 3) oxidation reduction (genes upregulated) (Table 3). Among glucocorticoid-sensitive mRNAs, the percentage of astrocyte-enriched mRNAs as a function of all glucocorticoid-regulated mRNAs was higher for shorter durations of glucocorticoid exposure compared with longer durations over the time course (e.g., 2 h = 42.4%, 24 h = 22.5%); this percentage consistently trended downward over the entire time course, reaching a value at the 24-h time point that was slightly below the overall percentage for the entire time course (Fig. 6D).
Fig. 6.
Certain mRNAs regulated by Dex are astrocyte enriched. A: table of genes found to be regulated ≥2-fold by Dex and Cort in both microarray and qPCR experiments and their reported enrichment in astrocytes relative to other neural cell types. Genes arranged by magnitude of enrichment. *Cahoy et. al., (8). B: proportional Venn diagram of overlap of total number of unique Dex-regulated mRNAs with 3 subsets of astrocyte-enriched mRNAs. C: table containing most statistically significant GO category associated with 3 astrocyte-enriched mRNA subsets of Dex-regulated mRNAs in B. D: plot of percentage of astrocyte-enriched mRNAs as a function of total mRNAs regulated ≥2-fold by Dex over time.
Table 3.
GO associations of glucocorticoid-sensitive astrocyte-enriched genes
| Illumina ID | Symbol | Gene Name | Astrocyte Enrichment* | Direction |
|---|---|---|---|---|
| GO term: sulfur metabolism | ||||
| ILMN_1253588 | Papss2 | 3′-phosphoadenosine 5′-phosphosulfate synthase 2 | 32.7 | upregulated |
| ILMN_2750558 | Mamdc2 | MAM domain containing 2 | 16.2 | downregulated |
| ILMN_1230318 | Cbs | cystathionine beta-synthase | 23.4 | upregulated |
| ILMN_2669416 | Mgst1 | microsomal glutathione S-transferase 1 | 19.2 | upregulated |
| ILMN_2776008 | Gstk1 | glutathione S-transferase kappa 1 | 4.7 | upregulated |
| GO term: nitrogen biosynthetic process | ||||
| ILMN_2722716 | Atp1a2 | ATPase, Na+/K+ transporting, alpha 2 polypeptide | 29.8 | upregulated |
| ILMN_1230318 | Cbs | cystathionine beta-synthase | 23.4 | upregulated |
| ILMN_2636666 | Prodh | proline dehy drogenase | 29.4 | upregulated |
| ILMN_1219442 | Slc7a2 | solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 | 21.2 | upregulated |
| ILMN_1243741 | Rora | RAR-related orphan receptor alpha | 4.8 | upregulated |
| ILMN_2644496 | Glul | glutamate-ammonia ligase (glutamine synthetase) | 8.5 | upregulated |
| GO term: oxidation reduction | ||||
| ILMN_1222765 | Adhfe1 | alcohol dehydrogenase, iron containing, 1 | 22.6 | upregulated |
| ILMN_1222734 | Aldh1a1 | aldehyde dehydrogenase family 1, subfamily A1 | 11.7 | upregulated |
| ILMN_1214498 | Cyp2d22 | cytochrome P450, family 2, subfamily d, polypeptide 22 | 16.8 | upregulated |
| ILMN_1251504 | Cyp4v3 | cytochrome P450, family 4, subfamily v, polypeptide 3 | 13.6 | upregulated |
| ILMN_2766037 | Dio2 | deiodinase, iodothyronine, type II | 56 | upregulated |
| ILMN_1250917 | Fmo1 | flavin containing monooxygenase 1 | 19.7 | upregulated |
| ILMN_2636666 | Prodh | proline dehydrogenase | 29.4 | upregulated |
| ILMN_2644092 | Aass | aminoadipate-semialdehyde synthase | 6.1 | upregulated |
| ILMN_1258158 | Aldh6a1 | aldehyde dehydrogenase family 6, subfamily A1 | 4.4 | upregulated |
GO, gene ontology.
Cahoy et al., (8).
DISCUSSION
Cell-specific mechanisms in the brain have long been of interest in an effort to understand normal neural processes and disease pathology. The goal of these studies was to characterize glucocorticoid-regulated mRNA regulation in astrocytes in vitro. Previous studies had identified genes regulated by glucocorticoids in astrocytes, but 1) mRNA regulation was observed on an individual gene basis and 2) few published reports have looked extensively at patterns of mRNA changes due to glucocorticoid exposure at time points less than 24 h. To extend current knowledge of GR-mediated transcriptional regulation to shorter durations of glucocorticoid exposure, we treated cortical astrocyte cultures with the synthetic GR agonist dexamethasone over eight time points from 15 min to 24 h. We chose a glucocorticoid concentration relevant to stress mechanisms (100 nM) to parallel 1) concentrations commonly used in other in vitro gene expression studies and 2) the range of corticosterone concentrations observed in the brain as measured by microdialysis following acute stress in rodents (e.g., Refs. 18, 59, 70).
Over the 24 h time course, we identified 854 unique genes that were regulated more than twofold at one or more time points by dexamethasone compared with vehicle control treatment. In an effort to validate the observed transcriptional regulation, we performed qPCR experiments on samples at the earliest time points that contained large numbers of regulated mRNAs (i.e., 2 h, 4 h, 6 h). All of the genes tested for glucocorticoid sensitivity by qPCR showed significant differences similar in magnitude and direction to the microarray data (18/18 mRNAs). We also observed that mRNAs previously reported to be glucocorticoid-regulated in astrocytes were regulated in manner consistent with the literature, e.g., Glul (37), Glt-1/Slc1a2 (76), MAO-B (10), Ndrg2 (69), GDH (29), with some exceptions, e.g., Fgf2 (43), GFAP (60). The exceptions may be the result of a number of factors, e.g., methods of cell preparation, species differences (e.g., mouse vs. rat), including primary vs. secondary effects of glucocorticoids based on length of glucocorticoid exposure in culture, e.g., GFAP glucocorticoid regulation sensitive to time of exposure (60). The overall consistency of our results with previous findings supports the validity of this data in terms of characterizing glucocorticoid regulation in astrocytes in vitro.
The dynamic temporal gene expression regulation by glucocorticoids observed over 24 h raises the possibility of coordinated pathway regulation. To examine relationships between the regulated mRNAs, we performed 1) a clustering analysis using eight clusters to group mRNAs according to glucocorticoid-mediated expression changes over time and 2) GO analyses on the mRNAs in each cluster. The clustering analysis identified novel temporal patterns of regulation among the glucocorticoid-regulated mRNAs. Clusters of mRNAs varied by the magnitude and rate of glucocorticoid-induced activation or repression (Fig. 3). Our results are similar to spatial patterns observed in previous reports characterizing time-dependent mRNA expression changes mediated by glucocorticoids in other cell culture systems (33, 57). From the GO analysis, the most statistically enriched biological processes changed between time points over the 24-h time course; different pathways were affected at earlier time points compared with later time points. Among the eight clusters, specific clusters associated with distinguishable sets of similar GO terms (cluster 4 = increased gene expression related to negative regulation of apoptosis, cluster 5 = increased gene expression related to cofactor binding and ion binding, cluster 7 = decreased gene expression related to DNA replication, cluster 8 = decreased gene expression related to cell cycle regulation). Negative regulation of apoptosis, DNA replication, and cell cycle pathways has often been associated with glucocorticoid regulation; glucocorticoids are known to inhibit proliferation in many cell types, including astrocytes (14). Cofactors and ions associated with mRNAs represented in the binding-related cluster included zinc (Bcl6b, Dpep1, Trp63, SceI, Cpm, Gm22, Lims2, Zhx3), pyridoxal phosphate (Cbs, Got1l1, Agxt2l1), and divalent cations (calcium: Dlk1, S100a7a, Arsj, Galntl2; magnesium: Pgm5, Acsl3, Ppm1k; iron: Cdo1, Cyp2d22). Together, these findings document that specific pathways in astrocytes are targeted by glucocorticoids across time in vitro.
To extend the observed synthetic glucocorticoid regulation to the endogenous ligand and further verify the GR-based mechanism of regulation on target mRNAs in astrocytes, we conducted microarray experiments on cortical astrocyte cell culture samples treated with 1) the endogenous ligand corticosterone and 2) corticosterone coadministered with RU486 (mifepristone), a GR-selective antagonist. Cotreatment of corticosterone and RU486 attenuated the regulation of almost all of the mRNAs regulated ≥twofold by corticosterone alone, suggesting they were regulated via GR. RU486 acted as an antagonist to corticosterone action for these robustly glucocorticoid-regulated mRNAs, consistent with reports involving glucocorticoid-antiglucocorticoid coadministration (e.g., Refs. 3, 7, 35). Although RU486 has been reported to have agonistic properties under select circumstances (e.g., Refs. 9, 53), we did not observe significant agonist activity by RU486 in these experiments (i.e., RU486 treatment alone). RU486 agonist activity has been suggested to be cell-type dependent (45). Although RU486 action has not been explicitly studied in astrocytes, the combination of GR concentration (74), corepressor expression (32, 42), and other cellular factors in astrocytes may not permit substantial RU486 agonist activity in this context.
Because both dexamethasone and corticosterone signal through the GR, we predicted the two steroids would regulate mRNAs in our astrocyte culture system in a similar manner. Previous comparative in vitro studies have found similar regulation between the two steroids for select mRNA targets (e.g., Ref. 66). Compared with dexamethasone treatment, corticosterone treatment regulated a smaller number of mRNAs ≥twofold at 2, 4, and 6 h of steroid exposure [absolute number of regulated genes: 341 (dexamethasone) vs. 141 (corticosterone)]. While a portion of the discrepancy may be due to experimental variation and astrocyte heterogeneity, this difference in the number of mRNAs regulated ≥twofold by these two glucocorticoids may be due in part to the higher GR affinity of dexamethasone vs. corticosterone (24, 35, 58). Differential responses to time-limited treatments of dexamethasone vs. corticosterone have been documented for other mRNAs (e.g., Refs. 19, 31, 40). In our experiments, the set of genes statistically regulated by corticosterone (P < 0.05) without the fold-change criteria included additional genes regulated ≥twofold by dexamethasone (133 additional genes, Fig. 4). These results are consistent with the interpretation that dexamethasone is a more robust regulator of initial mRNA expression changes than corticosterone at specific time points within our time course. The equivalence of dexamethasone and corticosterone regulation observed in previous studies may be due to longer duration of glucocorticoid exposures (e.g., 24+ h) that may overcome temporal, pharmacological distinctions between these molecules. Based on our findings, future temporal studies comparing regulation by dexamethasone and corticosterone may yield more precise comparisons of regulated mRNAs by matching physiological criteria other than absolute steroid concentration (e.g., specified IC50 or EC50 to GR).
We then compared the sets of 1) mRNAs regulated by dexamethasone with 2) mRNAs regulated by corticosterone and attenuated by RU486 cotreatment at any of the 2, 4, or 6 h time points. We found 69 mRNAs regulated more than twofold by both glucocorticoids that meet this criteria. Among these mRNAs, 33 had previously been associated with glucocorticoid regulation (31 upregulated, 2 downregulated; Table 1) and 36 displayed novel glucocorticoid regulation (35 up, 1 down; Table 2). Our findings extend the observed glucocorticoid regulation in other systems to astrocytes; among the mRNAs known to be regulated by glucocorticoids, all of the mRNA changes in this study were in the same direction as previously reported (data not shown). Both previously reported and the unreported glucocorticoid-regulated mRNAs may have unique actions in astrocytes; their functional roles in astrocytes remain to be investigated.
Comparisons of astrocyte gene expression between brain regions are infrequent despite the growing evidence for astrocyte heterogeneity and brain region-dependent astrocyte differences associated with both mRNA regulation and specific diseases (75, 76). Most astrocyte cell culture studies have derived cells in a brain region-independent manner (e.g., whole brain or hemispheres), yet astrocytes derived from different brain regions have been reported to have different gene expression profiles (73). To determine if the observed gene expression changes were specific to cortical astrocyte cultures, we measured the glucocorticoid sensitivity of select mRNAs in hippocampal astrocyte cultures. All of the genes measured were regulated by glucocorticoids in both cortical cultures and hippocampal cultures, indicating that these glucocorticoid effects occur in astrocytes derived from multiple brain regions (Fig. 5). This observed regulation could result from common mechanisms for robustly regulated mRNAs (i.e., directly mediated by GR), although we cannot exclude the possibility that astrocytes in vitro undergo cumulative cellular changes that disrupt or remove brain-region dependent differences. Whether the glucocorticoid regulation of mRNAs in astrocytes characterized in our experiments occurs in all astrocytes regardless of brain region source remains an open question. Additional experiments will be needed for comprehensive transcriptional comparisons of glucocorticoid regulation between astrocyte cell cultures derived from different brain regions (e.g., basal gene expression concordance for all mRNAs by brain region source, examining lower fold-change expression changes).
Some mRNAs regulated by glucocorticoids in our experiments have been previously reported to have enriched expression in astrocytes compared with other brain cell types (i.e., neurons, oligodendrocytes) (8) (Fig. 6, A and B). Transcriptional regulation of these astrocyte-enriched mRNAs may have particular functional significance for astrocytes. Many mRNAs previously associated with glucocorticoid regulation as well as mRNAs displaying novel regulation are also astrocyte-enriched, representing 205 of the 854 unique mRNAs regulated by glucocorticoids (24.0%). In addition, multiple mRNAs (Atp6v1b2, Gap43, Syn2) reported to be both 1) enriched in neurons (8) and 2) regulated by glucocorticoids (21, 65) were not regulated in our astrocyte culture system (data not shown), further suggesting that glucocorticoids act in a cell-specific manner among brain cell types.
What is the functional output of regulating these astrocyte-enriched mRNAs? GO analyses of mRNAs from the entire time course data set that were enriched in astrocytes ≥fourfold and ≥10-fold both identified the terms “sulfur metabolism” (Papss2, Mamdc2, Cbs, Mgst1, Gstk1), “nitrogen compound biosynthetic process” (Atp1a2, Cbs, Prodh, Slc7a2, Rora, Glul), and “oxidation reduction” (Adhfe1, Aldh1a1, Cyp2d22, Cyp4v3, Dio2, Fmo1, Prodh, Aass, Aldh6a1) as significantly enriched processes (Fig. 6C, Table 3). The regulated mRNAs associated with nitrogen biosynthetic processes and oxidation reduction encode for proteins associated with a range of cellular processes, including nitric oxide metabolism (e.g., Slc7a2, Rora) and amino acid metabolism and transport (e.g., Cbs, Prodh, Slc7a2, Glul, Aass, Aldh6a1). These pathways are associated with the cellular stress response, a reaction to glucocorticoid stimulation that is known to increase reactive oxygen species and cellular metabolic load (16).
We also observed striking basal and temporal trends in the percentage of glucocorticoid-regulated mRNAs that were astrocyte enriched. First, the percentage of glucocorticoid-regulated mRNAs that are astrocyte enriched was significantly higher for all lengths of glucocorticoid exposure than the average percentage for all detected mRNAs (range across all time points: 13–14.5%). All astrocyte-enriched percentages for glucocorticoid-regulated mRNAs were >1.5× greater and up to 3× greater than the astrocyte-enriched percentages for all expressed mRNAs. The difference in percentages implies that astrocyte-enriched mRNAs are on average more likely to be regulated by glucocorticoids compared with all mRNAs expressed in our astrocyte cultures. Our dataset is one of the first to demonstrate empirically a bias in transcriptional regulation in astrocytes toward astrocyte-enriched mRNAs at the level of the transcriptome in vitro. Second, we noticed that astrocyte-enriched mRNAs represented a greater percentage of glucocorticoid-regulated genes at earlier time points compared with later time points in the time course. This percentage steadily declined over the entire time course (e.g., for 1.5-fold enriched mRNAs, 42.4% at 2 h → 22.5% at 24 h). This temporal trend suggests an additional bias of glucocorticoid regulation toward astrocyte-enriched mRNAs in initial astrocyte responses to steroid exposure.
Generalizability of these findings to other brain cell types and additional transcriptional regulators is an intriguing potential extension of these findings. Glucocorticoids are thought to act in a cell-type dependent fashion based upon a number of factors, including 1) basal gene expression patterns (e.g., genes expressed only in astrocytes based on chromatin structure and gene availability/regulation) and 2) the compliment of transcriptional regulators present in the cell (e.g., cell-dependent transcription factor expression and interactions with GR in astrocytes) (54a, 67, 71). We have added empirical evidence suggesting that cell-enriched mRNAs may direct or guide initial cellular responses to glucocorticoids. Based on our findings, understanding the response of a specific cell type to a given signaling cascade may be enhanced by 1) knowing which mRNAs are enriched in that cell type relative to other cells in a given tissue and 2) investigating the regulation of cell type-enriched mRNAs. We currently do not know how the observed glucocorticoid regulation in astrocytes compares with glucocorticoid regulation in other brain cell types (e.g., neurons, oligodendrocytes), although there are data available on glucocorticoid mRNA regulation in hippocampal slices and a neuronal cell line (48, 49). As additional cell type-enriched expression data become available, comparing and contrasting our findings with glucocorticoid regulation in other cell types, both in the brain and in other tissues, may increase our understanding of the mechanisms of cell type-specific glucocorticoid regulation.
There are caveats to consider in interpreting these results. First, we used stringent criteria to define differential expression. mRNAs were considered differentially expressed in the microarray experiments when they were changed more than twofold by both dexamethasone and corticosterone vs. vehicle treatment. While this conservative criterion increases the likelihood of identifying authentic glucocorticoid regulation by limiting the identification of false positives, it also excludes mRNAs that are regulated at a lower magnitude by dexamethasone, corticosterone, or RU486. In addition, some genes were statistically regulated by one glucocorticoid and not the other; such genes may be authentic glucocorticoid targets but would not be identified as such based on these criteria (e.g., qPCR validation of Adora2b and Wnt7a). Second, all of these gene expression experiments were conducted on astrocytes in vitro. This approach isolated the glucocorticoid response in astrocytes from other cell types, but it does not assess the effects in vivo. As with many primary culture systems, cultured astrocytes are reported to have different gene expression profiles compared with astrocytes in vivo (8, 22). Additional experiments will be needed to determine how the glucocorticoid regulation in astrocytes in vitro translates to the in vivo environment and corresponding physiological influence. Third, astrocytes in the brain are regulated in the context of intercellular interactions; how glucocorticoids affect astrocytes and these mRNAs in their interactions with neurons and other glial cell types in the intact brain remains to be investigated. In addition, although a subset of regulated mRNAs are reportedly astrocyte enriched, these mRNAs may be expressed in other cell types. We are interested in knowing if the glucocorticoid regulation of these mRNAs observed in astrocytes also occurs in a similar manner in other cell types. Fourth, while the time course of regulation is consistent with primary transcriptional regulation, we have not demonstrated that GR directly modulates the transcription of these genes but rather that mRNA levels change. Future studies can be designed to assess whether GR binds the promoter domains near these the genomic origins of these glucocorticoid-regulated mRNAs. Finally, the associations mentioned in terms of astrocyte enrichment and gene ontology analyses are derived from transcriptome-level data sets. Individual gene connections and pathway enrichment must be further investigated to confirm the neurological relevance of these statistical connections.
In summary, we have characterized the responsiveness of mRNAs in astrocytes to glucocorticoids in vitro. To our knowledge, our study is the most extensive reported investigation of the temporal patterning of glucocorticoid-mediated mRNA regulation at the transcriptome level in a specific glial cell type. We find that 1) glucocorticoids regulate a specific set of mRNAs in astrocytes in vitro, 2) the observed dynamic gene expression regulation contains associations with specific biological pathways, 3) glucocorticoids regulate specific mRNAs in astrocyte cultures derived from multiple brain regions, and 4) a subset of glucocorticoid-regulated mRNAs is enriched in astrocytes. Together, these data add to the knowledge of glucocorticoid-mediated gene expression regulation and further enhance our understanding of glucocorticoid signaling and stress biology in the brain.
GRANTS
Funds used to support this work include National Institutes of Health Grants T32-EY-017878 (B. S. Carter), T32-MH-014279 (B. S. Carter), and R01-DA-025973 (R. C. Thompson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.S.C. and R.C.T. conception and design of research; B.S.C. performed experiments; B.S.C., F.M., and R.C.T. analyzed data; B.S.C. and R.C.T. interpreted results of experiments; B.S.C. prepared figures; B.S.C. drafted manuscript; B.S.C. and R.C.T. edited and revised manuscript; B.S.C., F.M., and R.C.T. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Audrey F. Seasholtz for helpful discussions on the manuscript. We thank Suzanne Smith for technical assistance on the microarray experiments.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte-and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophrenia Res 112: 54–64, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Baulieu E. The steroid hormone antagonist RU486. Mechanism at the cellular level and clinical applications. Endocrinol Metab Clin No Am 20: 873, 1991. [PubMed] [Google Scholar]
- 4. Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16: 634–646, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia 55: 1263–1271, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol 79: 77–89, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Ann Rev Med 48: 129–156, 1997. [DOI] [PubMed] [Google Scholar]
- 8. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cairns C, Cairns W, Okret S. Inhibition of gene expression by steroid hormone receptors via a negative glucocorticoid response element: evidence for the involvement of DNA-binding and agonistic effects of the antiglucocorticoid/antiprogestin RU486. DNA Cell Biol 12: 695–702, 1993. [DOI] [PubMed] [Google Scholar]
- 10. Carlo P, Violani E, Del Rio M, Olasmaa M, Santagati S, Maggi A, Picotti GB. Monoamine oxidase B expression is selectively regulated by dexamethasone in cultured rat astrocytes. Brain Res 711: 175–183, 1996. [DOI] [PubMed] [Google Scholar]
- 11. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58: 545, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55: 585–595, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Crossin KL, Tai MH, Krushel LA, Mauro VP, Edelman GM. Glucocorticoid receptor pathways are involved in the inhibition of astrocyte proliferation. Proc Natl Acad Sci USA 94: 2687, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4: 44–57, 2008. [DOI] [PubMed] [Google Scholar]
- 16. De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 14: 1276–1284, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Droste S, De Groote L, Lightman S, Reul J, Linthorst A. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: an in vivo microdialysis study in Wistar rats. J Neuroendocrinol 21: 132–140, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Eberwine JH, Roberts JL. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem 259: 2166–2170, 1984. [PubMed] [Google Scholar]
- 20. Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature 468: 223–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Federoff H, Grabczyk E, Fishman M. Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids. J Biol Chem 263: 19290–19295, 1988. [PubMed] [Google Scholar]
- 22. Foo LC, Allen NJ, Bushong EA, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA. Development of a method for the purification and culture of rodent astrocytes. Neuron 71: 799–811, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franklin RJM. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9: 839–855, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Funder JW, Feldman D, Edelman IS. Glucocorticoid receptors in rat kidney: the rinding of tritiated-dexamethasone. Endocrinology 92: 1005–1013, 1973. [DOI] [PubMed] [Google Scholar]
- 25. Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem 111: 522–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med 67, Suppl 1: S26–S28, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci 26: 9047–9056, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in endogenous depression: I. Basal plasma levels. Arch Gen Psychiatry 42: 904, 1985. [DOI] [PubMed] [Google Scholar]
- 29. Hardin-Pouzet H, Giraudon P, Belin M, Didier-Bazes M. Glucocorticoid upregulation of glutamate dehydrogenase gene expression in vitro in astrocytes. Mol Brain Res 37: 324–328, 1996. [DOI] [PubMed] [Google Scholar]
- 30. Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463: 232–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ihara H, Nakanishi S. Selective inhibition of expression of the substance P receptor mRNA in pancreatic acinar AR42J cells by glucocorticoids. J Biol Chem 265: 22441–22445, 1990. [PubMed] [Google Scholar]
- 32. Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol Endocrinol 11: 693–705, 1997. [DOI] [PubMed] [Google Scholar]
- 33. John S, Johnson TA, Sung MH, Biddie SC, Trump S, Koch-Paiz CA, Davis SR, Walker R, Meltzer PS, Hager GL. Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology 150: 1766–1774, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khelil M, Rolland B, Fages C, Tardy M. Glutamine synthetase modulation in astrocyte cultures of different mouse brain areas. Glia 3: 75–80, 1990. [DOI] [PubMed] [Google Scholar]
- 35. de Kloet ER. Hormones and the stressed brain. Ann NY Acad Sci 1018: 1–15, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Kumar S, Holmes E, Scully S, Birren B, Wilson R, De Vellis J. The hormonal regulation of gene expression of glial markers: glutamine synthetase and glycerol phosphate dehydrogenase in primary cultures of rat brain and in C6 cell line. J Neurosci Res 16: 251–264, 1986. [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-delta delta CT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 40. Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol 100: 1200–1208, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism 54: 20–23, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Meijer O, Van Der Laan S, Lachize S, Steenbergen P, De Kloet E. Steroid receptor coregulator diversity: what can it mean for the stressed brain? Neuroscience 138: 891–899, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Meisinger C, Zeschnigk C, Grothe C. In vivo and in vitro effect of glucocorticoids on fibroblast growth factor (FGF)-2 and FGF receptor 1 expression. J Biol Chem 271: 16520–16525, 1996. [DOI] [PubMed] [Google Scholar]
- 44. Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. J Neurosci 32: 5017–5023, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyer ME, Pornon A, Ji J, Bocquel MT, Chambon P, Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J 9: 3923, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry 48: 861–873, 2000. [DOI] [PubMed] [Google Scholar]
- 47. Milner R. Astrocytes: Methods and Protocols. SpringerLink, 2012. [Google Scholar]
- 48. Morsink M, Joels M, Sarabdjitsingh R, Meijer O, De Kloet E, Datson N. The dynamic pattern of glucocorticoid receptor-mediated transcriptional responses in neuronal PC12 cells. J Neurochem 99: 1282–1298, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Morsink M, Steenbergen P, Vos J, Karst H, Joels M, Kloet ER, Datson N. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 18: 239–252, 2006. [DOI] [PubMed] [Google Scholar]
- 50. Murphy B. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology 22: S125, 1997. [PubMed] [Google Scholar]
- 51. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet 367: 1605–1617, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Nichols NR, Osterburg HH, Masters JN, Millar SL, Finch CE. Messenger RNA for glial fibrillary acidic protein is decreased in rat brain following acute and chronic corticosterone treatment. Mol Brain Res 7: 1–7, 1990. [DOI] [PubMed] [Google Scholar]
- 53. Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor isoform expression, biochemical properties, and putative function. J Biol Chem 271: 9550–9559, 1996. [DOI] [PubMed] [Google Scholar]
- 54. Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev 63: 83–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54a. Phuc Le P, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet 1: e16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry 49: 741–752, 2001. [DOI] [PubMed] [Google Scholar]
- 56. Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45: 1085–1098, 1999. [DOI] [PubMed] [Google Scholar]
- 57. Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res 19: 2163–2171, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reul J, De Kloet E. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511, 1985. [DOI] [PubMed] [Google Scholar]
- 59. Reul JMHM, Gesing A, Droste S, Stec ISM, Weber A, Bachmann C, Bilang-Bleuel A, Holsboer F, Linthorst ACE. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol 405: 235–249, 2000. [DOI] [PubMed] [Google Scholar]
- 60. Rozovsky I, Laping NJ, Krohn K, Teter B, O'Callaghan JP, Finch CE. Transcriptional regulation of glial fibrillary acidic protein by corticosterone in rat astrocytes in vitro is influenced by the duration of time in culture and by astrocyte-neuron interactions. Endocrinology 136: 2066–2073, 1995. [DOI] [PubMed] [Google Scholar]
- 61. Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express μ, δ and κ opioid receptor mRNA. Mol Brain Res 34: 209–220, 1995. [DOI] [PubMed] [Google Scholar]
- 63. Ruzicka BB, Thompson RC, Watson SJ, Akil H. Interleukin-1β-mediated regulation of μ-opioid receptor mRNA in primary astrocyte-enriched cultures. J Neurochem 66: 425–428, 1996. [DOI] [PubMed] [Google Scholar]
- 64. Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucl Acids Res 37: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Skynner HA, Amos DP, Murray F, Salim K, Knowles MR, Munoz-Sanjuan I, Camargo LM, Bonnert TP, Guest PC. Proteomic analysis identifies alterations in cellular morphology and cell death pathways in mouse brain after chronic corticosterone treatment. Brain Res 1102: 12–26, 2006. [DOI] [PubMed] [Google Scholar]
- 66. Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem 271: 5301–5304, 1996. [DOI] [PubMed] [Google Scholar]
- 67. So AYL, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell-and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet 3: e94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophren Res 103: 71–82, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Takahashi K, Saitoh A, Yamada M, Iwai T, Inagaki M. Dexamethasone indirectly induces Ndrg2 expression in rat astrocytes. J Neurosci Res 90: 160–166, 2012. [DOI] [PubMed] [Google Scholar]
- 70. Thoeringer CK, Sillaber I, Roedel A, Erhardt A, Mueller MB, Ohl F, Holsboer F, Keck ME. The temporal dynamics of intrahippocampal corticosterone in response to stress-related stimuli with different emotional and physical load: an in vivo microdialysis study in C57BL/6 and DBA/2 inbred mice. Psychoneuroendocrinology 32: 746–757, 2007. [DOI] [PubMed] [Google Scholar]
- 71. Vardimon L, Ben-Dror I, Avisar N, Oren A, Shiftan L. Glucocorticoid control of glial gene expression. J Neurobiol 40: 513–527, 1999. [DOI] [PubMed] [Google Scholar]
- 72. Webster M, O'Grady J, Kleinman J, Weickert C. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133: 453–461, 2005. [DOI] [PubMed] [Google Scholar]
- 73. Yeh TH, Lee DY, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57: 1239–1249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids 72: 600–608, 2007. [DOI] [PubMed] [Google Scholar]
- 75. Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20: 588–594, 2010. [DOI] [PubMed] [Google Scholar]
- 76. Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem 280: 34924–34932, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.