Abstract
Maximal strength training (MST) reduces pulmonary oxygen uptake (V̇o2) at a given submaximal exercise work rate (i.e., efficiency). However, whether the increase in efficiency originates in the trained skeletal muscle, and therefore the impact of this adaptation on muscle blood flow and arterial-venous oxygen difference (a-vO2diff), is unknown. Thus five trained subjects partook in an 8-wk MST intervention consisting of half-squats with an emphasis on the rate of force development during the concentric phase of the movement. Pre- and posttraining measurements of pulmonary V̇o2 (indirect calorimetry), single-leg blood flow (thermodilution), and single-leg a-vO2diff (blood gases) were performed, to allow the assessment of skeletal muscle V̇o2 during submaximal cycling [237 ± 23 W; ∼60% of their peak pulmonary V̇o2 (V̇o2peak)]. Pulmonary V̇o2peak (∼4.05 l/min) and peak work rate (∼355 W), assessed during a graded exercise test, were unaffected by MST. As expected, following MST there was a significant reduction in pulmonary V̇o2 during steady-state submaximal cycling (∼237 W: 3.2 ± 0.1 to 2.9 ± 0.1 l/min). This was accompanied by a significant reduction in single-leg V̇o2 (1,101 ± 105 to 935 ± 93 ml/min) and single-leg blood flow (6,670 ± 700 to 5,649 ± 641 ml/min), but no change in single-leg a-vO2diff (16.7 ± 0.8 to 16.8 ±0.4 ml/dl). These data confirm an MST-induced reduction in pulmonary V̇o2 during submaximal exercise and identify that this change in efficiency originates solely in skeletal muscle, reducing muscle blood flow, but not altering muscle a-vO2diff.
Keywords: oxygen consumption, blood flow, work economy
during cycle exercise, at a given submaximal work rate (WR), pulmonary oxygen uptake (V̇o2) is similar between individuals of varying aerobic capacities [peak V̇o2 (V̇o2peak)] (34). This is true despite the complex, systemwide metabolic costs of exercise, such as ventilatory and cardiac muscle work, ion transport, and exercise-induced alterations in thermoregulation and metabolism, each of which may influence the V̇o2/WR relationship (6, 39, 43). Thus work efficiency, measured as the ratio of pulmonary V̇o2 to work accomplished during submaximal steady-state cycling, is a global assessment of metabolic demand and may be influenced by a change in any of these systems. Therefore, a perturbation or stressor, such as maximal strength training (MST), which has been determined to alter work efficiency (22, 26, 42), may not simply be attributed to a change in efficiency of the exercising muscle.
For over a decade, studies have documented that the use of MST, which consists of high loads and few repetitions, with an emphasis on the maximal rate of force development, improves work efficiency in both sedentary (22) and aerobically trained individuals (26, 42), and this MST-induced change is even more evident during high-intensity exercise (22). While it is reasonable to expect enhanced intramuscular efficiency to be a major contributor to this documented improvement in work efficiency, an attenuated O2 cost, external to the exercising muscle (i.e., respiratory and cardiac muscle work), might also play a role. However, there is some indirect evidence supporting functional adaptations within the exercising muscle which may contribute to an improvement in intramuscular efficiency. Specifically, following MST, there is an increase in the rate of force development (26). As previously documented during isometric exercise, the ATP cost of initiating muscle shortening is greater than that needed to maintain developed force (36). The ability to generate force more rapidly following MST could potentially prolong the duration of the contraction phase devoted to the maintenance of developed force, possibly reducing muscle metabolic rate. However, the effect of MST on muscle metabolism per se has not been directly measured and so remains unknown.
As described by the Fick equation, skeletal muscle V̇o2 is the product of blood flow and the difference between arterial and venous [O2] (a-vO2diff). Therefore, a given reduction in skeletal muscle V̇o2 may be achieved by a reduction in either a-vO2diff, blood flow, or reductions in both variables. As a major component of O2 delivery, which is tightly related to O2 demand (33), skeletal muscle blood flow typically follows the metabolic rate of the exercising muscle (9, 12). However, there is only a single study, performed by our group (19), investigating the contribution of altered skeletal muscle blood flow to the enhanced work efficiency following MST. Somewhat surprisingly, findings from this study revealed an MST-induced increase in work efficiency during arm exercise, assessed by pulmonary V̇o2, with no change in muscle blood flow. If MST does, in fact, increase muscular efficiency, this most recent finding adds an unexpected twist in the tale in terms of how the components of the Fick equation, blood flow and a-vO2diff, may respond to this reduction in skeletal muscle V̇o2.
Therefore, the purpose of this study was to determine if the MST-induced reduction in pulmonary V̇o2 during submaximal steady-state exercise is due to a decrease in the trained skeletal muscle V̇o2, and, if this is the case, document which components of the Fick equation, blood flow or a-vO2diff, contribute to this effect. Specifically, we hypothesized that improved intramuscular efficiency contributes significantly to the reduction in pulmonary V̇o2 at a submaximal WR following MST. Furthermore, based upon previous observations with this intervention (19), we hypothesized that the improvement in intramuscular efficiency would be achieved by maintaining skeletal muscle blood flow, but reducing muscle a-vO2diff.
METHODS
This study was approved by the University of California, San Diego, Human Subjects Committee. Each subject gave written informed consent before participating in the study. All studies were performed according to the Declaration of Helsinki.
Subjects.
Six healthy, nonsmoking, male cyclists who regularly rode 200–400 miles/wk, volunteered to participate in this study. The physical characteristics of the subjects were as follows: 26 ± 2 (SE) yr of age, 180 ± 1 cm height, and 70 ± 1 kg body weight.
Pre- and posttests.
Following initial familiarization visits to acquaint subjects with the testing procedures, all subjects reported to the laboratory for pre- and posttraining assessments on two separate days. During the initial visit, subject's one repetition maximum (1RM) for a half-squat, defined as the maximum weight that could be moved from an upright standing position to a knee angle of 90° and returning back to a knee angle of 180°, was determined. To assess the rate of force development, subjects performed maximal isometric knee extension with force measured by a force transducer (Revere Transducers, Tustin, CA) attached to the ankle. Prior to each study, the force transducer was calibrated using a dynamometer (Dynamometer 22, Dresden, Germany). All rate of force development measurements were performed with a knee angle of 90° and were measured from 10 to 90% of peak force. Data were collected at 2,000 Hz (Acknowledge, Biopac Systems, Goleta, CA). Subjects rested for 30 min, and then performed an incremental cycling exercise test to determine peak V̇o2 (V̇o2peak) and peak WR (WRpeak) on an electronically braked cycle ergometer (Lode Excaliber, Groningen, Netherlands). The test consisted of 1 min WR increments of 25 W at 60 rpm until volitional fatigue. Pulmonary V̇o2 was measured with a mixing chamber-based indirect open-circuit calorimetry system (Parvomedics, Sandy, UT) integrated with a mass spectrometry system (Perkin-Elmer MGA 1100).
Subject preparation, blood flow, and arterial blood pressure measurements.
Within 3 days of the initial visit, subjects returned to the laboratory. Two catheters (femoral artery and femoral vein; DS 400L, Cook, Bloomington, IN), each with an external diameter of 1.25 mm, and a thermocouple (femoral vein) were placed in the same leg using sterile techniques, as previously reported (30, 32). The 0.64-mm-diameter thermocouple (model IT-18, Physitemp Instruments, Clifton, NJ) was advanced from approximately the same location as the venous catheter, proximally 10 cm into the left femoral vein. During exercise, cold saline was infused through the venous catheter at flow rates sufficient to decrease blood temperature by ∼1°C. Infusions continued for 10–15 s, or until femoral vein temperature stabilized. Saline injection rate was measured by weight change in a reservoir bag suspended from a force transducer. Single-leg blood flow was calculated based on the thermal balance principle as described by Andersen and Saltin (3) and expressed as the average of duplicate measurements. Arterial blood pressure measurements were collected continuously from the indwelling catheter placed in the femoral artery with the pressure transducer placed at the level of the catheter (Transpac IV, Abbott Laboratories). Mean arterial pressure (MAP; mmHg) was calculated as diastolic arterial pressure + (arterial pulse pressure × 0.33).
Blood analyses.
Femoral arterial and venous blood samples (3–4 ml) were drawn anaerobically. Partial pressure of oxygen (Po2) was measured by a blood gas analyzer (IL 1306, Instrumentation Laboratories, Lexington, MA), and hemoglobin (Hb), oxyhemoglobin, saturation (SO2), and hematocrit (Hct) by CO-oximeter (IL 482, Instrumentation Laboratories, Lexington, MA). Arterial and venous blood oxygen content (CaO2 and CvO2) (ml/dl) were calculated as: blood oxygen content = 1.39 (Hb) × (SO2/100) + 0.003 × Po2. Single-leg V̇o2 (ml/min) was calculated as: single-leg V̇o2 = femoral a-vO2diff × single-leg blood flow, and doubled to determine two-legged V̇o2. O2 delivery (l/min) was calculated as: O2 delivery = CaO2 × single-leg blood flow.
Blood lactate concentration ([LA]blood) was measured by the enzymatic membrane method (model 1500, Yellow Springs Instruments). Using arterial and venous plasma lactate concentrations, with corrections for single-leg blood flow, “net lactate release” was calculated as: net lactate release = ([LA]vein − [LA]artery) × single-leg blood flow × (101 − (Hct/100)), where LA is lactate and Hct is hematocrit.
Experimental protocol.
After completion of the catheterization procedure, each subject performed submaximal exercise for 4–6 min, at a WR equivalent to 60% of their V̇o2peak, while maintaining a cycling cadence of 60 rpm. No blood gas or leg blood flow measurements were made within the first 2–3 min of exercise; blood gas and leg blood flow measurements were only taken when a plateau in pulmonary V̇o2, defined as no change in V̇o2 over time, was evident. On average, arterial and venous blood samples as well as single-leg blood flow took ∼1 min to acquire, and then these measurements were repeated. To determine work efficiency [WR/(V̇o2 − resting V̇o2) × 100], both pulmonary V̇o2 and WR were converted to kilojoules, as previously cited (17, 31), and expressed as percentage change.
Thigh volume measurement.
Thigh volume was calculated using thigh length, circumference, and skinfold measurements, allowing for an estimate of quadriceps femoris muscle mass, as previously suggested (3, 18).
Training intervention.
Subjects performed an 8-wk training regime (3 sessions per week, resulting in 24 total training sessions) that was individually monitored in the laboratory. During this time, subjects were instructed to continue with their standard cycle training. The strength training sessions consisted of four sets of four repetitions with a focus on the rate of force development during the concentric phase of the movement. The load corresponded to 85–90% of the subjects' respective 1RM, with 3 min of recovery between sets. Loads were increased by 2.5 kg each time a subject could perform a fifth repetition during a set.
Statistical analyses.
Pre- and posttest differences were analyzed using paired-sample t-tests. Statistics were computed using commercially available software (Graph Pad, San Diego, CA). Variables were considered significantly different when P < 0.05.
RESULTS
MST and adherence.
All subjects successfully completed the pretesting and attended all 24 training sessions over the 8-wk MST program (100% compliance); however, a single subject was unavailable for posttesting due to circumstances beyond their control that were unrelated to the study. Therefore, all data reflect only the five subjects who completed the entire study.
Strength parameters.
Following MST, 1RM of the subjects improved by ∼28%, in addition to a ∼23% improvement in the rate of force development. No differences in thigh volume, quadriceps muscle mass, or body mass were documented as a consequence of the 8 wk of MST (Table 1).
Table 1.
Alterations in strength parameters before and after 8 wk of maximal strength training
| Pre-MST | Post-MST | |
|---|---|---|
| 1 Repetition maximum, kg | 130 ± 8 | 166 ± 8* |
| Rate of force development, N/s | 729 ± 53 | 899 ± 52* |
| Thigh volume, liters | 7.1 ± 0.3 | 7.2 ± 0.6 |
| Quadriceps muscle-mass, kg | 2.6 ± 0.2 | 2.6 ± 0.4 |
| Body mass, kg | 69.8 ± 1.6 | 70.6 ± 1.6 |
Values are means ± SE; n = 5 subjects. MST, maximal strength training.
P < 0.05 vs. pre-MST.
Maximal cycling exercise.
Subjects exhibited subjective exhaustion (Borg rating of perceived exertion = 19–20) within 9–12 min during the graded exercise test. Following MST, there were no significant differences in either WRpeak or pulmonary V̇o2peak (Table 2).
Table 2.
Physiological responses at maximal cycling exercise before and after 8 wk of maximal strength training
| Pre-MST | Post-MST | |
|---|---|---|
| V̇o2peak, l/min | 4.11 ± 0.17 | 4.00 ± 0.19 |
| WRpeak, W | 355.0 ± 17 | 355.0 ± 17 |
Values are means ± SE; n = 5 subjects. V̇o2peak, peak O2 uptake; WRpeak, peak work rate. Values are means ± SE; n = 5 subjects. *P < 0.05 vs. pre-MST.
Submaximal cycling exercise.
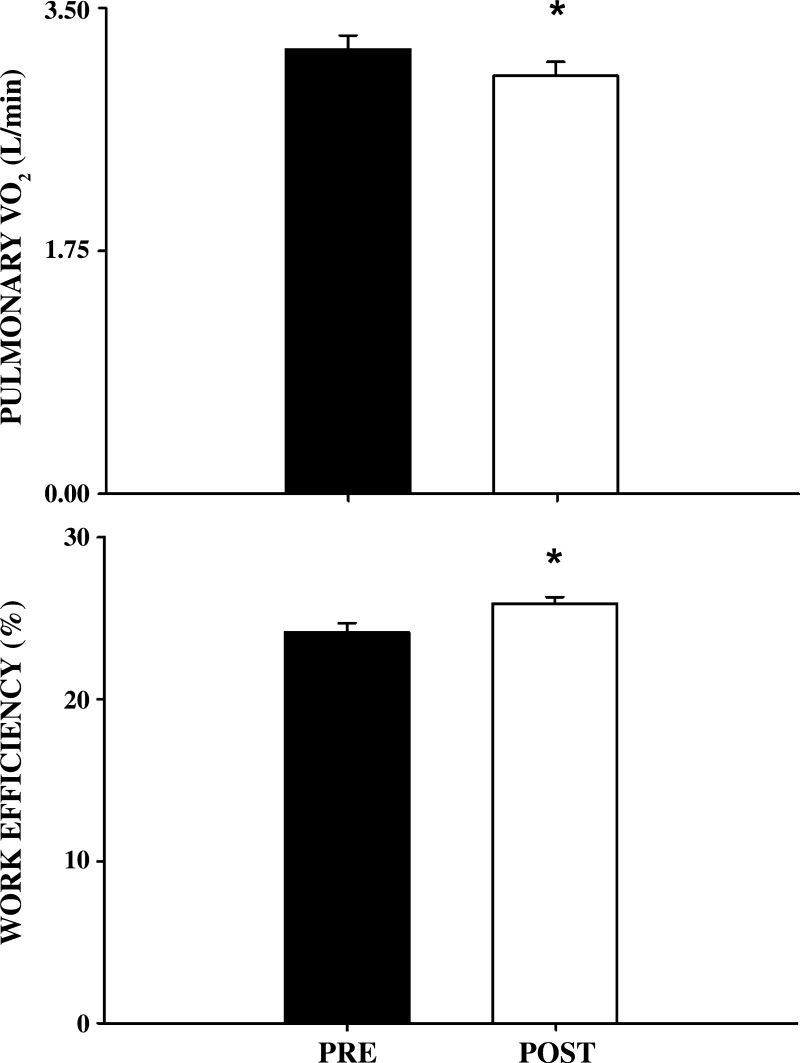
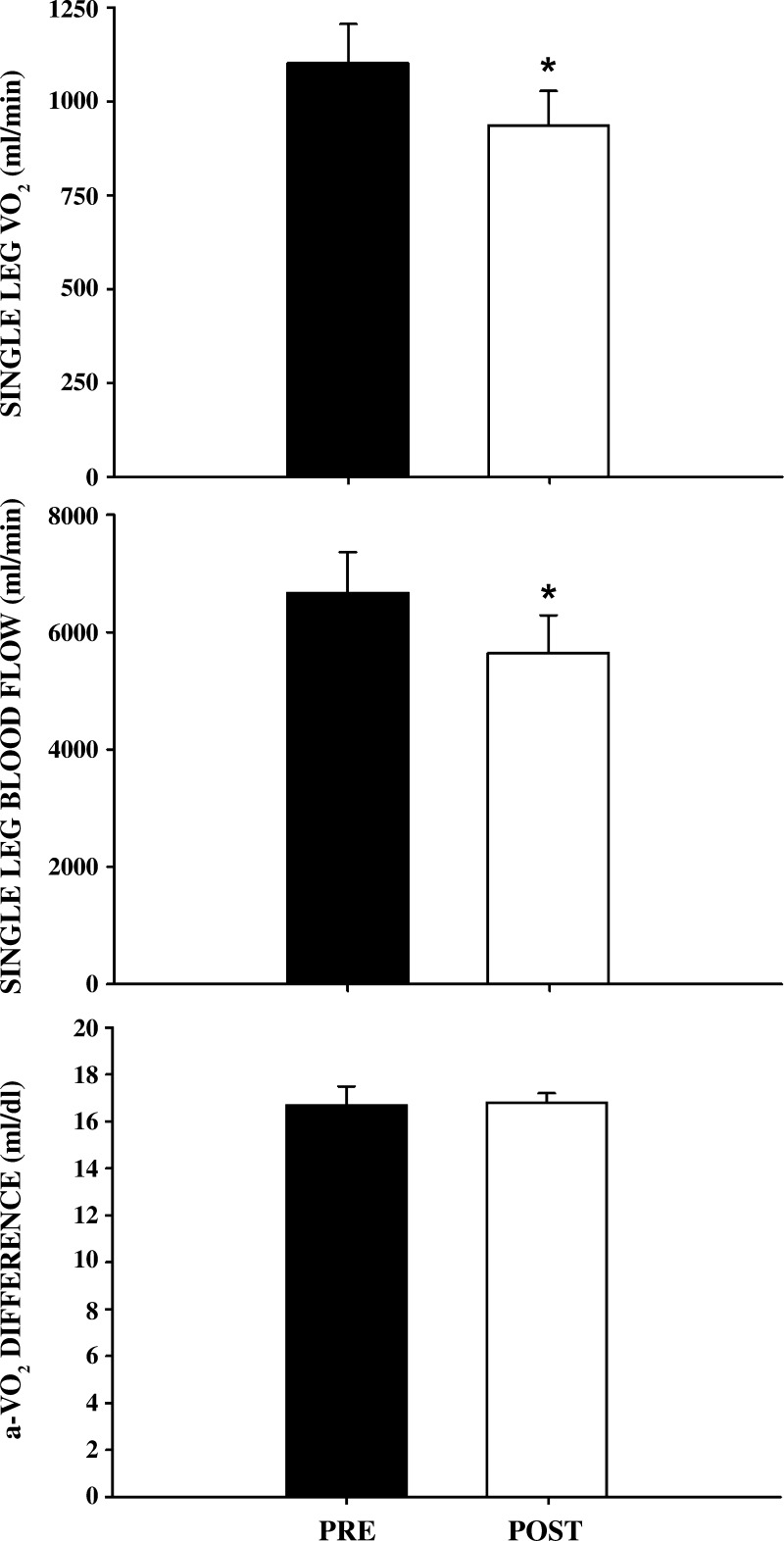
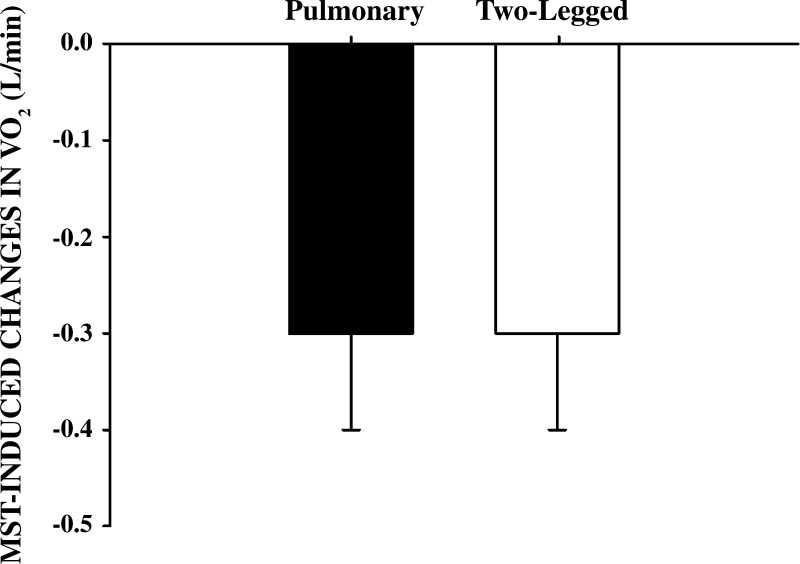
As neither WRpeak nor V̇o2peak was altered by the MST, 60% of V̇o2peak pre- and post- MST remained unchanged (237 ± 23 W). Post-MST, performing this submaximal WR, subjects exhibited a significantly lower pulmonary V̇o2 and a significantly enhanced work efficiency (Fig. 1). This was accompanied by a significantly lower single-leg blood flow and an unchanged a-vO2diff, which, in turn, resulted in a significantly lower single-leg V̇o2 (Fig. 2). The calculated change in two-legged V̇o2 was similar to the change in pulmonary V̇o2 (Fig. 3). Additionally, neither MAP (102 ± 4 to 103 ± 4 mmHg) nor lactate release (373 ± 33 to 352 ± 120 mmol/min) were significantly different from pre- to post-MST, respectively.
Fig. 1.
Pulmonary oxygen uptake (V̇o2) and percent work efficiency before and after 8 wk of maximal strength training (MST). Pulmonary V̇o2 (top panel) and work efficiency (bottom panel) are illustrated prior to (pre) and following (post) MST. Values are means ± SE. *P < 0.05 vs. pre-MST; n = 5.
Fig. 2.
Single-leg VO2, single-leg blood flow, and arterial-venous oxygen difference before and after 8 wk of maximal strength training (MST). Single-leg V̇o2 (top panel), single-leg blood flow (middle panel), and arterial-venous oxygen difference (bottom panel) are illustrated prior to (pre) and following (post) MST. Values are means ± SE. *P < 0.05 vs. pre-MST; n = 5.
Fig. 3.
Changes in pulmonary and 2-legged V̇o2 after 8 wk of MST. Values are means ± SE; n = 5.
DISCUSSION
Although MST has been documented to reduce pulmonary V̇o2 at a given submaximal exercise WR, the contribution of skeletal muscle V̇o2 to this change in efficiency is unclear. Therefore, the goal of this study was to determine to what extent the MST-induced increase in work efficiency is mediated by an improvement in intramuscular efficiency and, if muscle is a significant contributor, what is the impact on skeletal muscle blood flow and a-vO2diff. The major finding of this study was that the reduction in pulmonary V̇o2 following MST was exclusively the consequence of an improvement in the efficiency of the exercising skeletal muscle. As a consequence, a-vO2diff across the active muscle bed was unchanged while skeletal muscle blood flow was significantly reduced. Mechanistically, these data identify the adaptations to the trained skeletal muscle, and the subsequent improvements in work efficiency induced by MST, as the sole contributor to the post-MST reductions in pulmonary V̇o2 and rule out centralized changes in efficiency, such as adaptations in cardiac or respiratory muscle.
Skeletal muscle efficiency, blood flow, and a-vO2diff.
MST is associated with a reduction in pulmonary V̇o2 (Fig. 1). Whether this is due to improvements in efficiency of the trained skeletal muscle or adaptations external to this localized region was previously unknown. The trained skeletal muscle has now been identified as the site of this improved work efficiency (Figs. 2 and 3). This study employed direct a-v blood gas and blood flow measurements across the muscle bed to better understand the impact of MST on skeletal muscle oxidative metabolism. As described by the Fick equation, skeletal muscle V̇o2 is the product of blood flow and a-vO2diff, with skeletal muscle blood flow being tightly linked to O2 supply as documented by studies investigating the alterations in skeletal muscle V̇o2 and its components, in the presence of differing concentrations of inspired O2. For example, in mild hypoxic conditions, skeletal muscle blood flow has been documented to increase in order to compensate for the reduced O2 carriage while a-vO2diff remains constant and therefore V̇o2 remains unaltered (35). In hyperoxic conditions, the opposite occurs; skeletal muscle blood flow decreases while a-vO2diff remains constant and again muscle V̇o2 is unaltered (44). However, skeletal muscle blood flow is tightly linked not only to O2 supply but also to O2 demand (32, 33) and therefore the documented reduction in exercising muscle blood flow following MST is consistent with the conclusion that metabolic demand has been reduced in the skeletal muscle as a consequence of the MST.
Although more complete, the current data contrast with a prior study by our group in which we examined the effect of the MST-induced increases in work efficiency during arm exercise, assessed by pulmonary V̇o2, and found arm blood flow to be unchanged (19). Although, by experimental design, the prior study focused upon small muscle mass exercise training and testing, and thus the role of the centralized adaptations would be minimized, the lack of a direct assessment of muscle V̇o2 limited the confidence with which we could interpret the data. However, based upon the current conclusion that skeletal muscle is, indeed, the major contributor to the MST-induced increases in mechanical efficiency, these two studies raise the question as to whether there are limb-specific differences in the way skeletal muscle deals with such a change in work efficiency. Specifically, in the current study, comprehensive data collection reveals that MST resulted in decreased metabolic demand for a given work rate which resulted in a reduced skeletal muscle blood flow and subsequently a reduction in O2 delivery (Table 3). In contrast, with arm exercise (19), the unaltered blood flow, as a consequence of MST, suggests (although not measured) that a-vO2diff was reduced.
Table 3.
O2 transport and utilization variables measured during submaximal cycling exercise (237 ± 23 W; approximately 60% of V̇o2peak) before and after 8 wk of maximal strength training
| Pre-MST | Post-MST | |
|---|---|---|
| CaO2, ml/dl | 19.5 ± 0.9 | 18.9 ± 0.3 |
| CvO2, ml/dl | 3.4 ± 0.4 | 3.4 ± 0.5 |
| O2 delivery, l/min | 1.3 ± 0.1 | 1.1 ± 0.0* |
| Hb, g/dl | 14.7 ± 0.4 | 14.9 ± 0.2 |
| SaO2, % | 95.8 ± 0.5 | 95.9 ± 0.3 |
| SvO2, % | 16.3 ± 2.1 | 16.4 ± 1.9 |
| PaO2, Torr | 98.3 ± 3.7 | 98.4 ± 2.9 |
| PvO2, Torr | 18.8 ± 1.1 | 18.7 ± 1.1 |
| Hct, % | 43.7 ± 1.0 | 45.4 ± 0.3 |
Values are means ± SE; n = 5 subjects. CaO2, arterial O2 content; CvO2, venous O2 content; Hb, hemoglobin; SaO2, arterial Hb O2 saturation; SvO2, venous Hb O2 saturation; PaO2, arterial partial pressure of O2, PvO2, venous partial pressure of O2; Hct, hematocrit.
P < 0.05 vs. pre MST.
Although potentially complicated by the small versus large muscle mass approach in these two studies, this is not the first time that arm- and leg-specific differences have been found in terms of metabolic and vascular responses (4, 5, 24, 25, 45). Recent work, investigating limb-specific regulation of blood flow during incremental arm or leg exercise, revealed that for a given local V̇o2, leg vascular conductance was five to six times greater than arm vascular conductance (4). Interestingly, although the leg revealed the expected intensity-dependent increase in leg O2 delivery the arm did not, indicating that the arms rely much more upon an increase in oxygen extraction to achieve exercise-induced increases in VO2. In light of these limb-specific differences in vascular and metabolic regulation during exercise, further investigations are necessary to determine the mechanisms that may contribute differentially to MST-induced improvements in work economy in the upper and lower limbs.
The role of cardiac and respiratory muscle adaptations to MST.
While this study has documented skeletal muscle as apparently the only site contributing to the improvement work efficiency following MST, improvements in efficiency of other muscle groups, such as cardiac and respiratory muscle, could conceivably also have played a role. In terms of the heart, resistance training has been linked to structural alterations of the left ventricle such as increases in left ventricular wall thickness (8, 10) and left ventricular mass (8, 10, 37). However, these cardiac muscle adaptations appear to have little effect on systolic (21, 23, 28) and diastolic function (7, 8). Much like hemodynamic changes during resistance training stimulate adaptations to cardiac muscle, alterations in the work of breathing due to resistance training could lead to adaptations in respiratory muscle function that improve exercise performance. Indeed, our group has documented that in patients with chronic obstructive pulmonary disease, a population that could benefit greatly from improvements in pulmonary function, there was an MST-induced increase in expiratory capacity which correlated with the augmented rate of force development (17). However, the exercise performance benefits of improving pulmonary function have not been consistently documented in healthy people (38).
Although not unreasonable hypotheses to help explain the increased work economy afforded by MST, the current data do not support the involvement of cardiac and respiratory muscle in this phenomenon. Specifically, recognizing that two very different approaches were used to assess whole body and leg V̇o2 and therefore direct numerical comparisons may not be completely justifiable, the difference in two-legged V̇o2 attributed to the MST accounts for all of the effect recorded at the mouth in the form of pulmonary V̇o2 (Fig. 3). Thus this study reveals that the increase in work efficiency documented in the exercising muscle appears to exclusively account for the changes in pulmonary V̇o2. This tight relationship between pulmonary V̇o2 and leg V̇o2 was previously documented 20 years ago (20), and therefore, although interesting, the current determination that the change in pulmonary V̇o2 due to MST stems from the muscle should not be surprising.
MST and skeletal muscle adaptations.
Despite more than a decade since the recognition that MST can alter exercise economy and the application of this training approach to numerous healthy and diseased populations (14–17, 19, 27), to our knowledge, this study is the first to directly document the significant contribution of skeletal muscle. There are several adaptations which likely occur after high-intensity resistance training which might induce an improvement in the work efficiency of exercising skeletal muscle. First, following high-intensity resistance training there is a reduction in myosin heavy chain IIb and an increase in myosin heavy chain IIa (2, 40). This alteration in myosin heavy chain composition mirrors the change in fiber type composition, with a reduction in the percentage of type IIb fibers and an increase in the percentage of type IIa fibers (2). These alterations would reduce the metabolic cost related to muscle tension and enhance contractile efficiency (13). However, it should be noted that a shift in metabolism to a more aerobic fiber type is not supported by the unchanged lactate efflux from the exercising muscle bed in this study. Second, following high-intensity resistance training, less muscle is required to lift a given absolute load, as documented by a reduction in magnetic resonance image contrast shift (29). Such a reduction in muscle recruitment is associated with an alteration in metabolic demand (1, 11). Third, the kinetics of force development can also play a role in altering metabolic demand of the exercising muscle (36). Specifically, during the course of a muscle contraction, metabolic cost is greatest at the beginning of the contraction, while muscle shortening is being initiated compared with the maintenance phase of the contraction (36). As documented in this study (Table 1) and many others (15–17), MST induces an increase in the rate of force development. Possessing the capability to generate force more rapidly, lengthens the portion of the contraction phase devoted to the maintenance of developed force, thus, potentially reducing the metabolic demand of the muscle, and enhancing intramuscular efficiency during exercise. Although the current study cannot differentiate between these and MST-induced adaptations, it is highly likely that the changes in work efficiency in the skeletal muscle are, in fact, multifactorial in nature, but this remains to be documented.
Limitations.
A limitation of the present study is the fact that a control group was not incorporated into the study design. This was due to the invasiveness of the study, which would have required repeated insertions of femoral artery and venous catheters into a control group of subjects. However, it should be noted that in previous studies by members of our group, control groups have been incorporated into the study of MST-based responses (15, 41), and have documented no change in work efficiency as a consequence of pre- and posttesting and time. A further limitation to the study design was the fact that it did not unveil mechanistic insight into factors contributing to the reduction in exercising muscle blood flow following MST. Two potential mechanisms contributing the MST-induced changes in work economy are an improved matching of blood flow to V̇o2, as well as alterations in Group III-IV muscle-afferent activity and reflex changes in sympathetic outflow. However, MST-induced changes to the autonomic nervous system appear to be highly unlikely due to the lack of a change in arterial blood pressure during submaximal exercise following MST. Additionally, while the documented reduction in pulmonary V̇o2 during submaximal exercise seems to be primarily due to improvements in efficiency of the exercising skeletal muscle, we cannot rule out the possibility that MST resulted in changes in pulmonary and cardiac muscle efficiency during exercise. However, based on the similar magnitude of change in both leg and pulmonary V̇o2, such changes are likely of little significance.
Conclusions.
This study has confirmed that MST results in an improvement in work efficiency assessed by gas exchange across the mouth and that this is exclusively a consequence of reduced O2 utilization by the working skeletal muscle and cannot be attributed to changes in cardiac or respiratory muscle efficiency. Although this study was able to document that the change in skeletal muscle V̇o2 was achieved by a reduction in single-leg blood flow and no change in a-vO2diff, the actual intramuscular mechanism responsible for the increase in skeletal muscle efficiency remains to be elucidated.
GRANTS
This work was supported by National Institutes of Health Grants PO1-HL-091830 (to R. S. Richardson) and Veterans Affairs Rehabilitation Research and Development Service Grant E6910R (to R. S. Richardson), and American Heart Association Grant 0835209N (D. W. Wray).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.B.-O., J.H., P.D.W., and R.S.R. analyzed data; Z.B.-O., J.H., and R.S.R. interpreted results of experiments; Z.B.-O. and R.S.R. prepared figures; Z.B.-O. and R.S.R. drafted manuscript; Z.B.-O., J.H., P.D.W., and R.S.R. edited and revised manuscript; Z.B.-O., J.H., P.D.W., and R.S.R. approved final version of manuscript; J.H., P.D.W., and R.S.R. conception and design of research; J.H., P.D.W., and R.S.R. performed experiments.
REFERENCES
- 1. Adams GR, Duvoisin MR, Dudley GA. Magnetic resonance imaging and electromyography as indexes of muscle function. J Appl Physiol 73: 1578–1583, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol 74: 911–915, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol 103: 969–978, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 289: R1448–R1458, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cavanagh PR, Kram R. The efficiency of human movement—a statement of the problem. Med Sci Sports Exerc 17: 304–308, 1985 [PubMed] [Google Scholar]
- 7. Colan SD, Sanders SP, MacPherson D, Borow KM. Left ventricular diastolic function in elite athletes with physiologic cardiac hypertrophy. J Am Coll Cardiol 6: 545–549, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Effron MB. Effects of resistive training on left ventricular function. Med Sci Sports Exerc 21: 694–697, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjaer M, Sargeant AJ, Hellsten Y, Bangsbo J. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol 536: 261–271, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleck SJ. Cardiovascular adaptations to resistance training. Med Sci Sports Exerc 20: S146–S151, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Fleckenstein JL, Haller RG, Lewis SF, Archer BT, Barker BR, Payne J, Parkey RW, Peshock RM. Absence of exercise-induced MRI enhancement of skeletal muscle in McArdle's disease. J Appl Physiol 71: 961–969, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Hamann JJ, Kluess HA, Buckwalter JB, Clifford PS. Blood flow response to muscle contractions is more closely related to metabolic rate than contractile work. J Appl Physiol 98: 2096–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Han YS, Proctor DN, Geiger PC, Sieck GC. Reserve capacity for ATP consumption during isometric contraction in human skeletal muscle fibers. J Appl Physiol 90: 657–664, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Hickson RC, Dvorak BA, Gorostiaga EM, Kurowski TT, Foster C. Potential for strength and endurance training to amplify endurance performance. J Appl Physiol 65: 2285–2290, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Hoff J, Gran A, Helgerud J. Maximal strength training improves aerobic endurance performance. Scand J Med Sci Sports 12: 288–295, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hoff J, Helgerud J, Wisloff U. Maximal strength training improves work economy in trained female cross-country skiers. Med Sci Sports Exerc 31: 870–877, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Hoff J, Tjonna AE, Steinshamn S, Hoydal M, Richardson RS, Helgerud J. Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc 39: 220–226, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969 [PubMed] [Google Scholar]
- 19. Kemi OJ, Rognmo O, Amundsen BH, Stordahl S, Richardson RS, Helgerud J, Hoff J. One-arm maximal strength training improves work economy and endurance capacity but not skeletal muscle blood flow. J Sports Sci 29: 161–170, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg V̇o2 during maximal cycle ergometry. J Appl Physiol 73: 1114–1121, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Longhurst JC, Kelly AR, Gonyea WJ, Mitchell JH. Cardiovascular responses to static exercise in distance runners and weight lifters. J Appl Physiol 49: 676–683, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Loveless DJ, Weber CL, Haseler LJ, Schneider DA. Maximal leg-strength training improves cycling economy in previously untrained men. Med Sci Sports Exerc 37: 1231–1236, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Menapace FJ, Hammer WJ, Ritzer TF, Kessler KM, Warner HF, Spann JF, Bove AA. Left ventricular size in competitive weight lifters: an echocardiographic study. Med Sci Sports Exerc 14: 72–75, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Osteras H, Helgerud J, Hoff J. Maximal strength-training effects on force-velocity and force-power relationships explain increases in aerobic performance in humans. Eur J Appl Physiol 88: 255–263, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Paavolainen L, Hakkinen K, Hamalainen I, Nummela A, Rusko H. Explosive-strength training improves 5-km running time by improving running economy and muscle power. J Appl Physiol 86: 1527–1533, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Pearson AC, Schiff M, Mrosek D, Labovitz AJ, Williams GA. Left ventricular diastolic function in weight lifters. Am J Cardiol 58: 1254–1259, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Ploutz LL, Tesch PA, Biro RL, Dudley GA. Effect of resistance training on muscle use during exercise. J Appl Physiol 76: 1675–1681, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol 72: 805–810, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Powers SK, Howley ET. Exercise Physiology: Theory and Application to Fitness and Performance. Boston, MA: McGraw-Hill, 2007 [Google Scholar]
- 32. Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30: 28–33, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993, p. xv [Google Scholar]
- 35. Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Russ DW, Elliott MA, Vandenborne K, Walter GA, Binder-Macleod SA. Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am J Physiol Endocrinol Metab 282: E448–E457, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Snoeckx LH, Abeling HF, Lambregts JA, Schmitz JJ, Verstappen FT, Reneman RS. Echocardiographic dimensions in athletes in relation to their training programs. Med Sci Sports Exerc 14: 428–434, 1982 [DOI] [PubMed] [Google Scholar]
- 38. Sonetti DA, Wetter TJ, Pegelow DF, Dempsey JA. Effects of respiratory muscle training versus placebo on endurance exercise performance. Respir Physiol 127: 185–199, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Stainbsy WN, Gladden LB, Barclay JK, Wilson BA. Exercise efficiency: validity of base-line subtractions. J Appl Physiol 48: 518–522, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76: 1247–1255, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Storen O, Helgerud J, Stoa EM, Hoff J. Maximal strength training improves running economy in distance runners. Med Sci Sports Exerc 40: 1087–1092, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Sunde A, Storen O, Bjerkaas M, Larsen MH, Hoff J, Helgerud J. Maximal strength training improves cycling economy in competitive cyclists. J Strength Cond Res 24: 2157–2165, 2010 [DOI] [PubMed] [Google Scholar]
- 43. van Ingen Schenau GJ, Cavanagh PR. Power equations in endurance sports. J Biomech 23: 865–881, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977 [DOI] [PubMed] [Google Scholar]
- 45. Wray DW, Donato AJ, Nishiyama SK, Richardson RS. Acute sympathetic vasoconstriction at rest and during dynamic exercise in cyclists and sedentary humans. J Appl Physiol 102: 704–712, 2007 [DOI] [PubMed] [Google Scholar]