Abstract
In our preceding companion paper (Wang Y, Winters J, Subramaniam S. J Appl Physiol. doi: 10.1152/japplphysiol.01514.2011), we used extensive expression profile data on normal human subjects, in combination with legacy knowledge to classify skeletal muscle function into four models, namely excitation-activation, mechanical, metabolic, and signaling-production model families. In this paper, we demonstrate how this classification can be applied to study two well-characterized myopathies: amyotrophic lateral sclerosis (ALS) and Duchenne muscular dystrophy (DMD). Using skeletal muscle profile data from ALS and DMD patients compared with that from normal subjects, normal young in the case of DMD, we delineate molecular mechanisms that are causative and consequential to skeletal muscle dysfunction. In ALS, our analysis establishes the metabolic role and specifically identifies the mechanisms of calcium dysregulation and defects in mitochondrial transport of materials as important for muscle dysfunction. In DMD, we illustrate how impaired mechanical function is strongly coordinated with other three functional networks, resulting in transformation of the skeletal muscle into hybrid forms as a compensatory mechanism. Our functional models also provide, in exquisite detail, the mechanistic role of myriad proteins in these four families in normal and disease function.
Keywords: functional protein network, amyotrophic lateral sclerosis, Duchenne muscular dystrophy, pathological pathway
in the companion paper (81a), we developed four functional models of the human skeletal muscle and partitioned the key proteins responsible for skeletal muscle function into four model families: 1) excitation-activation (EA), forward pathways that transmit a transient motoneuronal command signal (the “source”) into the spatial volume to bind free Ca2+ to troponin C sites (“sinks”) on F-actin filaments, and pumps to transport ions back across membrane potential gradients; 2) mechanical transmission (MECH), a sophisticated three-dimensional mechanical transmission apparatus; 3) metabolic and bioenergetic (METB), organelles/pathways that manage and provide energy for the former two subsystems, as well as other cellular processes; and 4) signaling/production (SIGP) family, representing the proteins involved in various sensing, signal transduction, and nuclear processes that adaptively control the turnover of both structural and regulatory proteins. Each was further divided into functional subfamilies of proteins and associated genes. Impairment in one or more of these families, or altered inputs or cross talk between families, can reduce muscle capacity in various ways and cause adaptive myopathies. The models developed in our companion paper (81a), combined with data from myopathies, have the potential to provide mechanistic and systems-level insights into muscle pathophysiology. In this paper, we use our functional models to provide novel perspectives on two representative myopathies for which human quadriceps data is available, namely, amyotrophic lateral sclerosis (ALS) and Duchenne muscular dystrophy (DMD).
ALS is broadly considered to be the most common motoneuron disease, with progressive motoneuron degeneration and symptoms that include weakness, muscle atrophy (sometimes with adipose infiltration), fasciculation (muscle twitches), and in most cases moderate hypermetabolism (8). Most ALS cases are sporadic, but ∼10% of ALS patients are of familial forms, among which nearly 20% are identified with a direct link between ALS and the mutations in the SOD1 gene (84). While the conventional belief is that ALS originates from the nervous system, recent evidence suggests that skeletal muscle may be involved in the early stages (6, 83), that compromised neuromuscular junction (NMJ) function provides one of the earliest indicators of this disease (24), and that there is distinct mitochondrial involvement in even the early stages of ALS (14, 24, 46, 48, 82). There has been a sustained controversy regarding the sources of idiopathic ALS, including evidence for energy or materials transport deficit (14, 24) and for environmental factors [e.g., high exposure to the cyanobacterial toxin β-N-methylamino-l-alanine, or BMAA (51)]. Indeed, a remarkably large number of hypotheses that have been proposed (e.g., reviewed in Ref. 15) and successful biomarkers for ALS have been of limited value, but include the hallmark gradual loss of motor units as measured by electrodiagnosis, changes in several protein concentrations in cerebrospinal fluid (67), isometric muscle-joint strength testing to document weakness using a representative collection of strategic joints (15, 61), muscle atrophy scales based on images from muscle biopsies (61), and several validated functional well-being scales (15). The large number of failed clinical trials, implemented for a huge variety of promising therapeutic interventions, has been well-documented (39). There is a remarkable need for both more sensitive biomarkers of disease progression and for better understanding of the disease itself. A start along the former path is a recent study that classified ALS based on composite functional phenotype scores (from electrodiagnosis, muscle weakness, and atrophy) and used conventional (gene-fold) approaches for analysis of transcriptome data from deltoid muscle plus some correlation measures (61). The availability of Affymetrix Human Genome U133A Array expression profile data from ALS muscle biopsies for the quadriceps (Data Series 3307 in Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE3307. For detailed information on all of the data used in this paper, please see Supplement Table S1. The online version of this article contains supplemental data) offer us the opportunity to explore mechanisms of muscle degeneration. Based on use of our network models and the subfamilies within these models, we aim to provide mechanistic insights that will be valuable for future investigations.
While there are many distinct muscular dystrophies, the most common variants involve either the deletion of the dystrophin gene (DMD) or a genetically dysfunctional version of dystrophin (Becker muscular dystrophy) on the X chromosome, thus affecting young males. In DMD patients, gradual yet progressive muscle degeneration leads to death, usually before, or when the subjects are in their 20's (16, 26). Dystrophin is the longest identified human gene, covering 2.4 megabases. The NH2-terminal of this long protein links to the F-actin cytoskeleton, while the COOH-terminal anchors to the proteins dystroglycan and syntrophin. It is now recognized that dystrophin is a critical structural protein within the cell that is part of the dystrophin-dystroglycan transmembrane complex, which transmits forces to the structural protein laminin of the extracellular matrix (ECM), providing mechano-protection to the plasmalemma in the process (11, 50). There is also agreement that this complex colocates with the costamere structures (a collection of membrane scaffolding proteins that form a ring around the fiber in the proximity of Z-disks, some of which are part of our transmembrane subfamily of MECH). It is well documented through isometric muscle testing that muscle progressively weakens. Perturbation studies show that, despite the lower force capacity, there is higher stiffness (12), and that increased concentration of serum creatine kinase and abnormal muscle histology are always present (57). Classical molecular signatures in muscle include an inflammatory response with infiltration of mast and other inflammatory cells, extracellular matrix (ECM) remodeling with fibrosis, muscle regeneration (and its failure), and energy metabolism (57). The pathogenic processes associated with muscle degeneration are not entirely known, but manifold “downstream” changes are obvious, thus encouraging transcriptional studies (11). The previous microarray studies of DMD include two for quadriceps in the Gene Expression Omnibus database that use U133A arrays, including both DMD and young controls. These comprise our data set of 33 boys with a mean age of 2.8 yr (11, 57) and a range from 1.5 mo to 9 yr. Importantly, one of the studies (11) included extensive protein immunolocalization analysis to tie genotype to phenotype, which we can integrate into our analysis. Also, the other study clustered young age groups and found mostly a predictable progressive change in transcripts; they also compared their results on a single gene level with another study that used a slightly older DMD age population (30) and reported remarkable consistency across results, including 99% consistency of identified transcripts for a probability threshold of P < 0.05 (57). The challenge has been data interpretation, and these past studies have used null-model statistical approaches based on conventional methods, such as individual gene fold changes (11, 31, 57), ad hoc classification (11, 57), or gene ontology classification (78). The challenges with gene ontology classification were addressed in the companion paper (81a). The ad hoc classifications are worth noting: in Ref. 11, there were five “pathological processes” categories (cell surface and ECM, intracellular signaling and cell-cell communication, immune response, energy metabolism and mitochondria, muscle structure and development); and in Ref. 57, three categories (muscle genes, inflammation, and ECM remodeling). We suggest that, if the aim is to tie these statistical results to known functional deficits (especially beyond generic responses such as inflammation and immune response), our models and their underlying subfamilies provide a special opportunity to explore the underlying functional molecular mechanisms associated with DMD pathophysiology, especially given that there is a large collection of DMD and young age-matched muscle control biopsies from which expression data are available.
In the following, we use our model framework to examine the mechanisms associated with these two specific myopathies. For the most part, we refer to the companion paper (81a) for the methods, and present in detail the results of our analysis and discuss the diseases in the form of pathways associated with our functional families and subfamilies.
METHODS
The method used in this study is similar to the one described in in the companion paper (81a), and the methods are now extended to include muscle biopsy data sets from disease cases, specifically, ALS and DMD. The human skeletal muscle-related microarray raw data from experiments carried out on Affymetrix Human Genome U133A array were collected from the Gene Expression Omnibus database and normalized with MAS5 in Expression Console provided by Affymetrix, and then z-scores were calculated from the normalized data [see the companion paper (81a)]. Based on the biopsy information, all of the data were divided into two groups: normal and diseased. Following this procedure, we applied the k-means clustering method on the normalized microarrays based on key proteins of skeletal muscle identified in a previous study [see the companion paper (81a)].
While many disease data sets have been examined, in this study, we target differences in microarray data sets of mainstream normal [defined in the companion paper(81a)], and two disease groups: ALS and DMD (for patients' information, we refer to the Supplemental Table S1). These are presented in two forms: by the form of protein network models, in which Student's t-tests are used to illustrate family members that are statistically up- or downregulated in the disease condition through color-coding, and by graded heat map scales that are organized by protein subfamilies and compared side by side to the normal population. To make age-appropriate comparisons, we use muscle from young normal data to compare with those from DMD patients.
RESULTS
Figures 1A–4A summarize the significant changes in core skeletal muscle transcription levels for the four protein model families, with the EA model connectivity representing unidirectional signal connections, the MECH model using bidirectional mechanical links, and the METB model connected with unidirectional biochemical links, and the SIGP model with unidirectional signal/biochemical links. The first three models illustrate how “real-time” functional capacity associated with such connectivity will change, owing to longer term up- and downregulation; the fourth is responsible for such compensatory change. This picture provides insight into some key changes with ALS and DMD, albeit giving only a partial picture, as it does not show selective changes in isoforms, including, in some cases, transitions to nonmuscle isoforms.
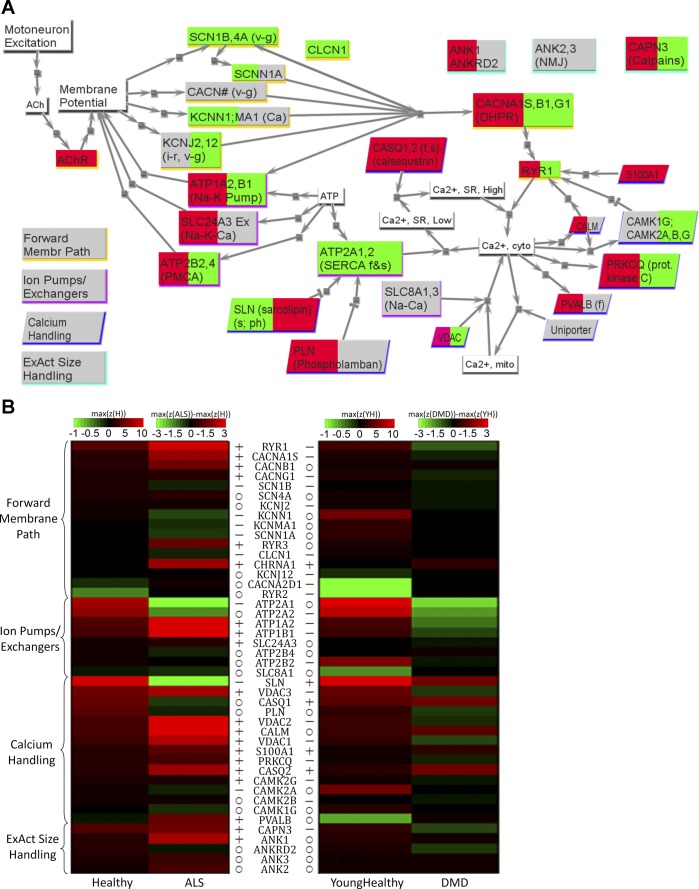
Fig. 1.
Excitation-activation (EA) family results for amyotrophic lateral sclerosis (ALS) and Duchenne muscular dystrophy (DMD). A: network view of changes for ALS (left background color) and DMD (right background color), with red implying significantly upregulated in diseased, green implying significantly downregulated in diseased, and gray implying no significant difference between diseased and healthy. Nodes with white background are signals or small molecules. Nodes with shadows are proteins, with the shadow color indicating to which one of the four subfamilies listed on the bottom left the protein belongs. For description of subfamilies, please see the companion paper (81a). B: heat map comparing the transcriptome data for healthy skeletal muscle to those of ALS and DMD. For each group, assuming there are N microarrays in the group, and in one microarray there are M probes targeting the same gene, then the corresponding z-score in this heat map is maxi=1 … M(1/N ∑j=1NZij). For healthy and young healthy, the color corresponds to the absolute z-score values. For ALS and DMD, the color corresponds to the difference (i.e., ALS − healthy, DMD − young healthy). If the difference is higher than 3 (or lower than −3), the color corresponding to 3 (or −3) is used. Genes are grouped based on subfamilies. For each subfamily, genes are sequenced according to the maximum mean z-scores in healthy. Left column: healthy and ALS. Right column: young healthy and DMD. The symbols before (for ALS and healthy) and after (for DMD and young healthy) the gene names indicate the results of t-test between healthy and diseased (P < 0.05). o, No significant difference; +, significantly higher in diseased than in healthy; −, significantly lower in diseased than in healthy. See text and supplemental tables for definitions of acronyms.
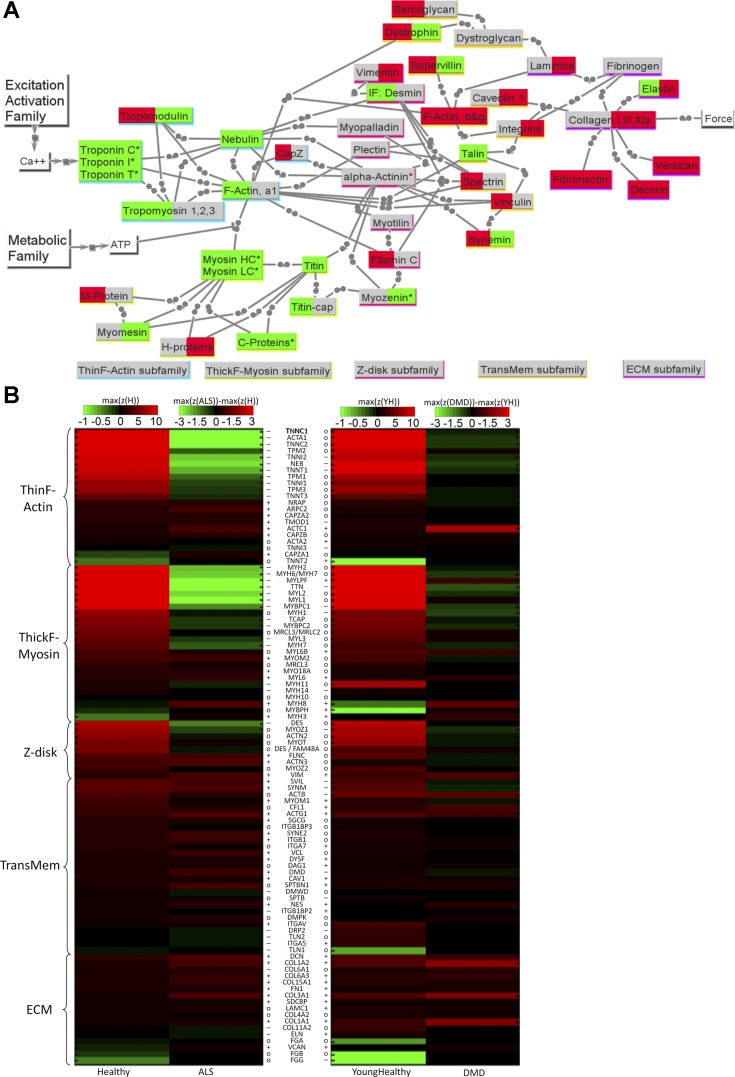
Fig. 2.
Mechanical transmission (MECH) family results for ALS and DMD. A: network view of changes for ALS (left background color) and DMD (right background color). The color scheme is the same as in Fig. 1A. Note that the connections depicted as two adjacent circles represent mechanical linkage. B: heat map comparing the transcriptome data of healthy skeletal muscle to those of ALS and DMD. Scaling and symbols are described in Fig. 1B legend. See text and supplemental tables for definitions of acronyms.
Fig. 3.
Metabolic and bioenergetic (METB) family results for ALS and DMD. A: network view of changes for ALS (left background color) and DMD (right background color). The color scheme is the same as in Fig. 1A. B: heat map comparing the transcriptome data of healthy skeletal muscle to those of ALS and DMD. Scaling and symbols are described in Fig. 1B legend. Voltage-dependent anion channel (VDAC) is also part of the EA model as well and is provided in Fig. 1B. See text and supplemental tables for definitions of acronyms.
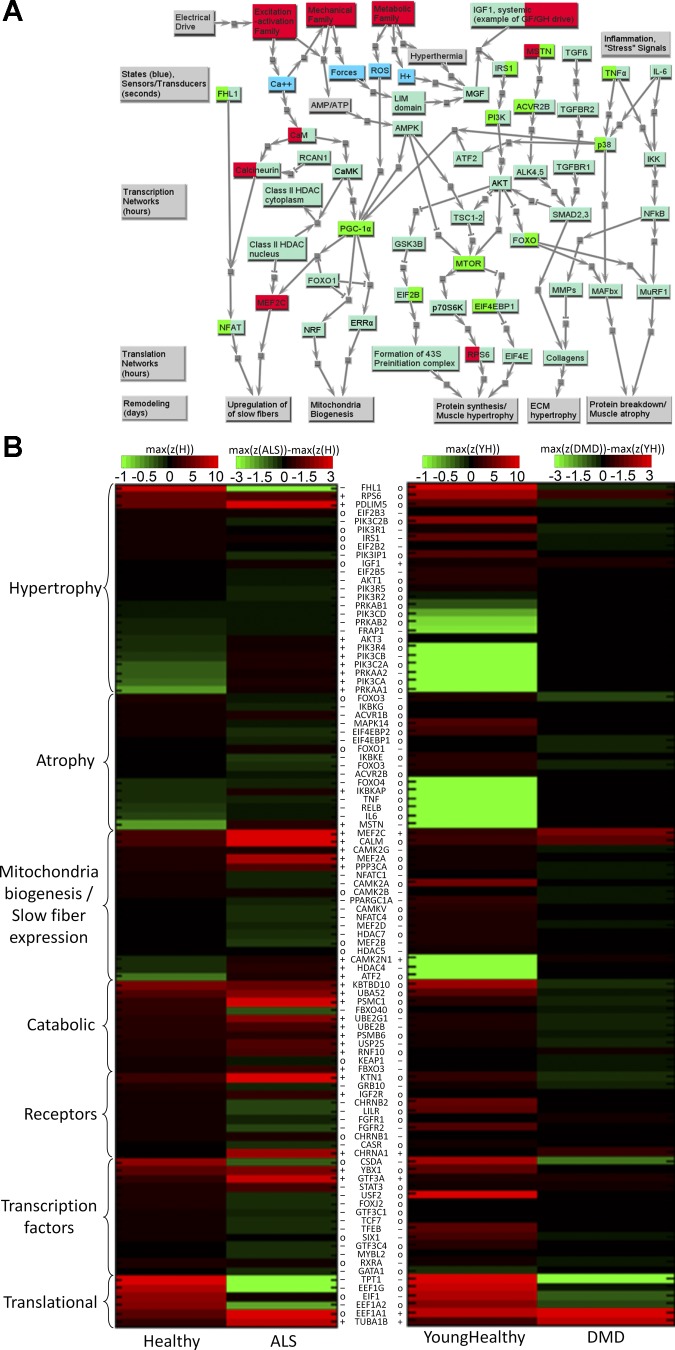
Fig. 4.
Signaling/production (SIGP) family results for ALS and DMD. A: network view of changes for ALS and DMD. Yellow nodes represent other protein families. Blue nodes are key dynamic states in the final muscle model framework. Along the bottom are gray nodes representing functional consequences related to phenotypic remodeling of skeletal muscle. The four big gray nodes on the left indicate different parts of the whole network and the time scale on which that part runs. The light green nodes are proteins in the SIGP family. If a gene is differentially expressed in diseased condition, either its left background color (differently expressed in ALS), or right background color (differently expressed in DMD), or both are changed to red or green, with red implying significantly upregulated in diseased, and green implying significantly downregulated in diseased. B: heat map comparing the transcriptome data of healthy skeletal muscle to those of ALS and DMD. Scaling and symbols are described in Fig. 1B legend. Only genes with significantly different z-scores between healthy and diseased are listed here, and results are classified in categories organized by functional consequences. See text and supplemental tables for definitions of acronyms.
The more detailed heat maps of Figs. 1B–4B, for all four models and their subfamilies, provide extended information for the various isoforms of the “core list” of proteins [see companion paper for definition (81a)]. It is clear that, except for a few transcripts [e.g., the immature myosin heavy chain (MYH) 11 and MYH3 myosin isoforms that are gradually replaced by mature myosin isoforms; and some fibronectin isoforms], there are few distinctions between adult and young normal data in these heat maps (of note is that, often the highest z-scores, already in the brightest red, tend to be even higher in young). Thus, while in Figs. 1B–4B, ALS is being compared with adult healthy data and DMD to young healthy data, in most cases a direct visual comparison of ALS to DMD is also possible. Importantly, we find the strong similarity between results for adult and young, from totally different data sets and studies, to provide strong support for the robustness of our approach. Complete data are provided in Supplemental Table S2.
In this section, the results of this study will be analyzed by each functional model and their subfamilies. For each functional family, the significantly differentially expressed genes between healthy and disease tissue are listed and briefly discussed in Tables 1–4. Synthesis across families and with existing literature, illustrating the advantages of this model-based framework, will be addressed in the discussion.
Table 1.
Key results of comparing the transcripts of skeletal muscle in ALS and DMD to those in healthy: EA model family
| Forward membrane path | |
| ALS | |
| Up | CHRNA1, DHPR, RYR1, RYR3 |
| Down | SCN1B, KCNN1, SCNN1A, CLCN1 |
| DMD | |
| Up | CHRNA1 |
| Down | DHPR, RYR1, SCN1B, CLCN1 |
| ALS | Upregulation of terminal regions of Forward Membrane Path [ACh receptor (CHRNA1) at NMJ or extra-junctional regions (58), DHPR and RYR1/3 at triads (55, 62)]. Downregulation of many transmembrane ion channels is consistent with the hypersensitivity in terminal regions (e.g., to encourage new NMJs or extract a weak signal), yet a remodeling toward lower use |
| DMD | Downregulation of most genes suggests a compensatory adaption to low use and perhaps decreased capability to transmit the excitation signal |
| Ion pumps/exchangers | |
| ALS | |
| Up | ATP1A2, ATP1B1, SLC24A3 |
| Down | ATP2A1F |
| DMD | |
| Up | None |
| Down | ATP2A2S, ATP1A2, ATP1B1, ATP2B2 |
| ALS | Less energy-efficient fast Ca2+ pump (ATP2A1), but not the slower pump, is downregulated. Upregulation of Na-K-ATPase (ATP1A2 and ATP1B1) is consistent with different mechanism in ALS from pure denervation (19) |
| DMD | Downregulation of Ca2+ pump (ATP2A1) and Na-K-ATPase activity (ATP1A2 and ATP1B1) (29) reflect compensatory adaption to low use |
| Calcium handling | |
| ALS | |
| Up | VDACs, CALMs, S100A1, PRKCQ, CASQ2S,Ca, PVALB |
| Down | SLN |
| DMD | |
| Up | SLN, CASQ1F, CASQ2S,Ca, S100A1 |
| Down | VDACs, PRKCQ |
| ALS | Downregulation of SLN (sarcolipin) is consistent with less need for regulating fast SERCA pump; PLN (more used for slower/cardiac pump) is not downregulated. Upregulation of VDACs (which include Ca2+ flux with these mitochondria channels) and of Ca2+ binding proteins suggests raised intracellular Ca2+ concentration |
| DMD | Upregulation of Ca2+ binding proteins is consistent with intracellular Ca2+ disregulation and of CASQs with elevated Ca2+ storage in SR |
| ExAct size handling | |
| ALS | |
| Up | CAPN3, ANK1 |
| Down | None |
| DMD | |
| Up | None |
| Down | CAPN3 |
| ALS | Upregulation of CAPN3 (a skeletal muscle-specific Ca2+-dependent protease) and ANK1 (ankyrin, which helps regulate EA physical space) suggests the apparatus is challenged by the disease) |
| DMD | As might be expected, little compensatory change is EA size is needed |
ALS, amyotrophic lateral sclerosis; DMD, Duchenne muscular dystrophy; EA, excitation-activation. The font of gene symbols in right column indicates the difference of the z-scores between healthy and disease: bold, difference > 5; italic, 3 < difference < 5. The superscripts of some gene symbols indicate the type of muscle fibers in which encoded proteins are expressed: S, slow fiber; F, fast fiber; FO, fast-oxidative fiber; FG, fast-glycolytic fiber; Ca, cardiac muscle; Sm, smooth muscle. See text and supplemental tables for definitions of acronyms.
Table 2.
Key results of comparing the transcripts of skeletal muscle in ALS and DMD to those in healthy: MECH model family
| Thin F-actin | |
| ALS | |
| Up | CapZs, TMOD1, ARPC2, ACTC1Ca |
| Down | ACTA1, NEB, TNNCs, TNNT1S, TNNI1S, TNNI2F, TPMs |
| DMD | |
| Up | ACTC1Ca, ACTA2Sm, TNNT2Ca |
| Down | NEB, TMOD1, TNNT1S, TNNI2F, TNNI3Ca |
| Thick F-myosin | |
| ALS | |
| Up | MYOM2, MYL6Sm, MYH3, MYH8 |
| Down | MYH2FO, MYLPFF, TTN, MYL1F, MYL2S,Ca, MYL3S, MYH7S, MYH6/MYH7S,Ca, MYBPC1S, MYBPC2F |
| DMD | |
| Up | MYH3, MYH8, MYL6Sm, MYL6BSm, MYLPFF, MYLBPH |
| Down | TTN, MYH1FG, MYBPC1S |
| ALS | Strong downregulation of most members of both thin and thick filaments is consistent with well-documented muscle weakness (15, 61), considerable atrophy of many and often most fibers (1, 61), led by profound downregulation (z-scores from over 11 to under −0.2, i.e., essentially off): ACTA1 (skeletal actin), TNNC2 (troponin C, fast), MYLPF (myosin light chain, fast), and TTN (titin). Also notable are all classic troponins, both fast and slow isoforms, consistent with ACTA1 loss. Fast isoforms were especially downregulated, except MYH1, which, along with upregulation of thick F-myosin linkers MYO18A and MYOM2, suggests there are still functional thick filaments. Yet MYBPCs, which recruit muscle-type creatine kinase to myosin filaments, are downregulated, consistent with energy deficiency and/or low “normal” use. Upregulation of a cardiac actin ACTC1 and “nonmuscle” or embryonic members in the thick F-myosin subfamily (i.e., MYL6, MYH3, and MYH8), consistent with Ref. 61 for deltoid muscle and with transformation into a “hybrid” fibers isoform substitutions. Also, upregulation of ARPC2 (which helps regulate actin assembly) and more mildly the actin capping proteins (CapZs and TMOD1) is consistent with a fluctuating thin-filament length |
| DMD | Upregulation of cardiac ACTC1Ca (strongly) and TNNT2Ca and smooth ACTA2Sm, and some members in the thick F-myosin subfamily related to smooth light chain (i.e., MYL6, MYL6B) and perinatal/embryonic heavy chain (MYH3 and MYH8) is consistent with a well-documented (11) trend toward fibers with a “hybrid” composition. Downregulation of TTN and NEB (the backbones of thick and thin filament) and some others [including titin (TTN) and MYH1FG] suggests a degree of compensatory atrophy and slower contractions |
| Z-disk | |
| ALS | |
| Up | FLNA, ACTN3F, VIM |
| Down | DES, MYOZ1F |
| DMD | |
| Up | VIM |
| Down | MYOZ1F |
| TransMem | |
| ALS | |
| Up | ACTG1, SYNM, SPTNB1, SVIL, MYOM1, NES, VCL, DMD |
| Down | DMWD, TLNs |
| DMD | |
| Up | MYOM1, ACTB, ACTG1, NES, SPTNB1 |
| Down | SVIL, SYNM, DMD |
| ECM | |
| ALS | |
| Up | COL3A1, DCN, COL6A3, COL1A1, FN1, VCAN |
| Down | COL11A2, ELN |
| DMD | |
| Up | COL1A1, COL1A2, COL3A1 and other collagens, DCN, FN1, ELN, VCAN |
| Down | FGG |
| ALS | Core members of Z-disk subfamily are not strongly changed, but downregulation of DES and MYOZ1 (fast) reflects a lower voluntary force and power capacity. Upregulation of genes in TransMem and ECM suggests a transformation into a hybrid active-passive “muscle” tissue, with a greater proportion of ECM (and fatty) tissue, trending toward transcripts seen for cardiac and smooth muscle tissue [compare to Fig. 6A in companion paper (81a)] |
| DMD | DMD gene is dramatically underexpressed, impairing the integrity of sarcolemma and the capacity of force transmission through the DGC linkage, a key TransMem subfamily pathway. Upregulation of some TransMem members (and of VIM, which participates for both Z-disk and TransMem) can be viewed as mechanical compensation (e.g., through the alternative integrin pathway) and greater use of nonmuscle cytoskeletal actin network. Strong upregulation of ECM subfamily collagens reflects passive force transmission that, in part, bypasses the muscle cells, consistent with literature documenting much stiffer (yet weaker) muscles (12) with a vulnerable plasmalemma (11, 30, 52, 57) and an increased risk of Ca2+ leakage (11, 33) |
MECH, mechanical transmission. See legend for Table 1.
Table 3.
Key results of comparing the transcripts of skeletal muscle in ALS and DMD to those in healthy: METB model family
| Glycolytic | |
| ALS | |
| Up | UGP2, SOD1, PGK1, AK1, CKMT2, CS, GYS1 |
| Down | CKM, GAPDH, ALDOA, ENO3, PYGM |
| DMD | |
| Up | AK1 |
| Down | CKM, GAPDH, ALDOA, ENO3, PYGM |
| ALS | Downregulated genes involved in glycolysis and glycogenolysis are consistent with lower use of fast isoforms in the EA and MECH models, and a degree of denervation. Upregulated genes involved in glycogenesis suggest more glycogen storage |
| DMD | Downregulated genes involved in glycolysis and glycogenolysis suggest limited or slower muscle energy usage |
| Oxidative phase 1 | |
| ALS | |
| Up | Nearly all |
| Down | ACADS |
| DMD | |
| Up | None |
| Down | One-half of them |
| Oxidative phase 2 | |
| ALS | |
| Up | Nearly all |
| Down | COX7A1, UCP1, UCP3 |
| DMD | |
| Up | None |
| Down | COX7A1, COX5A, COX6A2, ATP5H, UCP1, UCP3 |
| ALS | Upregulation of nearly all genes in oxidative subfamilies is consistent with elevated ATP production in mitochondria. However, dramatic downregulation of COX7A1 suggests a mitochondrial transformation process from skeletal-specific lattice to a more general type of mitochondria |
| DMD | Downregulation of nearly all genes in oxidative subfamilies (e.g., COX genes, PGC-1α) is consistent with less ATP production and decreased volume in mitochondria |
| Transporter | |
| ALS | |
| Up | VDAC1, VDAC2, VDAC3, SLC25A11, SLC25A12, SLC25A5, SLC25A6, SLC38A2 |
| Down | MB, HBA1/HBA2, HBB |
| DMD | |
| Up | None |
| Down | SLC25A4, SLC2A4 |
| ALS | Strong downregulation of MB and HB indicates diminished oxygen transportation and storage capacity, consistent with muscle disuse. Yet upregulation of VDAC for the outer membrane and SLC25A (mitochondria adenine nucleotide translocator, ANT) for the inner membrane suggests a degree of hypermetabolism |
| DMD | Downregulation of SLC2A4 (GLUT-4) is consistent with restrained glucose transportation and of SLC25A4 (ANT) restrained mitochondrial ATP production, both consistent with diminished muscle usage |
METB, metabolic and bioenergetics. See legend for Table 1.
Table 4.
Key results of comparing the transcripts of skeletal muscle in ALS and DMD to those in healthy: SIGP model family
| Hypertrophy | |
| ALS | |
| Up | RPS6, PDLIM5, PRKAA1, PRKAA2 |
| Down | FRAP1 (mTOR), PRKAB1, PRKAB2 |
| DMD | |
| Up | IGF1 |
| Down | FRAP1 (mTOR) |
| Atrophy | |
| ALS | |
| Up | MSTN |
| Down | ACVR1B, ACVR2B |
| DMD | |
| Up | None |
| Down | FOXO1, FOXO3, MSTN |
| ALS | Downregulation of mTOR is consistent with muscle atrophy (61). Upregulation of MSTN has been associated with muscle atrophy (38) |
| DMD | Downregulation of mTOR is associated with decreased muscle size (26). However, downregulation of FOXO1, FOXO3, and MSTN suggests a different muscle atrophy pathway than in ALS |
| Mito biogenesis/slow fiber expression | |
| ALS | |
| Up | MEF2C, MEF2A, PPP3CA, PPP3CB, CALMs |
| Down | FHL1, NFATC1, NFATC4, MEF2D, PPARGC1A (PGC-1α) |
| DMD | |
| Up | MEF2C |
| Down | NFATC1, MEF2B, MEF2D, PPARGC1A (PGC-1α) |
| ALS | Downregulation of PGC-1α, the key player in mitochondria biogenesis, is consistent with the observation of decreased mitochondrial volume (74). Downregulation of the highly expressed FHL1 plus NFATC's may lead to weakened calcinurin/NFAT pathway, despite upregulation of CALMs (probably caused by Ca2+ dysregulation, see EA family) and calcinurin (PPP3CA and PPP3CB), consistent with mild downregulation of slow fibers. Upregulation of MEF2C suggests ongoing muscle regeneration (56), as seen in denervated muscle (20) |
| DMD | Upregulation of MEF2C suggests ongoing muscle regeneration to repair damaged muscle (60) |
| Catabolic | |
| ALS | |
| Up | Nearly all |
| Down | HUWE1 |
| DMD | |
| Up | None |
| Down | One-half |
| ALS | Upregulation of nearly all catabolic subfamily members is consistent with muscle-wasting due to disuse |
| DMD | Downregulation of one-half of catabolic subfamily members indicates a gradual breakdown of muscle proteins, consistent with a protective response for muscle damage (26, 31) |
| Receptors | |
| ALS | |
| Up | KTN1, IGF2R |
| Down | GRB10, CHRNB2, CASR, LILR, FGFR1, FGFR2 |
| DMD | |
| Up | None |
| Down | GRB10, FGFR2, CHRNB1 |
| The alteration of the expression of these receptors may affect the signal pathways in which their corresponding ligands are involved | |
| Transcription factors | |
| ALS | |
| Up | YBX1, GTF3A, MYF6 |
| Down | More than one-half |
| DMD | |
| Up | GTF3A |
| Down | CSDA, TFEB, SIX1 |
| ALS | Upregulation of YBX1 and MYF6 promotes myogenesis (47, 87) |
| DMD | Underexpression of CSDA promotes angiogenesis in skeletal muscle (68). Downregulation of TFEB, a master gene for lysosomal biogenesis (70), indicates restrained autophagy |
| Translational | |
| ALS | |
| Up | TUBA1B |
| Down | TPT1, EEF1G, EEF1A2 |
| DMD | |
| Up | EEF1A1, TUBA1B |
| Down | TPT1, EIF1 |
| ALS and DMD | Strong downregulation of TPT1 suggests impaired intracellular Ca2+ homeostasis and microtubule stabilization (see http://www.uniprot.org/uniprot/P13693). Upregulation of EEF1A1 [lower in skeletal muscle than in other tissues, see the companion paper (81a)] and TUBA1B (part of tubulin), and downregulation of EEF1A2 [higher in skeletal muscle than in other tissues, see the companion paper (81a)] are consistent with transformation of skeletal muscle to hybrid tissue |
| Inflammatory | |
| ALS | |
| Up | IKBKAP |
| Down | IL6, IL6R, TNF, IKBKE, IKEKG, RELB |
| DMD | |
| Up | None |
| Down | None |
| ALS | Inflammatory pathways inhibition suggests muscle atrophy is probably not primarily caused by inflammation |
| DMD | No difference |
| Other biomarkers | |
| ALS | |
| Up | RTN4, SRPK3 |
| DMD | |
| Up | SPP1, SRPK3 |
| SRPK3 | Upregulation of SRPK3 is consistent with ongoing muscle degeneration (5) |
| ALS | Upregulation of RTN4 is correlated with the severity of ALS (36) |
| DMD | SPP1 is considered a determinant of disease severity in Duchenne muscular dystrophy (54) |
SIGP, signaling-production. See legend for Table 1.
EA Model Family
The transcriptional changes in the EA subfamilies associated with ALS and DMD are largely dissimilar (Fig. 1, Table 1). As a broad overview, most significantly up- or downregulated transcripts were of moderate value, except for two members of the network, both for ALS and for members most associated with fast muscle: ATP2A1 and SLN (see Table 1); otherwise more dramatic changes are seen for members of the MECH and METB families.
There are many notable distinctions between ALS and DMD in the transcriptional responses, suggesting very different EA phenotypes (see Table 1 and Fig. 1). For example, both the fast fiber Ca2+ to sarcoplasmic reticulum (SR) pump (ATP2A1F) and SLN (sarcolipin), which helps regulate (by slowing down) this pump, are strongly downregulated in ALS, but slightly upregulated in DMD, whereas the fast-isoform calsequestrin (CASQ1F) is slightly downregulated in ALS, but strongly upregulated in DMD. This pattern is consistent with the downregulation of fast isoforms of the thin F-actin (troponin, tropomyosin) in the MECH model family. Also upregulated with ALS (and mostly mildly downregulated in DMD) are voltage-dependent mitochondrial membrane channels [voltage-dependent anion channels (VDAC1/VDAC2/VDAC3)], here part of the calcium-handling subfamily, suggesting challenges with Ca2+ management that is consistent with the high upregulation of calmodulin (CALM). Only VDAC, the key component of a historically ancient regulatory control system that manages flux (including Ca2+) between the cytoplasm and mitochondria, is a member of multiple families (also METB transporter), and, in the discussion, we will propose a key role for VDAC in the dysfunction associated with ALS.
A hint of the dynamic conditions comes from consideration of key ExAct size handling members, with CAPN3 (a skeletal muscle-specific Ca2+-dependent protease) and ANK1 (ankyrin, which helps regulate the EA physical space) being slightly upregulated in ALS, and yet CAPN3 slightly downregulated in DMD. This suggests that, while DMD's EA apparatus is in a state of use-dependent decrease in capacity, in ALS its apparatus is challenged by the disease, with higher structural protein turnover. This also helps explain the upregulation of ANK1 (ankyrin), which helps regulate the EA physical space.
MECH Model Family
In the MECH model, the transcriptional changes in ALS and DMD (see Fig. 2A), compared with healthy, reflect the changes in mechanical bonds, and, as a consequence, forces are transmitted across model members. The heat maps shown in Fig. 2B illustrate the changes in the gene expression levels in members of the MECH family in ALS and DMD patients compared with normal patients.
In general, transitions in z-scores tend to be much more dramatic in ALS, especially for many of the classic functional proteins associated with the thin/F-actin and thick/F-myosin subfamilies (see Table 2, genes with bold or italic fonts). As seen in Table 2, some are so significantly downregulated as to be negligible, including skeletal muscle actin (ACTA1) and key fast muscle isoforms, resulting in utilization of an interesting mix of cytoskeletal, smooth, and cardiac isoforms. Given that muscle protein turnover rates are mostly on the same order of 1 mo (82), clearly the associated proteins in these subfamilies are similarly affected. The downregulation of the normal core of high z-score members of the contractile force generation and myofilament transmission subfamilies (thin F-actin, thick F-myosin, Z-disk) and upregulation of main TransMem and ECM subfamilies suggests that the MECH model exhibits many of the symptoms of a type of “disuse” that involves a lower neuromotor drive and, as we will see in the discussion section, remodeling to compensate for a diminished capacity for transient fluxes of ATP.
For DMD, the changes in MECH subfamilies are relate to progressive compensatory changes within certain strategy subfamilies. With the loss of the structural protein dystrophin, a key building block of the highly tuned dystrophin-sarcoglycan complex (DGC) linkage that mechanically transmits forces across the membrane and has been documented to enhance transmembrane stiffness (52), the transcripts for members of the alternative path of involving the integrin complex are mildly upregulated, a mechanical linkage mechanism that appears less optimized to the demands of transient muscle force transmission (77). The upregulation within TransMem of cytoskeletal actins (especially ACTB, to z-score = 8.3) and of CFL1 (cofilin 1) is consistent with this view. Finally, the finding of strong transcript upregulation of the cardiac actin (ACTC1) and embryonic myosin (MYH8) was verified in Ref. 11 via immunolocalization to correlate to high-level protein expression in dystrophic myofiber biopsies.
METB Model Family
Nearly all key genes/proteins in the oxidative phase 1 (e.g., TCA) and phase 2 subfamilies were upregulated in ALS compared with healthy, while most key genes/proteins were downregulated in DMD compared with young healthy (see Fig. 3). This suggests a degree of hypermetabolism in ALS and hypometabolism in DMD.
However, transcripts for the less efficient fast-energy pathways that we have lumped with our glycolytic subfamily were dramatically downregulated for ALS to a much greater extent than for DMD. Specifically, transcripts associated with glycolysis and glycogenolysis were downregulated in both ALS and DMD. Furthermore, AK1, the skeletal muscle-specific isoform of the enzyme that catalyzes the generation of ATP from ADP, was mildly upregulated in both ALS and DMD, indicating an energy deficiency in both diseases.
It is noteworthy that, within the oxidative phase 2 subfamily that was generally upregulated in ALS, muscle-specific isoforms of COXs (i.e., COX7A1 and COX6A2) were downregulated, with COX7A1 especially significant, both statistically (P = 0.000) and in change in z-score (see Table 3). Additionally, within the transporter subfamily, the nonmuscle isoforms of ATP/ADP translocases of the inner mitochondrial membrane [i.e., solute carrier family (SLC) 25A5 and SLC25A6] were upregulated in ALS. Together, these suggest that the mitochondrion is less muscle specific, which is consistent with a long history of literature (1); this suggests that these subfamilies reflect a network that is less capable of meeting the higher intensity and duration energy demands associated with skeletal muscle. In contrast, for DMD, all four subfamilies are mostly mildly downregulated (Fig. 3B, Table 3).
SIGP Family
Most of the transcripts associated with proteins included in the remodeling network (Fig. 4A) exhibited no significant difference between diseased and healthy (only differentially expressed genes were listed in Fig. 4B, heat map). See Table 4 for more details. However, several essential proteins [i.e., mammalian target of rapamycin (mTOR), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and myocyte enhancer factor 2C (MEF2C)], which dominate some typical pathways in skeletal muscle, were similarly differentially regulated in both ALS and DMD. There was one notable distinction, in which the highly expressed FHL1 (four and one-half LIM domain protein 1), recently associated with muscle mass (13), which binds to and enhances transcriptional activity of nuclear factor of activated T-cells (NFAT), was downregulated in ALS, but not in DMD. We also identified several biomarkers of ALS and DMD. For these genes, our results were consistent with other studies (see Table 4).
DISCUSSION
There are many types of myopathies, with different causes and symptoms. Our analysis of transcriptional changes in two examples of diseased muscle suggests that there will be manifold changes in up/downregulation of genes and as a consequence of muscle function, even when there is a known single source of pathology, such as the absence of dystrophin in DMD. This makes it challenging to identify causality and to predict consequences, as is reflected in past studies. We suggest that our functional model networks, each with subfamilies, provides a classification framework for helping address such challenges. For instance, it is useful to discriminate between sources of myopathies that are intrinsic to one or more members of our families (e.g., dysfunctional gene) from those where a dysfunctional input to a given model family causes gradual maladaptation [e.g., neuromotor-driven muscle dysfunction, such as with cerebral palsy (73)]. Most diseased muscle has a source in one model family, and usually one subfamily, but over time will functionally affect remodeling in others, or involves inflammatory myopathies, which may affect most of our four protein networks of families. But one distinction in our approach is our strong emphasis on members with high z-scores that are muscle specific and, as a consequence, on heat maps that emphasize magnitude changes rather than gene-fold changes. Thus, in contrast to most other studies that are often naturally biased toward high gene-fold changes, nonmuscle-specific genes that start with low z-scores, such as those associated with inflammatory responses, we focus on the changes in the networks of core constituents of the muscle tissue itself.
This section aims to 1) link genotype to phenotype based on model families corresponding to functional protein networks; and 2) create pathological network hypotheses that describe how the signal flows in myopathies from sources such as mutant genes to symptoms that include changes in muscle functional capacity.
ALS
As a context for interpreting our results, muscle biopsies for ALS will include some (often a majority) of muscle fibers that are clearly atrophic and smaller in cross section, and some that are of roughly normal size that are still likely to represent most of the tissue volume in a sample (61). Thus it is inherently a “hybrid” tissue with a mix of phenotypes. Our results indicated that muscle atrophy (i.e., downregulation of transcripts in MECH family, as seen in Table 2 especially for fast muscle) and general upregulation of transcripts in oxidative subfamilies (phases 1 and 2) in the METB model (with, as seen in Table 3, the important exceptions of the two muscle-specific COX members) could be major ALS symptoms, both of which are consistent with observations in ALS patients [with the latter often referred to as mild “hypermetabolism” (18)]. Our analysis also suggests mostly upregulation for all four EA subfamilies, with several notable exceptions that will be addressed. Based on these results and those for key members of the SIGP family, we augment an emerging perspective on how skeletal muscle is affected in ALS, with a novel hypothesis that is based on demand-supply communication between METB and other model families, as shown in Fig. 5A. Critical to our understanding is the role transporters, in particular, the VDACs, which, owing to the critical location and function, is unique in being a member of both EA and METB families.
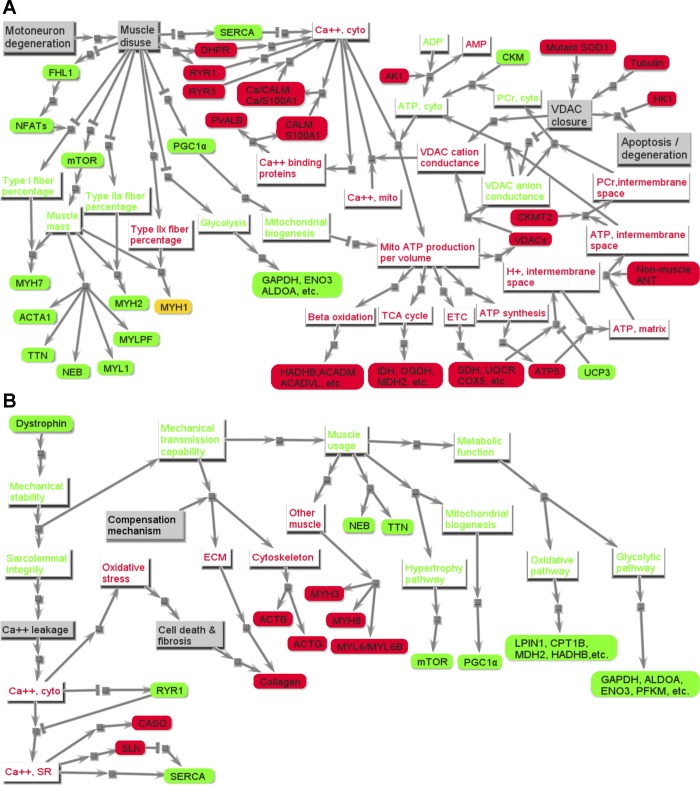
Fig. 5.
Hypothesized pathological pathways for ALS (A) and DMD (B). Nodes with gray background color represent physiological events. Rectangle nodes with red or green font are phenotypic features in skeletal muscle, with red font implying promoted or strengthened, and green font implying suppressed or weakened. The rounded rectangle nodes are genes, and their background colors indicate their relative expression level compared with those in healthy groups, with red implying overexpression (or upregulation), green implying underexpression (or downregulation), and yellow implying there is no significant difference. A: the signal starts from the node “Disuse” at the top left and “Mutant SOD1” at the top right and ends with the underexpression of MECH family genes (bottom left), the overexpression of genes in oxidative subfamily and underexpression of genes in glycolytic subfamily in METB family, and the elevated cytosolic Ca2+ concentration (the node “Ca2+, cyto” at top middle) and apoptosis (the node “Apoptosis” at middle left). B: the signal starts from the node “Mutant dystrophin” at the top left and ends with differentially expressed genes in EA family (bottom left), differentially expressed genes in MECH family (middle), and underexpression of genes in METB family (right). See text and supplemental tables for definitions of acronyms.
Calcium dysregulation.
Many studies have observed an elevated intracellular Ca2+ concentration in ALS (3), most commonly in neurons (72). In mice with the G93A human SOD1 mutation, elevated cytosolic Ca2+ concentration was also observed (10). Our analyses suggest that this may also be true in skeletal muscle (see Fig. 5A, top middle). The general trend in all EA subfamilies was upregulation or no change. Yet quite revealing, as seen in Fig. 1, is a dramatic difference between ALS and healthy, which is the downregulation of energy-demanding fast isoform for sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps that rapidly remove Ca2+ out of cytosol, but is less energy efficient and more demanding of ATP flux than the slow isoform (4); the dramatic downregulation of calcium-handling subfamily member SLN is consistent with this loss. All of the forward membrane path subfamily members [dihydropyridine receptor (DHPR), ryanodine receptor (RYR) 1, RYR3, and VDACs] that release Ca2+ into cytosol were upregulated, with the former two often upregulated in nearly denervated skeletal muscle (38) and, therefore, capable of releasing more Ca2+ into cytosol if stimulated, resulting in what is commonly called the hypersensitivity of ALS muscle (or of some of the fiber phenotypes within it). On the other hand, RYR3 and VDAC could be the key players in calcium dysregulation in ALS. Unlike RYR1, the Ca2+ release activity of RYR3 is voltage independent (42). Overexpression of RYR3, which is also observed in ALS skeletal muscle in another study (37), can lead to the higher resting cytosolic Ca2+ concentration in myotubes (56). This overexpression of VDACs and RYR3, along with other two significantly upregulated calcium-handling members, S100 family proteins [S100A1 (P = 0.020) and calmodulin (P = 0.001)], can raise cytosolic Ca2+ through a cascade of reactions initiated by the dysregulation of VDAC (see Fig. 5A).
In skeletal muscle in ALS, VDAC may close due to mutant SOD1 or overexpressed tubulin (see Hypothesized mechanism in skeletal muscle in ALS, including signaling changes below). When in the closed state, VDAC conductance to cations such as Ca2+ actually increases (76). Owing to the increased Ca2+ in mitochondria in ALS (72), the upregulation of VDAC, and its higher permeability to Ca2+, Ca2+ can diffuse from mitochondria into cytosol and bind to S100A1 and calmodulin, both upregulated. Upon Ca2+ binding, these can bind to RYRs and enhance their Ca2+ release, although they compete with each other for the same binding site (32). Elevated cytosolic Ca2+ also promotes the shift of RYR3 to the open conformation (75), causing low-intensity, calcium-induced Ca2+ release. Therefore, Ca2+ release from SR is elevated, but the Ca2+ uptake flux by pumps into SR is constrained, thus resulting in an elevated cytosolic Ca2+ concentration. At some stage, this may help trigger a transition of some fibers into an atrophic phenotype.
Altered metabolism in ALS.
As developed in the companion paper (81a), among three energy systems in skeletal muscle, two were lumped in the glycolytic subfamily [creatine phosphate (PCr) process, and short-term glycolytic], and one addressing longer term (oxidative) energy demand was lumped into two oxidative subfamilies. Similar to other disuse cases (such as immobilization and microgravity), most proteins in PCr system [e.g., CKM (creatine kinase, muscle)] and glycolytic system [e.g., GAPDH and aldolase A fructose-bisphosphate (ALDOA)] were downregulated (see Fig. 3); however, nearly all members of the oxidative subfamilies involved in lipid metabolism were upregulated (Fig. 3), with the exception of several muscle-specific COX members. The upregulated yet dysfunctional oxidative system can be related to the following: 1) the mild “hypermetabolism” seen in most ALS patients, as demonstrated in controlled studies (8, 18); 2) the finding that a fat-rich diet can extend the expected life of ALS patients (23); 3) the emerging role of mitochondrial functional signaling in apoptotic signaling in neurodegenerative diseases (59); and 4) the reality that rapid fatigue is a hallmark of ALS. Our results are consistent with the likelihood that skeletal muscle in ALS contributes to the increased resting metabolism and yet, ironically, with a METB model family and cannot provide an appropriate on-demand ATP energy flux capacity.
Hypothesized mechanism in skeletal muscle in ALS, including signaling changes.
VDAC, a key member of the METB transporter subfamily and also the EA calcium-handling subfamily that locates at the outer membrane of mitochondria, serves as the main regulator of metabolite transport between cytosol and mitochondria. When in an open state, it is highly permeable to anions such citrate3− and ATP4−. When in a closed state, it is impermeable to anions, but permeable to cations such as K+. The closure of VDAC itself may lead to the initiation or promotion of cell death (64, 65). Although the connection between the VDAC closure and apoptosis is still unclear, several studies connect the decreased VDAC regulation of conductance and expression of important apoptosis biomarkers (43, 76, 79, 80). There are also recent studies that emphasize ties between mitochondria pore regulation, materials transport, and morphological structure in ALS (22).
Figure 5A, right, shows a VDAC-centered signaling pathway that can explain the cytosol energy deficiency and elevated mitochondrial activity. In SOD1-mutation-caused ALS, if the mutant SOD1 protein moves close to mitochondria and enters the outer membrane, it binds directly to VDAC, which can lead to its closure (35). SIGP translational subfamily member tubulin, the main cytoskeletal building protein for microtubules, is also an inherent component of mitochondrial membrane design and interacts with VDACs (9), increasing VDAC voltage sensitivity and making reversible closure more likely (66). Supporting this, use of anti-tubulin agents can cause cytochrome c release and induce apoptosis (2). While mitochondrial tubulin represents only ∼2% of cellular tubulin, dimeric tubulin, by binding to VDAC, can restrict the mitochondrial outer membrane permeability to ADP and ATP (49). Considering the upregulation of α-, β-, and δ-tubulins in ALS, it is logical to assume that the probability that VDAC is in a closed state increases, resulting in its decreased conductance to anions. Based on this assumption and our modeling framework, we propose a novel mechanism to explain physiological changes in skeletal muscle in ALS, as shown in Fig. 5A, that are consistent with known symptoms.
1) METB transporter subfamily dysfunction. Reduced VDAC conductance to anions leads to energy demand deficiency within the cytosol, with high-energy-containing molecules generated within mitochondria, such as ATP and PCr, having difficulty diffusing into the cytosol. The downregulation of CKM from the highest z-score in healthy to a negative z-score in ALS also indicates that PCr is close to absent in cytosol. Without PCr, the main source for immediate energy replenishment, skeletal muscle will have to take advantage of another immediate energy supply: generating ATP from ADP, which is catalyzed by myoadenylate kinase (AK1). The upregulation of AK1 in ALS supports this assumption of cytosolic energy deficiency in ALS.
2) Subsequent oxidative phases 1 and 2 subfamily dysfunction. Reduced control of VDAC conductance, along with the elevated cytosolic calcium and the cytosolic energy deficiency, can explain the altered metabolism in ALS. Elevated Ca2+ increases the oxidative stress and stimulates mitochondrial ATP production. The capability of mitochondria to produce ATP depends on the volume of mitochondria, the amount of enzymes within it, and effective transport of materials and energy. However, the observed downregulation of PGC-1α (a key protein promoting the mitochondria biogenesis) suggests limited volume of mitochondria. Correspondingly, enzymes in the oxidative phase 1 and 2 subfamilies, including β-oxidation, the TCA cycle, and the electron transport chain, will be upregulated, as seen in Figs. 3A, 3B, and 5A. However, notably not upregulated are the muscle-specific COX71A (dramatically downregulated) and the COX6A2 (trends toward downregulation) isoforms. This suggests a transition toward a nonmuscle type of mitochondria of lower capacity. Combined with morphological abnormalities that include less of a coordinated lattice ultrastructure with the myofilaments (1, 46, 81), this results in longer distances to reach the sites of highest ATP flux demand within the MECH and EA families. Upregulated oxidative phase 2 subfamily (electron transport chain members, except muscle-specific members) strengthens the potential gradient across the inner membrane of mitochondria. Given the downregulated uncoupling proteins (UCP1/UCP3) of this subfamily, which cause generation of heat instead of ATP during the electron transport process by leaking protons from the intermembrane space into the matrix, and upregulated ATP synthase, it is reasonable to conclude that there is excessive ATP in the matrix. There is evidence that muscle mitochondrial uncoupling (e.g., overexpressing UCP1, which is under the control of the promoter MCK gene) induces abnormal energy homeostasis and may trigger NMJ dismantling (24). Furthermore, upregulated METB transporter subfamily member ANT (SLC25A) suggests that those ATP housed within the matrix were translated into the intermembrane space. Normally within the intermembrane space, ATP becomes ADP and contributes energy and phosphate for the generation of PCr, a process catalyzed by CKMT2 (creatine kinase mitochondrial 2) of the gylcolytic subfamily, which was also upregulated in ALS. PCr not entering the cytosol helps explain the most significant downregulation of CKM (z-score = −0.42 in ALS, z-score = 13.40 in healthy, P = 0.000) since there is minimal demand for it. PCr thus accumulates at the intermembrane space due to blocked VDAC (80).
This analysis, based on the use of our network framework, may help explain the paradoxical situation of elevated metabolism and yet reduced access to energy for recurring muscle force production: while elevated demands for ATP during and after actuator use (e.g., cytosolic Ca2+ increases the oxidative stress) promotes the ATP production from METB, ATP, and PCr accumulation within the intermembrane space leaves key transient ATP consumption sites, such as SERCA pumps and myosin, with insufficient access to energy and challenges to the calcium-handling subfamily. This, in turn, leads to a transition toward slow and hybrid muscle fibers, where the isoforms are more efficient. As a result, despite the intensified oxidative process to create ATP, excessive oxidative stress is maintained in skeletal muscle, which can cause damage to muscle cells, both within the myofibrillar space and, importantly, near the NMJ, where there is also a high demand for energy.
The muscle fibers in ALS appear to evolve into hybrid types with reduced energy flux (METB) capacity and, as a consequence, a reduced capability to meet EA and MECH demands, which, in turn, induces adaptive compensatory changes to EA and MECH capacity. Furthermore, METB contribution to maintenance of NMJs, another region of the cell with high energy demands and normally supported with extra local myonuclei, will diminish, which can compromise the effectiveness of NMJs (commonly viewed as compromised early in ALS). Our hypothesis of a metabolic contribution to ALS is also consistent with degeneration of motoneurons [which, owing to their long axons, can be expected to be especially vulnerable to unmet energy flux demands arising near the proximal end of their axons and at their NMJ end plates (14, 24)].
Compensatory muscle atrophy of MECH family, into a functionally “hybrid” muscle.
We suggest that the dramatic changes in the MECH family are consequences of a compromised neuromotor drive to the EA family (and the EA changes described above) and the diminished ATP flux capacity due to dysfunction of the METB family. This represents a gradual but severe skeletal muscle dysfunction and phenotype, and atrophy is inevitable in ALS patients. Our analysis demonstrates that core proteins in both the thin/F-Actin and thick/myosin subfamilies, and a few of the main Z-disk subfamily scaffolding proteins (desmin, myozenin 1), were very strongly downregulated. This included the downregulation of titin and nebulin, which mark the functional deterioration of normal skeletal muscle. Yet other actin and myosin isoforms were upregulated, including cardiac actin, as were many members of especially the TransMem but also ECM subfamilies. Many of these structural members also possess transduction sites that pass mechanical stresses to corresponding sensors and, therefore, stimulate the production of contractile proteins through signaling pathways. Consistent under-expression of a key SIGP hypertrophy subfamily member (mTOR) suggests that machinery to produce structural proteins in skeletal muscle was weakened in ALS, but with alternative compensatory adjustments, such as fewer fast-fiber isoforms that are more energy consuming. Thus what emerges is a tissue of hybrid fiber composition but still, in most cases, functionally contractile tissue.
The magnitude of force that skeletal muscle can generate depends on two parts: the neural drive and the muscle capacity, with the latter frequently associated with mass that is still functionally contractile. In ALS, the deteriorating NMJs and EA model apparatus (Altered metabolism in ALS above) provides reduced or diminished neural drive capability. However, as seen in Hypothesized mechanism in skeletal muscle in ALS, including signaling changes above, we also propose that it is a function of METB functional capacity.
Possible molecular protective responses in skeletal muscle in ALS.
Compared with neural tissue, the death of muscle fibers in ALS is seldom reported, probably due to two reasons: 1) skeletal muscle cells are multinucleated, and it is difficult to determine the death of a muscle cell, with these giant cells tending to atrophy (sometimes severe) and take on hybrid properties rather than die; and 2) several molecular protective mechanisms are probably activated in ALS, such as the upregulation of hexokinase 1 (HK1) (P = 0.000) and parvalbumin (P = 0.032). The former prevents apoptosis when binding to VDAC (53), and the latter might be a protective response to elevated intracellular Ca2+, which may enhance the production of free radicals and lead to alterations of mitochondrial function, as shown in Fig. 5A. Parvalbumin, a member in the calcium-handling subfamily, is a calcium-binding protein in cytosol and acts like Ca2+ buffer. Its upregulation may alleviate the stress due to extracytosolic Ca2+. Clinically, it serves as one of the therapeutic targets to slow down the loss of motoneurons in ALS (21) and as a biochemical marker of ALS-resistant motoneurons (25).
DMD
In DMD patients, the absence of MECH TransMem subfamily member dystrophin leads to mechanical dysfunction that causes faulty mechanical transmission of forces when muscle is activated and compensatory mechanisms throughout the MECH subfamilies (Fig. 1). That ECM is upregulated is consistent with a large body of literature; indeed, both Refs. 11 and 57 had ECM as a category, and our model structure illustrates how this will increase passive stiffness. But we go further and tie it to the mechanics associated with TransMem: the lack of dystrophin is known to lower TransMem stiffness capacity (52) and explains the compensatory (but eventually futile) upregulation of the integrin complex pathway. This repeated mechanical vulnerability during voluntary action leads to secondary events, such as Ca2+ penetration across damaged sarcolemma, as reflected in the EA calcium-handling subfamily. This eventually results in the wasting of muscle cells, which are gradually replaced by a weaker hybrid tissue that includes more adipose and connective tissue and greater expression of embryonic and prenatal myosin heavy chains (Fig. 1). This basic causality between lack of dystrophin and DMD has been established for more than 15 yr, and our analysis displays trends that are consistent with that of others analyzed by other methods (11, 31). However, the mechanisms leading from the absence of dystrophin to muscular degeneration, and how this could affect therapeutic interventions, still remain elusive. Many mechanisms, such as calcium misregulation, relevance of nitric oxide, and glycosylation, are hypothesized to relate to the pathogenesis of DMD (17). Based on the transcriptomic comparison of skeletal muscle within the framework of our functional protein families, we propose a hypothesized pathogenic network to link the absence of dystrophin to DMD, as shown in Fig. 5B. We note that muscle wasting and atrophy in DMD is progressive, and all of the symptoms of DMD are not fully expressed until the age of 2–6 yr. As the mean age of DMD subjects in this study was 2.8 yr, the difference of z-scores between DMD and age-matched healthy groups could be expected to be larger for older children with DMD (78), but findings in Ref. 57, one source for our data, suggest that this will not change our core findings.
Abnormal calcium regulation.
Calcium dysregulation in skeletal muscle has been tied with the pathogenesis of DMD (33). Normally, a large gradient (roughly 10,000 times lower than intracellular Ca2+ concentration) is actively maintained in a resting muscle, and during excitation only small transient amounts of extracellular Ca2+ pass across the sarcolemma (e.g., through voltage-dependent DHPR Ca2+ channels) that trigger a larger transient influx from SR (11). In DMD, as Fig. 5B, left, shows, without dystrophin the mechanical transmission capability leads to impaired sarcolemma integrity and the leakage of extracellular Ca2+, especially as a consequence of force-producing events.
The Ca2+ leakage through impaired sarcolemma leads to the elevated cytoplasmic residual Ca2+ (34). To accommodate this, there are changes in all EA subfamilies, but especially (see also Fig. 5B) the following.
1) Terminal end of forward membrane path. The main Ca2+ channels, DHPR at the sarcolemma and RYR1 at the SR membrane, were both mildly downregulated in DMD compared with young healthy. It is arguable that it may be due to the muscle deconditioning in DMD (62, 69); however, it limits the further entry of Ca2+ into the cytosol [for example, the amplitude of the action potential-evoked Ca2+ transients is significantly decreased in mdx mouse muscle fibers (86)].
2) Ion pumps/exchangers and calcium-handling subfamilies. Excess cytosolic Ca2+ and downregulated RYR1 can lead to the extra Ca2+ storage in SR, which is consistent with upregulation of both isoforms of CASQ. The extra Ca2+ storage in the SR inhibits further Ca2+ pumping into the SR through the downregulation of the bidirectional SERCA pumps and upregulation of SERCA inhibitor SLN, which, together, suggest why intracellular Ca2+ in dystrophic muscle returns more slowly to its resting level following a contractile stimulation (64).
Strong upregulation of CASQ and mild upregulation of calmodulin (CALM) helps buffer Ca2+ and trigger adaptive signaling pathways of the SIGP family.
3) Excess cytosolic Ca2+ induces Ca2+ uptake by mitochondria through mitochondrial Ca2+ uniporter, which can increase oxidative stress and lead to apoptosis and inflammatory response (7, 40), and thus the METB family is also impacted.
Muscle mechanics compensation spread across all subfamilies.
In DMD patients, the muscle is voluntarily controlled and used, despite lack of dystrophin, with the TransMem subfamily providing a lower local stiffness and force capacity and lateral force transmission (63), and a higher overall compensatory stiffness (12). Clearly, this will directly affect members of the ECM and Z-disk subfamilies as well and involve greater use of the integrin complex as an alternative pathway for transmembrane force transmission (with key members mildly upregulated).
Sufficient evidence indicates the occurrence of ECM remodeling and ECM transcript upregulation in skeletal muscle in DMD patients (31), as also shown in our analysis (see Fig. 2) and illustrated in Fig. 5B. Collagens are the main component in ECM, providing structural support to resident muscle cells, as well as compensatory force pathways that bypass muscle cells, and matrix metalloproteinase 2 (MMP2) from the SIGP (Fig. 4A) is a key regulator controlling the formation, remodeling, and degradation of ECM.
ECM remodeling in DMD can be induced by two mechanisms (Fig. 5B). First, the lack of dystrophin and a functional DGC cause a substantial reduction in the local stiffness of affected muscle fibers (52), causing compensatory adaptive responses, such as greater use of the integrin complex and nonmuscle actins of the TransMem subfamily (ACTB, ACTG1) as a cytoskeletal network (both upregulated). The second is via a degree of fibrosis of damaged or dead fibers (or parts of long fibers), as a consequence of the inflammatory response and the multiple stages in wound healing. In our analysis, S100A4 and S100A6, two biomarkers correlated with the proliferation of fibroblasts, had higher z-scores in DMD, indicating the fibroblast activation and the incidence of fibrosis in dystrophin-absent skeletal muscle. The end product of fibrosis can be scar tissue, which contains mostly collagens, and mainly type I collagen (both COL1A1 and COL1A2 were significantly upregulated, as were 3 of the other 6 collagen markers) and elastin.
Finally, the mild downregulation of certain traditional high-use members of thin/F-actin and thick/F-myosin subfamilies (e.g., fast MYH1, supporting members nebulin and titin) and upregulation toward greater use of alternative components (e.g., upregulation of cardiac actin and of certain light chain and embryonic myosins relative to young healthy) are indicative of a compensatory disuse paradigm, leading to a hybrid composition of parts, one that is confirmed in dystrophic fibers (11). The tendency is clearly toward a more protective mix: less use of the faster myosin isoforms (e.g., MYH1) that would generate high force transients across the membrane and challenge the SERCA pump capacity of the EA family, and greater use of actins that are normally more associated with nonskeletal muscles and with cytoskeletal structures that can modulate stiffness. This results in functionally weaker, yet mechanically stiffer, muscles, as seen experimentally (12).
Metabolic family compensation to disuse.
As shown in Fig. 3B, the z-scores of most of the proteins in the METB model were either lower or not higher in DMD than in young healthy, indicating the reduced metabolic level in skeletal muscle in DMD. Proteins in both the glycolytic subfamily and oxidative phase 1 and 2 subfamilies were downregulated, consistent with the decreased concentration of metabolites such as glycolytic substrates glucose, gluconeogenic amino acids such as glutamine and alanine, and glycolytic products lactate, creatine, choline, and acetate (71).
The pattern of reduced metabolism in DMD skeletal muscle is similar to that in a homeostatic disuse condition, i.e., with reduced usage, both energy storage such as glucose and PCr and energy production measured such as mitochondrial ATP product rate are lower than in control groups (44, 85). Most clinical protocols for DMD suggest that the daily activity of DMD patients should be limited to prevent the large forces or stretch of skeletal muscle, which not only increases the mechanical stress but also results in the opening of stretch-activated ion channels at the cell membrane (27). Since the muscle-specific COX7A1 and COX6A2 are moderately yet significantly downregulated, there is also indication of less use of muscle-specific mitochondria.
Conclusion
This work demonstrates how our functional network-based model framework for skeletal muscle tissue, derived from transcriptional changes in normal and diseased muscle, provides a foundation for understanding mechanisms leading to disease and disease response in ALS and DMD. We show that ALS can be characterized as a unique form of atrophy and hybrid muscle tissue formation, with a tendency for mildly higher mitochondrial metabolic activity, and propose a novel set of mechanisms for understanding ALS that emphasizes dysfunction in METB materials transport, as well as neuromotor disuse signaling to EA. This motivates well-characterized experimental investigations, especially those focused on adaptive remodeling of the actual muscle components rather than tissue-independent phenomena, such as transcripts directly associated with inflammatory or immune or tumor responses. For DMD, whose origins are established through the deletion of dystrophin gene, our framework helps us understand the unique types of compensatory mechanisms that appear to unfold, including atrophy with excessive ECM, a reversion to certain embryonic and cardiac isoforms, and reduced metabolic capacity based on reduced demand. For both pathologies, the use of our protein models and their subfamilies was able to help classify and illuminate the role of the many up- and downregulated transcripts on the observed phenotypes. Our models help illustrate how different mechanistic origins can induce gradual remodeling changes via dysfunctional signaling; for instance, MECH changes for ALS reflect disuse, owing to a source outside the MECH model family, while DMD reflects a compensatory “redistribution” strategy with a source primarily within this model family. For EA, both ALS and DMD display Ca2+ dysregulation, through more Ca2+ binding/buffering proteins in ALS and less Ca2+ channels/pumps in DMD. This functional model framework can be extended to study other skeletal muscle disorders, with the aim of providing an understanding of the corresponding pathology that can more easily bridge the continuum between molecular changes within muscle tissue and muscle functional capacity as an actuator.
GRANTS
This work is supported by the following grants: National Heart, Lung, and Blood Institute Grant 5 R33 HL087375-02 (S. Subramaniam), National Science Foundation (NSF) Grant DBI-0641037 (S. Subramaniam), NSF Collaborative Grant DBI-0835541 (S. Subramaniam), and NSF Collaborative Grant STC-0939370 (S. Subramaniam).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., J.M.W., and S.S. conception and design of research; Y.W., J.M.W., and S.S. performed experiments; Y.W., J.M.W., and S.S. analyzed data; Y.W., J.M.W., and S.S. interpreted results of experiments; Y.W., J.M.W., and S.S. prepared figures; Y.W., J.M.W., and S.S. drafted manuscript; Y.W., J.M.W., and S.S. edited and revised manuscript; Y.W., J.M.W., and S.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Robert Byrnes for assistance in figure generation, Dr. Brian Saunders for assistance in data management, and Richard L. Lieber for valuable discussions.
REFERENCES
- 1. Afifi AK, Aleu FP, Goodgold J, MayKay B. Ultrastructure of atrophic muscle in amyotrophic lateral sclerosis. Neurology 16: 475–481, 1966. [DOI] [PubMed] [Google Scholar]
- 2. André N, Braguer D, Brasseur G, Gonçalves A, Lemesle-Meunier D, Guise S, Jordan MA, Briand C. Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells. Cancer Res 60: 5349–5353, 2000. [PubMed] [Google Scholar]
- 3. Appel SH, Beers D, Siklos L, Engelhardt JI, Mosier DR. Calcium: the Darth Vader of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2: S47–S54, 2001. [PubMed] [Google Scholar]
- 4. Barton K, MacLennan D. The proteins of sarcotubular system. In: Myology: Basic and Clinical (3rd Ed.), edited by Engel AG, Franzini-Armstrong C. New York: McGraw Hill, 2004, p. 307–323. [Google Scholar]
- 5. Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Beal MF. Mitochondria and the pathogenesis of ALS. Brain 123: 1291–1292, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem 45: 481–506, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, Couratier P. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol 256: 1236–1242, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Carré M, André N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem 277: 33664–33669, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Carrμ MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett 414: 365–368, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol 151: 1321–1336, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornu C, Goubel F, Fardeau M. Muscle and joint elastic properties during elbow flexion in Duchenne muscular dystrophy. J Physiol 533: 605–616, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowling BS, McGrath MJ, Nguyen MA, Cottle DL, Kee AJ, Brown S, Schessl J, Zou Y, Joya J, Bönnemann CG, Hardeman EC, Mitchell CA. Identification of FHL1 as a regulator of skeletal muscle mass: implications for human myopathy. J Cell Biol 183: 1033–1048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cozzolino M, Carri MT. Mitochondrial dysfunction in ALS. Prog Neurobiol 97: 54–66, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Cudkowicz M, Qureshi M, Shefner J. Measures and markers in amyotrophic lateral sclerosis. NeuroRx 1: 273–283, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darras BT, Korf BR, Urion DK. Dystrophinopathies. In: Gene Reviews (edited by Pagon RA, Bird TD, Dolan CR, Stephens K.). Seattle, WA: University of Washington, 2000. [Google Scholar]
- 17. Deconinck N, Dan B. Pathophysiology of Duchenne muscular dystrophy: current hypotheses. Pediatr Neurol 36: 1–7, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Desport JC, Torney F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis 2: 202–207, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Ditor DS, Hamilton S, Tarnopolsky MA, Green HJ, Craven BC, Parise G, Hicks AL. Na+,K+-ATPase concentration and fiber type distribution after spinal cord injury. Muscle Nerve 29: 38–45, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Doppler K, Mittelbronn M, Bornemann A. Myogenesis in human denervated muscle biopsies. Muscle Nerve 37: 79–83, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Dreessen J, Lutum C, Schäfer BW, Heizmann CW, Knöpfel T. Alpha-parvalbumin reduces depolarization-induced elevations of cytosolic free calcium in human neuroblastoma cells. Cell Calcium 19: 527–533, 1996. [DOI] [PubMed] [Google Scholar]
- 22. Duffy LM, Chapman AL, Shaw PJ, Grierson AJ. Review: the role of mitochondria in the pathogenesis of amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 37: 336–352, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Dupuis L, Corcia P, Fergani A, Gonzales de Aguilar JL, Bonnefont-Rousselot D, Bittar R, Scihean D, Hauw JJ, Lacomblez L, Loeffler JP, Meininger V. Dylipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70: 1004–1009, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Dupuis L, Gonzales de Aguillar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, Halter B, Huze C, Schaeffer L, Bouillaund F, Loeffler JP. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One 4: e5390, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elliott JL, Snider WD. Parvalbumin is a marker of ALS-resistant motor neurons. Neuroreport 6: 449–452, 1995. [DOI] [PubMed] [Google Scholar]
- 26. Engel AG, Ozawa E. Dystrophinopathies. In: Myology: Basic and Clinical (3rd Ed.), edited by Engel AG, Franzini-Armstrong C. New York: McGraw Hill, 2004, p. 961–1026. [Google Scholar]
- 27. Franco A, Jr, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344: 670–673, 1990. [DOI] [PubMed] [Google Scholar]
- 28. Glass DJ. A signaling role for dystrophin: inhibiting skeletal muscle atrophy pathways. Cancer Cell 8: 351–352, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Godin DV, Bridges MA, MacLeod PJ. Erythrocyte membrane enzyme abnormalities in two hereditary disorders of muscle. J Med 10: 287–302, 1979. [PubMed] [Google Scholar]
- 30. Goldstein JA, McNally EM. Mechanisms of muscle weakness in muscular dystrophy. J Gen Physiol 136: 29–34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haslett JN, Sanoudou D, Kho AT, Han M, Bennett RR, Kohane IS, Beggs AH, Kunkel LM. Gene expression profiling of Duchenne muscular dystrophy skeletal muscle. Neurogenetics 4: 163–171, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Heiny JA. S100A1: a physiological modulator of RYR1, Ca2+ release, and contractility in skeletal muscle. Focus on “S100A1 promotes action potential-initiated calcium release flux and force production in skeletal muscle”. Am J Physiol Cell Physiol 299: C882–C884, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Hopf FW, Turner PR, Steinhardt RA. Calcium misregulation and the pathogenesis of muscular dystrophy. Subcell Biochem 45: 429–464, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Imbert N, Vandebrouck C, Duport G, Raymond G, Hassoni AA, Constantin B, Cullen MJ, Cognard C. Calcium currents and transients in co-cultured contracting normal and Duchenne muscular dystrophy human myotubes. J Physiol 534: 343–355, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Israelson A, Arbel N, Da Cruz S, Ilieva H, Yamanaka K, Shoshan-Barmatz V, Cleveland DW. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron 67: 575–587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jokic N, Gonzalez de Aguilar JL, Pradat PF, Dupuis L, Echaniz-Laguna A, Muller A, Dubourg O, Seilhean D, Hauw JJ, Loeffler JP, Meininger V. Nogo expression in muscle correlates with amyotrophic lateral sclerosis severity. Ann Neurol 57: 553–556, 2005. [DOI] [PubMed] [Google Scholar]
- 37. Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14: 2189–2200, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Kraner SD, Wang Q, Novak KR, Cheng D, Cool DR, Peng J, Rich MM. Upregulation of the CaV 1.1-ryanodine receptor complex in a rat model of critical illness myopathy. Am J Physiol Regul Integr Comp Physiol 300: R1384–R1391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lanka V, Cudkowicz M. Therapy development for ALS: lessons learned and path forward. Amyotroph Lateral Scler 9: 131–140, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Lawler JM. Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J Physiol 589: 2161–2170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SJ, McPherron AC. Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev 9: 604–607, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Legrand C, Giacomello E, Berthier C, Allard B, Sorrentino V, Jacquemond V. Spontaneous and voltage-activated Ca2+ release in adult mouse skeletal muscle fibres expressing the type 3 ryanodine receptor. J Physiol 586: 441–457, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator–thinking outside the box. Biochim Biophys Acta 1762: 181–190, 2006. [DOI] [PubMed] [Google Scholar]
- 44. MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol 43: 700–703, 1977. [DOI] [PubMed] [Google Scholar]
- 45. McDonough MA, Loenarz C, Chowdhury R, Clifton IJ, Schofield CJ. Structural studies on human 2-oxoglutarate dependent oxygenases. Curr Opin Struct Biol 20: 659–672, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Mancuso M, Piazza S, Carlesi C, Siciliano G. Mitochondrial involvement in amyotropic lateral sclerosis. In: Motor Neuron Disease Research Progress, edited by Mancini RL. New York: Nova Science, 2008, p. 159–174. [Google Scholar]
- 47. Mastroyiannopoulos NP, Nicolaou P, Anayasa M, Uney JB, Phylactou LA. Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLos One 7: e29896, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menzies FM, Ince PG, Shaw PJ. Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem Int 40: 543–551, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Monge C, Beraud N, Kuznetsov AV, Rostovtseva T, Sackett D, Schlattner U, Vendelin M, Saks VA. Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol Cell Biochem 318: 147–165, 2008. [DOI] [PubMed] [Google Scholar]
- 50. Ozawa E. The muscle fibre cytoskeleton: the dystrophin system. In: Myology: Basic and Clinical (3rd Ed.), edited by Engel AG, Franzini-Armstrong C. New York: McGraw Hill, 2004, p. 443–454. [Google Scholar]
- 51. Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand 120: 216–225, 2009. [DOI] [PubMed] [Google Scholar]
- 52. Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol 128: 355–361, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 40: 171–182, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM; Cooperative International Neuromuscular Research Group. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology 76: 219–226, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Péréon Y, Sorrentino V, Dettbarn C, Noireaud J, Palade P. Dihydropyridine receptor and ryanodine receptor gene expression in long-term denervated rat muscles. Biochem Biophys Res Commun 240: 612–617, 1997. [DOI] [PubMed] [Google Scholar]
- 56. Perez CF, López JR, Allen PD. Expression levels of RyR1 and RyR3 control resting free Ca2+ in skeletal muscle. Am J Physiol Cell Physiol 288: C640–C649, 2005. [DOI] [PubMed] [Google Scholar]
- 57. Pescatori M, Broccolini A, Minetti C, Bertini E, Bruno C, D'amico A, Bernardini C, Mirabella M, Silvestri G, Giglio V, Modoni A, Pedemonte M, Tasca G, Galluzzi G, Mercuri E, Tonali PA, Ricci E. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J 21: 1210–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 58. Pestronk A, Drachman DB, Griffin JW. Effect of muscle disuse on acetylcholine receptors. Nature 260: 352–353, 1976. [DOI] [PubMed] [Google Scholar]
- 59. Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83: 84–92, 2007. [DOI] [PubMed] [Google Scholar]
- 60. Potthoff MJ, Arnold MA, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol Cell Biol 27: 8143–8151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pradat PF, Dubourg O, de Tapia M, di Scala F, Dupuis L, Lenglet T, Bruneteau G, Salachas F, Lacomblez L, Corvol JC, Demougin P, Primig M, Meininger V, Loeffler JP, Gonzalez de Aguilar JL. Muscle gene expression is a marker of amyotrophic lateral sclerosis severity. Neurodegener Dis 9: 38–52, 2012. [DOI] [PubMed] [Google Scholar]
- 62. Radzyukevich TL, Heiny JA. Regulation of dihydropyridine receptor gene expression in mouse skeletal muscles by stretch and disuse. Am J Physiol Cell Physiol 287: C1445–C1452, 2004. [DOI] [PubMed] [Google Scholar]
- 63. Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rivet-Bastide M, Imbert N, Cognard C, Duport G, Rideau Y, Raymond G. Changes in cytosolic resting ionized calcium level and in calcium transients during in vitro development of normal and Duchenne muscular dystrophy cultured skeletal muscle measured by laser cytofluorimetry using indo-1. Cell Calcium 14: 563–571, 1993. [DOI] [PubMed] [Google Scholar]
- 65. Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr 40: 163–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A 105: 18746–18751, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ryberberg H, An J, Darko S, Lustgarten JL, Jaffa M, Gopalakrishnan V, Lacomis D, Cudkowicz M, Bowser R. Discovery and verification of amyotrophic lateral sclerosis biomarkers by proteomics. Muscle Nerve 42: 104–111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saito Y, Nakagami H, Azuma N, Hirata S, Sanada F, Taniyama Y, Morishita R, Kaneda Y, Sasajima T. Critical roles of cold shock domain protein A as an endogenous angiogenesis inhibitor in skeletal muscle. Antioxid Redox Signal 15: 2109–2120, 2011. [DOI] [PubMed] [Google Scholar]
- 69. Salanova M, Schiffl G, Rittweger J, Felsenberg D, Blottner D. Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem Cell Biol 130: 105–118, 2008. [DOI] [PubMed] [Google Scholar]
- 70. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sharma U, Atri S, Sharma MC, Sarkar C, Jagannathan NR. Skeletal muscle metabolism in Duchenne muscular dystrophy (DMD): an in-vitro proton NMR spectroscopy study. Magn Reson Imaging 21: 145–153, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Siklós L, Engelhardt J, Harati Y, Smith RG, Joó F, Appel SH. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Ann Neurol 39: 203–216, 1996. [DOI] [PubMed] [Google Scholar]
- 73. Smith LR, Chambers HG, Subramaniam S, Lieber RL. Transcriptional abnormalities of hamstring muscle contractures in children with cerebral palsy. PLos One 7: 1–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Song W, Song Y, Kincaid B, Bossy B, Bossy-Wetzel E. Mutant SOD1(G93A) triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1α. Neurobiol Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sonnleitner A, Conti A, Bertocchini F, Schindler H, Sorrentino V. Functional properties of the ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO J 17: 2790–2798, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta 1768: 2510–2515, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res 56: 831–841, 2004. [DOI] [PubMed] [Google Scholar]
- 78. Timmons JA, Larsson O, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Peyrand-Janvid M, Wahlestedt C, Sundberg CJ. Human muscle gene expression responses to endurance training provide a novel perspective on Duchenne muscular dystrophy. FASEB J 19: 750–760, 2005. [DOI] [PubMed] [Google Scholar]
- 79. Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A 97: 4666–4671, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem 276: 19414–19419, 2001. [DOI] [PubMed] [Google Scholar]
- 81. Vielhaber S, Kunz D, Winkler K, Weidemann FR, Kirches E, Feistner H, Heinze HJ, Elger CE, Schubert W, Kunz WS. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain 123: 1339–1348, 2000. [DOI] [PubMed] [Google Scholar]
- 81a. Wang Y, Winters J, Subramaniam S. Functional classification of skeletal muscle networks. I. Normal physiology. J Appl Physiol; doi: 10.1152/japplphysiol.01514.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Waterlow JC. Protein Turnover. Oxfordshire, UK: CABI, 2006. [Google Scholar]
- 83. Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, Kunz WS. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci 156: 65–72, 1998. [DOI] [PubMed] [Google Scholar]
- 84. Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol 73: 2004–2010, 1992. [DOI] [PubMed] [Google Scholar]
- 86. Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol 557: 59–75, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang A, Liu X, Cogan JG, Fuerst MD, Polikandriotis JA, Kelm RJ, Jr, Strauch AR. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta 1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol Biol Cell 16: 4931–4940, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.