Abstract
Prior exercise by rats can induce a sustained increase in muscle Akt substrate of 160 kDa (AS160) phosphorylation on Thr642 (pAS160Thr642). Because phosphorylation of AS160 on both AS160Thr642 and AS160Ser588 is important for insulin-stimulated glucose transport (GT), we determined if exercise would also induce a sustained increase in pAS160Ser588 concomitant with persistently elevated pAS160Thr642 and GT. Given that the mechanisms for sustained postexercise (PEX) effects on pAS160 were uncertain, we also studied the four kinases known to phosphorylate AS160 (Akt, AMPK, RSK, and SGK1). In addition, because the serine/threonine phosphatase(s) that dephosphorylate muscle AS160 were previously unidentified, we assessed the ability of four serine/threonine phosphatases (PP1, PP2A, PP2B, and PP2C) to dephosphorylate AS160. We also evaluated exercise effects on posttranslational modifications (Tyr307 and Leu309) that regulate PP2A. In isolated epitrochlearis muscles from rats, GT at 3hPEX with insulin significantly (P < 0.05) exceeded SED controls. Muscles from 0hPEX vs. 0hSED and 3hPEX vs. 3hSED rats had greater pAS160Thr642 and pAS160Ser588. AMPK was the only kinase with greater phosphorylation at 0hPEX vs. 0hSED, and none had greater phosphorylation at 3hPEX vs. 3hSED. Each phosphatase was able to dephosphorylate pAS160Thr642 and pAS160Ser588 in cell-free assays. Exercise did not alter posttranslational modifications of PP2A. Our results revealed: 1) pAMPK as a potential trigger for increased pAS160Thr642 and pAS160Ser588 at 0hPEX; 2) PP1, PP2A, PP2B, and PP2C were each able to dephosphorylate AS160; and 3) sustained PEX-induced elevations of pAS160Thr642 and pAS160Ser588 were attributable to mechanisms other than persistent phosphorylation of known AS160 kinases or altered posttranslational modifications of PP2A.
Keywords: Akt, AMP-activated protein kinase, glucose transport, insulin sensitivity, serine/threonine phosphatase
a single exercise session can induce a persistent increase in insulin-stimulated glucose transport for up to 3–48 h postexercise (6, 7, 48, 49, 59). This increase is attributable to greater insulin-induced recruitment of GLUT4 to the cell surface GLUT4 (23) without increased total GLUT4 abundance (5). A great deal of evidence indicates that elevated insulin-stimulated glucose transport is not accompanied by enhanced insulin signaling at proximal steps ranging from the insulin receptor to Akt (2, 18, 23, 57, 65, 68), implicating a more distal event.
Sano et al. (50) identified Akt substrate of 160 kDa (also known as AS160 or TBC1D4) as a distal insulin signaling protein that is a link between insulin's activation of Akt and the subsequent increase in cell-surface GLUT4 in 3T3-L1 adipocytes. Insulin leads to increased AS160 phosphorylation in skeletal muscle (3), and this increase is important for insulin-stimulated glucose transport (33). Thr642 and Ser588 of AS160 are the two primary sites that regulate cell-surface GLUT4 content upon insulin stimulation (50). Phosphorylation of these sites is predicted to inhibit AS160's activation of Rab-GTPase proteins and favor greater exocytosis of GLUT4 vesicles, leading ultimately to greater glucose transport (50).
We previously reported that AS160 phosphorylation is increased immediately postexercise and remains elevated for up to 3 and 27 h after cessation of exercise (2, 17, 18). Furthermore, the sustained AS160 phosphorylation on the Thr642 site tracked closely with the postexercise increase in insulin-stimulated glucose transport in rat skeletal muscle (17, 18). We proposed the idea that the persistent AS160 phosphorylation postexercise may be important for the increase in insulin-stimulated glucose transport after acute exercise. The first aim of this study was to determine in rat skeletal muscle if the phosphorylation of the second key site of AS160 (Ser588) is also enhanced both immediately and 3 h after exercise.
A plausible mechanism that might lead to the persistent elevation in AS160 phosphorylation after acute exercise is a long-lasting activation of kinases. Using a cell-free assay, Geraghty et al. (21) identified four kinases (Akt, AMPK, SGK, and RSK) that were able to phosphorylate AS160. Therefore our second aim was to determine if SGK and/or RSK phosphorylation in skeletal muscles were increased immediately and/or 3 h postexercise. The consensus sequences for substrate phosphorylation are nearly identical for p70S6K compared with Akt (41), and some studies have indicated that exercise can increase activation of p70S6K in skeletal muscle (11, 62). Accordingly, we also evaluated the phosphorylation of p70S6K in skeletal muscle in response to an exercise protocol previously demonstrated to cause a sustained increase in the phosphorylation of AS160 (2, 17, 18).
Protein phosphorylation status depends on the balance of actions by kinases and phosphatases, but nothing is currently known about which of the protein phosphatases are able to dephosphorylate AS160. To overcome this gap in knowledge, our third aim was to determine if any of the four most highly expressed serine/threonine protein phosphatases in skeletal muscle [protein phosphatases 1 (PP1), 2A (PP2A), 2B (PP2B), and 2C (PP2C) (12, 24, 28, 52, 61)] were able to dephosphorylate AS160 using cell-free assays.
PP2A has been shown to be regulated via posttranslational modifications on its catalytic subunit (Tyr307 phosphorylation and Leu309 methylation). Each of these modifications has been suggested as potential modulator of PP2A's function (16, 29, 36, 39, 56, 67). Our fourth aim was to determine if tyrosine phosphorylation and/or leucine methylation on PP2A's catalytic subunit were altered immediately or 3 h postexercise.
METHODS
Materials.
Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for SDS-PAGE and immunoblotting were purchased from Bio-Rad (Hercules, CA). Bicinchoninic acid protein assay reagent (no. 23227), T-PER tissue protein extraction reagent (no. 78510), and West Dura Extended Duration Substrate (no. 34075) were purchased from Pierce Biotechnology (Rockford, IL). Goat anti-rabbit IgG horseradish peroxidase conjugate (no. 7074), anti-Akt (no. 9272), anti-phospho-Ser473 Akt (no. 9271), anti-phospho-Thr308 Akt (no. 9275), anti-p70 ribosomal S6 Kinase (p70S6K; no. 9202), anti-phospho-Thr389 p70S6K (no. 9205), anti-ribosomal S6 kinase 1 antibody (RSK1; no.9333), anti-phospho-Ser380 RSK1 (no. 9341), anti-AMPKα (no. 2532), anti-phospho-Thr172 AMPKα (no. 2531) were purchased from Cell Signaling Technology (Danvers, MA). Anti-phospho-Thr642 AS160 (no. 07-802), anti-serum- and glucocorticoid-induced protein kinase 1 (SGK1; no. 07-315), anti-phospho-Thr256 SGK1 (no. 36-002), anti-PP1α (no. 07-273), anti-calcineurin pan A (anti-PP2B pan A) (no. 07-1491), anti-AS160-serum (no. 07-741), anti-AS160-purified (no. ABS54), and protein G agarose beads (no. 16-266) were purchased from Millipore (Billerica, MA). Protein A sepharose beads 6MB (17-0469-01) were purchased from GE Healthcare (Piscataway, NJ). Anti-PP2A catalytic α (no. 610555) was purchased from BD Biosciences (San Jose, CA). Recombinant PP2A (no. V6311) was purchased from Promega (Madison, WI). Recombinant PP1 (no. P0754) was purchased from New England Biolabs (Ipswich, MA). Recombinant PP2B/calcineurin and calmodulin was included in the Calcineurin Activity Kit (no. BML-AK816-0001) and was purchased from Enzo Life Sciences (Farmingdale, NY). Recombinant PP2Cα isoform (no. 539569) was purchased from EMD Chemical (Gibbstown, NJ). Anti-PP2Cα/β (no. ab27267) and anti-phospho-Thr642 AS160 (no. ab65753) used in cell-free assay experiments were purchased from Abcam (Cambridge, MA). Anti-phospho-Tyr307 PP2A-Cα/β (no. sc-12615), Anti-methyl-PP2A-Cα/β (no. sc-81603) and donkey anti-goat IgG-HRP (no. sc-2020) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Ser588 AS160 (no. 3028-P2) was purchased from B-Bridge International (Mountain View, CA), 3-O-methyl-[3H]glucose ([3H]3-MG) was purchased from Sigma-Aldrich (St. Louis, MO), and [14C]mannitol was purchased from Perkin-Elmer (Waltham, MA). Other reagents were purchased from Sigma-Aldrich or Fisher Scientific (Pittsburgh, PA).
Animal treatment.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (∼135–200 g; Harlan, Indianapolis, IN) were provided with rodent chow (Lab Diet; PMI Nutritional International, Brentwood, MO) and water ad libitum. Starting at 1700 on the night before the experiments, rats were fasted. On the following day, rats were randomly assigned to a postexercise (PEX) or sedentary (SED) treatment. Beginning at ∼0900, PEX rats swam in a barrel filled with water (35°C) to a depth of ∼60 cm (seven or eight rats per barrel) for 4 × 30 min bouts, with a 5-min rest period between each bout. Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g body wt) either immediately postexercise (0hPEX) or 3–4 h postexercise (3hPEX) along with time-matched sedentary rats. After exercise, the 3hPEX rats and their time-matched sedentary controls (3hSED) were allowed access to drinking water. While the rats were under deep anesthesia, both epitrochlearis muscles [myosin heavy chain composition of 8% type I, 13% type IIA, 51% type IIB, and 28% type IIX (8)] were rapidly dissected out and either freeze-clamped immediately or transferred to vials for subsequent incubation.
Muscle dissection and incubations.
For the 0hPEX experiment, following anesthetization, an epitrochlearis muscle from each rat was rapidly dissected out, trimmed, freeze-clamped using aluminum clamps cooled to the temperature of liquid N2, and stored at −80°C until analyzed. The contralateral muscle from each rat in the 0hPEX experiment (including both 0hPEX and time-matched SED treatment groups) underwent a two-step incubation as previously described (2) in which they were first incubated for 10 min at 30°C in flasks containing solution 2 [Krebs-Henseleit buffer (KHB) with 0.1% bovine serum albumin (BSA), 2 mM pyruvate, and 6 mM mannitol] at 30°C for 10 min and then transferred to flasks containing solution 3 [KHB with 0.1% BSA, 8 mM 3-MG (including 0.25 mCi/mmol [3H]3-MG), and 2 mM mannitol (including 0.1 mCi/mmol [14C]mannitol)]. For all incubation steps, flasks were continuously gassed from above with 95% O2-5% CO2 and shaken in a heated water bath.
For the 3hPEX experiment, rats were dried following the final exercise bout and returned to their cage for 3 h before being anesthetized. Time-matched SED rats were also anesthetized, and then epitrochlearis muscles were dissected out. Both epitrochlearis muscles from each animal were incubated in solution 1 (KHB with 0.1% BSA, 8 mM glucose, and 2 mM mannitol) for 30 min in a water bath at 35°C. During this step, one muscle from each rat was incubated in solution 1 supplemented with 50 μU/ml of insulin, and the contralateral muscle was incubated in solution 1 without insulin. The same insulin concentration was used for each muscle during all subsequent incubations. After the initial incubation, muscles were transferred to vials containing solution 2 with or without 50 μU/ml insulin at 30°C for 10 min. Finally, muscles were transferred to flasks containing solution 3 with or without 50 μU/ml insulin at 30°C for 10 min for determination of glucose transport rate. For all incubation steps, flasks were continuously gassed from above with 95% O2-5% CO2 and shaken in a heated water bath. After incubation with 3-MG for 10 min, the muscles were rapidly blotted on filter paper dampened with incubation medium, trimmed, freeze-clamped, and stored at −80°C until being processed as described below.
Homogenization and glucose transport measurement.
Frozen muscles were homogenized in 1 ml ice-cold homogenization buffer (1% Triton X-100, 1 mM activated Na3VO4, 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethanesulfonyl fluoride, and 1 μg/ml leupeptin in water) using glass-on-glass tubes (Kontes, Vineland, NJ) for all samples except those used for phosphatase cell-free assays which were homogenized using the same homogenization buffer by high-speed tissue disruption with the TissueLyser II (Qiagen, Valencia, CA). Homogenates were subsequently solubilized by rotating at 4°C at 50 rpm for 1 h before being centrifuged (15,000 g for 15 min at 4°C). Aliquots of the supernatant from muscles used for the 3-MG transport measurement were pipetted into vials with scintillation cocktail for scintillation counting, and 3-MG transport were determined as previously described (4). A portion of the supernatant were used to determine protein concentration by the bicinchoninic acid assay (53) according to the manufacturer's instructions (Pierce Biotechnology; no. 23227). The remaining supernatant was stored at −80°C until further analyzed.
Immunoprecipitation.
Aliquots of the homogenates prepared as described above (300 μg protein) were brought to the same volume to equalize the concentration of muscle lysates and were precleared with 100 μl of protein G agarose beads for 1 h. When using protein G agarose beads for the immunoprecipitation of SGK (for samples that were subsequently used for immunoblotting with anti-phospho-Thr256 SGK1), the muscle lysates were mixed with an immunomatrix of 100 μl of protein G agarose beads that had been gently rotated with 3 μg/μl of anti-AS160 for 1 h. The lysate-antibody-protein G mixture was gently rotated overnight at 4°C at 5 rpm. When using protein A sepharose beads for immunoprecipitation of AS160, the supernatant from the muscle lysates were gently rotated with 2 μg of AS160 antibody for 3 h, after which 25 μl of a 50/50 slurry of protein A sepharose beads were added. The lysate-antibody-protein A mixture was gently rotated overnight. Following overnight rotation in both protein G agarose and protein A sepharose immunoprecipitation assays, the immunoprecipitation mix was centrifuged (4,000 g) and supernatant were aspirated. After washing (four times with 500 μl PBS), immunoprecipitated proteins were eluted with 2X Laemmli sample buffer, boiled, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). For the cell-free assays, after washing (three times with 500 μl PBS and two times with the appropriate phosphatases buffer, described below), the immunoprecipitated AS160 was eluted with 2X Laemmli sample buffer (1:1 volume), boiled, and subjected to SDS-PAGE.
Immunoblotting and protein phosphorylation.
Homogenized muscle lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Blots were then rinsed with Tris-buffered saline plus Tween (TBST) (0.14 mol/l NaCl, 0.02 mol/l Tris base, pH 7.6, and 0.1% Tween), blocked with 5% BSA in TBST for 1 h at room temperature, washed 3 × 5 min at room temperature, and incubated with the relevant primary antibody overnight at 4°C. Blots were then washed 3 × 5 min with TBST, incubated with the relevant secondary antibody (goat anti-rabbit, goat anti-mouse, goat anti-sheep) IgG horseradish peroxidase conjugate for 1 h at room temperature, washed again 3 × 5 min with TBST, and developed with SuperSignal reagent. Protein bands were quantified by digital densitometry (ProteinSimple, Santa Clara, CA). The mean values for sedentary samples without insulin on each blot were normalized to equal 1.0, and then all samples on the blot were expressed relative to the normalized sedentary without insulin value.
Cell-free dephosphorylation assay using recombinant PP1, PP2A, PP2B, or PP2C.
AS160 that had been immunoprecipitated from lysates prepared from epitrochlearis muscles that had been incubated with insulin (2,000 μU/ml for 30 min to induce high levels of AS160 phosphorylation) were used to assess the ability of recombinant PP1, PP2A, PP2B, and PP2C to dephosphorylate AS160Thr642 and AS160Ser588. The immunoprecipitated AS160 (as described above) was incubated for 2 h at 30°C with gentle shaking in the appropriate buffer for each phosphatase. PP1 buffer was 50 mM HEPES, 100 nM NaCl, 2 mM DTT, and 1 mM MnCl2 in 0.01% Brij35 (pH 7.5). PP2A buffer was 50 mM Tris-HCl (pH 8.5), 20 mM MgCl2, and 1 mM DTT. PP2B buffer was 50 mM Tris (ph7.5), 100 mM NaCl, 6 mM MgCl2, 0.5 mM DTT, 1 mM CaCl2, 0.05% NP-40, and 0.5 μM calmodulin. PP2C buffer was 20 mM Tris-HCl (pH 7.4), 10 mM MnCl2, 0.5 mM EGTA (pH 8.0), and 3 mM 2-mercaptoethanol in 0.2 mg/ml BSA. The reactions were terminated by adding SDS loading buffer (2X) to the reaction and boiling the sample for 5 min. Samples were subsequently subjected to SDS-PAGE, immunoblotted to nitrocellulose membranes, and blots were probed with anti-phospho-Thr642 AS160 and anti-phospho-Ser588 AS160 (10).
Statistical analyses.
Statistical analyses were done using Sigma Stat version 2.0 (San Rafael, CA). Data were expressed as means ±SE. When an analysis comparing four groups was required, a two-way ANOVA was used to determine significant differences, and a Tukey post hoc test was used to identify the source of significant variance. When an analysis comparing two groups was required, a t-test was used to determine significant differences. A P value ≤0.05 was considered statistically significant.
RESULTS
3-MG transport.
Insulin-independent glucose transport measured immediately postexercise was three- to fourfold greater than time-matched sedentary controls (P < 0.05; Fig. 1A). This effect was lost in muscles dissected from rats that were returned to their cage following exercise without access to food for 3 h (3hPEX). Muscles stimulated with a submaximally effective insulin dose (50 μU/ml) had greater glucose transport than paired muscles incubated without insulin for both 3hSED (∼2-fold greater; P < 0.05) and 3hPEX (3- to 4-fold greater; P < 0.05; Fig. 1) rats. The glucose transport for insulin-stimulated muscles was significantly greater for the 3hPEX group vs. the 3hSED group (P < 0.05; Fig. 1).
Fig. 1.
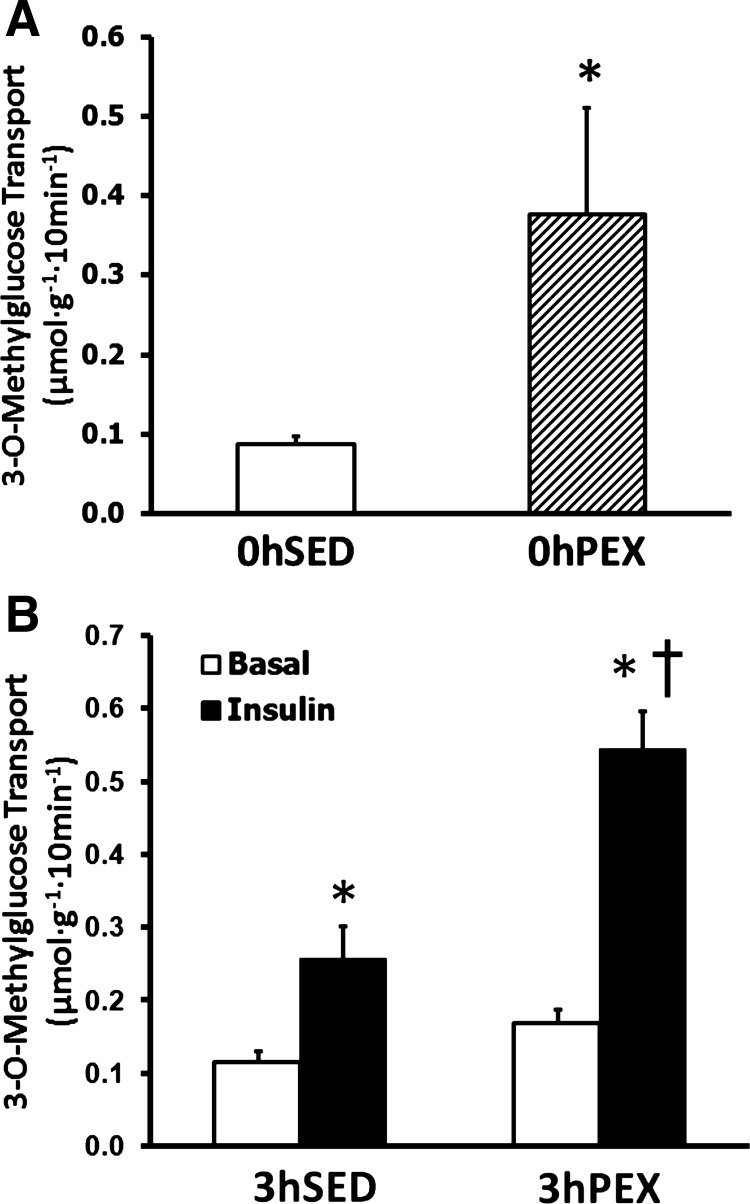
Rate of 3-O-methylglucose (3-MG) transport in isolated rat epitrochlearis muscles. A: rats anesthetized immediately postexercise (0hPEX) with time-matched sedentary controls (0hSED). B: rats anesthetized 3 h postexercise (3hPEX) with time-matched sedentary controls (3hSED). For rats in the 0hPEX and 0hSED groups, all muscles that were used to measure 3-MG transport were incubated without insulin to determine the insulin-independent effect of exercise. For rats in the 3hPEX groups and sedentary controls (3hSED), one of the paired muscles was incubated without insulin, and the contralateral muscle was incubated with 50 μU/ml insulin. A: data are means ±SE; n = 8/group. *P < 0.05 (exercise effect, t-test). Open bars indicate 0hSED and hatched bars indicate 0hPEX. B: data are means ±SE; n = 15–16/group. *P < 0.05 (insulin effect, post hoc test); †P < 0.05 (exercise effect, post hoc test). Open bars indicate muscles incubated without insulin and filled bars indicate muscles incubated with insulin.
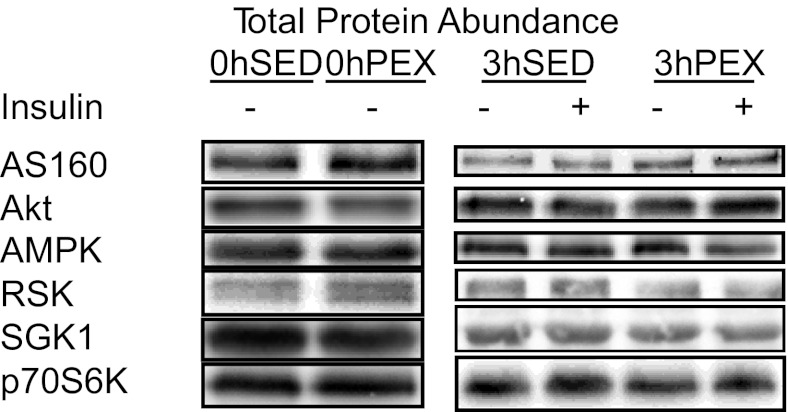
Total abundance of proteins.
There were no significant effects of exercise or insulin on the total abundance of any of the proteins that were assessed (AS160, AMPKα, Akt, RSK, SGK1, and p70S6K; Fig. 2).
Fig. 2.
Total protein abundance (AS160, AMPK, Akt, RSK, SGK1, and p70S6K) in rat epitrochlearis muscles. A: rats anesthetized immediately postexercise (0hPEX) with time-matched sedentary controls (0hSED). B: rats anesthetized 3 h postexercise (3hPEX) with time-matched sedentary controls (3hSED). Data are means ±SE; n = 4/group. There were no statistically significant effects of exercise or insulin for any of the proteins.
AS160 phosphorylation.
AS160Thr642 and AS160Ser588 phosphorylation were increased in 0hPEX vs. 0hSed rats (P < 0.05; Fig. 3, A and B). AS160Thr642 and AS160Ser588 were increased with insulin in both 3hSED and 3hPEX rats (P < 0.05; Fig. 3, C and D). Additionally, for 3hPEX rats vs. 3hSED rats in the absence of insulin, there were residual and significant differences in AS160Thr642 and AS160Ser588 phosphorylation (P < 0.05; Fig. 3, C and D).
Fig. 3.
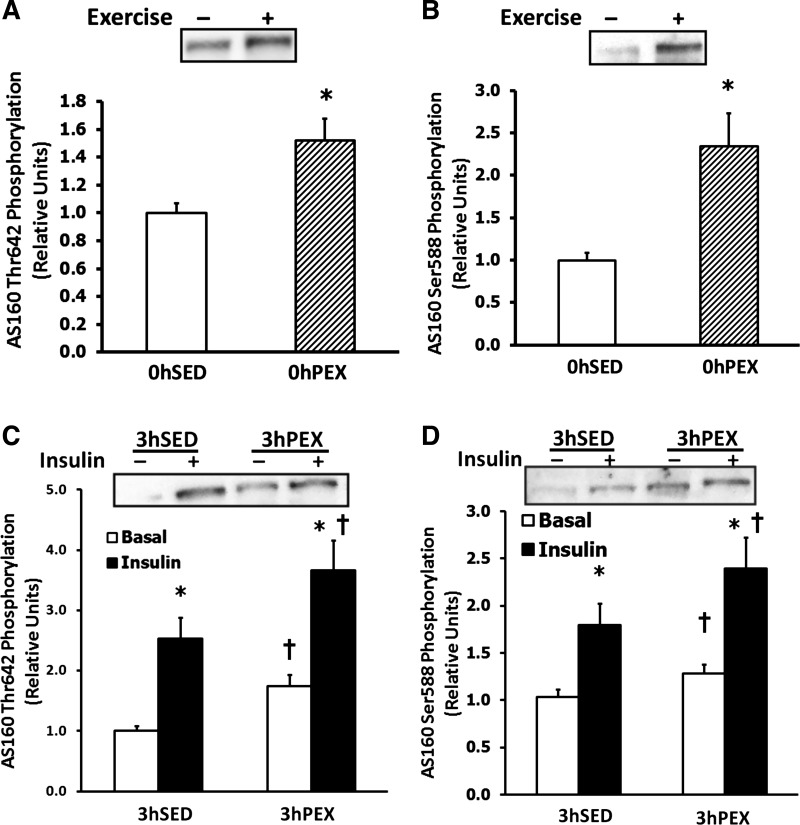
AS160Thr642 and AS160Ser588 phosphorylation in isolated rat epitrochlearis muscles. A and B: rats anesthetized immediately postexercise (0hPEX) with time-matched sedentary controls (0hSED); C and D: rats anesthetized 3 h postexercise (3hPEX) with time-matched sedentary controls (3hSED). A and C: samples immunoblotted for AS160Thr642 phosphorylation. B and D: samples immunoblotted for AS160Ser588 phosphorylation. For rats in the 0hPEX and 0hSED groups, all muscles that were used to measure AS160 phosphorylation were frozen immediately after dissection to determine the insulin-independent effect of exercise. For rats in the 3hPEX groups and sedentary controls (3hSED), one of the paired muscles was incubated without insulin, and the contralateral muscle was incubated with 50 μU/ml insulin. A and B: data are means ±SE; n = 19–24/group. *P < 0.05 (exercise effect, t-test). Open bars indicate 0hSED and hatched bars indicate 0hPEX. C and D: data are means ± SE; n = 19–21/group. *P < 0.05 (insulin effect, post hoc test); †P < 0.05 (exercise effect, post hoc test). Open bars indicate muscles incubated without insulin and filled bars indicate muscles incubated with insulin.
Kinases.
Among all of the kinases that were assessed for phosphorylation in the absence of insulin, there was only a significant difference for AMPKαThr172 at 0hPEX vs. 0hSED (P < 0.05; Fig. 4A), and there were no exercise-related differences in AktThr308, AktSer473, RSKSer380, SGK1Thr256, and p70S6KThr389 at 0hPEX (Fig. 4, B–F).
Fig. 4.
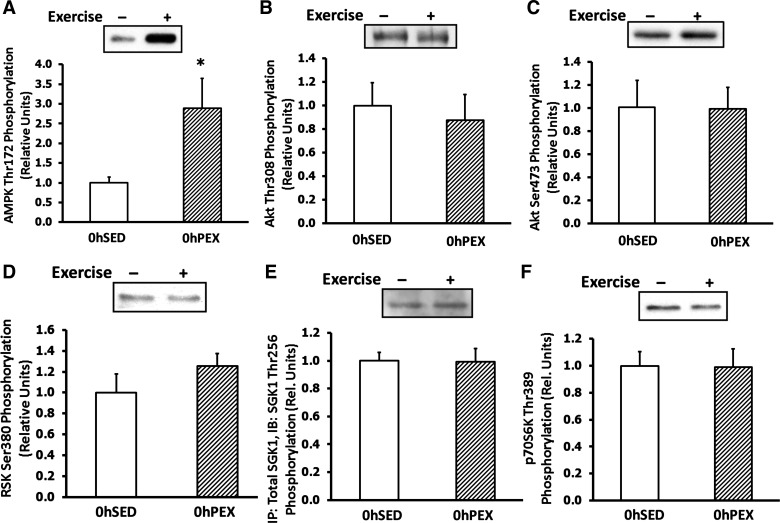
Immediate postexercise kinase phosphorylation in isolated rat epitrochlearis muscles. Rats anesthetized immediately postexercise (0hPEX) with time-matched sedentary controls (0hSED), and muscles were used to and measure phosphorylation of the following kinases. A: AMPKThr172, B: AktThr308, C: AktSer473, D: RSKSer380, E: SGKThr256, and F: p70S6KThr389. All muscles that were used to measure kinase phosphorylation were frozen immediately after dissection to determine the insulin-independent effect of exercise. A–F: data are means ± SE; n = 4–12/group. *P < 0.05 (exercise effect, t-test). Open bars indicate 0hSED and hatched bars indicate 0hPEX.
AktThr308 phosphorylation was increased with insulin in both 3hSED and 3hPEX rats (P < 0.05), and AktThr308 phosphorylation in muscles incubated with insulin was greater for 3hPEX vs. 3hSED (P < 0.05; Fig. 5B), consistent with our previous results (2, 18). However, there was no residual increase in AktThr308 phosphorylation in 3hPEX vs. 3hSED rats in muscles incubated without insulin (Fig. 5B). AktSer473 phosphorylation was increased with insulin in both 3hSED and 3hPEX rats (P < 0.05), but there was no difference in insulin-stimulated AktSer473 phosphorylation in 3hPEX rats vs. 3hSED rats (Fig. 5C).
Fig. 5.
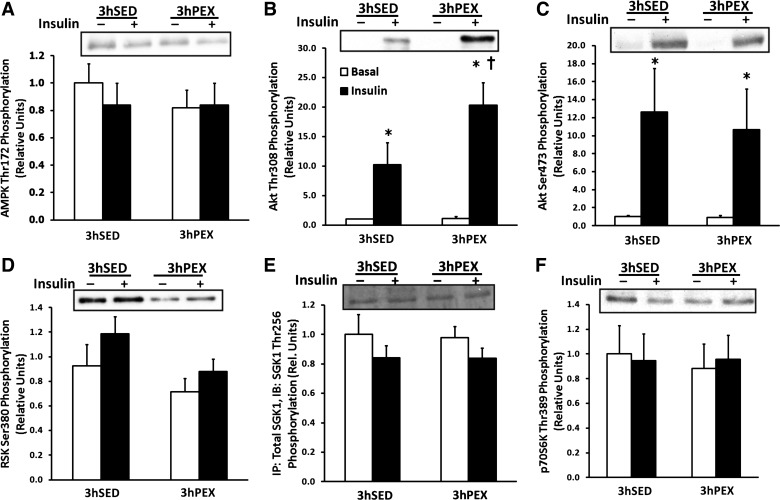
Three-hour postexercise kinase phosphorylation in isolated rat epitrochlearis muscles. Phosphorylation was measured for the following kinase phosphosites. A: AMPKThr172, B: AktThr308, C: AktSer437, D: RSKSer380, E: SGKThr256, and F: p70S6KThr389. For rats in the 3hPEX groups and sedentary controls (3hSED), one of the paired muscles was incubated without insulin, and the contralateral muscle was incubated with 50μU/ml insulin. A–F: data are means ± SE; n = 8–12/group. *P < 0.05 (insulin effect, post hoc test); †P < 0.05 (exercise effect, post hoc test). Open bars indicate muscles incubated without insulin and filled bars indicate muscles incubated with insulin.
RSKSer380 phosphorylation was not significantly altered with insulin in either 3hSED or 3hPEX rats, and there was a trend for a decrease in RSKSer380 phosphorylation in 3hPEX vs. 3hSED rats (P = 0.15; Fig. 5D). AMPKαThr172, SGK1Thr256, and p70S6KThr389 phosphorylation were not significantly different with or without insulin in either 3hSED or 3hPEX rats, and there was no significant difference for each of these kinases at 3hPEX vs. 3hSED (Fig. 5, A, E, and F, respectively).
Cell-free phosphatase assays with AS160.
PP1, PP2A, PP2B, and PP2C are expressed in skeletal muscle (12, 24, 52, 61). Recombinant PP1, PP2A, PP2B, and PP2C were each able to reduce the level phosphorylation of insulin-stimulated AS160Thr642 and AS160Ser588 phosphorylation in rat epitrochlearis muscle under cell-free conditions (Fig. 6, A–D).
Fig. 6.
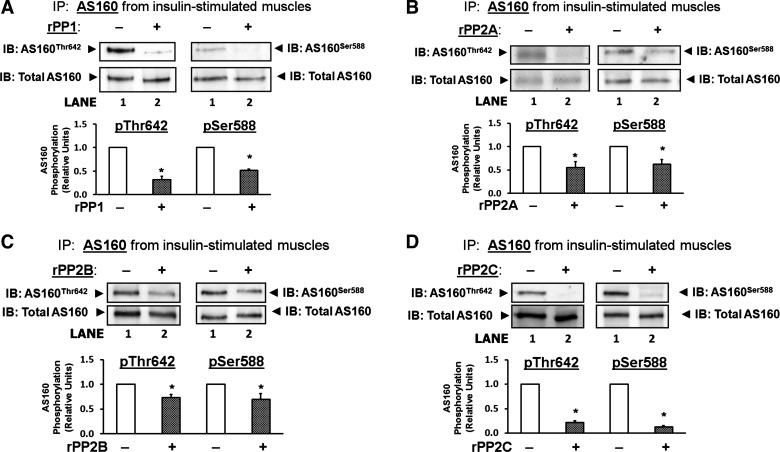
Cell-free AS160 dephosphorylation assay. AS160 was immunoprecipitated (IP) from rat epitrochlearis muscles that had been incubated with 2,000 μU/ml insulin. IB: AS160Thr642 and IB: AS160Ser588. Blots for immunoprecipitated AS160 that had been incubated in the absence of recombinant phosphatase (−) and that were probed with antibodies against phosphorylated AS160Thr642 and AS160Ser588 demonstrated a relatively high level of AS160 phosphorylation (the values for AS160Thr642 and AS160Ser588 were normalized to equal 1.0; lane 1). Blots for immunoprecipitated AS160 that had been incubated in the presence (+) of rPP1 (A), rPP2A (B), rPP2B (C), or rPP2C (D), demonstrated a significantly lower level of AS160Thr642 and AS160Ser588 phosphorylation (lane 2). IB: Total AS160. Blots that were stripped and reprobed with an antibody against total AS160 to demonstrate equal loading. Data are means ± SE; n = 3–7/group. *P < 0.05 (recombinant phosphatase effect). Open bars indicate muscles incubated without recombinant phosphatase and cross-hatched bars indicate muscles incubated with recombinant phosphatase. IB: immunoblot; IP: immunoprecipitated; rPP1: recombinant PP1; rPP2A: recombinant PP2A; rPP2B: recombinant PP2B; rPP2C: recombinant PP2C.
Posttranslational modifications of PP2A.
There was no difference in PP2ATyr307 phosphorylation or PP2ALeu309 methylation in 0hSED vs. 0hPEX rats (Fig. 7, A and B). PP2ATyr307 phosphorylation was not altered by incubation with insulin or by prior exercise (Fig. 7C). PP2ALeu309 methylation was also not altered by incubation with insulin or by prior exercise (Fig. 7D).
Fig. 7.
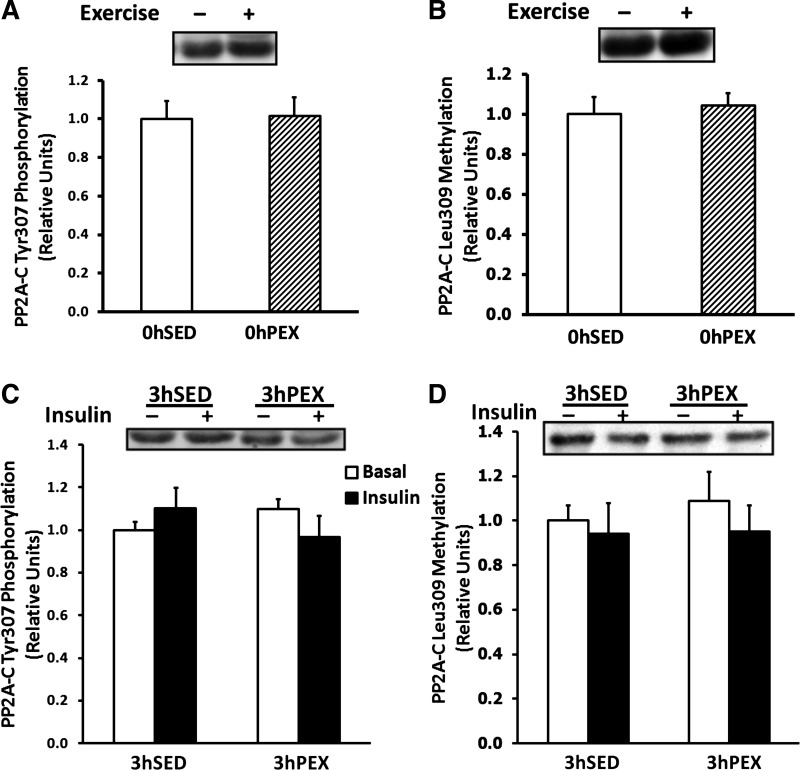
PP2ATyr307 phosphorylation and PP2ALeu309 methylation in isolated rat epitrochlearis muscles. A and B: rats anesthetized immediately postexercise (0hPEX) with time-matched sedentary controls (0hSED). C and D: rats anesthetized 3 h postexercise (3hPEX). A and C: samples immunoblotted for PP2ATyr307 phosphorylation. B and D: samples immunoblotted for PP2ALeu309 methylation. For rats in the 0hPEX and 0hSED groups, all muscles that were used to measure AS160 phosphorylation were frozen immediately after dissection to determine the insulin-independent effect of exercise. For rats in the 3hPEX groups and sedentary controls (3hSED), one of the paired muscles was incubated without insulin, and the contralateral muscle was incubated with 50μU/ml insulin. A and B: data are means ± SE; n = 7/group. Open bars indicate 0hSED and hatched bars indicate 0hPEX. C and D: data are means ± SE; n = 8–12/group. Open bars indicate muscles incubated without insulin and filled bars indicate muscles incubated with insulin.
DISCUSSION
A sustained increase in muscle AS160 phosphorylation after exercise has been suggested to be important for the greater insulin-stimulated glucose transport that can persist for many hours postexercise, but the mechanisms for the long-lasting AS160 phosphorylation are unknown. Consistent with earlier studies (2, 18), exercise resulted in elevated insulin-dependent glucose transport at 3hPEX. The most important new results were that in rat epitrochlearis: 1) both phosphosites of AS160 that are important for insulin-stimulated glucose transport (Thr642 and Ser588) have increased phosphorylation both immediately and 3 h postexercise; 2) AMPKα phosphorylation, but none of the other known AS160 kinases, was increased immediately postexercise; 3) phosphorylation was not increased for any of the four known AS160 kinases or p70S6K at 3 h postexercise; 4) all four of the serine/threonine phosphatases that were studied were able to dephosphorylate AS160 on both the Thr642 and Ser588 phosphosites; and 5) prior exercise did not alter posttranslational modifications of PP2A.
Current understanding of the biological basis for greater insulin sensitivity after acute exercise has greatly benefitted from research using a variety of experimental approaches. The first study that clearly demonstrated that insulin sensitivity was elevated after a single exercise session used the perfused rat hindlimb to document the effect in the absence of factors in the plasma (48). Subsequently, other studies established that acute exercise can improve insulin-stimulated glucose uptake by skeletal muscle in vivo (19, 37, 44, 63, 64). By studying isolated rat epitrochlearis muscles, Wallberg-Henriksson et al. (60) demonstrated that elevated insulin-stimulated glucose transport after one exercise session was attributable, at least in large part, to sustained exercise effects on the intrinsic properties of the muscle itself rather than being exclusively the direct consequence of systemic factors (e.g., exercise-induced changes in circulating factors in the plasma, altered muscle blood flow, etc.). The magnitude and persistence of the acute exercise effect on insulin-stimulated glucose transport in the isolated rat epitrochlearis corresponds to the substantial and sustained enhancement of insulin-stimulated glucose uptake that has been repeatedly observed in vivo (19, 37, 44, 63, 64). After the landmark study by Wallberg-Henriksson et al. (60) was published, the isolated rat epitrochlearis became one of the most widely used and valuable models for probing mechanisms that contributed to greater insulin-stimulated glucose transport in skeletal muscle after acute or chronic exercise (6, 7, 15, 20, 22, 23, 25, 30, 31, 35, 38, 47, 51, 54). In the context of the extensive knowledge base on exercise-induced changes in insulin signaling and glucose transport in this model, we recently used the isolated rat epitrochlearis to study, for the first time, the effects of acute exercise on subsequent AS160 phosphorylation and insulin-stimulated glucose transport in skeletal muscle. We discovered that the increased insulin-stimulated glucose transport observed at 3–4 h after exercise was accompanied by greater phosphorylation of AS160 (2, 18). The current research builds upon our earlier observations with the goal of advancing understanding of the mechanisms that accounted for the greater phosphorylation of AS160 that we previously found concomitant with greater insulin-stimulated glucose transport after exercise in the isolated rat epitrochlearis muscle. Accordingly, in the current study, we used experimental conditions that corresponded to those that were used in the previous studies (2, 18).
Sano et al. (50) provided compelling evidence that phosphorylation on AS160Thr642 and AS160Ser588 accounts for AS160's effects on insulin-stimulated GLUT4 translocation (50). Accordingly, we assessed exercise effects on both of these crucial phosphosites. The current study confirmed earlier results (17, 18) showing that AS160Thr642 phosphorylation is increased immediately and 3 h postexercise in rat skeletal muscle and extended the previous studies by demonstrating that AS160Ser588 phosphorylation is also increased both immediately and 3 h postexercise in rat muscle. The current results demonstrate that both of the phosphosites of AS160 that are important for insulin-mediated glucose transport have sustained phosphorylation in rat muscle 3 h after exercise concomitant with increased insulin-stimulated glucose transport. Treebak et al. (58) evaluated human skeletal muscle at 4 h after exercise and reported that exercise significantly increased phosphorylation of AS160Ser318, AS160Ser341, and AS160Ser751 with a trend for increased phosphorylation on AS160Ser588 but not AS160Thr642. Although the reason for the differing results for AS160Thr642 in rats vs. the results for humans after exercise is unknown, it is clear that a single bout of exercise can induce a sustained increase in AS160 phosphorylation of skeletal muscle in either species.
Increased kinase-mediated AS160 phosphorylation is a plausible mechanism to explain sustained AS160 phosphorylation after exercise. Therefore all the known AS160 kinases (Akt, AMPKα, RSK, and SGK1) were studied (21). Phosphorylation of AMPKα on the site essential for its kinase activity (55) was increased immediately postexercise. Because we found no immediate postexercise increases in the phosphorylation of any of the other known AS160 kinases on sites essential for their kinase activity (1, 13, 34, 40), these results implicate AMPK as the leading candidate to account for the exercise-induced increase in AS160 phosphorylation. However, AS160 phosphorylation remained elevated at 3 h postexercise despite no concomitant exercise-induced elevation in phosphorylation of AMPKα, Akt, RSK, or SGK1. We also evaluated p70S6K because its consensus substrate phosphorylation motif is similar to that of SGK1 and RSK (14, 41). However, phosphorylation of p70S6K on a site required for its increased kinase activity (42) was unaltered either immediately or 3 h postexercise compared with sedentary controls. Neither AktThr308 nor AktSer473 was increased in rat skeletal muscle immediately after exercise. Although the current results did not provide evidence linking prolonged activation of any of the kinases studied to sustained AS160 phosphorylation, it remains possible that one or more of these kinases can influence AS160 phosphorylation [e.g., via altered subcellular localization of the kinase(s) and/or AS160]. However, the exercise protocol used in the current study leads to greater phosphorylation of both acetyl-CoA carboxylase (ACC, a well-known AMPK substrate) and TBC1D1 [a paralog protein of AS160 and substrate of both AMPK and Akt (9)] immediately postexercise (2, 18), but there was not a sustained increase in either ACC or TBC1D1 phosphorylation 3 to 4 h postexercise. Furthermore, there was also no effect of this exercise protocol on phosphorylation of glycogen synthase kinase-3 (an Akt substrate) immediately or 3 to 4 h postexercise (2). These results argue against sustained activation of AMPK or Akt. Nonetheless, it remains possible that a currently unknown kinase is also able to phosphorylate AS160 and that this putative kinase could potentially have sustained effects on AS160 after exercise.
Protein phosphorylation status represents the balance between the actions of kinases and phosphatases. In the absence of evidence supporting increased phosphorylation of AS160 by any of the known AS160 kinases, it was logical to consider that decreased phosphatase activity may contribute to the sustained AS160 phosphorylation after acute exercise. Ingebritsen et al. (27) reported that the four major serine/threonine phosphatases represented the following relative contributions to total enzyme activity in skeletal muscle: PP1 (61%), PP2A (18%), PP2B (20%), and PP2C (0.1%). No previous studies have attempted to identify which of the serine/threonine phosphatases are responsible for AS160 dephosphorylation in skeletal muscle. Accordingly, as a first step to address this important aspect of the regulation of AS160 function, we assessed each of four phosphatases that together account for over 99% of the serine/threonine phosphatase activity in skeletal muscle (PP1, PP2A, PP2B, and PP2C) to determine if they are able to dephosphorylate AS160 (12, 24, 28, 52, 61). Under cell-free conditions, each of the four phosphatases was found to dephosphorylate AS160 at both the Thr642 and Ser588 phosphosites. These results provided the first information about the specific phosphatases that are able to dephosphorylate AS160 in skeletal muscle.
We performed additional analysis to evaluate the potential influence of exercise on PP2A which has been reported to be regulated by posttranslational modifications on the catalytic subunit, including PP2ATyr307 phosphorylation (29, 36, 56) and PP2ALeu309 methylation (16, 39, 67). Neither PP2ATyr307 phosphorylation nor PP2ALeu309 methylation were altered immediately or 3 h postexercise, arguing against a role for these posttranslational modifications in the sustained AS160 phosphorylation postexercise.
If the sustained increase in AS160 phosphorylation after exercise were attributable to a global decline in the total activity of one or more of the serine/threonine phosphatases, then a reasonable prediction would be that prior exercise would be characterized by sustained serine/threonine phosphorylation of multiple proteins. In contrast to this prediction, under identical experimental conditions that have been repeatedly resulted in a sustained AS160 phosphorylation after exercise, we have found that several proteins (e.g., TBC1D1, AMPK, and acetyl CoA carboxylase) with elevated phosphorylation immediately after exercise do not have a persistently increased phosphorylation at 3–4 h postexercise (2, 17, 18). Taken together, these data are consistent with the idea that the mechanism for sustained AS160 phosphorylation involves specific modulation of AS160 rather than a global attenuation of the activity of serine/threonine phosphatases. One possibility for such an AS160-specific mechanism is that AS160 itself is somehow modified, e.g., by a posttranslational modification (such as acetylation or methylation). In this speculative model, the putative modification of AS160 might induce a conformational change that makes its phosphosites less susceptible to dephosphorylation. Another possible mechanism is that dephosphorylation of AS160 may modulated by AS160's binding to a regulatory protein. For example, experiments using cultured cells have documented that AS160 can bind to 14-3-3 proteins (21, 32, 45). Although the direct binding of 14-3-3 to AS160 has apparently not been reported for skeletal muscle tissue, Howlett et al. (26) used an overlay assay and found that 14-3-3 binding capacity of immunoprecipitated AS160 prepared from human skeletal muscle was elevated immediately after exercise but not at 3hPEX. Other proteins (insulin-regulated amino peptidase, ClipR-59, and RUVBL2) have also been reported to bind to AS160 in cultured cells (43), and there is evidence supporting the idea that RUVBL2 or ClipR-59 binding to AS160 may modulate AS160 phosphorylation (46, 66).
The current study demonstrated for the first time that both of the AS160 phosphosites that are known to be crucial for regulating insulin-stimulated GLUT4 translocation (AS160Thr642 and AS160Ser588) have sustained phosphorylation in rat skeletal muscle after exercise that leads to increased insulin-stimulated glucose transport, and the long-lasting AS160 phosphorylation was evident without concomitant phosphorylation of the four kinases known to be able to phosphorylate AS160. The current study also revealed that each of the four most abundant serine/threonine phosphatases in skeletal muscle is capable of dephosphorylating AS160 from skeletal muscle. It will be important for future studies to identify which of these enzymes are responsible for regulating AS160 phosphorylation in skeletal muscle under physiologic conditions. The results of this study have led to our working hypothesis that posttranslational modifications of AS160 and/or binding of AS160 to regulatory proteins contribute to the sustained postexercise increase in AS160 phosphorylation that accompanies the persistent increase in insulin-stimulated glucose transport in skeletal muscle.
GRANTS
The study was supported by R01-DK077171 from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.G.S. and G.D.C. conception and design of research; G.G.S. and E.B.A. performed experiments; G.G.S., E.B.A., and G.D.C. analyzed data; G.G.S. and G.D.C. interpreted results of experiments; G.G.S. prepared figures; G.G.S. and G.D.C. drafted manuscript; G.G.S., E.B.A., and G.D.C. edited and revised manuscript; G.G.S., E.B.A., and G.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Carlos Castorena for his technical assistance with exercise experiments.
REFERENCES
- 1. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 2. Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol 75: 972–978, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol Endocrinol Metab 258: E390–E393, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Castorena CM, Mackrell JG, Bogan JS, Kanzaki M, Cartee GD. Clustering of GLUT4, TUG, and RUVBL2 protein levels correlate with myosin heavy chain isoform pattern in skeletal muscles, but AS160 and TBC1D1 levels do not. J Appl Physiol 111: 1106–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chiang CW, Yan L, Yang E. Phosphatases and regulation of cell death. Methods Enzymol 446: 237–257, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20: 190–192, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem 58: 453–508, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem 273: 1496–1505, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Dubois F, Vandermoere F, Gernez A, Murphy J, Toth R, Chen S, Geraghty KM, Morrice NA, Mackintosh C. Differential 14-3-3-affinity capture reveals new downstream targets of PI 3-kinase signaling. Mol Cell Proteomics 8: 2487–2499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Etgen GJ, Jr, Jensen J, Wilson CM, Hunt DG, Cushman SW, Ivy JL. Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Am J Physiol Endocrinol Metab 272: E864–E869, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Favre B, Zolnierowicz S, Turowski P, Hemmings BA. The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J. Biol Chem 269: 16311–16317, 1994 [PubMed] [Google Scholar]
- 17. Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab 298: E999–E1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J, Ren J, Gulve EA, Holloszy JO. Additive effect of contractions and insulin on GLUT-4 translocation into the sarcolemma. J Appl Physiol 77: 1597–1601, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Roves PM, Han DH, Song Z, Jones TE, Hucker KA, Holloszy JO. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol Endocrinol Metab 285: E729–E736, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gulve EA, Cartee GD, Zierath JR, Corpus VM, Holloszy JO. Reversal of enhanced muscle glucose transport after exercise: roles of insulin and glucose. Am J Physiol Endocrinol Metab 259: E685–E691, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85: 1218–1222, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev 80: 173–210, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 301: E779–E784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 294: E401–E407, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Ingebritsen TS, Cohen P. The protein phosphatases involved in cellular regulation 1 Classification and substrate specificities. Eur J Biochem 132: 255–261, 1983 [DOI] [PubMed] [Google Scholar]
- 28. Ingebritsen TS, Stewart AA, Cohen P. The protein phosphatases involved in cellular regulation 6 Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. Eur. J Biochem 132: 297–307, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci 33: 113–121, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Kawanaka K, Tabata I, Katsuta S, Higuchi M. Changes in insulin-stimulated glucose transport and GLUT-4 protein in rat skeletal muscle after training. J Appl Physiol 83: 2043–2047, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Kinnick TR, Youngblood EB, O'Keefe MP, Saengsirisuwan V, Teachey MK, Henriksen EJ. Modulation of insulin resistance and hypertension by voluntary exercise training in the TG(mREN2)27 rat. J Appl Physiol 93: 805–812; 2002 [DOI] [PubMed] [Google Scholar]
- 32. Koumanov F, Richardson JD, Murrow BA, Holman GD. AS160 phosphotyrosine-binding domain constructs inhibit insulin-stimulated GLUT4 vesicle fusion with the plasma membrane. J Biol Chem 286: 16574–16582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Kumar A, Chaudhry I, Reid MB, Boriek AM. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem 277: 46493–46503, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu R, Zhou XW, Tanila H, Bjorkdahl C, Wang JZ, Guan ZZ, Cao Y, Gustafsson JA, Winblad B, Pei JJ. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med 12: 241–257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab 254: E248–E259, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Palanivel R, Veluthakal R, Kowluru A. Regulation by glucose and calcium of the carboxylmethylation of the catalytic subunit of protein phosphatase 2A in insulin-secreting INS-1 cells. Am J Physiol Endocrinol Metab 286: E1032–E1041, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18: 3024–3033, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J 14: 5279–5287, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peck GR, Ye S, Pham V, Fernando RN, Macaulay SL, Chai SY, Albiston AL. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol Endocrinol 20: 2576–2583, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Ren W, Cheema S, Du K. The association of ClipR-59 with AS160 modulates AS160 phosphorylation and adipocyte Glut4 membrane translocation. J Biol Chem 287: 26890–26900, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reynolds TH, Brozinick JT, Jr, Rogers MA, Cushman SW. Effects of exercise training on glucose transport and cell surface GLUT-4 in isolated rat epitrochlearis muscle. Am J Physiol Endocrinol Metab 272: E320–E325, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruderman NB, Balon T, Zorzano A, Goodman M. The postexercise state: altered effects of insulin on skeletal muscle and their physiologic relevance. Diabetes Metab Rev 1: 425–444, 1986 [DOI] [PubMed] [Google Scholar]
- 50. Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Schweitzer GG, Cartee GD. Postexercise skeletal muscle glucose transport is normal in kininogen-deficient rats. Med Sci 43: 1148–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shenolikar S. Protein serine/threonine phosphatases–new avenues for cell regulation. Annu Rev Cell Biol 10: 55–86, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985 [DOI] [PubMed] [Google Scholar]
- 54. Steen MS, Foianini KR, Youngblood EB, Kinnick TR, Jacob S, Henriksen EJ. Interactions of exercise training and ACE inhibition on insulin action in obese Zucker rats. J Appl Physiol 86: 2044–2051, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437–443, 2000 [PMC free article] [PubMed] [Google Scholar]
- 56. Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 291: E849–E859, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Thong FS, Derave W, Kiens B, Graham TE, Urso B, Wojtaszewski JF, Hansen BF, Richter EA. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 51: 583–590, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Treebak JT, Frosig C, Pehmoller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl 564: 1–80, 1987 [PubMed] [Google Scholar]
- 60. Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol 65: 909–913, 1988 [DOI] [PubMed] [Google Scholar]
- 61. Weiser DC, Shenolikar S. Use of protein phosphatase inhibitors. Curr Protoc Mol Biol 18: 18 10, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49: 325–331, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes 46: 1775–1781, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest 104: 1257–1264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xie X, Chen Y, Xue P, Fan Y, Deng Y, Peng G, Yang F, Xu T. RUVBL2, a novel AS160-binding protein, regulates insulin-stimulated GLUT4 translocation. Cell Res 19: 1090–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Zhu T, Matsuzawa S, Mizuno Y, Kamibayashi C, Mumby MC, Andjelkovic N, Hemmings BA, Onoe K, Kikuchi K. The interconversion of protein phosphatase 2A between PP2A1 and PP2A0 during retinoic acid-induced granulocytic differentiation and a modification on the catalytic subunit in S phase of HL-60 cells. Arch Biochem Biophys 339: 210–217, 1997 [DOI] [PubMed] [Google Scholar]
- 68. Zorzano A, Balon TW, Garetto LP, Goodman MN, Ruderman NB. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol Endocrinol Metab 248: E546–E552, 1985 [DOI] [PubMed] [Google Scholar]