Abstract
Dynamic remodeling of mitochondrial morphology through membrane dynamics are linked to changes in mitochondrial and cellular function. Although mitochondrial membrane fusion/fission events are frequent in cell culture models, whether mitochondrial membranes dynamically interact in postmitotic muscle fibers in vivo remains unclear. Furthermore, a quantitative assessment of mitochondrial morphology in intact muscle is lacking. Here, using electron microscopy (EM), we provide evidence of interacting membranes from adjacent mitochondria in intact mouse skeletal muscle. Electron-dense mitochondrial contact sites consistent with events of outer mitochondrial membrane tethering are also described. These data suggest that mitochondrial membranes interact in vivo among mitochondria, possibly to induce morphology transitions, for kiss-and-run behavior, or other processes involving contact between mitochondrial membranes. Furthermore, a combination of freeze-fracture scanning EM and transmission EM in orthogonal planes was used to characterize and quantify mitochondrial morphology. Two subpopulations of mitochondria were studied: subsarcolemmal (SS) and intermyofibrillar (IMF), which exhibited significant differences in morphological descriptors, including form factor (means ± SD for SS: 1.41 ± 0.45 vs. IMF: 2.89 ± 1.76, P < 0.01) and aspect ratio (1.97 ± 0.83 vs. 3.63 ± 2.13, P < 0.01) and circularity (0.75 ± 0.16 vs. 0.45 ± 0.22, P < 0.01) but not size (0.28 ± 0.31 vs. 0.27 ± 0.20 μm2). Frequency distributions for mitochondrial size and morphological parameters were highly skewed, suggesting the presence of mechanisms to influence mitochondrial size and shape. In addition, physical continuities between SS and IMF mitochondria indicated mixing of both subpopulations. These data provide evidence that mitochondrial membranes interact in vivo in mouse skeletal muscle and that factors may be involved in regulating skeletal muscle mitochondrial morphology.
Keywords: skeletal muscle mitochondria, myofiber, outer mitochondrial membrane, scanning and transmission electron microscopy, mitochondrial reticulum
skeletal muscle fibers perform energetically demanding functions (contraction, rapid cycles of ion transport, gene expression, and protein synthesis) requiring large amounts of ATP supplied in large part by mitochondria.1 A unique and relatively recently discovered feature of mitochondria is their ability to fuse and divide, collectively known as mitochondrial dynamics (6, 39). Dynamic remodeling of mitochondria from spheroid, bean-shaped structures to elongated tubules, and vice versa, is now understood as an integral aspect of mitochondrial biology. Mitochondrial fusion and fission regulate the autophagy quality control system (35, 37) and apoptotic sensitivity (19, 38) and may act as a metabolic sensing mechanism influencing cell survival in response to nutritional stress (11, 25, 30) and possibly muscle atrophy (31). Furthermore, the genes encoding mitochondrial dynamics proteins have been identified and genetic disruptions of either mitochondrial fusion or fission components impair embryonic development (7, 14), cause the accumulation of mitochondrial DNA impairments in muscle fibers (8), and lead to neuromuscular disorders in humans (40, 41). Other work also suggests that low levels of the mitochondrial fusion protein mitofusin 2 (Mfn2) impair skeletal muscle insulin sensitivity in diabetes (2, 12). Collectively, this evidence has been taken to imply that mitochondria normally undergo fusion and fission in vivo and that disrupting these processes, and therefore mitochondrial morphology, negatively impairs the function of skeletal muscle.
However, although mitochondria continuously undergo morphological changes in the order of seconds to minutes in the cytosol of proliferating cultured cells (36), direct evidence of mitochondrial membrane interactions (a prerequisite for mitochondrial dynamics) in skeletal muscle fibers is scarce (24). Furthermore, methods to examine and quantify mitochondrial shape in intact myofibers are required to determine whether changes in mitochondrial morphology are linked to specific physiological functions of skeletal muscle fibers.
Here, building from previous work by others (4, 16, 17, 22), we used a combination of freeze-fracture scanning electron microscopy (SEM) and resin-embedded transmission EM (TEM) in both longitudinal and transverse planes of mouse soleus muscle to characterize mitochondria topology and quantify their morphology. Our novel quantitative approach revealed that standard morphological parameters for both subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria differed extensively and were generally skewed in their distributions. In addition, several sites of mitochondrial membranes interaction were apparent among and between IMF and SS compartments, contributing new evidence that mitochondrial membranes dynamically interact in vivo, possibly to accomplish fusion/fission (34), kiss-and-run (20), or for other physiological processes within intact muscle fibers.
METHODS
Animals and tissue collection.
Eight-week-old female C57BL/6 mice were studied. Mice were group housed with free access to food and water until the morning preceding the experiments, when three mice derived from different litters were killed by cervical dislocation during the dark phase of the light cycle (22:15). Within 1–1.5 min following death, the soleus was dissected, cut into two equal halves, and fixed by immersion into fixative for either transmission electron microscopy (TEM) or scanning electron microscopy (SEM). Tissues thereafter remained in fixative overnight at room temperature and >48 h at 4°C before processing for electron microscopy. All animal procedures were approved by the University of Newcastle Animal Care and Ethics Committee.
TEM.
For TEM, half of the mouse soleus was immediately fixed in a 2% glutaraldehyde solution in 0.1 M cacodylate (TAAB Lab Equipment) buffer, pH 7.4. Muscle samples were then postfixed in 1% osmium tetroxide (OsO4, Agar Scientific) for 1 h and dehydrated in sequential steps of acetone (25%, 50%, 75%, and 100% twice) prior to impregnation in increasing concentrations (25%, 50%, 75%, and 100% three times) of resin (TAAB Lab Equipment) in acetone over a 24-h period. The half soleus was cut into smaller segments and embedded either in transverse (TS) or longitudinal (LS) orientation in 100% resin at 60°C for 24 h. To verify orientation and section quality, 1-μm-thick sections were cut and stained with 1% toluidine blue in 1% borax. Ultrathin sections of 70 nm were subsequently cut using a diamond knife on a Leica EM UC7 ultramicrotome. Sections were stretched with chroloform to eliminate compression and mounted on Pioloform filmed copper grids prior to staining with 2% aqueous uranyl acetate and lead citrate (Leica). Ultrathin sections were examined on a Phillips CM 100 Compustage (FEI) transmission electron microscope, and digital micrographs were captured by an AMT CCD camera (Deben).
To analyze SS mitochondrial morphology, muscle in the LS orientation was photographed at ×19,000 magnification. Regions containing numerous SS mitochondria were selected for analysis. For IMF mitochondria, muscle in the TS orientation was imaged at ×7,900 magnification. To ensure optimal transverse orientation at the subcellular level (relative to the Z-line) (see Fig. 1D), muscle fibers presenting no more than two Z-lines separated by a minimum of 10–15 μm were selected for analysis. For each animal, five to six mitochondria-rich muscle fibers were analyzed in both TS and LS, for which at least two micrographs were captured, allowing the analysis of ∼40 IMF and 40 SS mitochondria per myofiber. A total of 505 SS and 653 IMF mitochondria were analyzed. The coefficient of variation (CV) for average mitochondrial size between myofibers was of 48.6% for SS and 15.6% for IMF mitochondria, whereas the CV between animals was 34.0% and 8.4%, respectively. For aspect ratio, a measure of mitochondrial shape, the CV between myofibers was of 12.4% for SS and 14.4% for IMF mitochondria, whereas the CV between animals was 2.5% and 5.9%, respectively.
Fig. 1.
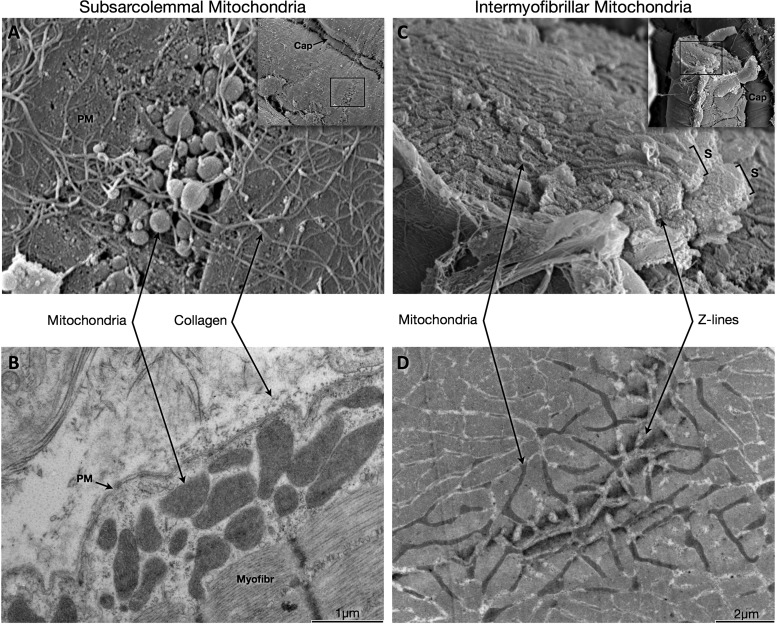
Subsarcolemmal and intermyofibrillar mitochondrial morphology in intact mouse soleus muscle fibers. Scanning electron microscopy (EM) of a freeze-fractured muscle sample (A) and transmission EM in the longitudinal plane (B), showing a myofiber's outer surface lined with collagen fibers forming the extracellular matrix. A breach within the plasma membrane exposes globular subsarcolemmal (SS) mitochondria in A, which can be observed in B between the plasma membrane and myofibrils. Scanning EM of a freeze-fractured myofiber (C) and transmission EM in the transverse plane (D) exposing elongated intermyofibrillar (IMF) mitochondria clustering around Z-lines between sarcomeres. Insets represent lower magnification micrographs with regions magnified in the main panels. PM, plasma membrane; Myofibr, myofibrils; S, sarcomeres; Cap, capillary. Scanning electron microscopy (SEM) images were captured at magnifications of ×10,300 (A) and ×1,140 (A, inset), and ×5,800 (B) and ×1,310 (C, inset).
To quantify mitochondrial interactions among SS and IMF mitochondria, micrographs of muscle photographed in the longitudinal orientation at ×19,000 (SS) and ×13,500 (IMF) were used. In the IMF compartment, a total of 750 Z-lines possessing mitochondria on both sides, either as pairs of discrete organelles (Fig. 3A), as a continuous mitochondrion (Fig. 3B), or as interacting organelles (Fig. 3C), were analyzed, and the proportion of Z-lines spanned by a continuous or interacting mitochondria is reported as a percentage of the total. In addition, 437 SS and 667 IMF physical contacts sites between adjacent mitochondria were evaluated for the presence of electron density. The proportion of all mitochondrial contact sites between organelles that were electron dense is reported as a percentage.
Fig. 3.
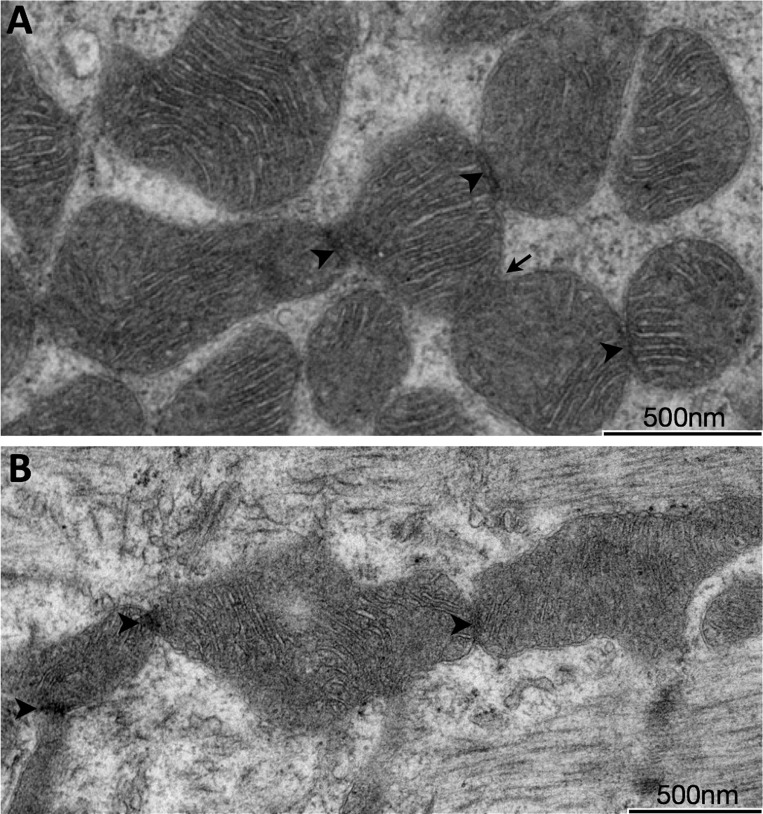
Electron dense mitochondrial contact sites among SS and IMF mitochondria. Transmission EM showing representative electron dense mitochondrial contact sites among subsarcolemmal (SS; A) and intermyofibrillar (IMF; B) mitochondria. Arrowheads indicate electron densities associated with membrane juxtaposition; the arrow indicates a restricted but continuous neck between two mitochondrial units.
SEM.
For SEM, the other half of the mouse soleus was immediately fixed in a 1% glutaraldehyde (TAAB Lab Equipment) and 0.5% paraformaldehyde (Sigma 158127) solution in a 0.060 M cacodylate (TAAB Lab Equipment) buffer, pH 7.4. For processing, fixed samples were rinsed twice in 0.067 M cacodylate buffer and subsequently immersed in DMSO (Sigma D2650) for 5–10 min. Then, the fixed samples were snap frozen in liquid nitrogen-cooled isopentane (Sigma 277258) for 5–10 s and immediately fractured by applying lateral pressure within a custom designed liquid nitrogen-cooled cracking instrument. The resulting fractured specimens, ∼0.5–3 mm in size, were rinsed three times in 0.067 M cacodylate buffer and postfixed in osmium tetroxide (OsO4) 1% for 1 h. Samples were then transferred to OsO4 0.1% for 72–96 h to partially extract cytoplasmic components (22). Then, muscle samples were dehydrated in sequential steps of ethanol (25%, 50%, 75%, and 100% twice) and dried with CO2 in a Baltec critical point dryer. Specimens were mounted on stubs covered with carbon disks, gold coated (15 nm) in Polaron SEM coating unit, and examined on a Stereoscan 240 scanning electron microscope. Fracture sites were observed for exposed mitochondria, and digital micrographs were captured at different magnifications with the Orion 6.60.6 software.
Morphological analysis and statistics.
Mitochondrial shape descriptors and size measurements were obtained using Image J (version 1.42q, National Institutes of Health, Bethesda, MD) by manually tracing only clearly discernible outlines of SS and IMF mitochondria on TEM micrographs, as shown in Fig. 5. Mitochondria coexisting in both SS and IMF compartments (representing less than 1% of total) were excluded from these analyses. Surface area (mitochondrial size) is reported in squared micrometers; perimeter in micrometers; aspect ratio (AR) is computed as [(major axis)/(minor axis)] and reflects the “length-to-width ratio”; form factor (FF) [(perimeter2)/(4π·surface area)] reflects the complexity and branching aspect of mitochondria; circularity [4π·(surface area/perimeter2)] and roundness [4·(surface area)/(π·major axis2)] are two-dimensional indexes of sphericity with values of 1 indicating perfect spheroids; and Feret's diameter represents the longest distance (μm) between any two points within a given mitochondrion (18). Computed values were imported into Microsoft Excel and Prism 5 (GraphPad Software) for data analysis. Statistical significance was evaluated based on 99% confidence interval (CI) of the mean. To produce frequency distributions of each morphological parameter, each mitochondrion was assigned to one of 20 bins of equal sizes and proportions were determined, yielding frequency histograms and level of skewness. A summary of the major statistical tests for each morphological parameter in SS and IMF mitochondria is presented in Table 1.
Fig. 5.
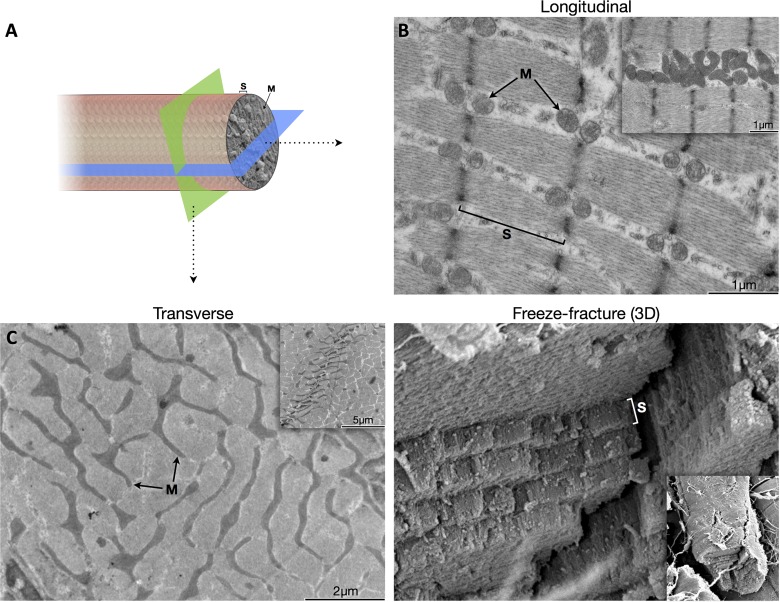
Three-dimensional visualization of intermyofibrillar mitochondrial morphology in muscle fibers. A: schematic representation of a myofiber sectioned in the two major planes. A combination of transmission EM in the longitudinal (B) and transverse planes (C) reveals the complexity of intermyofibrillar (IMF) mitochondria within the natural organization of sarcomeres. The Z-line is not visible in C and highly branched mitochondria are apparent due to the almost perfect transverse orientation of the muscle fiber. D: scanning EM micrograph showing how the freeze-fracture process tends to rupture sarcomeres at the I band near the Z-line, where thick filaments are absent and most IMF mitochondria are located. Myofibers are composed of multiple sarcomeres arranged in series, where each sarcomeric plane is populated by reticular mitochondria along its transverse axis (see also Fig. 1C). Insets show B: IMF mitochondria in a zone of high mitochondrial density, note also sarcomeric shortening due to myofiber contraction. C: a minimally inclined transverse section showing an expanded Z-line (dark line) and mitochondria on either sides; D: lower magnification of the main image showing the whole-width myofiber after freeze-fracture. SEM images were captured at magnifications of ×5,800 (D), ×2,900 (D, inset). S, sarcomere; M, mitochondria.
Table 1.
Detailed morphological parameters for SS and IMF mitochondria in mouse soleus
| Median | Mean (SD) | Min | Max | Skewness | |
|---|---|---|---|---|---|
| Surface area, μm2 | |||||
| SS | 0.19 | 0.28 (0.31) | 0.01 | 2.72 | 3.33 |
| IMF | 0.22 | 0.27 (0.20) | 0.02 | 1.39 | 1.67 |
| Roundness (0–1) | |||||
| SS | 0.57 | 0.57 (0.18) | 0.14 | 1 | −0.01 |
| IMF | 0.33 | 0.37 (0.20)* | 0.08 | 1 | 0.78 |
| Circularity (0–1) | |||||
| SS | 0.80 | 0.75 (0.16) | 0.22 | 0.97 | −0.94 |
| IMF | 0.41 | 0.45 (0.22)* | 0.07 | 0.97 | 0.46 |
| Feret's Diameter, μm | |||||
| SS | 0.69 | 0.79 (0.48) | 0.08 | 3.8 | 1.84 |
| IMF | 1.06 | 1.20 (0.68)* | 0.18 | 5.49 | 1.37 |
| Perimeter, μm | |||||
| SS | 1.77 | 2.05 (1.20) | 0.22 | 8.21 | 1.71 |
| IMF | 2.54 | 3.00 (1.84)* | 0.48 | 16.06 | 1.78 |
| Form factor | |||||
| SS | 1.26 | 1.41 (0.45) | 0.97 | 4.55 | 2.60 |
| IMF | 2.44 | 2.89 (1.76)* | 0.92 | 14.77 | 2.05 |
| Aspect ratio | |||||
| SS | 1.75 | 1.97 (0.83) | 1 | 7.18 | 2.16 |
| IMF | 3.03 | 3.63 (2.13)* | 1 | 13.16 | 1.26 |
Skewness is a measure of asymmetry in distributions. A value of 0 = normally or randomly distributed population; a value >0 = more smaller values than expected; and a value <0 = more larger values than expected. See Fig. 6B-for a visual representation of distributions and their skewness.
Intermyofibrillar (IMF) significantly different (P < 0.01) from subsarcolemmal (SS) mitochondria, based on 99% confidence interval of the mean. n = 505 SS and 563 IMF mitochondria.
RESULTS
Skeletal muscle mitochondria and outer membrane interactions.
We studied the morphology of the two distinct mitochondrial populations in mouse skeletal muscle: subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria. SS mitochondria reside between the sarcolemma and myofibrils, whereas IMF mitochondria are located between myofibrils (21). The combination of freeze-fractured myofibers imaged with SEM (Fig. 1, A and C) and resin-embedded ultrathin sections imaged with TEM (Fig. 1, B and D) revealed that mitochondrial morphology in skeletal muscle fibers is highly diverse.
To determine whether mitochondrial membranes interact in vivo, mitochondria were examined at high resolution. The classical dyad of IMF mitochondria across the Z-line was commonly observed (Fig. 2A), although continuous or interacting mitochondria across the Z-line were observed in 12.5% of cases within mitochondria-rich myofiber segments (Fig. 2, B and C). The outer mitochondrial membranes of adjacent IMF mitochondria were found to interact, forming mitochondrial contact sites. Mitochondrial contact sites were occasionally characterized by increased electron density (Fig. 2, C and D), likely from the presence of large electron-dense proteins. At electron-dense contact sites, two adjacent outer mitochondrial membranes came into close juxtaposition to become indistinguishable (Fig. 2, C' and D'). Electron densities were present at contact sites with similar prevalence among SS and IMF mitochondria, consisting of 8.7% and 7.5% of all contact sites, respectively. Irregularly shaped mitochondria with distinct inner mitochondrial membranes surrounded by continuous outer membranes were also observed (Fig. 2E), possibly representing midpoint events of incomplete mitochondrial fusion events in which the outer mitochondrial membranes are known to fuse first, followed by the inner membranes (34). Electron-dense contact sites of varying aspects were observed among both IMF (Fig. 3A) and SS (Fig. 3B) subpopulations.
Fig. 2.
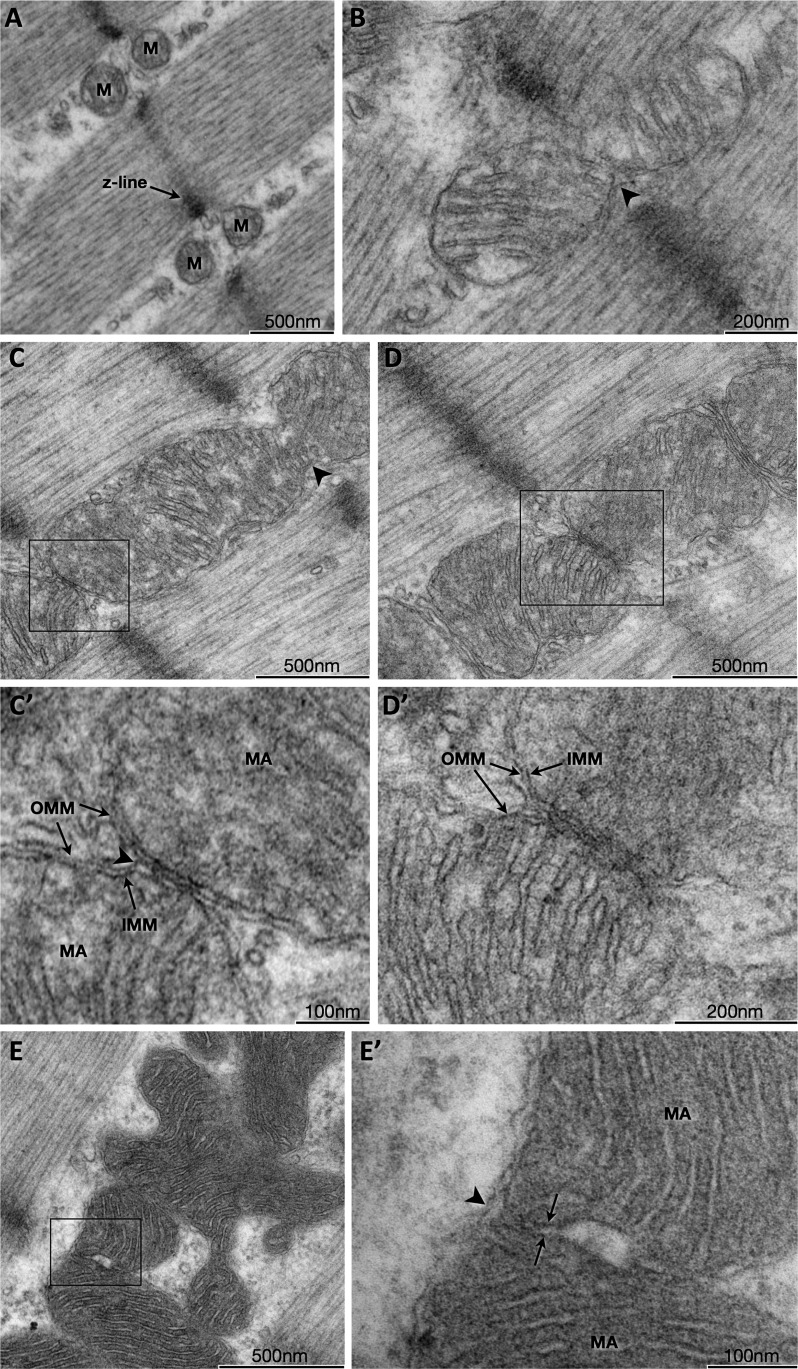
Interactions among intermyofibrillar mitochondrial membranes. Transmission EM of myofibers in the longitudinal plane showing distinct mitochondria on either sides of the Z-line (A) or continuous mitochondria spanning the Z-lines (B and C; arrowheads). C and D: mitochondrial contact sites characterized by electron densities localized at the outer mitochondrial membranes. C' and D': higher magnifications demonstrating electron-dense outer membranes of adjacent mitochondria that become indistinguishable at the contact sites. The arrowhead in C' indicates separation of the outer and inner mitochondrial membranes suggestive of forces acting upon the outer membrane. E: an irregularly shaped mitochondrion with distinct matrix spaces bound by (E') distinct inner mitochondrial membranes (arrows), but surrounded by a common outer mitochondrial membrane (arrowhead). OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; MA, mitochondrial matrix.
Interactions between SS and IMF mitochondria (16, 17) were also investigated. Although SS and IMF mitochondria were generally distinct groups with distinct morphology (Fig. 4A), we observed continuous organelles coexisting in both compartments (Fig. 4, B and C). These organelles were characterized by more globular morphology in the SS compartment, whereas they adopted more elongated morphology within the IMF space.
Fig. 4.
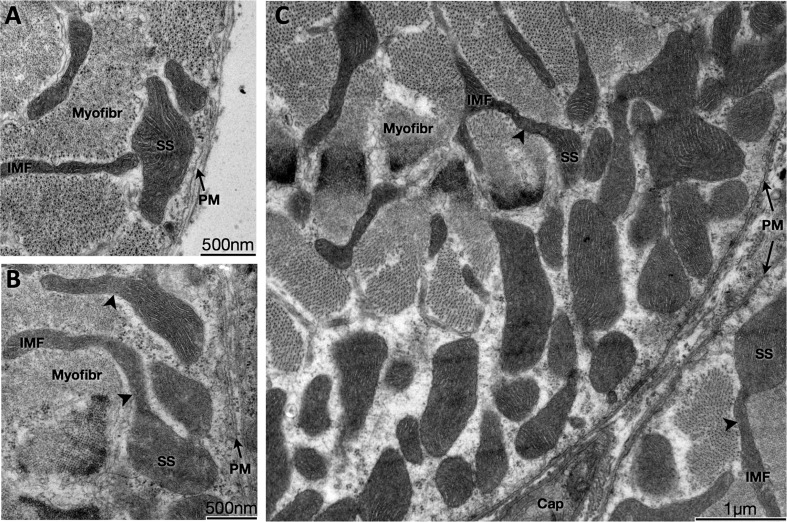
Physical interactions between SS and IMF mitochondria. Transmission electron micrograph of myofibers in the transverse plane. A: SS and IMF mitochondria are distinct organelles. B and C: some SS and IMF mitochondria form continuous organelles (arrowheads) that coexist in both subcellular compartments. SS, subsarcolemmal; IMF, intermyofibrillar; PM, plasma membrane (sarcolemma); Myofibr, Myofibrils; Cap, capillary.
Quantification of mitochondrial morphology in myofibers.
Three-dimensional mitochondrial morphology was analyzed by using a combination of transmission EM in both the longitudinal and transverse planes (Fig. 5A). Whereas SS mitochondria are mostly spherical (see Fig. 1A) and can be visualized with similarly accurate representation in either planes, IMF mitochondrial morphology differs extensively depending on the plane selected. Whereas the longitudinal plane presented IMF mitochondria as more or less spheroid structures positioned on either sides of the Z-lines (Fig. 5B), microscopic analysis of the transverse plane (Fig. 5C) revealed the mitochondrial reticulum (4, 17). During freeze fracture, myofibers were ruptured at regular intervals in a “staircase” manner to partially expose sarcomeres lined on either sides by reticular mitochondria (Fig. 5D and Fig. 1C).
To quantify mitochondrial morphology, mitochondria were individually traced from transmission EM. The “form factor”/“aspect ratio” distribution among SS and IMF mitochondria differed markedly (Fig. 6, A and B). Further analysis of the major mitochondrial shape descriptors indicated that distribution of roundness, circularity, Feret's diameter, perimeter, form factor, and aspect ratio differed significantly between SS and IMF mitochondria (Fig. 7 and Table 1). For instance, circular mitochondria (roundness >0.8) constituted 48.5% of SS mitochondria, but represented only 8.9% of IMF mitochondria. In contrast, mitochondrial size was relatively similar between SS and IMF mitochondria (Table 1). Furthermore, the frequency distributions for mitochondrial size (Fig. 7B) and several morphological parameters (Fig. 7, C–H) were highly (>1.0) skewed (Table 1), suggesting the presence of mechanisms to actively preserve small mitochondrial sizes and specific morphology.
Fig. 6.
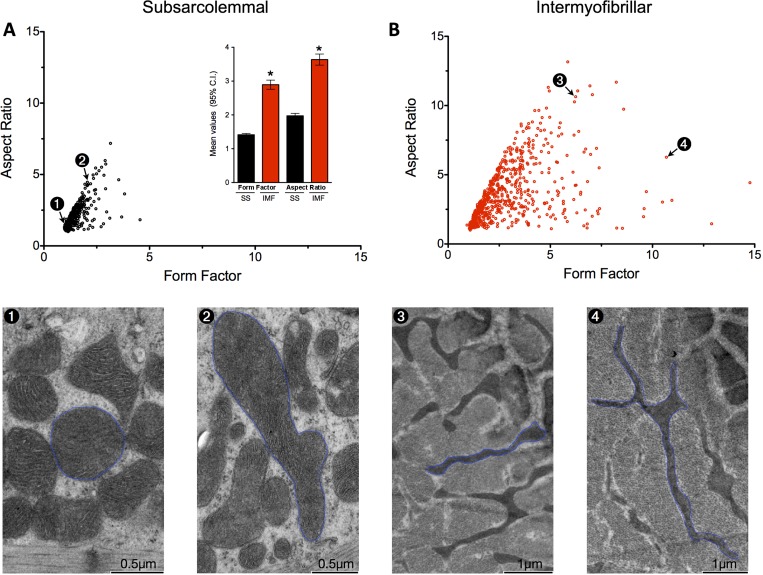
Quantification of form factor and aspect ratio among SS and IMF mitochondria. Mitochondrial form factor and aspect ratio were quantified by manually tracing SS (A) and IMF (B) mitochondria, and plotted against each another. Electron micrographs numbered 1–4 correspond to individual mitochondria plotted above. Inset in A shows mean values and 95% confidence internal (CI) for form factor and aspect ratio among both SS and IMF subpopulations. *Denotes statistical significance (P < 0.01) compared to SS. n = 505 SS and 563 IMF mitochondria.
Fig. 7.
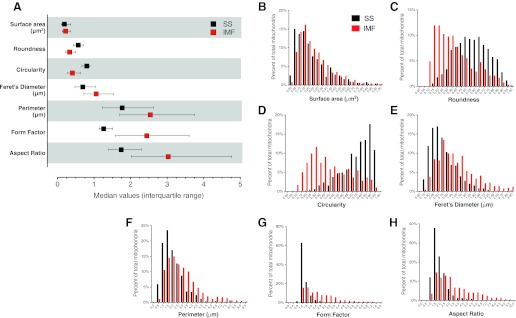
Analysis of morphological parameters in SS and IMF mitochondria. A: multiple shape descriptors were determined for manually traced SS and IMF mitochondria. Bars represent the range of values that contain 50% of all mitochondria (interquartile range) for each parameter. B–H: frequency distributions (%total mitochondria) were plotted for each shape descriptor with 20 bins of equal sizes. n = 505 SS and 563 IMF mitochondria.
DISCUSSION
The existence of mitochondrial dynamics in intact skeletal muscle fibers largely remains to be established. Likewise, although mitochondrial morphology is known to be diverse in skeletal muscle fibers (4, 22), the extent to which SS and IMF mitochondria differ is underappreciated and no prior study has systematically quantified normal parameters of mitochondrial morphology in both subpopulations. In this study, we report and quantify the extent to which outer mitochondrial membranes of adjacent organelles interact in mouse soleus muscle, forming electron dense contact sites [or junction sites (3, 4)]. Furthermore, using a combination of scanning and transmission EM in two orthogonal planes, we define for the first time distributions of morphological parameters among SS and IMF mitochondria, suggesting that mitochondrial morphology is not randomly distributed and therefore likely to be actively regulated.
Mitochondrial dynamics.
Mitochondrial dynamics—or the ability of mitochondria to undergo fusion and fission processes—has emerged as a fundamental biological mechanism essential to mitochondrial function and cellular health (6, 39). Following the identification of the proteins involved in mitochondrial morphology transitions in mammals—mitofusins 1 and 2 (Mfn1, Mfn2) and optic atrophy 1 (OPA1) for fusion; dynamin-related protein 1 (DRP1), mitochondrial fission factor (Mff) and fission protein 1 (Fis1) for fission—molecular genetic defects in some of these proteins have been shown to cause neuromuscular pathology in mouse and in human (8, 42). These findings indicated that disrupting mitochondrial dynamics in vivo causes neuromuscular pathology. The prevailing interpretation of these results has been that mitochondria must therefore normally undergo regular fusion events (and fission) in myofibers in vivo. However, mitochondrial fusion proteins also perform other physiological function (e.g., tether mitochondria with endoplasmic reticulum for Ca2+ dynamics, transcriptional regulation of respiratory chain genes) that are independent of mitochondrial morphology transitions (9, 10, 29). Thus the pathological manifestations of altered fusion and fission proteins could arise independently from abnormal mitochondrial shape changes and should therefore not be regarded as definite proof of mitochondrial dynamics in vivo. Except for one preliminary report demonstrating the exchange of a mitochondria-targeted photo-activable probe between neighboring skeletal muscle mitochondria (24), direct evidence of mitochondrial fusion in mature myofibers is lacking. Establishing whether mitochondrial membranes physically interact in vivo is a first step in addressing this point.
In the cytosol of cultured cells, including myoblasts [myogenic precursor cells (2)], mitochondria continuously undergo morphological changes on the order of seconds to minutes (36). However, mitochondrial dynamics may differ, both qualitatively and quantitatively, between proliferating cultured cells and mature differentiated muscle fibers. Unique intracellular features in myofibers may prevent extensive mitochondrial dynamics, particularly the densely packed myofibrils and other organelles that populate the cytosolic space, which may hinder free organelle movement and extensive network remodeling as in cultured cells. In addition, the presence of soluble cytosolic factors has been proposed to inhibit mitochondrial fusion in differentiated cells. The observations from the current study [and previously (4)] of mitochondrial contact sites, characterized by electron densities and complex organelle morphology, suggest that mitochondrial membranes do interact in vivo. On the basis of the recent finding that adjacent mitochondria in 8-wk-old mouse skeletal muscle can exchange matrix-located molecules (24), this suggests that at least a portion of the contact sites observed could be the result of mitochondrial fusion processes. However, mitochondrial membrane contacts sites such as those reported herein could be the result of kiss-and-run behavior (20), mitochondrial division (although unlikely), or other processes (e.g., ion or lipid transfer) that would involve contact between adjacent membranes (18a).
SS and IMF morphology and shape.
Because of known topological, functional and morphological differences between SS and IMF mitochondria, a significant portion of our analysis compared both mitochondrial subpopulations. Topologically, SS mitochondria are located beneath the plasma membrane often in proximity to myonuclei and capillaries; whereas IMF mitochondria are located at the core of myofibers between myofibrils (15, 21). Functionally, differences have been observed when these two subpopulations are mechanically isolated and studied, although the isolation process yields only spherical organelles and may thus alter native mitochondrial morphology and function (27, 28). Nevertheless, in preparations of isolated organelles, compared with IMF, SS mitochondria typically have lower oxidative capacity, produce lower levels of reactive oxygen species (rate of H2O2 efflux), and are more sensitive to the proapoptotic event of permeability transition (1, 23, 32). Morphologically, consistent with previous findings (16, 17, 22), we observed that whereas SS mitochondria were for the most part isolated spherical units (although relatively elongated mitochondria were also observed), IMF mitochondria organize into a reticulum interconnected by transverse mitochondrial tubules near the Z-line (4, 17).
Elongated mitochondrial tubules have been likened to “power-transmitting cables” capable of transmitting membrane potential and possibly other molecules (e.g., O2, ATP) along their length (33). Continuous organelles coexisting within both SS and IMF compartments—mitochondrial linkages (17)—might fulfill such a role, allowing the exchange of information between SS mitochondria (which are in close contact with nuclei and capillaries) and IMF mitochondria. Another possibility to explain the existence of continuous SS-IMF mitochondrial linkages is that mitochondrial biogenesis principally takes place within the SS space and that “newly synthesized” mitochondria thereafter migrate to the IMF region.
The most novel aspect of our study lies in the detailed characterization of SS and IMF mitochondrial morphology, which reinforces the notion that SS and IMF mitochondrial morphology differ substantially and expand upon previous studies by providing detailed analysis of size and several morphological parameters. The skewed distributions of mitochondrial sizes and morphological parameters shown in Fig. 7 strongly suggest the presence of factors to regulate mitochondrial morphology. Considering mitochondrial size, if no factor controlled mitochondrial volume, one would expect that most mitochondria would be of a given “optimal” size (the mean), with an equal distribution of sizes on either side to produce a normal distribution (skewness = 0, not skewed). Statistically, the random sampling of mitochondria from micrographs across the thickness of myofibers would likewise yield an equal distribution of values for sizes and morphological parameters. However, distribution of SS and IMF mitochondrial sizes were highly skewed (3.33 and 1.67, respectively), as were several other morphological parameters (see Table 1), indicating that mechanisms likely exist to regulate mitochondrial size and shape. Factors involved in determining mitochondrial shape could be passive, such as the presence of densely packed myofibrils that limit the lateral enlargement of IMF mitochondria, potentially explaining their larger aspect ratio (length/width ratio) compared with SS mitochondria. The fact that SS mitochondria, which are not constrained by myofibrils exhibit normal distribution in roundness (skewness = −0.01) but that IMF mitochondria exhibit significant skewness (0.78) supports this notion. In turn, active factors, such as selective mitochondrial fusion and fission processes (34a, 35), or other unknown mechanisms, could actively participate in shaping nonnormal distributions of mitochondrial sizes and morphology observed among both SS and IMF mitochondria.
In our study, the mouse soleus was chosen because it is of relatively homogenous oxidative fiber-type composition (55% type I, 51% type IIa) (13) and also because it is extensively studied and thus well characterized biochemically and physiologically. Although mitochondrial topology may be preserved between oxidative and glycolytic fiber types (15), fiber type differences in mitochondrial density (particularly SS) and reticular morphology (17, 22) and function (26) exist and should be taken into consideration when extrapolating these results to other models.
Methodological considerations.
Significant obstacles to the study of mitochondrial morphology and mitochondrial dynamics through microscopy relates to space and time resolution. Although light microscopy generally enables the imaging of a larger number of events/cells over time, conventional approaches employing fluorescent molecules are limited by the natural resolution limits of 200 nm (down to 70 nm on super-resolution platforms) of light microscopy, cannot discriminate between adjacent organelles or membranes, and therefore do not enable accurate quantification of mitochondrial morphology or interactions in skeletal muscle fibers. In turn, electron microscopy allows precisely to resolve mitochondrial membranes and to qualitatively assess interorganelle contact sites. However, EM-based approaches require tissue fixation and are thus bound to static observations of dynamic processes, a limitation that recent in vivo imaging techniques employing photo-activatable mitochondrial dyes are aiming to overcome (24). Quantitative electron microscopy analyses similar to those described here, repeated at multiple consecutive time points through an intervention, should allow tracking of population-wide changes in mitochondrial morphology and contact site dynamics.
Historically, the complexities of skeletal muscle mitochondrial morphology and topology have been qualitatively described (4, 5, 16, 17, 22), building from elegant studies performed 20–40 years ago involving serial sections and three-dimensional reconstructions of small portions of the mitochondrial reticulum in skeletal muscles of various species (4, 16). Chronologically, these observations were made over two decades before mitochondrial dynamics emerged as a field of research and thus before it became established that mitochondrial interactions and dynamics bear functional significance for the cell (39). The present study combined established electron microscopic approaches and modern tools derived from light microscopy to quantify mitochondrial morphology and interpreted its findings cognizant of the molecular mechanisms and potential functional consequences of mitochondrial dynamics. Although our results do not unequivocally establish the existence of mitochondrial dynamics in the intact muscle, they demonstrate that mitochondrial membranes interact and assume configurations consistent with mitochondrial dynamics. We hope that this work will contribute to establish that mitochondrial morphology (both SS and IMF) is unique and diverse in skeletal muscle and that systematic quantification of mitochondrial morphology and interactions in intact myofibers can be accomplished using standard TEM procedures and morphometric analysis.
Summary
In conclusion, this study reveals that mitochondrial morphology in mature myofibers consists of two divergent but interacting populations of mitochondria, for which we systematically quantified size and morphological parameters. This study provides new evidence that mitochondrial membranes interact in vivo and that factors may be involved in regulating SS and IMF mitochondrial size and morphology in the intact muscle. The molecular composition of mitochondrial contact sites remains to be established. Likewise, future studies will be required to determine the relationship between mitochondrial contact sites, potential remodeling of SS and/or IMF mitochondrial morphology, and skeletal muscle physiology.
GRANTS
M. Picard was supported by a Ph.D. Canada Graduate Scholarship and a Michael Smith Foreign Study Supplement from the National Science and Engineering Research Council of Canada (NSERC), was a Canadian Institute of Health Research (CIHR) Fellow in Psychosocial Oncology and in Systems Biology, and is now supported by a CIHR Postdoctoral Fellowship from the Institute of Neuroscience as part of the Canadian Epigenetics, Environment and Health Research Consortium (CEEHRC). This work was performed in the Newcastle University Centre for Brain Ageing and Vitality supported by grants from the Biotechnology and Biological Sciences Research Council (BBRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), Medical Research Council (MRC), the Wellcome Trust Centre for Mitochondrial Research and National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals National Health Service Foundation Trust and Newcastle University to Douglass M. Turnbull.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.P. conception and design of research; M.P. and K.W. performed experiments; M.P. analyzed data; M.P. and D.M.T. interpreted results of experiments; M.P. prepared figures; M.P. drafted manuscript; M.P., K.W., and D.M.T. edited and revised manuscript; M.P., K.W., and D.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Tracey Davey (EM Research Services, Newcastle University) for expert technical assistance for the preparation and visualization of samples; to Christopher Huggins for assistance with animal handling; to Heidi McBride, Orian Shirihai, Yan Burelle and Benoit Gentil for valuable discussions; and to Gilles Gouspillou for providing critical comments on a previous version of this manuscript.
Footnotes
This article is the topic of an Invited Editorial by David Hood and Sobia Iqbal (12a).
REFERENCES
- 1. Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289: C994–C1001, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bakeeva LE, Chentsov Yu S, Skulachev VP. Intermitochondrial contacts in myocardiocytes. J Mol Cell Cardiol 15: 413–420, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501: 349–369, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Bubenzer HJ. [The thin and the thick muscular fibers of the rat diaphragm]. Z Zellforsch Mikrosk Anat 69: 520–550, 1966 [PubMed] [Google Scholar]
- 6. Chen H, Chan DC. Physiological functions of mitochondrial fusion. Ann NY Acad Sci 1201: 21–25, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1[alpha]/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33: 645–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a. Hood D, Iqbal S. Muscle mitochondrial ultrastructure: New insights into morphological divergences. J Appl Physiol; doi:10.1152/japplphysiol.01428.2012 [DOI] [PubMed] [Google Scholar]
- 13. Hughes SM, Chi MM, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J Cell Biol 145: 633–642, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11: 958–966, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Kayar SR, Hoppeler H, Essen-Gustavsson B, Schwerzmann K. The similarity of mitochondrial distribution in equine skeletal muscles of differing oxidative capacity. J Exp Biol 137: 253–263, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Kayar SR, Hoppeler H, Mermod L, Weibel ER. Mitochondrial size and shape in equine skeletal muscle: a three-dimensional reconstruction study. Anat Rec 222: 333–339, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol Cell Physiol 251: C395–C402, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Koopman WJ, Visch HJ, Smeitink JA, Willems PH. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A 69: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 18a. Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Weaver D, Shirihai O, Hajnoczky G. Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J 28: 3074–3089, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller W. Subsarcolemmal mitochondria and capillarization of soleus muscle fibers in young rats subjected to an endurance training. A morphometric study of semithin sections. Cell Tissue Res 174: 367–389, 1976 [DOI] [PubMed] [Google Scholar]
- 22. Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739, 1977 [PubMed] [Google Scholar]
- 24. Pham AH, Michael J, Chan DC. Mouse lines with photo-activatable mitochondria (PhAM) to study mitochondrial dynamics. Genesis 50: 833–843, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Picard M, Burelle Y. Mitochondria: starving to reach quorum?: Insight into the physiological purpose of mitochondrial fusion. Bioessays 34: 272–274, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Picard M, Hepple RT, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol 302: C629–C641, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Picard M, Taivassalo T, Gouspillou G, Hepple RT. Mitochondria: isolation, structure and function. J Physiol 589: 4413–4421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MT, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6: e18317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14: 1405–1415, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 108: 10190–10195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Servais S, Couturier K, Koubi H, Rouanet JL, Desplanches D, Sornay-Mayet MH, Sempore B, Lavoie JM, Favier R. Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic Biol Med 35: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26: 23–29, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a. Sukhorukov VM, Dikov D, Reichert AS, Meyer-Hermann M. Emergence of the mitochondrial reticulum from fission and fusion dynamics. PLoS Comput Biol 8: e1002745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Twig G, Liu X, Liesa M, Wikstrom JD, Molina AJ, Las G, Yaniv G, Hajnoczky G, Shirihai OS. Biophysical properties of mitochondrial fusion events in pancreatic beta-cells and cardiac cells unravel potential control mechanisms of its selectivity. Am J Physiol Cell Physiol 299: C477–C487, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14: 1939–1951, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wasilewski M, Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab 20: 287–294, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Yu-Wai-Man P, Chinnery PF. Dysfunctional mitochondrial maintenance: what breaks the circle of life? Brain 135: 9–11, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W, Jr, Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133: 771–786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu-Wai-Man P, Sitarz KS, Samuels DC, Griffiths PG, Reeve AK, Bindoff LA, Horvath R, Chinnery PF. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Hum Mol Genet 19: 3043–3052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


