Abstract
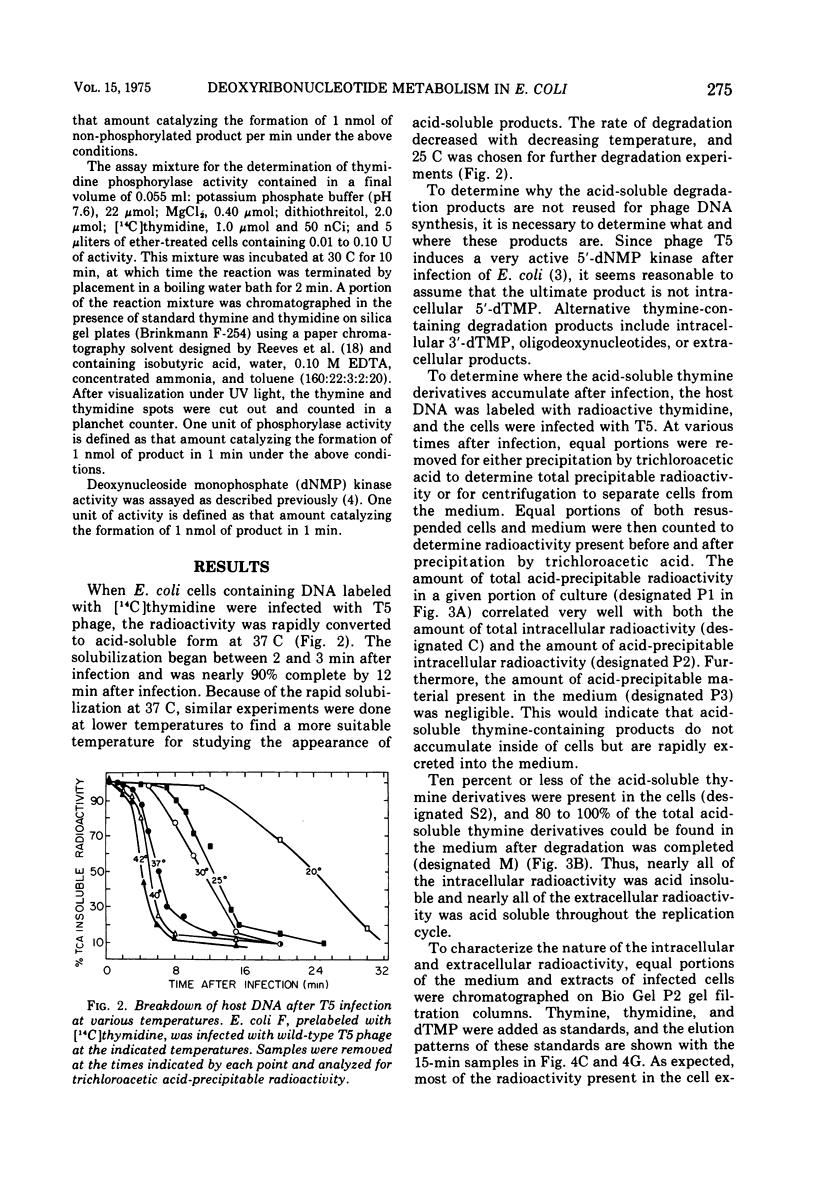
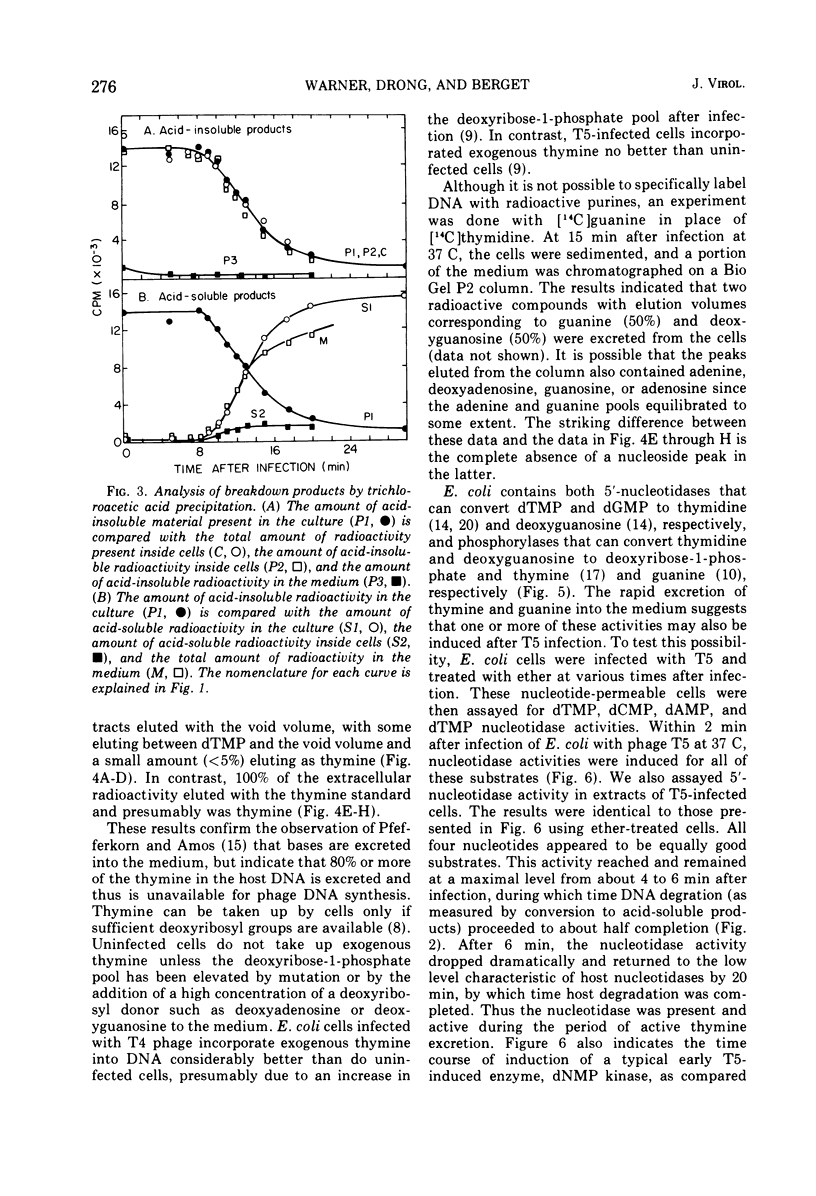
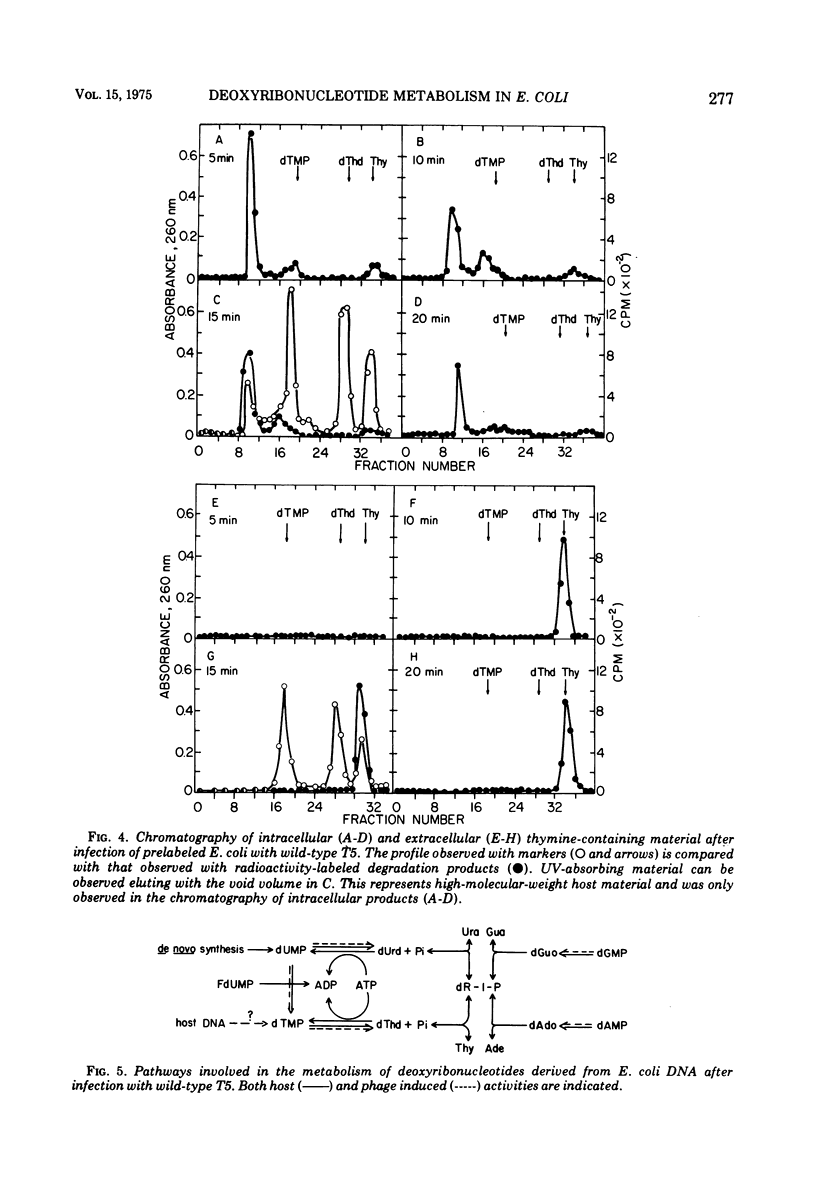
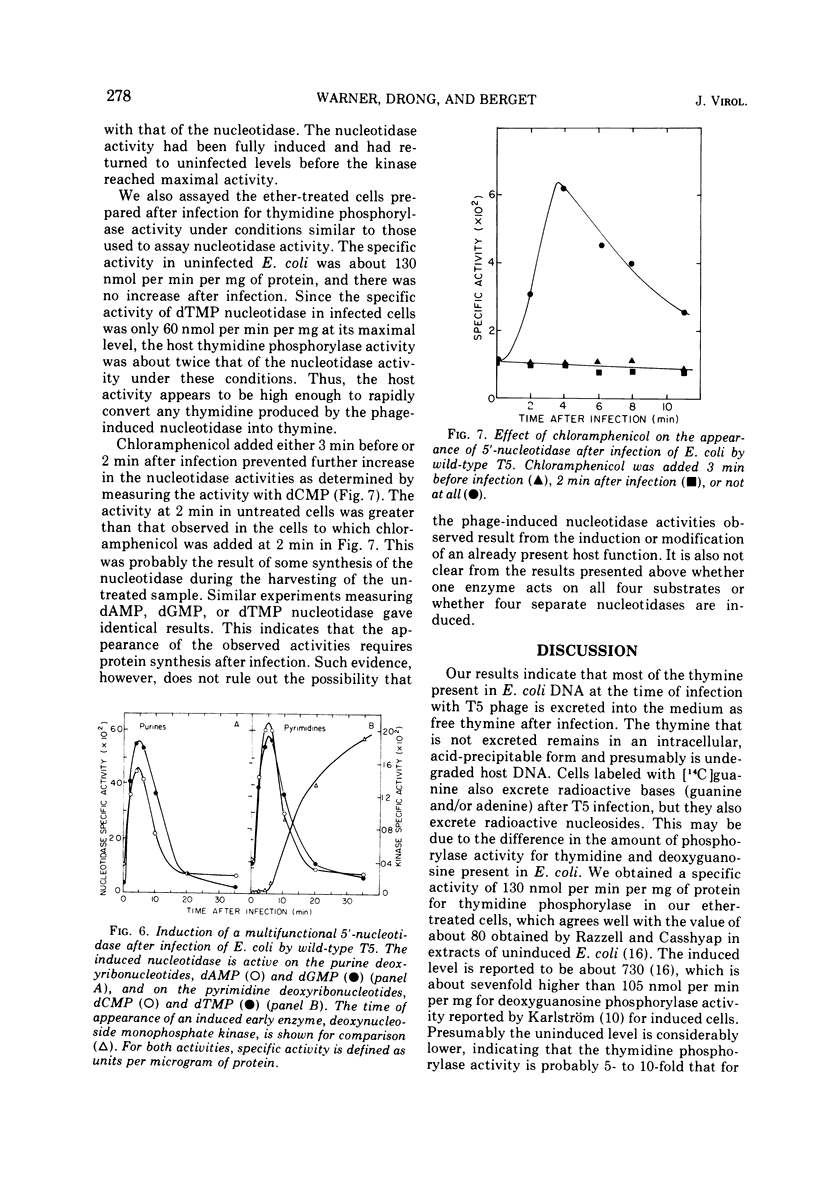
Thymine-containing compounds, produced degradation of Escherichia coli DNA after infection of the cells with bacteriophage T5, did not accumulate in the cell but were excreted into the medium as the DNA was degraded. The ultimate degradation product was extracellular thymine that was not reutilized when T5 DNA synthesis began. This excretion of thymine may have been due in part to the induction of 5'-nucleotidase activity within 3 min after T5 infection. The level of this activity reached a maximum between 4 to 6 min after infection and then rapidly declined to its preinfection level by 10 to 15 min after infection. Chloramphenicol added before or soon after infection prevented the appearance of the nucleotidase. The induced nucleotidase activity was active not only on dTMP but also on dAMP, dGMP, and dCMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSMAN M. J., HERRIOTT S. T., ORR M. J. THE ENZYMOLOGY OF VIRUS-INFECTED BACTERIA. VI. PURIFICATION AND PROPERTIES OF THE DEOXYNUCLEOTIDE KINASE INDUCED BY BACTERIOPHAGE T5. J Biol Chem. 1965 Jan;240:439–445. [PubMed] [Google Scholar]
- Becker A., Hurwitz J. The enzymatic cleavage of phosphate termini from polynucleotides. J Biol Chem. 1967 Mar 10;242(5):936–950. [PubMed] [Google Scholar]
- Berget S. M., Warner H. R., McCorquodale D. J. Isolation and partial characterization of bacteriophage T5 mutants deficient in the ability to induce deoxynucleoside monophosphate kinase. J Virol. 1974 Jul;14(1):78–85. doi: 10.1128/jvi.14.1.78-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V. Nucleic acid metabolism in Escherichia coli infected with phage T5. Virology. 1959 Apr;7(4):359–374. doi: 10.1016/0042-6822(59)90065-0. [DOI] [PubMed] [Google Scholar]
- DUKES P. P., KOZLOFF L. M. Phosphatases in bacteriophages T2, T4, and T5. J Biol Chem. 1959 Mar;234(3):534–538. [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Kammen H. O., Strand M. Thymine metabolism in Escherichia coli. II. Altered uptake of thymine after bacteriophage infection. J Biol Chem. 1967 Apr 25;242(8):1854–1863. [PubMed] [Google Scholar]
- Karlström O. Mutants of Escherichia coli defective in ribonucleoside and deoxyribonucleoside catabolism. J Bacteriol. 1968 Mar;95(3):1069–1077. doi: 10.1128/jb.95.3.1069-1077.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- PFEFFERKORN E., AMOS H. Deoxyribonucleic acid breakdown and resynthesis in T5 bacteriophage infection. Virology. 1958 Aug;6(1):299–301. doi: 10.1016/0042-6822(58)90083-7. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., CASSHYAP P. SUBSTRATE SPECIFICITY AND INDUCTION OF THYMIDINE PHOSPHORYLASE IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1789–1793. [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. J., Jr, Seid A., Greenberg D. M. A new paper chromatography solvent system resolving pyrimidine-pyrimidine riboside-pyrimidine deoxyriboside mixtures. Anal Biochem. 1969 Sep;30(3):474–477. doi: 10.1016/0003-2697(69)90145-6. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uerkvitz W., Karlström O., Munch-Petersen A. A deoxyuridine monophosphate phosphatase detected in mutants of Escherichia coli lacking alkaline phosphatase and 5'-nucleotidase. Mol Gen Genet. 1973 Mar 19;121(4):337–346. doi: 10.1007/BF00433232. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Rosenkranz H. S., Morgan C. Development of coliphage T5: ultrastructural and biochemical studies. J Virol. 1972 Mar;9(3):526–543. doi: 10.1128/jvi.9.3.526-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



