Abstract
The transverse carpal ligament (TCL) serves as the origin of the thenar muscles and is integral to thenar muscle contraction anatomically and biomechanically. TCL hypertrophy has been observed in patients with carpal tunnel syndrome and is potentially caused by repetitive hand use. The purpose of this study was to investigate the biomechanical interaction between the TCL and the thenar muscles. Specifically, the morphological changes of the carpal arch, formed by the TCL, in response to thenar muscle contractions were examined during isometric tip pinch between the thumb and index finger. Ultrasound videos of the carpal tunnel were recorded from 13 healthy subjects and were synchronized with the forces measured by a pinch dynamometer. The thenar muscles' ulnar point, trapezium, and hamate were tracked by a pattern-matching program. The pinch force significantly affected the carpal arch height, width, and area (P < 0.005). As the pinch force increased from 0 to 100% maximum voluntary contraction force, the carpal arch height increased from 1.8 ± 1.0 to 2.3 ± 1.3 mm, the carpal arch width decreased from 23.9 ± 2.4 to 23.1 ± 2.4 mm, and the carpal arch area increased from 22.2 ± 13.6 to 27.3 ± 16.3 mm2. The TCL was pulled volarly during thenar muscle contractions, providing evidence for the biomechanical interaction between the ligament and muscles. Repetitive biomechanical stimulation on the TCL from thenar muscle contractions could lead to tissue remodeling and then TCL hypertrophy. This study sheds light on the potential cause of TCL hypertrophy, which may be an etiological factor for carpal tunnel syndrome.
Keywords: ultrasound imaging, transverse carpal ligament, thenar muscles, carpal tunnel, pattern tracking
the carpal tunnel is formed by the transverse carpal ligament (TCL) as its volar boundary and the interconnected carpal bones as its medial, lateral, and dorsal boundaries. The TCL plays an important role in carpal tunnel mechanics. It stabilizes the carpal bones in the transverse direction (26, 33), serves as a pulley for the flexor tendons (13, 15), and functions as an anchor for the thenar and hypothenar muscles. In particular, 68% of the thenar muscle origin area is from the volar aspect of the TCL (16). This indicates that the TCL is anatomically and biomechanically integral to proper thenar muscle contractions, which are essential for pinching and gripping tasks. The importance of the ligament-muscle biomechanical interaction is further illustrated by weakened grip strength after carpal tunnel release surgery, during which the TCL is transected (4).
It is well known that soft connective tissues such as ligaments and tendons adapt to mechanical loading conditions (e.g., physiological stress levels and range of motion), leading to changes in tissue mass and material properties (14, 29, 30, 32). Therefore, thickening and stiffening of the TCL may occur when the TCL is repeatedly subjected to contraction forces generated by the thenar muscles. Indeed, TCL hypertrophy has been observed in individuals with carpal tunnel syndrome (3, 10, 34) and has been proposed as an etiological factor of this prevalent hand disorder (20). However, the association of TCL hypertrophy with carpal tunnel syndrome remains controversial, and the cause of such a tissue change is unknown. It has been postulated that repetitive hand use leads to mechanical stimulation for tissue remodeling, resulting in hypertrophic changes (9, 20). This postulation is supported by positive association between carpal tunnel syndrome and manual jobs that require high forces and high repetition (24). It has also been pointed out that certain hand activities (e.g., pinching, gripping, and using hand-held vibrating tools) are particularly provocative for the development of carpal tunnel syndrome (1).
Several studies have investigated the morphological (21, 23, 25) and mechanical properties (7, 8, 19) of the TCL, but the biomechanical environment surrounding the TCL as well as its influence on TCL properties is not clear. The purpose of this study was to investigate the biomechanical interaction between the TCL and the thenar muscles. Specifically, we examined the morphological changes of the carpal arch, formed by the TCL, in response to thenar muscle contractions. The thenar muscles were activated during isometric tip pinching, while ultrasound videos of the carpal tunnel were simultaneously recorded. We hypothesized that thenar muscle contractions would cause a displacement of the TCL in the volar direction, leading to increased carpal arch height and area.
MATERIALS AND METHODS
Subjects.
Fifteen healthy, right-handed female subjects were recruited for this study, with approval of the institutional review board. The means (±SD) age, height, and weight were 29 ± 7 yr, 1.65 ± 0.05 m, and 58.1 ± 10.3 kg, respectively. Written, informed consent was obtained from all subjects before the study. Exclusion criteria for subjects were self-reported musculoskeletal or neuromuscular disorders to the hand and wrist.
Subject positioning.
Each subject sat in an upright position next to a testing table. The right hand was secured in a custom-made thermoplastic splint using Velcro straps (Fig. 1). The wrist was in a supinated, anatomically neutral position. The thumb and index finger were in a tip pinch posture. The middle, ring, and little fingers were fixed to the splint in a slightly flexed position. The splint was attached to a metal plate using Velcro to stabilize the forearm. The arm position was adjusted to allow the shoulder to be abducted at ∼30° and the elbow flexed at ∼90°. The hand and forearm together with the splint were submerged in water to facilitate ultrasound imaging.
Fig. 1.
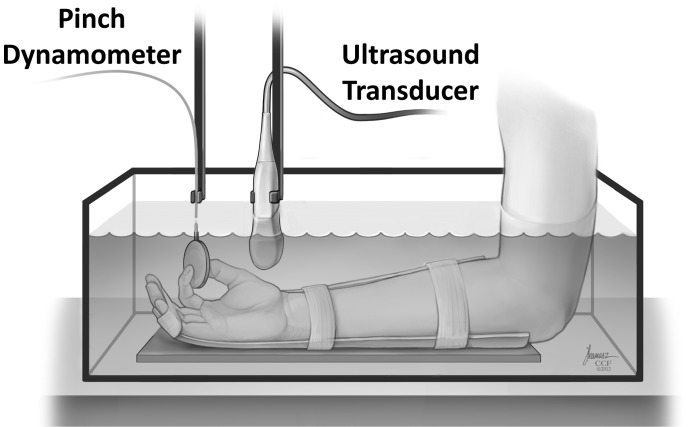
A schematic of the experimental setup for ultrasound imaging of the carpal tunnel during isometric tip pinch. [Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2012. All Rights Reserved.]
Ultrasound examination.
A single operator conducted ultrasound examinations on all subjects. The operator was specifically trained for imaging the carpal tunnel. An ultrasound system (Acuson S2000, Siemens Medical Solutions USA, Mountain View, CA) with a 18L6 HD linear array transducer was used for this study. The system was operated in the two-dimensional mode with tissue harmonic imaging and tissue equalization at an imaging frequency of 12 MHz, and with an image field depth of 4 cm. First, the subject's hand was imaged in air following a protocol used in our previous study (23). The ultrasound transducer was orientated along the transverse axis of the hand. The position of the transducer was adjusted until the ridge of the trapezium and the hook of the hamate were clearly identified from the ultrasound image. The transducer was held in place while the outline of the transducer was traced on the palmar wrist with a permanent marker. Next, the subject's hand was imaged in water (12). An articulated positioning arm (Manfrotto Distribution, Ramsey, NJ) was used to hold the ultrasound transducer and to place the transducer in the water tank. The transducer was placed on the hand following the drawn outline to reproduce the desired view of the carpal tunnel. Then, the transducer was slowly lifted while maintaining its orientation and tilt angle so that the ridge of the trapezium and the hook of the hamate could be clearly identified from the ultrasound image. A space of 10–15 mm was kept between the transducer and the skin to prevent direct contact. The positioning arm was then tightened to hold the transducer in place.
Experimental protocol.
Isometric tip pinch was chosen as the experimental task because it specifically targets the thenar musculature (5) that originates from the TCL. Another articulated positioning arm (Manfrotto Distribution, Ramsey, NJ) was used to hold a pinch dynamometer (P200, Biometrics, Cwmfelinfach, Gwent, UK). The position of the pinch dynamometer was adjusted so the subject was able to comfortably pinch it using the tips of the thumb and index finger. Care was taken to ensure that the posture of the wrist, thumb, and fingers was kept the same as explained in Subject positioning. The testing protocol consisted of two parts: 1) determination of the maximum voluntary contraction (MVC) pinch force, and 2) isometric tip pinch from zero to MVC force in 4 s at a constant rate. In the first part, each subject was instructed to gradually squeeze the pinch dynamometer and reach to the maximum in ∼4 s. Three MVC trials were performed with a 2-min rest period in between to prevent muscle fatigue (2). The average of the maximum forces measured during the three contractions was defined as the MVC force for each subject. In the second part, to control the pace of pinching, a custom LabVIEW (National Instruments, Austin, TX) program was used to generate a ramp-up function that constantly increased from zero to the predetermined MVC force in 4 s. Each subject was instructed to gradually squeeze the pinch dynamometer following the ramp-up function and relax after reaching the MVC force. The force-time curve was plotted by LabVIEW on a monitor, allowing the subject to match the real-time pinch force with the ramp-up function. Two practice trials were performed, followed by five testing trials. A 2-min rest period was given between consecutive contractions.
During the five testing trials, the ultrasound system was first initiated to capture video clips at a rate of 60 frames/s. Next, the LabVIEW program was started to record force measurements from the pinch dynamometer at a frequency of 600 Hz and to send a 5-V DC trigger signal to the ultrasound system. The trigger signal was recorded on the ultrasound video via the physio-mode (Fig. 2), permitting synchronization of force measurements and dynamic ultrasound data for further analysis.
Fig. 2.
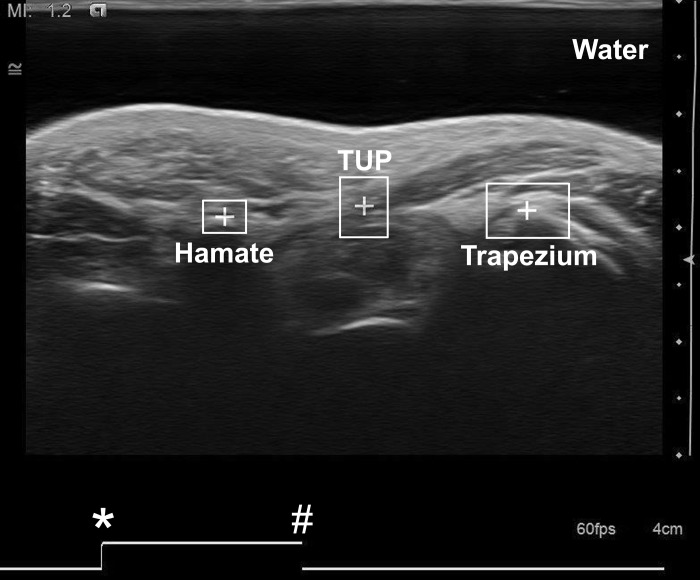
Ultrasound image of the carpal tunnel recorded at the hook of the hamate level. Three region-of-interest boxes were drawn with the centroids (+) placed on the thenar muscles' ulnar point (TUP) and the most volar points of the trapezium and hamate. *Starting point of the trigger signal for synchronization. #The time point at which the current image frame was taken.
Data analysis.
The ultrasound videos were recorded as AVI files and then decomposed into individual JPEG images. The image frame corresponding to the impulse of the trigger signal served as the synchronization time point. Eleven ultrasound images from each testing trial were identified corresponding to pinch forces from 0 to 100% MVC force in 10% increments. Another custom LabVIEW program was used to identify and track regions of interest through pattern matching. Three region-of-interest boxes were drawn on the ultrasound image, with the centroids placed on the thenar muscles' ulnar point (TUP), the most volar point of the trapezium, and the most volar point of the hamate (Fig. 2). The TUP was defined as the most ulnar aspect of the thenar muscle attachment to the TCL and was typically located in the middle of the TCL. The TUP was chosen because it was a unique anatomical feature that can be reliably identified from the ultrasound image (23). The most volar points of the trapezium and hamate were chosen as the TCL bony insertion sites.
The position coordinates of the centroids of the three regions of interest served as inputs to a custom MATLAB (The Mathworks, Natick, MA) program to assess the interaction between the TCL and the thenar muscles. The coordinates were translated and rotated so that the line connecting the trapezium and hamate served as the x-axis, and its perpendicular bisector served as the y-axis. Three carpal arch morphological parameters were calculated, including the carpal arch height (CAH) at the TUP, the carpal arch width (CAW), and the carpal arch area (CAA). The CAH at the TUP was defined as the perpendicular distance between the TUP and the line connecting the trapezium and hamate, i.e., the y coordinate of the TUP. The CAW was defined as the distance between the most volar points of the trapezium and hamate. The CAA was estimated by calculating the area of the triangle formed by the TUP, trapezium, and hamate, i.e., CAA = CAH × CAW/2. Only the first three testing trials in the second part of the experiment were used to calculate the morphological parameters, whereas the fourth and fifth testing trials were backups. The mean of the first three testing trials was used to represent each subject in the statistical analysis.
SPSS for Windows 20.0 (IBM SPSS, Chicago, IL) was used to perform statistical analysis. One-way repeated-measures ANOVA with a Greenhouse-Geisser correction were used to determine the effects of pinch force on the carpal arch morphological parameters (CAH, CAW, and CAA). Post hoc tests with the least significant difference were used for all pairwise comparisons. A significance level of 0.05 was used. Least-squares curve fitting was also performed between the average pinch force (independent variable) and carpal arch morphological parameters (dependent variables) to estimate the trend of the outcomes.
RESULTS
Only 13 subjects (n = 13) were used for data analysis as ultrasound images from two subjects were not trackable due to the bony landmarks moved out of the imaging plane. Generally, the TCL was pulled volarly and the carpal bones moved closer to each other with an increasing pinch force (Fig. 3). The ANOVA tests showed that the pinch force significantly affected the carpal arch height, width, and area (P < 0.005).
Fig. 3.
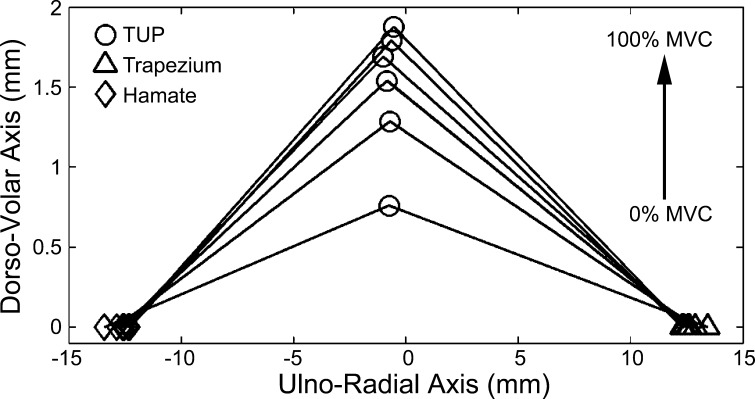
Movement of the thenar muscles' ulnar point (TUP) relative to the carpal bones during a single trial for 0–100% maximal voluntary contraction (MVC) force in 20% increments. The TUP was pulled volarly, and the trapezium and hamate moved closer to each other with an increasing pinch force.
The CAH increased linearly with an increasing pinch force (R2 = 0.94; Fig. 4A). Pairwise comparisons showed that the CAHs at 10–100% MVC were significantly greater than that at 0% MVC (P < 0.05). The initial CAH at 0% MVC was 1.8 ± 1.0 mm. The CAH at 100% MVC was 2.3 ± 1.3 mm, representing an increase of 28% relative to the initial CAH. The difference between the initial and final CAHs indicated that the TCL at the TUP moved volarly 0.5 mm due to the maximum contractions of the thenar muscles.
Fig. 4.
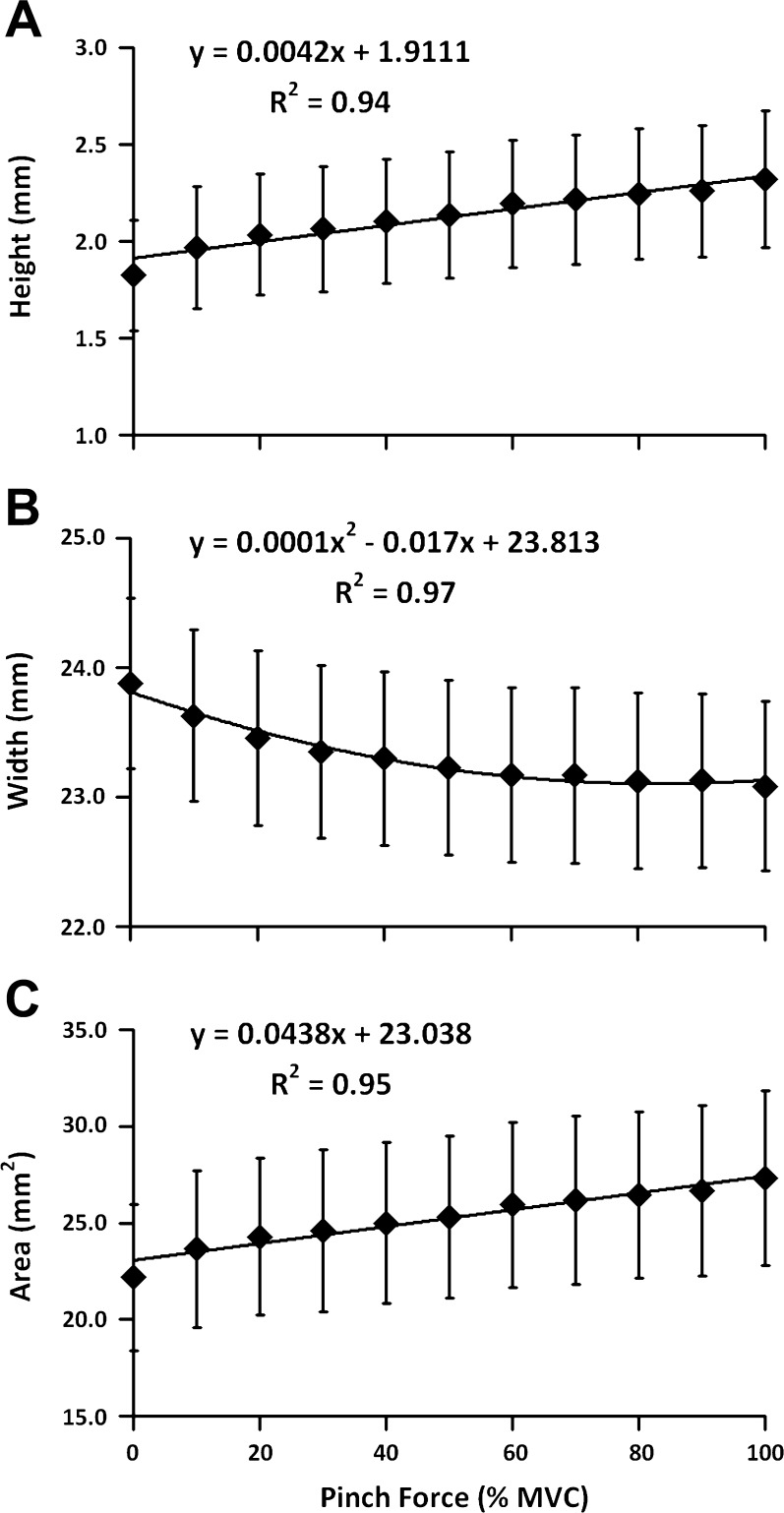
Height (A), width (B), and area (C) of the carpal arch as a function of the pinch force during thenar muscle contractions. Means and standard error bars are shown.
In contrast to the CAH, the CAW decreased nonlinearly with an increasing pinch force (R2 = 0.97; Fig. 4B). Pairwise comparisons revealed that the CAWs at 60–100% MVC were smaller than those at 0–50% MVC, whereas the CAWs at 60–100% MVC did not significantly differ from each other. As the pinch force increased from 0 to 100% MVC, the CAW decreased from 23.9 ± 2.4 to 23.1 ± 2.4 mm, a narrowing of 0.8 mm or a 3.3% decrease.
Similar to the CAH, a linear relationship was found between the CAA and the pinch force (R2 = 0.95; Fig. 4C). Pairwise comparisons demonstrated that the CAAs at 10–100% MVC were significantly larger than that at 0% MVC (P < 0.05). Starting with the initial CAA of 22.2 ± 13.6 mm2 at 0% MVC, the CAA increased to 27.3 ± 16.3 mm2 at 100% MVC. The total increase in CAA was 5.1 mm2, an increase of 23% compared with the initial CAA.
DISCUSSION
From an anatomical standpoint, biomechanical interactions within the musculoskeletal system usually occur between ligaments and bones or between muscles and tendons. However, the anatomical arrangement between the TCL and the thenar muscles lead to a unique biomechanical and physiological phenomenon of ligament-muscle interaction. In the present study, we investigated this interaction by examining the morphological changes of the carpal arch, formed by the TCL, in response to thenar muscle contractions.
One special technical feature of this study was that the forces measured by the pinch dynamometer were synchronized with the ultrasound videos of the carpal tunnel in real time. This allowed us to investigate the force-morphology relationship of the carpal arch, formed by the TCL, during thenar muscle contractions. Another unique feature was that the ultrasound images were collected in a noncontact manner by submerging the hand in water. This prevented direct contact between the transducer and the skin that might affect the biomechanical interaction between the TCL and the thenar muscles while the water served as an ultrasound coupling medium.
The main finding of the present study was that the TCL was pulled volarly due to thenar muscle contractions. The CAH at 0% MVC force was 1.8 ± 1.0 mm, indicating that palmar bowing of the TCL exists under normal physiological conditions for the unloaded state. It agreed well with previously reported palmar bowing of 1.29 ± 0.78 mm in healthy subjects (28). As the pinch force increased to 100% MVC, the CAH increased to 2.3 ± 1.3 mm. This confirmed our hypothesis that thenar muscle contractions caused a displacement of the TCL in the volar direction. This finding can be explained by the anatomical arrangement that approximately two-thirds of the thenar muscle origin area is from the volar boundary of the TCL (16). Furthermore, muscle-ligament junctions usually have a smooth transition from striated muscle fibers to ligament's collagen fibers (22), so the force generated by thenar muscle contractions can be gradually transferred to the TCL.
As for the carpal arch morphological parameters, the CAH and the CAA increased while the CAW decreased in response to thenar muscle contractions. Our results agreed well with two previous cadaveric studies investigating the morphological changes of the carpal arch (17, 18). In one study, a palmarly directed force was applied from inside the tunnel to the TCL (18), whereas in the other study a pressure was applied to the carpal tunnel by a balloon device (17). The amount of changes in the carpal arch morphological parameters and the relationship among the parameters in these cadaveric studies compared well with our in vivo findings. The relationship among the carpal arch morphological parameters was examined previously by a geometric model, showing that the increase in CAA was mainly attributed to the narrowing of CAW and the formation of a greater carpal arch by the TCL (18). The model demonstrated that a decrease of 0.2 mm in CAW caused an increase of 1.5 mm in CAH and an increase of 16.1 mm2 in CAA. In the present study, the 100% MVC force led to a decrease of 0.8 mm in CAW, an increase of 0.5 mm in CAH, and an increase of 5.1 mm2 in CAA. The amount of increases in CAH and CAA was smaller than the model prediction, which could be explained by the difference in the initial CAH. The model assumed no initial arch formation, whereas our data showed an initial CAH of 1.8 mm.
The volar displacement of the TCL due to thenar muscle contractions provides evidence for biomechanical interaction between the TCL and the thenar muscles. It is well accepted that mechanical loading conditions, such as the stress and strain levels, affect soft tissue homeostasis (14, 29, 30, 32). Previous studies demonstrated that both the amount of collagen content and the diameter of the collagen fiber bundles in the ligaments increased after exercise (6, 27). It was postulated that the stressing of ligaments results in microtears and the tissue remodeling process ultimately strengthens the ligamentous tissue (31). Although previous findings were from bone-ligament junction, similar tissue remodeling process is expected for the muscle-ligament junction. In other words, repetitive biomechanical stimulation on the TCL from thenar muscle contractions could have similar effects that induce tissue remodeling and lead to a thickened and stiffened TCL. Such changes in the morphological and mechanical properties of the TCL could reduce carpal tunnel volume and compress the median nerve, ultimately leading to carpal tunnel syndrome. This suggests that TCL hypertrophy, a potential etiological factor for carpal tunnel syndrome, is likely caused by repetitive loading of the TCL. The TCL-muscle interaction also helps explain why people whose jobs involve high-force and high-repetition tasks are susceptible to carpal tunnel syndrome (24).
This study has a few limitations. First, only female subjects were recruited for this study. It is likely that findings are applicable to men given the anatomical arrangements of the TCL and the thenar muscles are similar for both sexes. Second, tip pinch may stretch the tendons within the carpal tunnel, such as the flexor pollicis longus of the thumb and the flexor digitorum profundus and superficialis of the index finger. The tendons under tension may elevate the carpal tunnel pressure (11) and contribute to the increase of the carpal tunnel area (17). Third, carpal arch area was approximated by a triangle formed by the TUP, trapezium, and hamate. Although the CAA was likely underestimated, the trend in the changes of CAA in response to thenar muscle contractions was valid.
In summary, this study provided evidence for biomechanical interaction between the TCL and the thenar muscles under normal physiological conditions. As the pinch force increased, the TCL was pulled volarly by the thenar muscles, causing a decrease in CAW and increases in CAH and CAA. This study sheds light on the biomechanical stimulation of the TCL and its subsequent changes in morphological and mechanical properties, which may lead to the compression of the median nerve and eventually carpal tunnel syndrome.
GRANTS
The project described was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R21 AR-062753.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.L.S. and Z.-M.L. conception and design of research; Z.L.S. performed experiments; Z.L.S. and Z.-M.L. analyzed data; Z.L.S. and Z.-M.L. interpreted results of experiments; Z.L.S. prepared figures; Z.L.S. drafted manuscript; Z.L.S. and Z.-M.L. edited and revised manuscript; Z.L.S. and Z.-M.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Jason Frampton for contribution in a pilot study and Lara Schoeffler for assistance in designing LabVIEW tracking programs.
REFERENCES
- 1. Armstrong T, Dale AM, Franzblau A, Evanoff BA. Risk factors for carpal tunnel syndrome and median neuropathy in a working population. J Occup Environ Med 50: 1355–1364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caldwell LS, Chaffin DB, Dukes-Dobos FN, Kroemer KH, Laubach LL, Snook SH, Wasserman DE. A proposed standard procedure for static muscle strength testing. Am Ind Hyg Assoc J 35: 201–206, 1974 [DOI] [PubMed] [Google Scholar]
- 3. Ferrari FS, Della Sala L, Cozza S, Guazzi G, Belcapo L, Mariottini A, Bolognini A, Stefani P. [High-resolution ultrasonography in the study of carpal tunnel syndrome]. Radiol Med (Torino) 93: 336–341, 1997 [PubMed] [Google Scholar]
- 4. Fuss FK, Wagner TF. Biomechanical alterations in the carpal arch and hand muscles after carpal tunnel release: a further approach toward understanding the function of the flexor retinaculum and the cause of postoperative grip weakness. Clin Anat 9: 100–108, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord 8: 114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heikkinen E, Vuori I. Effect of physical activity on the metabolism of collagen in aged mice. Acta Physiol Scand 84: 543–549, 1972 [DOI] [PubMed] [Google Scholar]
- 7. Holmes MW, Howarth SJ, Callaghan JP, Keir PJ. Biomechanical properties of the transverse carpal ligament under biaxial strain. J Orthop Res 30: 757–763, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Holmes MW, Howarth SJ, Callaghan JP, Keir PJ. Carpal tunnel and transverse carpal ligament stiffness with changes in wrist posture and indenter size. J Orthop Res 29: 1682–1687, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Horiguchi G, Aoki T, Ito H. Characteristics of the electrophysiological activity of muscles attached to the transverse carpal ligament in carpal tunnel syndrome. J Nihon Med Sch 78: 208–213, 2011 [DOI] [PubMed] [Google Scholar]
- 10. John V, Nau HE, Nahser HC, Reinhardt V, Venjakob K. CT of carpal tunnel syndrome. AJNR Am J Neuroradiol 4: 770–772, 1983 [PMC free article] [PubMed] [Google Scholar]
- 11. Keir PJ, Wells RP, Ranney DA, Lavery W. The effects of tendon load and posture on carpal tunnel pressure. J Hand Surg Am 22: 628–634, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Kim DH, Marquardt TL, Gabra JN, Shen ZL, Evans PJ, Seitz WH, Li ZM. Pressure-morphology relationship of a released carpal tunnel. J Orthop Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiritsis PG, Kline SC. Biomechanical changes after carpal tunnel release: a cadaveric model for comparing open, endoscopic, and step-cut lengthening techniques. J Hand Surg Am 20: 173–180, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kline SC, Moore JR. The transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am 74: 1478–1485, 1992 [PubMed] [Google Scholar]
- 16. Kung J, Budoff JE, Wei ML, Gharbaoui I, Luo ZP. The origins of the thenar and hypothenar muscles. J Hand Surg Br 30: 475–476, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Li ZM, Masters TL, Mondello TA. Area and shape changes of the carpal tunnel in response to tunnel pressure. J Orthop Res 29: 1951–1956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng 131: 081011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Main EK, Goetz JE, Baer TE, Klocke NF, Brown TD. Volar/dorsal compressive mechanical behavior of the transverse carpal ligament. J Biomech 45: 1180–1185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore JS. Biomechanical models for the pathogenesis of specific distal upper extremity disorders. Am J Ind Med 41: 353–369, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Pacek CA, Chakan M, Goitz RJ, Kaufmann RA, Li ZM. Morphological analysis of the transverse carpal ligament. Hand NY 5: 77–81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarzkopf R, DeFrate LE, Li G, Herndon JH. The quantification of the origin area of the deep forearm musculature on the interosseous ligament. Bull NYU Hosp Jt Dis 66: 9–13, 2008 [PubMed] [Google Scholar]
- 23. Shen ZL, Li ZM. Ultrasound assessment of transverse carpal ligament thickness: a validity and reliability study. Ultrasound Med Biol 38: 982–988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med 11: 343–358, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Stecco C, Macchi V, Lancerotto L, Tiengo C, Porzionato A, De Caro R. Comparison of transverse carpal ligament and flexor retinaculum terminology for the wrist. J Hand Surg Am 35: 746–753, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Tengrootenhuysen M, van Riet R, Pimontel P, Bortier H, Van Glabbeek F. The role of the transverse carpal ligament in carpal stability: an in vitro study. Acta Orthop Belg 75: 467–471, 2009 [PubMed] [Google Scholar]
- 27. Tipton CM, James SL, Mergner W, Tcheng TK. Influence of exercise on strength of medial collateral knee ligaments of dogs. Am J Physiol 218: 894–902, 1970 [DOI] [PubMed] [Google Scholar]
- 28. Uchiyama S, Itsubo T, Yasutomi T, Nakagawa H, Kamimura M, Kato H. Quantitative MRI of the wrist and nerve conduction studies in patients with idiopathic carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 76: 1103–1108, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang JH. Mechanobiology of tendon. J Biomech 39: 1563–1582, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol 5: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Weisman G, Pope MH, Johnson RJ. Cyclic loading in knee ligament injuries. Am J Sports Med 8: 24–30, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology 19: 397–408, 1982 [DOI] [PubMed] [Google Scholar]
- 33. Xiu KH, Kim JH, Li ZM. Biomechanics of the transverse carpal arch under carpal bone loading. Clin Biomech (Bristol) 25: 776–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamagami T, Higashi K, Handa H, Minouchi K, Fujii M, Nishihara K, Kaji R. [Carpal tunnel syndrome: clinical experience of 61 cases]. No Shinkei Geka 22: 617–620, 1994 [PubMed] [Google Scholar]


