Abstract
Telomeres protect chromosome ends and shorten with age in most tissues. Integral to the maintenance of telomeres is the protein complex shelterin. The gene expression regulation of shelterin proteins to physiological stressors is not understood in vivo. We have recently reported increased telomere-repeat binding factor 1 (TRF1) protein expression and longer telomere length in skeletal muscle of sedentary compared with chronically active mice. These provocative observations led us to examine the effects of acute physiological stress on shelterin expression in vivo in mice and to further define potential mechanisms associated with gene regulation of shelterin. Three groups of female C57Bl/6 mice were studied: one control group and two groups that underwent a 30-min treadmill running bout and were killed either immediately following or 1-h after the exercise. Following the exercise bout, mRNA expression of Trf1 was significantly reduced in the plantaris muscle, and this reduction was paralleled by significant increases in p38 MAPK phosphorylation. To determine if p38 mediated the decreases in Trf1 mRNA expression, C2C12 myotubes were treated with the calcium ionophore, A23187. In response to the A23187, Trf1 gene expression was significantly reduced, coupled with significant increases in p38 phosphorylation, similar to in vivo data. C2C12 myotubes pretreated with a p38 inhibitor (SB-202190) prevented the A23187-induced decrease in Trf1 mRNA expression, indicating a link between Trf1 gene expression and p38 MAPK activation. While it is too early to definitively report the effect of exercise on telomere biology in rodents or humans, these data provide important mechanistic insights into the paradoxical telomere shortening that occurs in skeletal muscle in response to chronic exercise in mice.
Keywords: shelterin, treadmill exercise, telomeres, gene expression, signaling
telomeres are specialized repetitive DNA sequences (5′-TTAGGGn-3′) that play a protective role at the ends of linear chromosomes (7). Current dogma is that, in response to cellular replication, telomere DNA is lost, resulting in telomere shortening, potentially contributing to the induction of cellular senescence (6, 21). In this sense, telomeres are considered a biomarker of aging. Telomeres act as an important contributor to genome stability and transcriptional regulation (e.g., telomere position effect), thus indicating an important role for telomere length in postmitotic tissues (such as skeletal muscle) as well (32).
Telomeres also function to protect the ends of chromosomes from end-to-end fusions and from being recognized as damaged DNA (12). To protect chromosome ends, telomeres form a T-loop chromatin structure (22) that is maintained by a six-protein complex termed shelterin (13). Shelterin consists of three telomere-specific binding proteins and three accessory proteins that link the complex together and stabilize the DNA-protein interactions (13). The three DNA binding proteins are as follows: telomere-repeat binding factors 1 and 2 (TRF1 and TRF2), and protection of telomeres 1 (POT1; POT1a and b in the mouse) (23). TRF1 is critical in telomere length homeostasis and regulates cell cycle progression, whereas TRF2 helps form the T-loop structure and has telomere length-regulating functions (43). POT1 functions in regulating telomerase action at the telomere and also in maintaining telomere structure (20). Additional linker and bridging proteins tie together the shelterin components and stabilize telomere ends (13).
In skeletal muscle, the telomere shortening dogma does not hold true: skeletal muscle telomere length does not change with age, likely stemming from the postmitotic nature of skeletal muscle (14, 30, 40). However, certain disease states, such as Duchenne's muscular dystrophy, have been associated with shortened skeletal muscle telomere lengths from the degeneration-regeneration cycles, causing satellite cell telomeres to shorten (1). In addition to telomere shortening, Aguennouz et al. (1) also observed increased TRF1 protein levels in skeletal muscle from Duchenne's patients, which they hypothesized was related to telomere shortening. However, in a study of skeletal muscle inflammatory diseases with increased regenerative cycles, which one would hypothesize to shorten mean telomere length, no effect on telomere length was observed in diseased skeletal muscle compared with controls (38). In this same study, components of the shelterin complex were upregulated, indicating telomere length protective mechanisms in skeletal muscle in response to telomere shortening stressors. Collectively, these studies point to complex regulation of shelterin and telomere length, dependent on the cellular context in skeletal muscle. Unaccustomed or extreme exercise bouts are also potent physiological stressors, and the impact of such stressors on telomere length and shelterin expression is not clearly defined.
Recent evidence has shown that endurance exercise training may shorten telomere length in skeletal muscle (11, 30, 39), which contrasts significantly from other tissues in which chronic exercise appears to have a “telo-protective” effect and slow age-related telomere shortening (9, 15, 29, 31, 46, 47). Collins et al. (11) were the first to show that, in age- and training-matched endurance athletes, skeletal muscle telomere length was shorter in those displaying symptoms of fatigued athlete myopathic syndrome compared with healthy athletes. However, these results may be confounded by small sample size or other factors, as subject characterization was not comprehensive. In a follow-up study of healthy endurance-trained individuals, those who had trained for the greatest number of years and the greatest number of hours per week had the shortest skeletal muscle telomere lengths (39), although endurance athletes and age-matched controls had similar telomere lengths. A third study investigating telomere length and resistance training reported no difference in telomere length between trained and control individuals (25). Recently, Laye et al. (27) examined the effect of seven marathons in 7 days on telomere length and shelterin in skeletal muscle. The authors observed no change in telomere length, but did observe an increase in shelterin expression in immune cells. Furthermore, expression of DNA damage response proteins Ku70 and Ku80, which are known to interact with telomeres, increased in immune cells and skeletal muscle (27). These studies provide equivocal results, and longitudinal studies are needed to determine the role of physiological stress on human skeletal muscle telomere biology.
While no longitudinal study, to date, has been performed in humans, several groups have recently examined the effect of long-term exercise on telomere biology in rodents (29). Werner et al. (46, 47) have shown in two studies of heart and vascular tissues that long-term exercise training maintains telomere length and increases the expression of shelterin components and telomerase in active compared with sedentary mice. Our laboratory (30) has recently shown in CAST/Ei mice (short telomere strain of mice) that 1 yr of chronic exercise exposure resulted in shorter skeletal muscle telomere length compared with both age-matched sedentary and young (8 wk) mice, while maintaining telomere length in the liver and heart. In addition to telomere shortening in the exercised animals, we observed differences in gene expression and protein content of telomere length-regulating genes (i.e., shelterin components). Specifically, our laboratory (30) observed an upregulation of TRF1 gene expression and telomere length maintenance in the sedentary animals compared with the active animals at 1 yr of age, indicating unique gene expression regulation of shelterin following physiological stress in mouse skeletal muscle. Together, these data indicate that telomere length dynamics and in vivo regulation of shelterin expression in skeletal muscle are unique and do not follow the pattern typical of other tissues in response to chronic exercise.
The links between exercise, telomere length, and shelterin gene expression are unknown, as are the initial signaling events induced by endurance exercise in skeletal muscle that could lead to telomere length alterations (i.e., shortening). It is well established that exercise, specifically skeletal muscle contraction, is known to activate several mitogen-activated protein kinases (MAPK) (41). The terminal kinases in the MAPK signaling cascade are extracellular regulated kinase (ERK1/2), p38 MAPK, and c-Jun NH2-terminus kinase (JNK1/2)/stress-activated protein kinase (41). MAPK are broadly involved in processes such as cell death, differentiation, apoptosis, and cell cycle regulation (3). Recently, the regulation of expression of the shelterin complex was linked to the activation patterns of the MAPK family in cardiomyocytes (44). Thus we hypothesized that MAPK activation (phosphorylation) in skeletal muscle could regulate gene expression patterns of specific members of the shelterin complex.
Given the data from our laboratory and others demonstrating that chronic exercise results in changes in expression of shelterin proteins (11, 30, 39), we sought to determine the possible regulatory signaling pathway by which an acute bout of exercise regulates components of the shelterin complex, independent of changes in telomere length. The purpose of our investigation was twofold: 1) to determine the effects of an acute treadmill exercise bout on skeletal muscle gene expression of shelterin components; and 2) to assess a potential relationship between MAPK signaling and any detected alterations in gene expression of the shelterin components. We hypothesized that acute exercise would result in decreased skeletal muscle shelterin mRNA expression associated with activation of MAPK.
METHODS
Animals
All animal experiments were approved by the University of Maryland Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Use and Care of Laboratory Animals (NIH publication no. 85–23, revised 1996). Twenty-two female 6-wk-old C57Bl/6 mice were purchased (Jackson Laboratories, Bar Harbor, ME). Animals were acclimated to the animal facility for 1 wk before being randomly assigned to treatment groups. The animals were housed at 25°C on a 12:12-h light-dark cycle. Animals were fed ad libitum laboratory mouse chow (Prolab RMH 3000, 5P00, LabDiet by Purina, Nestlé, Vevey, Switzerland) and given free access to water. Animals were separated into three groups for the experiment: baseline (BL; n = 6) animals were exposed to a motionless treadmill for 30 min before being euthanized. The remaining 16 animals underwent a 10-day treadmill acclimation protocol and peak speed test, were separated into two groups that underwent a 30-min treadmill exercise bout, and were euthanized either immediately following [time point 1 (TP1), n = 8] or 1 h after [time point 2 (TP2), n = 8]. Plantaris (PLT) and tibialis anterior (TA) skeletal muscles were dissected and flash frozen in liquid nitrogen until processed and analyzed for gene expression and protein content.
Treadmill Acclimation Protocol
The treadmill was set at a 7% incline for all treadmill sessions. The animals were acclimated to the treadmill over a period of 10 days, consisting of four different sessions and separated by 1 day of rest (Supplemental Table S1; the online version of this article contains supplemental data.)
Incremental Treadmill Exercise Test
Forty-eight hours after the last acclimation session, animals from the exercise groups completed an incremental exercise test for assessment of their peak treadmill running speed. First, the mouse was placed for 2 min on a motionless treadmill with the test beginning at a speed of 6 m/min. The treadmill speed was then increased 3 m/min every 2 min until running ability was visually impaired, as evidenced by the animal sitting on the shock pad for more than 30 s. The speed of the last stage completed was recorded as the peak treadmill running speed.
Acute Treadmill Exercise Bout
Forty-eight hours after the incremental exercise test, the mice were exposed to 30 min of treadmill running at 65% [22.8 (3.6) m/min; mean (SD)] of their peak speed [34.7 (4.6) m/min] and were killed immediately (TP1; n = 8) or 1 h following (TP2; n = 8) the running bout.
Gene Expression
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Briefly, 1 μg of total RNA was reverse transcribed (Applied Biosystems, High-capacity cDNA kit, Carlsbad, CA), followed by PCR and gel electrophoresis using previously published primers (30, 47).
Immunoblotting Procedures
Total protein was extracted from skeletal muscle samples with lysis buffer containing protease inhibitor cocktail [50 mM HEPES (pH 7.4), 0.1% Triton X-100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7·H2O, 100 mM glycerophosphate with a protease inhibitor cocktail, complete mini EDTA-free, Roche, Indianapolis, IN]. Protein concentration was determined with a commercially available bicinchoninic acid protein assay (Pierce, Rockford, IL). Thirty micrograms of total protein from each sample were separated on 7.5% SDS-PAGE gels, transferred onto polyvinylidene difluoride membranes (Immunobilon-P, Millipore, Billericka, MA) blocked in 5% nonfat dry milk for 30 min and exposed to primary antibodies overnight at 4°C as follows: phosphorylated p38 MAPK (Thr180/Tyr182, Cell Signaling 9211, 1:500) and phosphorylated ERK1/2 (p44 ERK1/ p42 ERK2, Thr202/Tyr204, Cell Signaling 9101, 1:1,000). For total protein isoforms and GAPDH analyses, membranes from phosphorylated immunoblots were stripped and reprobed with the following antibodies for total proteins: total p38 MAPK (Cell Signaling 9212, 1:500), total ERK1/2 (Cell Signaling 9102, 1:1,000), and GAPDH (Cell Signaling, Danvers, MA, 14C10 rabbit MAb no. 2118, 1: 1,000). Products were visualized using enhanced chemiluminescence on the Gene Gnome (Syngene Bio Imaging). Band intensities were analyzed by densitometry using Image J software (NIH, Betesda, MD).
In Vitro Study
Myoblasts (C2C12, ATCC CRL-1772, Manassas, VA) were seeded onto six-well plates (Greiner bio-one 657 160, Frickenhausen, Germany) and cultured in growth media (10% FBS, Gibco 10082–189, Carlsbad, CA), 1% penicillin and streptomycin (Invitrogen Carlsbad, CA, 15070–063, 5,000 units penicillin, 5,000 μg streptomycin) in DMEM, (ATCC 30–2002, Manassas, VA) for 48 h before being switched to differentiation media, which contained 2% horse serum (ATCC 30–2040, Manassas, VA) and 1% penicillin and streptomycin in DMEM for 72 h. After cells were confirmed to be differentiated (visual confirmation of myotube formation, data not shown), myotubes were treated for various time periods with calcium ionophore (A23187, 1 μM, Sigma-Aldrich, St. Louis, MO, C7522 in ethanol) or in combination with a pharmacological inhibitor of p38 MAPK [10 μM; this inhibitor binds to the ATP binding pocket and does not directly alter the phosphorylation status of p38 MAPK (16) at Thr180-X-Tyr182; SB-202190, Sigma S7067 in DMSO]. Gene expression and immunoblotting were performed as above.
Statistical Analysis
Gene expression targets were normalized to the reference gene Gapdh and then expressed relative to the BL group. Gapdh and GAPDH were not different between groups and thus, in our hands, was a sufficient loading control and normalization gene (data not shown). All values are presented as means ± SE. One-way ANOVA was performed with a post hoc analysis using Tukey's honestly significant difference, with significance accepted at P ≤ 0.05.
RESULTS
In Vivo Shelterin Gene Expression Results
PLT.
To test the hypothesis that acute exercise would decrease shelterin gene expression levels, we measured Trf1, Trf2, Pot1a, and Pot1b in PLT at BL, TP1, and TP2. Compared with BL, Trf1 gene expression was significantly decreased at TP1 (P = 0.02). BL and TP2 Trf1 gene expression was not different (P = 0.07), whereas TP1 and TP2 Trf1 gene expression was similar (P = 0.58; Fig. 1A). Trf2 gene expression was not different at TP1 compared with BL (P = 0.10) and was not different between any other groups (Fig. 1B). Pot1a and Po1tb gene expression were not different from BL at either time point (Fig. 1, C and D, respectively). We measured peroxisome proliferator-activated receptor gamma co-activator-1 alpha (Ppargc1alpha) gene expression. BL and TP1 animals were similar, while TP2 resulted in an increase that was not statistically significant at TP2 (Fig. 1E). Other studies have found increases in Ppargc1alpha to occur at later time points (i.e., 2 h or more) following treadmill running (28, 45).
Fig. 1.

Acute exercise reduced shelterin component gene expression in the plantaris. A: Trf1 gene expression was significantly reduced in time point 1 (TP1) animals compared with baseline (BL) animals (P = 0.02), tended to remain reduced at time point 2 (TP2) (P = 0.07), and was not different between TP1 and TP2 (P = 0.58). *TP1 significantly different than BL (P < 0.05). B: Trf2 mRNA expression tended to decrease in TP1 animals compared with BL (P = 0.1), but was not different between BL and TP2 (P = 0.3) or between TP1 and TP2 (P = 0.5). C and D: Pot1a and Po1tb mRNA expression, respectively, was not different between any groups (P = 0.3 and P = 0.4, respectively). E: Pgc1a was not different between BL and TP1 or TP2 (P = 0.4 for both), but tended to be higher at TP2 compared with TP1 (P = 0.08). Values are means ± SE. mRNA abundance was assessed with RT-PCR, corrected for Gapdh, and expressed relative to the BL group. BL, n = 6; TP1, animals killed immediately following acute exercise bout, n = 8; TP2, animals killed 1 h after the acute exercise bout, n = 8. See text for definition of genes.
TA.
To investigate if shelterin gene response was muscle specific, we measured mRNA expression in the TA muscle. No significant changes in gene expression were observed for shelterin components Trf1, Pot1a, or Pot1b (Fig. 2, A, C, and D, respectively) in the TA. Gene expression of Trf2 in TP1 animals was significantly higher compared with that for TP2 animals (P = 0.03, Fig. 2B), was not different from BL animals (P = 0.08), and was similar between TP2 and BL animals (P = 0.80). To assess the animal's response to treadmill running, we measured Ppargc1alpha. BL and TP1 animals were similar, while TP2 animals had significantly greater Ppargc1alpha gene expression compared with TP1 animals (Fig. 2E; P < 0.05).
Fig. 2.
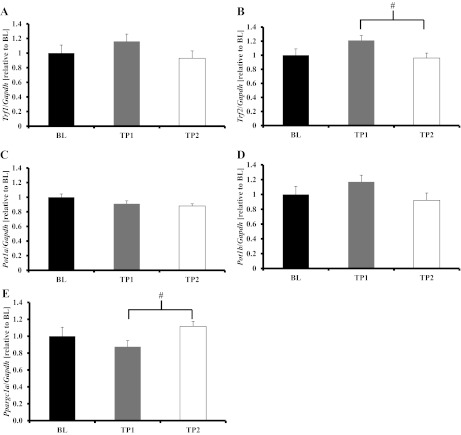
Gene expression of shelterin components in tibialis anterior (TA) muscle following acute exercise. A: Trf1 gene expression was not different in TP1 or TP2 animals compared with BL animals (P = 0.3 and P = 0.7, respectively) and was not different between TP1 and TP2 (P = 0.1). B: Trf2 gene expression was higher in TP1 compared with TP2 animals and tended to be higher than BL (P = 0.03 and P = 0.08, respectively), but was similar between BL and TP2 (P = 0.8). C and D: Pot1a and Po1tb mRNA expression, respectively, was not different between any groups (P = 0.2 and P = 0.2, respectively). E: Pgc1a gene expression was not different in BL compared with TP1 and TP2 (P = 0.8), but TP2 had significantly higher expression compared with TP1 (P = 0.02). Values are means ± SE. mRNA abundance was assessed with RT-PCR, corrected for Gapdh, and expressed relative to the BL group. BL, n = 6; TP1, n = 8; TP2, n = 8. #TP1 significantly different than TP2 (P < 0.05).
In Vivo MAPK Results
PLT.
We hypothesized that the stress-responsive MAPK protein family could be associated with altered levels of shelterin gene expression; thus we measured the phosphorylation status of ERK1/2 and p38 in skeletal muscles. Phosphorylated levels of ERK1 or ERK2 were not different between any groups (P = 0.50 and P = 0.80, respectively, Fig. 3A). Phosphorylated levels of p38 MAPK were significantly higher in the TP1 group compared with the BL animals (P = 0.03, Fig. 3B), but were not different compared with TP2 (P = 0.20). No differences were detected between BL and TP2 (P = 0.30).
Fig. 3.

p38 MAPK is activated following acute treadmill exercise in the plantaris. We measured the phosphorylation status of the MAPKs ERK1/2 (A) and p38 (B) following acute treadmill exercise. ERK1/2 was not different among any groups (ERK1 p44, P = 0.6 and ERK2 p42, P = 0.8). p38 MAPK had significantly increased phosphorylation at TP1 compared with BL (P = 0.03), TP1 and TP2 were similar (P = 0.2), as were BL and TP2 (P = 0.3). Densitometric analysis and representative immunoblot images (right) are shown. Values are means ± SE. Phosphorylated (p)-to-total (t) protein content ratios were derived and then expressed relative to the BL group for comparisons. GAPDH was a loading reference. BL, n = 6; TP1, n = 8; TP2, n = 8. *TP1 significantly different than BL (P < 0.05).
TA.
In the TA, phosphorylated levels of ERK1 were not different between any time points; however, phosphorylated levels of ERK2 were significantly higher in TP1 animals compared with TP2 (P = 0.04), but not different than BL animals (P = 0.08, Fig. 4A). No differences were detected between BL and TP2. No differences were observed in the phosphorylation status of p38 MAPK for any time point (Fig. 4B).
Fig. 4.

p38 MAPK is not activated following acute treadmill exercise in the TA. A: ERK1 (p44) phosphorylation was not significantly altered in the TA due to treadmill exercise (P = 0.4). ERK2 (p42) phosphorylation was greater at TP1 compared with TP2 (P = 0.04) and tended to be greater than BL (P = 0.08), but BL and TP2 were similar (P = 0.8). #Significantly different than TP2 (P < 0.05). B: p38 MAPK phosphorylation was not significantly altered by treadmill exercise (P = 0.6). Densitomeric analysis and representative immunoblot images (right) are shown. Values are means ± SE. Phosphorylated (p)-to-total (t) protein content ratios were derived and then expressed relative to the BL group for comparisons. GAPDH was a loading reference. BL, n = 6; TP1, n = 8; TP2, n = 8.
In Vitro Results
Due to the critical importance of Trf1 in telomere length regulation and homeostasis (26, 43), we conducted cell culture experiments in an attempt to delineate the signaling mechanisms that regulate Trf1 gene expression in skeletal muscle. Our intent with the in vitro experiments was not to mimic exercising muscle, but to delineate the importance of p38 MAPK to Trf1 gene regulation. The purpose of these experiments in C2C12 murine skeletal muscle cells was to activate the same signaling pathway that was activated following exercise and observe if Trf1 gene expression changes in a similar fashion. Previous experiments have indicated that p38 MAPK activation is induced by increased calcium levels in the skeletal muscle cell (49), and activated p38 MAPK can influence telomere biology by altering the expression of shelterin components Trf1 and Trf2 (44). Thus we treated C2C12 myotubes with calcium ionophore (A23187) to induce increases in intracellular calcium levels, as previously described (4, 5, 17, 18, 24) and then determined Trf1 gene expression. Treatment with 1 μM A23187 induces an approximately fourfold increase in cytosol calcium levels in skeletal muscle myotubes (37). Treatment with A23187 for 24 h resulted in a significant decrease in Trf1 gene expression (P = 0.003, Fig. 5). We then determined that 3 h was the shortest period of exposure to A23187 that significantly increased p38 MAPK phosphorylation (P = 0.02, Fig. 6), which returned to near BL levels 24 h posttreatment. To determine whether p38 MAPK regulates Trf1 gene expression, we inhibited p38 MAPK activity with SB-202190, as previously described (19, 34). Treatment of the C2C12 myotubes with A23187 resulted in a significant decrease in Trf1 gene expression, which was completely prevented by inhibition of p38 MAPK with SB-202190 (P < 0.001, Fig. 7).
Fig. 5.
Calcium ionophore stimulation in C2C12 myotubes results in downregulation of Trf1 gene expression. Myotubes after 72 h of differentiation were treated with calcium ionophore (A23187, 1 μM) for 24 h to induce a similar reduction in Trf1 gene expression, as observed in vivo following treadmill running. All experiments were performed in duplicate on at least 3 separate occasions, in serial passage. Values are means ± SE. mRNA abundance of Trf1 and Gapdh were assessed with RT-PCR, corrected for Gapdh, and expressed relative to the BL group. *A23187 treated significantly different than BL (ethanol, P < 0.05).
Fig. 6.
Calcium ionophore stimulation results in p38 MAPK phosphorylation in C2C12 myotubes. To determine whether calcium ionophore treatment resulted in activation/phosphorylation of p38 MAPK, myotubes were treated for 3 h with 1 μM A23187 or control, and protein was isolated 30 min and 24 h following treatment. Calcium ionophore treatment resulted in significant phosphorylation of p38 MAPK compared with BL. Densitometric analysis (top) and representative immunoblot images (bottom) are shown. Values are means ± SE. Phosphorylated-to-total protein content ratios were derived and then expressed relative to the BL group for comparisons. GAPDH was a loading reference. BL, vehicle treated; A23187, treated for 3 h, and protein isolated 30 min posttreatment; 24H post, myotubes treated for 3 h, protein isolated 24 h posttreatment. All experiments were performed in duplicate on at least 3 separate occasions, in serial passage. *A23187 significantly different than BL (P < 0.05). #A23187 significantly different than 24H post (P < 0.05).
Fig. 7.
Inhibition of p38 MAPK prevents reduction of Trf1 gene expression. To determine whether p38 MAPK activation was directly related to Trf1 gene expression, we treated myotubes with calcium ionophore and in combination with p38 MAPK inhibitor SB-202190 (note: this inhibitor does not affect phosphorylation at Thr180-X-Tyr182, but inhibits p38 activity by competitively binding to the ATP binding site). Myotubes were treated with control (vehicle), calcium ionophore (A23871, 1 μM), or pretreated with SB-202190 p38 MAPK inhibitor (10 μM) for 30 min before 24 h of treatment with A23187. Treatment with p38 MAPK inhibitor prevented calcium ionophore-induced decrease in Trf1 gene expression. All experiments were performed in duplicate on at least 3 separate occasions. Values are means ± SE. mRNA abundance of Trf1 and Gapdh was assessed with RT-PCR, corrected for Gapdh, and expressed relative to the BL group. BL, vehicle treated (DMSO and ETOH); A23187, ionophore treated; SB-202190, myotubes treated in combination with A23187 and p38 MAPK inhibitor. *A23187 significantly different than BL. #A23187 significantly different than SB-202190.
DISCUSSION
Our laboratory has previously determined that long-term voluntary wheel-running exercise in mice resulted in telomere shortening and unique gene expression of shelterin in skeletal muscle (30). The purpose of the present research was to delineate the effects of an acute bout of exercise on the skeletal muscle shelterin protein complex and to begin to elucidate mechanisms regulating gene expression patterns of the shelterin complex. We describe, for the first time, that acute exercise reduces the gene expression of a key shelterin component, Trf1. Our data also indicate that Trf1 mRNA expression is regulated by calcium-related activation of p38 MAPK in skeletal muscle. Understanding how telomere length and shelterin are regulated in a tissue such as skeletal muscle, where telomere length does not decrease with aging, provides novel insights into telomere biology.
Chronic exercise training slows age-related telomere shortening in several tissues (9, 30, 31, 46, 47). A recent study by our group indicates that long-term exercise training results in shorter skeletal muscle telomere lengths compared with young and age-matched sedentary animals (30). These results are supported by two cross-sectional studies in humans, indicating a similar tendency (11, 39), but no longitudinal study in human skeletal muscle has been performed to date. In our previous work, we also found that chronic voluntary exercise (1 yr) attenuated an age-induced increase in mouse skeletal muscle TRF1 protein content, suggesting that exercise may negatively regulate Trf1 gene expression (30). Based on those results, it could be interpreted that the telomere complex in skeletal muscle is regulated in a unique fashion compared with other tissues (30).
Evidence from in vitro studies of mouse and human cancer cell lines indicate that TRF1 is a negative regulator of telomere length (43). When TRF1 is overexpressed in vivo in epithelial cells, telomere shortening occurs, as would be predicted from the in vitro studies (35). However, this overexpression is not telomere protective, but rather is detrimental to the telomere and results in end-to-end fusions, chromosomal aberrations, and telomere recombination (35). This is contrasted by data from tissue-specific knockout models of TRF1, showing no detectable telomere shortening, but other telomere dysfunctional phenotypes (33). Combined, these data indicate the TRF1 levels and likely the entire shelterin complex are tightly regulated in a cell-type and tissue-specific fashion; thus determination of the function and regulation of TRF1 in vivo in multiple tissues is important.
For example, Werner et al. (47) found, in cardiac muscle, that wheel running for 21 days in mice resulted in increased Trf1 gene expression and no telomere length changes compared with sedentary mice in cardiac tissue. To the best of our knowledge, only three other studies have examined Trf1 gene expression in skeletal muscle, where it was determined that Duchenne's muscular dystrophy patients have increased Trf1 protein expression compared with controls [along with shorter telomeres (1)], and that multiple consecutive exercise bouts resulted in no changes in Trf1 in highly trained individuals (27). More recently, Ponsot et al. (38) found in skeletal muscle that telomerase and shelterin components were increased and telomere length maintained in skeletal muscle of patients with two different inflammatory diseases. This indicates that shelterin is tightly regulated, depending on the stimulus and the cellular context of that stimulus making understanding the gene expression (mRNA) regulation of shelterin components vital in health and disease states related to telomere length. These findings, in combination with our own training data (30), led us to examine the role of acute exercise as a means to identify potential signals that may alter Trf1 gene expression.
Numerous cellular signaling pathways are activated by contraction of skeletal muscle, including the MAPK pathway (8). Due to the unique shelterin gene expression response observed in exercised and aged skeletal muscle (30), we hypothesized that shelterin gene expression may be a stress response induced by the initial bouts of unaccustomed exercise in untrained individuals. Thus we targeted the MAPK signaling pathway, as numerous studies have shown that components of this family are induced by external stresses, and recent evidence in cardiomyocytes has linked MAPK signaling proteins to regulation of shelterin gene expression (44). We observed that an acute bout of treadmill running resulted in enhanced phosphorylation of p38 MAPK in the PLT muscle, which was associated with a decrease in Trf1 mRNA expression. The activation of p38 MAPK is known to occur in response to muscle contraction, and the amount of activation is dependent on the type, time, and intensity of the contractions (41). We saw increased phosphorylation of p38 in the PLT, but not the TA muscle. We speculate that this is a response to the treadmill running, since previous studies have shown greater neuromuscular activation of the PLT in rodents compared with the TA (42, 50). These preliminary results give us a good idea of what pathways are important in the gene regulation of Trf1 in rodent skeletal muscle following exercise. When viewed in conjunction with our laboratory's previous longitudinal findings (30), we hypothesize that the initial bouts of exercise training, the onset of a stress response, may reduce TRF1 protein levels and lead to reduced protection of telomeres, potentially contributing to an eventual telomere shortening response detected in individuals training for a long period of time.
Previously, in vitro studies have found that elevations in intracellular calcium levels can activate p38 MAPK (36, 49), and intracellular resting calcium levels are known to be elevated in fatigued skeletal muscle (10). Thus elevations in intracellular calcium may act as an upstream signal, resulting in reduced Trf1 mRNA expression. Based on prior work (17), we exposed cultured C2C12 myotubes to A21387 to increase intracellular calcium (37) and observed a significant increase in p38 MAPK phosphorylation. Similar to our in vivo results, we found that, when p38 MAPK was phosphorylated, there was a subsequent decrease in Trf1 mRNA content. To determine whether p38 MAPK was critical for the A21387-induced reduction of Trf1 gene expression, we inhibited p38 MAPK activity using SB-202190, which prevented the reduction in Trf1 gene expression under these conditions. By doing the in vitro inhibitor experiments, we were able to show a direct link between the activation of the pathway and changes in Trf1 gene expression. Further experiments in rodent models would be needed, and eventually an experiment with humans involving acute and longitudinal exercise measuring Trf1 gene expression would be needed. These data indicate a mechanistic linkage between p38 MAPK and Trf1 gene expression and support our in vivo observations that p38 MAPK activation may be regulating the reduced Trf1 gene expression. These data are in agreement with previous results from Spallarossa et al. (44), who observed decreased Trf1 gene expression when treating cardiomyocytes with doxorubicin, which resulted in simultaneous increases in phosphorylation of p38 MAPK. Our results provide a rationale for future experiments to test possible mechanisms by which long-term aerobic exercise training results in shortened telomeres in skeletal muscle.
Our data indicate that contraction/exercise-dependent decreases in Trf1 gene expression may be the result of p38 MAPK activation. We speculate that intracellular elevations in calcium may be an important signal in this activation, although it is equally possible that other signals could also activate p38 MAPK during exercise (e.g., reactive oxygen species). We hypothesize that physiological stressors (e.g., repeated bouts of exercise), such as chronic endurance training, whereby each bout results in transient decreases in mRNA expression of Trf1 could over time lead to reduced TRF1 protein content. We further hypothesize that there is a threshold of contractile stimuli that could result in either activation of gene expression (observed response in TA) or gene repression (observed response in PLT), indicating unique regulation dependent on the level of stress signaling for shelterin components. Activation of stress signaling in skeletal muscle may repress gene expression of Trf1 initially, and, following rest, expression returns to BL or homeostasis. Thus, with Trf1, exercise-induced p38 activation reduces gene expression, and following rest and reduced activation (by TP2) Trf1 expression returns to similar levels as BL. Over time, (i.e., with repeated exercise bouts), a significant reduction of TRF1 protein could lead to altered telomere length homeostasis and a loss of telomere length in chronically trained skeletal muscles, which is supported by our laboratory's previous work (30). These results combined suggest that repeated bouts of exercise would result in decreased Trf1, which, over time, could lead to telomere shortening in trained individuals, suggesting unique regulation of mouse skeletal muscle Trf1 in response to exercise. While the data from Laye et al. (27) showed that endurance exercise should maintain or increase Trf1 gene expression, one should consider the fact that the authors investigated individuals with a long history of endurance training (e.g., marathon running) and did not compare subjects to a body mass, composition, or age-matched group. Thus the findings from Laye et al. (27) cannot be compared directly to our present results, but rather should be interpreted as how Trf1 gene expression responds to extreme acute exercise in highly trained individuals. The present study investigated the gene expression response of Trf1 in untrained animals and showed different responses between muscle groups likely dependent on neuromuscular activation during exercise.
Several limitations to our investigation should be noted. First, we did not assess the protein levels of TRF1 in our tissues, nor did we measure telomere length as a functional measure of TRF1. These variables were not central to our hypothesis or aims, which were to delineate potential mechanisms controlling mRNA gene expression of Trf1, rather than to delineate short-term protein changes that are unlikely to impact short-term changes in telomere length. Telomere length would not be expected to change in response to such a short exercise bout, and any such change is not likely physiologically important or even detectable with the present methods. Second, we did not assess the full MAPK family of proteins, omitting JNK due to the fact that this pathway is related to extreme physiological stress (i.e., marathon running), rather than more typical exercise-related stress. Also, the type of muscle contraction plays a role in the activation of MAPK signaling molecules. Fatiguing muscle contractions activate JNK (48); since our animals were not exercised to fatigue, we focused our investigation on p38 and ERK rather than on JNK. While we did observe several tendencies, our sample size was sufficient to detect a significant difference for Trf1 gene expression and was sufficiently powered to detect a 30% difference for other targets. Finally, we were unable to conclusively determine which p38 MAPK isoform is responsible for the observed changes. Nevertheless, these data provide the first evidence of skeletal muscle Trf1 regulation by p38 MAPK in response to an acute bout of exercise.
In summary, these results represent a first step in understanding the mechanism behind how skeletal muscle telomere length is reduced in chronically trained skeletal muscle (rodent or human). We provide insight into how unaccustomed exercise may initially result in telomere de-protection (i.e., downregulation of Trf1) and thus lead to unrepaired telomere DNA damage and telomere length loss. Future studies are needed to determine the timeline of de-repression of Trf1 gene expression in response to exercise training, the timing and mechanism of telomere shortening in response to exercise training, and the physiological and cellular consequences of having short telomeres in skeletal muscles.
GRANTS
This work was supported by the Department of Kinesiology Graduate Research Initiative Fund and National Institutes of Health predoctoral training Grant AG000268 (A. T. Ludlow) and by Brazilian CAPES Foundation PDEE scholarship BEX1714090 (L. C. J. Lima).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.T.L., E.E.S., and S.M.R. conception and design of research; A.T.L., L.C.J.L., J.W., E.D.H., and L.M.G. performed experiments; A.T.L., L.C.J.L., and E.D.H. analyzed data; A.T.L., E.D.H., E.E.S., and S.M.R. interpreted results of experiments; A.T.L., L.M.G., and S.M.R. prepared figures; A.T.L. and L.C.J.L. drafted manuscript; A.T.L., L.C.J.L., J.W., E.D.H., L.M.G., E.E.S., and S.M.R. edited and revised manuscript; A.T.L., L.C.J.L., J.W., E.D.H., L.M.G., E.E.S., and S.M.R. approved final version of manuscript.
REFERENCES
- 1. Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Rodolico C, Di Giorgio RM, Vita G. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging 32: 2190–2197, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem 277: 45323–45330, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM. Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol 298: R198–R210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579: 859–862, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol 3: 640–649, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chambers MA, Moylan JS, Smith JD, Goodyear LJ, Reid MB. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol 587: 3363–3373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 168: 154–158, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chin ER, Balnave CD, Allen DG. Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol Cell Physiol 272: C550–C559, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Collins M, Renault V, Grobler LA, St. Clair Gibson A, Lambert MI, Wayne Derman E, Butler-Browne GS, Noakes TD, Mouly V. Athletes with exercise-associated fatigue have abnormally short muscle DNA telomeres. Med Sci Sports Exerc 35: 1524–1528, 2003 [DOI] [PubMed] [Google Scholar]
- 12. de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol 75: 167–177, 2010 [DOI] [PubMed] [Google Scholar]
- 13. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19: 2100–2110, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 8: 1429–1438, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, De Vivo I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol 175: 414–422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 23: 40–45, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Freyssenet D, Di Carlo M, Hood DA. Calcium-dependent regulation of cytochrome c gene expression in skeletal muscle cells. Identification of a protein kinase c-dependent pathway. J Biol Chem 274: 9305–9311, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Freyssenet D, Irrcher I, Connor MK, Di Carlo M, Hood DA. Calcium-regulated changes in mitochondrial phenotype in skeletal muscle cells. Am J Physiol Cell Physiol 286: C1053–C1061, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Geiger PC, Wright DC, Han DH, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 288: E782–E788, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Ghosh A, Rossi ML, Aulds J, Croteau D, Bohr VA. Telomeric D-loops containing 8-oxo-2′-deoxyguanosine are preferred substrates for Werner and Bloom syndrome helicases and are bound by POT1. J Biol Chem 284: 31074–31084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb Symp Quant Biol 71: 225–229, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell 97: 503–514, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Hockemeyer D, Daniels JP, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell 126: 63–77, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 284: C1669–C1677, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Kadi F, Ponsot E, Piehl-Aulin K, Mackey A, Kjaer M, Oskarsson E, Holm L. The effects of regular strength training on telomere length in human skeletal muscle. Med Sci Sports Exerc 40: 82–87, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Karlseder J, Kachatrian L, Takai H, Mercer K, Hingorani S, Jacks T, de Lange T. Targeted deletion reveals an essential function for the telomere length regulator Trf1. Mol Cell Biol 23: 6533–6541, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laye MJ, Solomon TP, Karstoft K, Pedersen KK, Nielsen SD, Klarlund Pedersen B. Increased shelterin mRNA expression in peripheral blood mononuclear cells and skeletal muscle following an ultra-long-distance running event. J Appl Physiol 112: 773–781, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294: E463–E474, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Ludlow AT, Roth SM. Physical activity and telomere biology: exploring the link with aging-related disease prevention. J Aging Res 2011: 790378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ludlow AT, Witkowski S, Marshall MR, Wang J, Lima CJ, Guth LM, Spangenburg EE, Roth SM. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J Gerontol A Biol Sci Med Sci 67: 911–926, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc 40: 1764–1771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinez P, Thanasoula M, Carlos AR, Gomez-Lopez G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG, Tarsounas M, Blasco MA. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat Cell Biol 12: 768–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev 23: 2060–2075, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298: C542–C549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Munoz P, Blanco R, de Carcer G, Schoeftner S, Benetti R, Flores JM, Malumbres M, Blasco MA. TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol Cell Biol 29: 1608–1625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J 17: 675–681, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol Endocrinol Metab 283: E1040–E1045, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Ponsot E, Echaniz-Laguna A, Delis AM, Kadi F. Telomere length and regulatory proteins in human skeletal muscle with and without ongoing regenerative cycles. Exp Physiol 97: 774–784, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Rae DE, Vignaud A, Butler-Browne GS, Thornell LE, Sinclair-Smith C, Derman EW, Lambert MI, Collins M. Skeletal muscle telomere length in healthy, experienced, endurance runners. Eur J Appl Physiol 109: 323–330, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 1: 132–139, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60: 145–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol 70: 2522–2529, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20: 1659–1668, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A, Brunelli C. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol 297: H2169–H2181, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Spangenburg EE, Brown DA, Johnson MS, Moore RL. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem 332: 225–231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120: 2438–2447, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Bauersachs J, Thum T, Pfreundschuh M, Muller P, Haendeler J, Bohm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol 52: 470–482, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem 107: 171–178, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282: 18793–18799, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20: 6414–6423, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]





