Abstract
Exchange transfusion of large volumes of hemoglobin (Hb)-based oxygen carriers can protect the brain from middle cerebral artery occlusion (MCAO). Hb in the carboxy state (COHb) may provide protection at relatively low volumes by enhancing vasodilation. We determined whether transfusion of rats with 10 ml/kg PEGylated COHb [polyethylene glycol (PEG)-COHb] at 20 min of 2-h MCAO was more effective in reducing infarct volume compared with non-carbon monoxide (CO) PEG-Hb. After PEG-COHb transfusion, whole blood and plasma COHb was <3%, indicating rapid release of CO. PEG-COHb transfusion significantly reduced infarct volume (15 ± 5% of hemisphere; mean ± SE) compared with that in the control group (35 ± 6%), but non-CO PEG-Hb did not (24 ± 5%). Chemically dissimilar COHb polymers were also effective. Induction of MCAO initially produced 34 ± 2% dilation of pial arterioles in the border region that subsided to 10 ± 1% at 2 h. Transfusion of PEG-COHb at 20 min of MCAO maintained pial arterioles in a dilated state (40 ± 5%) at 2 h, whereas transfusion of non-CO PEG-Hb had an intermediate effect (22 ± 3%). When transfusion of PEG-COHb was delayed by 90 min, laser-Doppler flow in the border region increased from 57 ± 9 to 82 ± 13% of preischemic baseline. These data demonstrate that PEG-COHb is more effective than non-CO PEG-Hb at reducing infarct volume, sustaining cerebral vasodilation, and improving collateral perfusion in a model of transient focal cerebral ischemia when given at a relatively low dose (plasma Hb concentration < 1 g/dl). Use of acellular Hb as a CO donor that is rapidly converted to an oxygen carrier in vivo may permit potent protection at low transfusion volumes.
Keywords: carbon monoxide, cerebral blood flow, pial artery, rat, stroke
hemoglobin (hb)-based oxygen carriers have been investigated for use in ischemic conditions. Exchange transfusion of cell-free Hb with subunit cross-linking to stabilize the tetramer has been reported to reduce infarct volume from experimental focal cerebral ischemia (6–8). Large transfusion volumes and high concentrations of cross-linked tetrameric Hb were most effective (5). More recently, polymers of tetrameric Hb, which are less likely to extravasate and scavenge nitric oxide surrounding vascular smooth muscle (16), have been studied in focal cerebral ischemia. Exchange transfusion of a zero-link Hb (ZL-Hb) polymer was more effective at a 6% concentration than at a 3% concentration in reducing infarct volume (17). In addition, the ZL-Hb polymer and recombinant Hb polymers (2, 19) were more effective than cross-linked tetrameric Hb in reducing infarct volume (17). To achieve the minimal effective plasma Hb concentration of ∼2 g/dl in these studies, an exchange transfusion was used. However, an exchange transfusion would be difficult to implement for early clinical use in stroke patients; a simple infusion without removal of blood (topload transfusion) would be preferable. Thus designing an oxygen therapeutic with enhanced efficacy would be advantageous.
Polymerization is one means of limiting extravasation. Another strategy is conjugation of the Hb tetramer with side chains of polyethylene glycol (PEG) to enlarge the molecular radius. PEGylated Hb also has reduced antigenicity and an increased circulating half-life. However, because of its increased oncotic pressure, it is generally transfused at a Hb concentration of 4%. Unexpectedly, we found that a topload transfusion with only 10 ml/kg of a 4% solution of PEGylated Hb during 2 h of middle cerebral artery (MCA) occlusion (MCAO) markedly reduced infarct volume in rats at 1 day of reperfusion, despite achieving a plasma Hb concentration ([Hb]) of only 0.6 g/dl (12). However, this Hb was transfused in the carboxy state because storage in this state reduces autooxidation to methemoglobin (metHb) and thereby extends the shelf life. This finding raised the possibility that carbon monoxide (CO) released from the transfused Hb may enhance its efficacy in reducing injury from ischemic stroke. In support of this possibility, transfusion of PEGylated Hb bound with CO (COHb) (PEG-COHb) before coronary artery occlusion was found to be more efficacious in reducing myocardial infarct size than transfusion of PEG-Hb without CO (23).
Because CO is capable of dilating pial arteries (9), one way by which transfusion of PEG-COHb may be beneficial during MCAO is by increasing cerebral vasodilation and improving collateral blood flow. Previous work demonstrated that pial arterioles in the distal MCA region near the anterior cerebral artery border region dilate after the onset of MCAO, but that the dilation gradually subsides over a 2-h period (4). Exchange transfusion with ZL-Hb without COHb did not prevent this loss of dilation.
The present study had four aims. First, we examined whether topload transfusion of PEG-COHb during MCAO was more efficacious in reducing cerebral infarct size than transfusion of PEG-Hb without CO. Moreover, we extended the duration of reperfusion from 1 day, as used in the previous study (12), to 3 days to ensure that tissue salvage was sustained. Second, we determined whether an effect of Hb transfusion in the carboxy state was specific for PEG-COHb by evaluating the effect of topload transfusion of the chemically dissimilar ZL-Hb polymer in the carboxy state (ZL-COHb). Third, we determined whether topload transfusion of PEG-COHb at 20 min of MCAO better preserved pial arterioles in the dilated state throughout 2 h of MCAO compared with transfusion of PEG-Hb. Fourth, we determined whether delaying transfusion by 90 min, when pial arterioles are no longer fully dilated, improved perfusion in the ischemic border region.
METHODS
Surgical preparation.
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Male Wistar rats (250–350 g) were anesthetized initially with 5% isoflurane and maintained with ∼2% isoflurane during surgery. Isoflurane concentration was decreased to ∼1.5% after surgery. Inspired O2 was ∼25–30%. In survival studies for infarct volume determination, isoflurane was administered via nose cone with spontaneous ventilation. In nonsurvival studies of pial arteriole diameter, the lungs were mechanically ventilated via a tracheostomy. In all experiments, a femoral vein was catheterized for transfusion, and a femoral artery was catheterized for measuring arterial blood pressure and sampling arterial blood. Laser-Doppler flowmetry was used to assess the adequacy of MCAO. A small burr hole was made in the lateral parietal bone until only a thin amount of bone remained, and a 1-mm-diameter fiber-optic probe was secured against the thinned bone. Rectal temperature was maintained at ∼37°C with a heating lamp throughout the surgical procedure, MCAO, and early reperfusion.
The intraluminal filament technique was used to produce 2 h of MCAO (1, 12). The right common carotid artery was exposed through a lateral incision and occluded. The right external carotid artery was ligated, the occipital artery branch of the external carotid artery was coagulated, and the proximal pterygopalatine artery branch of the right internal carotid artery was ligated. A 4–0 monofilament nylon suture with a rounded tip was advanced ∼2 cm into the internal carotid artery. The filament position was adjusted to produce at least a 60% reduction in the laser-Doppler flux (LDF) signal. To permit reperfusion, the intraluminal suture was withdrawn. In survival studies, catheters were removed, incisions were closed with suture, and anesthesia was discontinued after 30 min of reperfusion.
Mean arterial blood pressure (MABP), the percent change in LDF, and rectal temperature were recorded at 15-min intervals during MCAO. Arterial blood (∼0.7 ml) was analyzed for pH, Pco2, and Po2 on a blood-gas analyzer (ABL80, Radiometer, Copenhagen, Denmark) and for Hb concentration, O2 saturation, metHb, and COHb on a hemoximeter (OSM3, Radiometer). Plasma from the samples was analyzed for Hb concentration.
Modified Hbs.
Purified bovine Hb was conjugated with 5,000-molecular weight residues of PEG on surface lysine residues (20) and stored in either the deoxy state (PEG-Hb) or CO-bound state (PEG-COHb) in phosphate-buffered saline as a 4% Hb solution at ∼4°C (Prolong Pharmaceuticals, South Plainfield, NJ). This product has a P50 (Po2 at 50% oxyhemoglobin saturation) of ∼11 mmHg.
Adipyl-cross-linked tetrameric Hb was produced as previously described (3, 14). Purified bovine Hb was treated with the cross-linking reagent bis(3,5-dibromosalicyl)-adipate in 0.5 M borate buffer at pH 9.0 in the oxygenated state. The reaction produces one tetramer component with cross-links across the two β-chains and another tetramer component with cross-links across the α- and β-chains. Each cross-linked component displays cooperativity and low O2 affinity (14). For infusion solutions, the purified tetramers were pasteurized and transferred into a lactated Ringer solution at 6% concentration by dialysis.
To produce ZL-Hb polymers, the bovine adipyl-cross-linked tetramers underwent additional treatment with the polymerization reagent 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, as previously described (16). This reaction produces covalent amide linkages between adjacent tetramers without binding the chemical linker and is thus defined as a zero-link polymer. The pasteurized product was filtered to remove molecular mass species <300 and >14,000 kDa and transferred into a lactated Ringer solution at 6% concentration by dialysis, whereupon it was sterilized by passage through a 0.45-μm filter and treated to remove any endotoxin. The product was stored at −80°C in either the oxy state (ZL-Hb) or carboxy state (ZL-COHb). ZL-Hb has a P50 of 4 mmHg and no cooperativity.
The various Hb solutions were transfused intravenously at a rate of 0.5 ml/min. Unless otherwise noted, the volume of the topload transfusion was 10 ml/kg body wt, and the transfusion occurred over ∼6 min. Control groups underwent no transfusion or were transfused with a 5% solution of human serum albumin.
Infarct volume.
At 3 days, the brain was harvested for measurement of infarct volume in a group that was not transfused (n = 10), and in groups that were transfused with PEG-Hb (n = 10), PEG-COHb (n = 9), albumin (n = 10), ZL-Hb (n = 10), and ZL-COHb (n = 10) solutions. Seven coronal sections (2 mm thick) were stained with a 1% solution of triphenyltetrazolium chloride. The pale, nonviable areas were measured on the anterior and posterior surfaces of each section. The infarct volume of each section was calculated from the average of the infarct area on the anterior and posterior surfaces and the section thickness. The total infarct volume was obtained by summing the volume from each section. To correct for swelling, we multiplied the infarct volume by the ratio of the total contralateral volume to total ipsilateral volume.
Pial arteriole diameter.
Closed cranial windows were constructed as previously described (4). In brief, a plastic ring with inlet and outlet ports was cemented to the skull around a 3- to 4-mm-diameter craniotomy and filled with artificial cerebrospinal fluid. The dura was gently retracted, and the ring was sealed with a glass coverslip. Through other side ports in the plastic ring, fluid pressure in the window was monitored, and fluid temperature in the window was measured with a thermistor.
The diameter of pial arterioles in the distal MCA territory near the anterior cerebral artery territory was measured at three to six sites. The percent change in diameter from baseline was calculated for each site, and the percent changes were averaged for each rat. In one experiment in which MCAO was not induced, the diameter response was measured at 15-min intervals after topload transfusion with 10 ml/kg solutions of 5% albumin (n = 7), 4% PEG-Hb (n = 7), or 4% PEG-COHb (n = 7). In another experiment, the diameter response was measured at 15-min intervals during 2 h of MCAO. Groups included no transfusion (n = 10) and topload transfusion with 10 ml/kg solutions of 5% albumin (n = 7), 4% PEG-Hb (n = 7), 4% PEG-COHb (n = 7), or 6% adipyl-cross-linked tetrameric Hb (n = 5). The transfusions were carried out 20 min after the onset of MCAO.
Blood flow in the ischemic border region.
To determine whether delaying transfusion increased blood flow in the ischemic border region, we measured LDF at a site 4 mm caudal and 3 mm lateral from bregma. LDF was also measured at a second site (0 mm rostral and 10 mm lateral from bregma) that represents the cortical ischemic core. A topload transfusion of 10 ml/kg albumin (n = 8), PEG-Hb (n = 9), and PEG-COHb (n = 7) was performed at 90 min of MCAO. Blood flow at 120 min of MCAO was compared with that at 90 min of MCAO.
Statistical analysis.
Infarct volume was analyzed by two-way ANOVA, with transfusion treatment as a between-subject factor and the seven coronal sections as a within-subject factor (as the infarct area at each coronal level will be correlated within subjects). Comparisons with the control group that did not receive transfusion were made at each coronal level and for total infarct volume with a Bonferroni correction. Arterial blood values, MABP, and LDF were compared among groups at each time point by one-way ANOVA and the Newman-Keuls multiple-range test. In the pial arteriole diameter experiments, the percent change in diameter was analyzed by two-way ANOVA with repeated measures. If a significant effect of treatment or interaction with time occurred, comparisons among groups were made with the Newman-Keuls multiple-range test. To analyze the effect of delayed transfusion on LDF, we compared LDF values at 90 and 120 min of MCAO by paired t-tests. The family-wise error rate was set at 0.05 in all tests. Data are presented as means ± SE.
RESULTS
COHb dynamics.
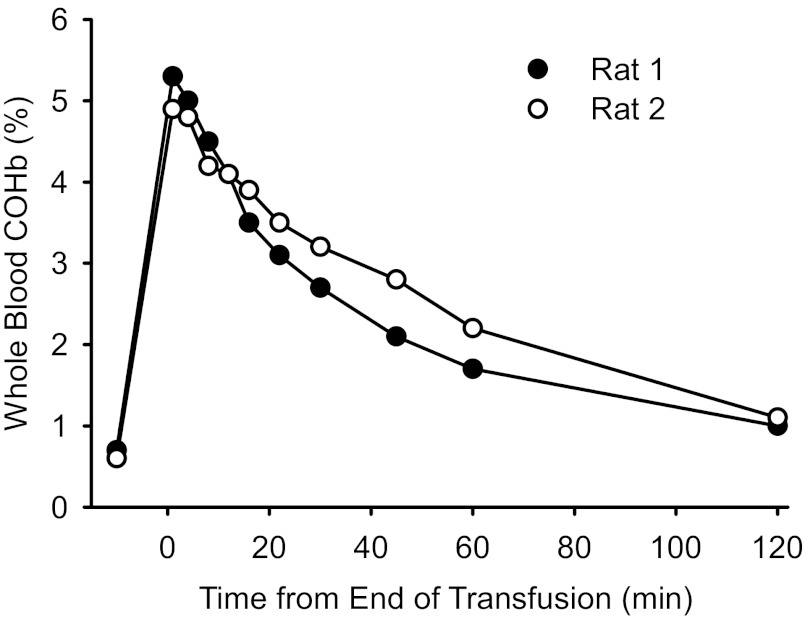
We obtained serial arterial blood samples from two unanesthetized rats to assess the time course of COHb after topload transfusion with a 4% PEG-COHb solution. We used a transfusion volume of 20 ml/kg, twice that used in the remaining experiments, to increase the sensitivity of the measurements at low COHb values. The duration of transfusion was 6 min. The transfusion produced little change in MABP (from 89 to 89 mmHg in the first rat and from 83 to 85 mmHg in the second rat). Whole blood COHb increased to 5.3 and 4.9% in the two rats at 1 min after the transfusion was complete and then decreased exponentially to 1.0 and 1.1%, respectively, by 2 h (Fig. 1). Blood samples were centrifuged for 3 min, and COHb was measured in the plasma. Plasma COHb values in the samples drawn at 1 min after transfusion were 6.6 and 4.9% in the two rats, and the plasma [Hb] was 0.9 and 1.0 g/dl, respectively. Although some exchange of CO between plasma-based Hb and red blood cell (RBC)-based Hb can occur ex vivo during centrifugation, the results indicate that the exchange of CO between compartments occurs within several minutes. This analysis is consistent with results seen after in vitro mixing of blood and PEG-COHb (23).
Fig. 1.
Time course of the percentage of carboxy-hemoglobin (COHb) in arterial blood and plasma after topload transfusion of two unanesthetized rats with 20 ml/kg polyethylene glycol (PEG)-COHb. Time at 0 min denotes the end of the 6-min transfusion.
Infarct volume.
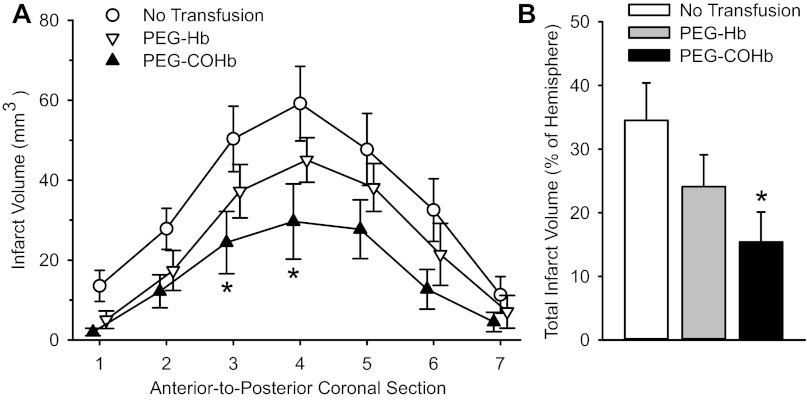
Topload transfusion of rats with 10 ml/kg PEG-Hb solution (without CO) or PEG-COHb solution at 20 min of a 2-h period of MCAO increased plasma [Hb] to 0.7 ± 0.1 g/dl in both groups. Infarct volume was measured at 3 days of reperfusion and was compared with that in a nontransfused control group. Analyses of infarct volume in individual coronal sections and in the total hemispheric infarct volume both indicated significant reductions in the group transfused with PEG-COHb (Fig. 2). Values in the group transfused with PEG-Hb were not significantly different from those in the control group.
Fig. 2.
Infarct volume in seven individual coronal sections (A) and total infarct volume (B) in nontransfused rats (n = 10) or rats transfused with 10 ml/kg PEG-Hb (n = 10) or PEG-COHb (n = 9) at 20 min of a 2-h middle cerebral artery occlusion (MCAO). Values are means ± SE. *P < 0.05 from the nontransfused control group.
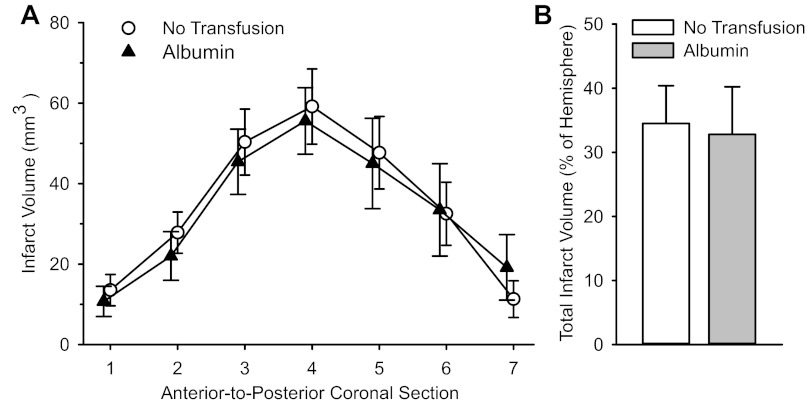
To determine whether the blood volume effect of transfusion contributed to the reduction in infarct volume, we compared a group transfused with albumin to the nontransfused control group. Infarct volumes were nearly the same at individual coronal levels and for the total hemisphere between the two groups (Fig. 3). A similar lack of effect was previously observed with topload transfusion of PEGylated albumin (12).
Fig. 3.
Infarct volume in seven individual coronal sections (A) and total infarct volume (B) in nontransfused rats (n = 10) or rats transfused with 10 ml/kg albumin (n = 10) at 20 min of a 2-h MCAO. Values are means ± SE. No significant differences were present between groups.
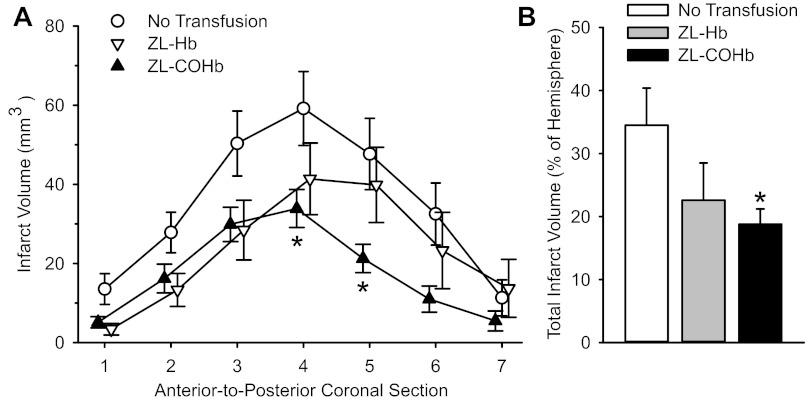
To determine whether the beneficial effect of transfusing COHb was specific for PEGylated Hb, we also tested the effect of transfusing polymeric Hb with and without COHb. Topload transfusion of ZL-COHb at 20 min of MCAO significantly decreased infarct volume at some of the individual coronal levels and in the total hemisphere compared with those in the nontransfused control group (Fig. 4). Transfusion of ZL-Hb without CO did not produce statistically significant reductions in infarct volume.
Fig. 4.
Infarct volume in seven individual coronal sections (A) and total infarct volume (B) in nontransfused rats (n = 10) or rats transfused with 10 ml/kg zero-link (ZL)-Hb (n = 10) or ZL-COHb (n = 10) at 20 min of a 2-h MCAO. Values are means ± SE. *P < 0.05 from the nontransfused control group.
At 3 days of reperfusion, the volume of the ischemic hemisphere was larger than the nonischemic hemisphere. However, the percent swelling in the control (10.9 ± 2.3%) and albumin (9.9 ± 2.0%) groups was not significantly different from that in the ZL-Hb (7.9 ± 1.8%) and ZL-COHb (6.3 ± 1.0%) groups or from the PEG-Hb (7.7 ± 1.6%) and PEG-COHb (7.6 ± 1.0%) groups. Because the circulating half-lives of these acellular Hbs are <1 day in the rat, plasma oncotic effects of transfusion on cerebral edema should have largely dissipated, and the nonsignificant but numerically smaller swelling 3 days after Hb transfusion might be secondary to smaller volumes of injured tissue with cytotoxic edema.
MABP was not significantly different among the groups transfused with albumin and PEG-COHb and the nontransfused group during MCAO and early reperfusion (Fig. 5A). MABP in the PEG-Hb group was significantly greater than that in the non-transfused group at 30, 60, and 75 min after MCAO and at 5 min of reperfusion, but the values were not different from those in groups transfused with albumin or PEG-COHb. MABP in the groups transfused with ZL-Hb and ZL-COHb was not different from MABP in the groups that received no transfusion or transfusion with albumin during MCAO (Fig. 5B). MABP in the ZL-COHb group was significantly greater than that in the nontransfused group at 5, 15, and 30 min after reperfusion, but these values were not significantly different from those in groups transfused with albumin or ZL-Hb. In addition, LDF measured over parietal lateral cortex, representing the primary MCA distribution area, decreased to a similar extent throughout MCAO in the groups transfused with PEG-Hb and PEG-COHb (Fig. 5C) and in the groups transfused with ZL-Hb and ZL-COHb (Fig. 5D) compared with the groups with no transfusion and albumin transfusion. After reperfusion, LDF recovered to a similar extent in all groups. Thus the severity of the insult in the ischemic core was equivalent among groups.
Fig. 5.
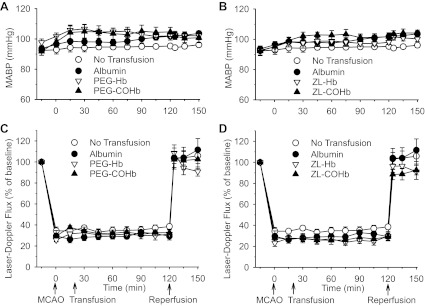
Mean arterial blood pressure (MABP; A and B) and laser-Doppler flux (C and D) during 120 min of MCAO and 30 min of reperfusion in nontransfused rats (n = 10); rats transfused with 10 ml/kg albumin (n = 10), PEG-Hb (n = 10), or PEG-COHb (n = 9) at 20 min of MCAO (A and C); or rats transfused with ZL-Hb (n = 10) or ZL-COHb (n = 10) at 20 min of MCAO (B and D). Values are means ± SE. MABP in the PEG-Hb group was significantly greater than that in the nontransfused group at 30, 60, 75, and 125 min after MCAO. MABP in the ZL-COHb group was significantly greater than that in the nontransfused group at 125, 135, and 150 min after MCAO (5, 15, and 30 min of reperfusion). Laser-Doppler flux was not different among groups.
Arterial pH, Pco2, Po2, and rectal temperature were similar among groups during MCAO and 30 min of reperfusion (Table 1). Although the topload transfusion produced small decreases in arterial hematocrit, Hb concentration, and O2 content in all of the transfused groups, values were not significantly different from those in the nontransfused group (Table 2). As expected, COHb was elevated at 90 min after MCAO and 30 min of reperfusion in the groups transfused with PEG-COHb and ZL-COHb. Values of metHb remained <2% in all groups.
Table 1.
Arterial pH, blood gases, and rectal temperature of rats during 120 min of middle cerebral artery occlusion and 30 min of reperfusion (150 min after middle cerebral artery occlusion)
| No Transfusion | Albumin | PEG-Hb | PEG-COHb | ZL-Hb | ZL-COHb | |
|---|---|---|---|---|---|---|
| pH | ||||||
| Baseline | 7.42 ± 0.01 | 7.43 ± 0.01 | 7.45 ± 0.01 | 7.42 ± 0.01 | 7.41 ± 0.01 | 7.40 ± 0.02 |
| 90 min | 7.42 ± 0.01 | 7.40 ± 0.01 | 7.42 ± 0.01 | 7.41 ± 0.02 | 7.39 ± 0.01 | 7.40 ± 0.01 |
| 150 min | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.40 ± 0.01 | 7.39 ± 0.02 | 7.37 ± 0.01 | 7.38 ± 0.01 |
| Pco2, Torr | ||||||
| Baseline | 41 ± 1 | 43 ± 2 | 40 ± 2 | 43 ± 2 | 43 ± 1 | 45 ± 2 |
| 90 min | 44 ± 1 | 45 ± 2 | 46 ± 2 | 45 ± 2 | 47 ± 2 | 46 ± 1 |
| 150 min | 49 ± 1 | 48 ± 1 | 48 ± 2 | 45 ± 2 | 48 ± 2 | 49 ± 2 |
| Po2, Torr | ||||||
| Baseline | 146 ± 5 | 147 ± 5 | 153 ± 7 | 143 ± 5 | 147 ± 4 | 142 ± 5 |
| 90 min | 140 ± 5 | 148 ± 5 | 150 ± 6 | 143 ± 8 | 136 ± 8 | 137 ± 6 |
| 150 min | 132 ± 6 | 147 ± 7 | 140 ± 8 | 135 ± 6 | 136 ± 7 | 147 ± 6 |
| Temperature, °C | ||||||
| Baseline | 36.8 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.7 ± 0.2 | 36.9 ± 0.1 |
| 90 min | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 |
| 150 min | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.3 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 |
Values are means ± SE (n = 9–10 per group). PEG, polyethylene glycol; Hb, hemoglobin; COHb, carboxyhemoglobin; ZL-Hb, zero-link hemoglobin; ZL-COHb, zero-link carboxyhemoglobin. Measurements were made in rats that received no transfusion or topload transfusion of albumin, PEG-Hb, PEG-COHb, ZL-Hb, or ZL-COHb solution. There were no significant differences among groups at any time point.
Table 2.
Hemoglobin analysis of arterial whole blood in rats during 120 min of middle cerebral artery occlusion and 30 min of reperfusion
| No Transfusion | Albumin | PEG-Hb | PEG-COHb | ZL-Hb | ZL-COHb | |
|---|---|---|---|---|---|---|
| Hematocrit, % | ||||||
| Baseline | 36 ± 1 | 38 ± 1 | 37 ± 1 | 38 ± 1 | 38 ± 1 | 38 ± 1 |
| 90 min | 36 ± 1 | 36 ± 1 | 34 ± 1 | 36 ± 1 | 37 ± 1 | 37 ± 1 |
| 150 min | 38 ± 1 | 37 ± 1 | 35 ± 1 | 36 ± 1 | 37 ± 1 | 37 ± 1 |
| Hemoglobin, g/dl | ||||||
| Baseline | 12.9 ± 0.2 | 13.2 ± 0.4 | 13.5 ± 0.2 | 13.4 ± 0.4 | 13.6 ± 0.2 | 13.3 ± 0.2 |
| 90 min | 12.4 ± 0.2 | 12.2 ± 0.4 | 12.1 ± 0.2 | 12.8 ± 0.3 | 12.6 ± 0.2 | 13.0 ± 0.3 |
| 150 min | 12.0 ± 0.2 | 12.1 ± 0.4 | 12.3 ± 0.1 | 12.6 ± 0.3 | 12.2 ± 0.2 | 12.3 ± 0.2 |
| O2 content, ml O2/dl | ||||||
| Baseline | 17.4 ± 0.2 | 17.7 ± 0.5 | 18.1 ± 0.3 | 17.6 ± 0.6 | 18.2 ± 0.3 | 17.5 ± 0.3 |
| 90 min | 16.8 ± 0.3 | 16.4 ± 0.5 | 16.2 ± 0.2 | 16.7 ± 0.4 | 16.9 ± 0.2 | 17.1 ± 0.4 |
| 150 min | 16.6 ± 0.4 | 16.7 ± 0.4 | 16.7 ± 0.3 | 16.8 ± 0.4 | 16.8 ± 0.3 | 17.1 ± 0.3 |
| COHb, % | ||||||
| Baseline | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| 90 min | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 1.8 ± 0.2* | 1.2 ± 0.2 | 2.0 ± 0.1† |
| 150 min | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.5 ± 0.2* | 1.0 ± 0.2 | 1.7 ± 0.1† |
| MetHb, % | ||||||
| Baseline | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.1 |
| 90 min | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 |
| 150 min | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.6 ± 0.2‡ | 1.4 ± 0.1 |
Values are means ± SE (n = 9–10 per group). Measurements were made in rats that received no transfusion or topload transfusion of albumin, PEG-Hb, PEG-COHb, ZL-Hb, or ZL-COHb solution.
P < 0.001 from no transfusion, albumin, and PEG-Hb groups.
P < 0.001 from no transfusion, albumin, and ZL-Hb groups.
P < 0.05 from albumin group. MetHb, methemoglobin.
Pial arteriole diameter.
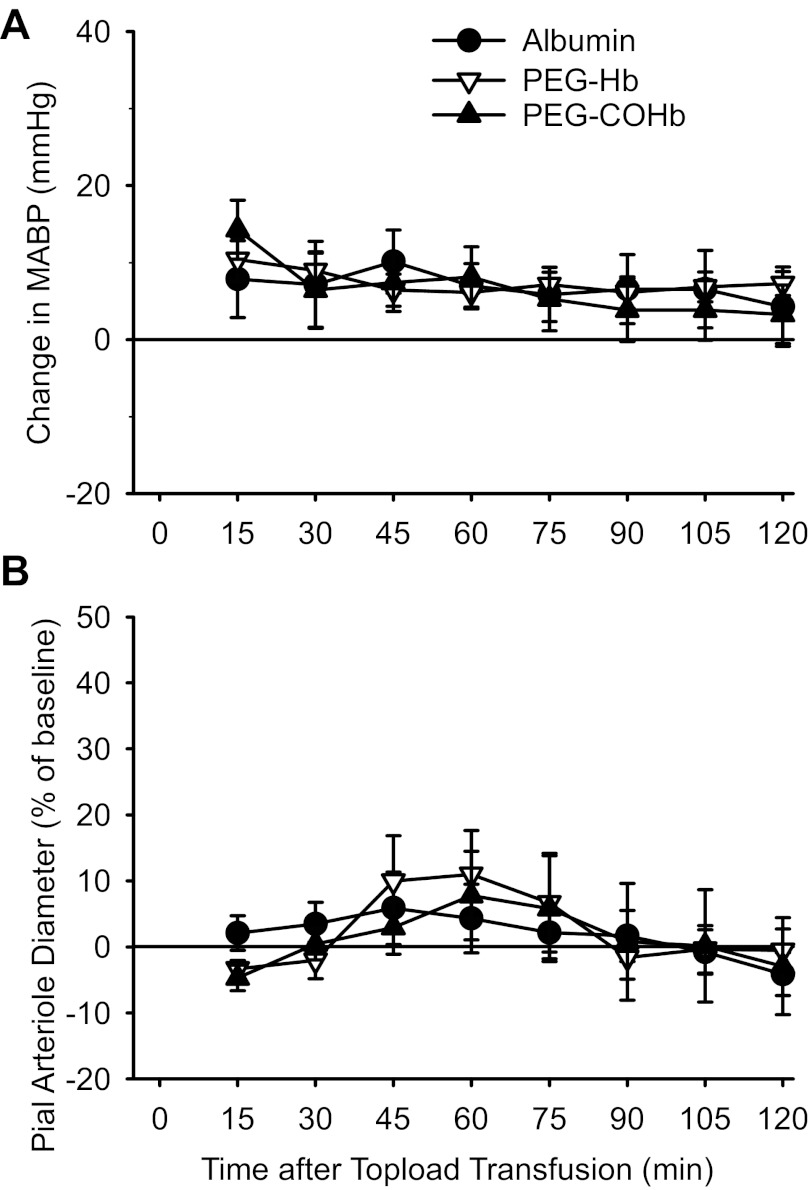
Topload transfusion of 10 ml/kg albumin, PEG-Hb, and PEG-COHb in the absence of ischemia produced small increases in arterial pressure, but these changes were not significantly different among groups at any time point (Fig. 6A). In addition, no major differences in arterial Pco2, hematocrit, or [Hb] were present among groups after transfusion, whereas whole blood COHb was elevated after transfusion of PEG-COHb (Table 3). Analysis of the percent change in diameter over time after transfusion (Fig. 6B) by two-way ANOVA with repeated measures did not indicate a significant overall effect of the infusion solution (P = 0.98) or interaction of the infusion solution with time (P = 0.73). Thus PEG-Hb and PEG-COHb transfusion did not produce significant vasoconstriction or vasodilation in the pial circulation in the absence of ischemia.
Fig. 6.
Change in MABP from pretransfusion baseline (A) and percent change in pial arteriole diameter (B) in rats without induced ischemia after 10 ml/kg topload transfusion with albumin, PEG-Hb, and PEG-COHb. Values are means ± SE (n = 7 per group).
Table 3.
Arterial blood analysis in nonischemic rats with cranial windows for pial arteriole diameter measurements after topload transfusion of albumin, PEG-Hb, and PEG-COHb solutions
| Albumin | PEG-Hb | PEG-COHb | |
|---|---|---|---|
| Pco2, Torr | |||
| Baseline | 42 ± 1 | 44 ± 1 | 40 ± 1* |
| 5 min | 43 ± 1 | 44 ± 1 | 43 ± 1 |
| 120 min | 40 ± 1 | 41 ± 1 | 39 ± 1 |
| Hematocrit, % | |||
| Baseline | 40 ± 1 | 37 ± 1 | 36 ± 1† |
| 5 min | 35 ± 1 | 34 ± 1 | 34 ± 1 |
| 120 min | 35 ± 1 | 35 ± 1 | 35 ± 1 |
| Hemoglobin, g/dl | |||
| Baseline | 13.1 ± 0.4 | 12.2 ± 0.4 | 11.8 ± 0.2† |
| 5 min | 12.0 ± 0.4 | 11.4 ± 0.5 | 11.3 ± 0.2 |
| 120 min | 11.7 ± 0.5 | 11.8 ± 0.4 | 12.0 ± 0.3 |
| COHb, % | |||
| Baseline | 1.0 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.3 |
| 5 min | 1.0 ± 0.4 | 1.2 ± 0.4 | 3.5 ± 0.5*† |
| 120 min | 0.8 ± 0.3 | 1.1 ± 0.5 | 2.1 ± 0.4† |
Values are means ± SE (n = 7 per group).
P < 0.05 from PEG-Hb group.
P < 0.05 from albumin group.
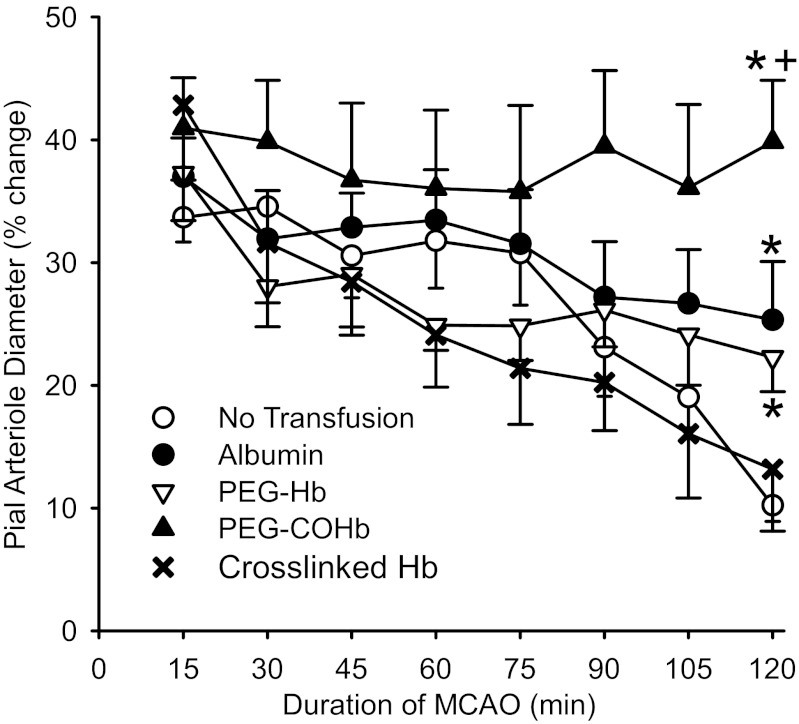
To determine whether these agents influence pial arteriole diameter during MCAO, we carried out topload transfusion at 20 min of MCAO. Upon initiation of MCAO, pial arterioles in the distal MCA territory dilated. Without subsequent transfusion, the dilation subsided over a 2-h period (Fig. 7). With transfusion of albumin or PEG-Hb solution, the diameter response became significantly greater than that in the group without transfusion at 2 h of MCAO. With transfusion of the PEG-COHb solution, the dilation seen initially after MCAO was sustained throughout the 2 h of MCAO, and the dilation was significantly greater than that in the other groups at 2 h of MCAO. In contrast, topload transfusion of adipyl-cross-linked tetrameric Hb solution failed to prevent the loss of pial arteriole dilation during MCAO (Fig. 7), despite producing an increase in MABP (Table 4). MABP and arterial Pco2 were not significantly different among the other groups. All groups remained well oxygenated without evidence of significant acidemia.
Fig. 7.
Percent change in pial arteriole diameter from preischemic baseline during 2 h of MCAO in groups of rats with no transfusion (n = 10) and rats transfused at 20 min of MCAO with 10 ml/kg albumin (n = 7), PEG-Hb (n = 7), PEG-COHb (n = 7), or adipyl-cross-linked Hb (n = 5). Values are means ± SE. *P < 0.05 from the nontransfused control group at that time point. +P < 0.05 from the albumin and PEG-Hb groups at that time point.
Table 4.
Arterial blood gases and blood pressure in rats with cranial windows for pial arteriole diameter measurements during 120 min of middle cerebral artery occlusion
| No Transfusion | Albumin | Crosslinked Hb | PEG-Hb | PEG-COHb | |
|---|---|---|---|---|---|
| pH | |||||
| Baseline | 7.41 ± 0.01 | 7.38 ± 0.01 | 7.39 ± 0.02 | 7.39 ± 0.01 | 7.39 ± 0.01 |
| 15 min | 7.41 ± 0.02 | 7.39 ± 0.01 | 7.39 ± 0.01 | 7.39 ± 0.01 | 7.40 ± 0.01 |
| 120 min | 7.40 ± 0.02 | 7.37 ± 0.01 | 7.43 ± 0.01* | 7.41 ± 0.01 | 7.43 ± 0.01* |
| Pco2, Torr | |||||
| Baseline | 39 ± 1 | 42 ± 1 | 40 ± 2 | 42 ± 1 | 41 ± 2 |
| 15 min | 39 ± 1 | 42 ± 1 | 38 ± 1 | 41 ± 1 | 41 ± 1 |
| 120 min | 39 ± 1 | 40 ± 1 | 38 ± 1 | 41 ± 1 | 38 ± 2 |
| Po2, Torr | |||||
| Baseline | 145 ± 3 | 130 ± 9 | 132 ± 11 | 122 ± 6 | 129 ± 10 |
| 15 min | 152 ± 5 | 130 ± 6 | 137 ± 9 | 123 ± 5† | 131 ± 9 |
| 120 min | 144 ± 3 | 132 ± 5 | 158 ± 6* | 137 ± 4 | 142 ± 6 |
| MABP, mmHg | |||||
| Baseline | 106 ± 2 | 106 ± 2 | 115 ± 6 | 101 ± 2 | 108 ± 1 |
| 15 min | 104 ± 2 | 107 ± 4 | 106 ± 5 | 103 ± 2 | 111 ± 5 |
| 120 min | 103 ± 2‡ | 111 ± 4‡ | 139 ± 13*† | 117 ± 5‡ | 116 ± 5‡ |
Values are means ± SE (n = 5–10 per group). MABP, mean arterial blood pressure.
P < 0.05 from albumin group.
P < 0.05 from no transfusion group.
P < 0.05 from crosslinked Hb group.
Blood flow in the ischemic border region.
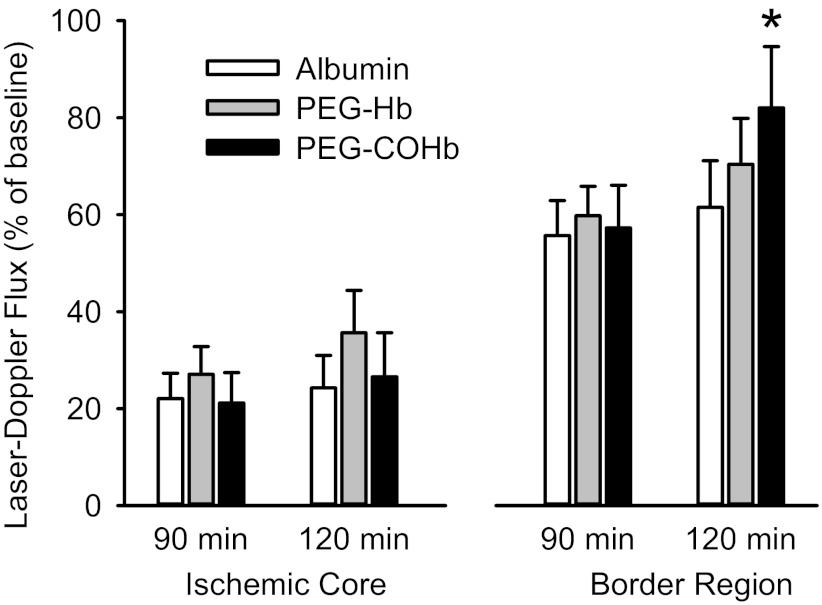
In another experiment, LDF was measured at two sites, and transfusion was delayed until 90 min of MCAO, at which time pial arteriole dilation had begun to subside in the nontransfused control rats. In the ischemic border region, LDF was not significantly changed at 120 min of MCAO compared with the pretransfusion values at 90 min of MCAO in groups transfused with albumin or PEG-Hb solutions (Fig. 8). However, in the group transfused with PEG-COHb, LDF was significantly increased from 57 to 82% of the preischemic baseline. LDF was also measured in the ischemic core. No significant changes in LDF occurred in the ischemic core after transfusion of any of the solutions. The latter findings are consistent with the LDF recordings in the ischemic core in the previous groups that were allowed to survive for 3 days (Fig. 5).
Fig. 8.
Percent change in laser-Doppler flux from preischemic baseline in the cortical ischemic core and border region at 90 and 120 min of MCAO in groups of rats transfused after 90 min of MCAO with 10 ml/kg albumin (n = 8), PEG-Hb (n = 9), or PEG-COHb (n = 7). Values are means ± SE. *P < 0.05 from value at 90 min within the same group.
DISCUSSION
This study demonstrated that topload transfusion of rats with 10 ml/kg of two types of cell-free Hb bound with CO produces more consistent reductions in infarct volume than does transfusion with the corresponding Hb lacking bound CO. The mechanism of protection may be multifactorial. Here, we showed that transfusion of PEG-Hb in the carboxy state at 20 min of MCAO better maintained dilation of pial arterioles in the MCA border region throughout 2 h of MCAO, and that delaying transfusion until 90 min of MCAO significantly improved perfusion in the border region. However, early or delayed transfusion did not substantially improve perfusion in the ischemic core. Thus one mechanism whereby transfusion of Hb in the carboxy state reduces infarct volume is promotion of vasodilation and collateral flow in the border region.
Because CO-bound Hb does not carry O2, the protective effect of COHb transfusion in an ischemic state appears counterintuitive. However, mixing PEG-COHb with blood samples ex vivo is known to produce a rapid equilibrium of CO between the compartments (23). Using a higher dose of 20 ml/kg PEG-COHb to better resolve the kinetics, we found that COHb rapidly fell from ∼80–90% in the stock solution to ∼5% in the plasma sample obtained 1 min after the 6-min transfusion was complete. This observation implies a large volume of CO distribution comprising RBC-based Hb, other heme proteins, and dissolved CO throughout the body. Whole blood COHb increased to a peak of 5% and then exponentially declined toward baseline levels over a 2-h period. The latter decline presumably reflects pulmonary clearance of CO. Thus the plasma-based COHb and RBC-based COHb appear to reach near equilibrium in a time scale of minutes. We could not determine a precise time from our data, because CO exchange between plasma and RBCs may have continued ex vivo, while the blood samples were being centrifuged. Nevertheless, with the 10 ml/kg dose used in the MCAO experiments, whole blood COHb remained <3%, thereby indicating that the O2-carrying capacity was not markedly impaired. Because of the slow pulmonary clearance of CO, RBC-based Hb and plasma-based Hb act as a reservoir to support an increase in the partial pressure of CO above normal in tissue for several hours after transfusion. In contrast with ventilation of external CO gas, transfusion of acellular COHb permits administration of a precise number of moles of CO.
Previous studies in which chemically modified and recombinant acellular Hb lacking bound CO were transfused after MCAO have demonstrated reductions in infarct volume (2, 6, 17, 19). However, those studies used exchange transfusion to achieve greater plasma [Hb] than those achieved in the present study. For example, exchange transfusion with a 6% solution of bovine ZL-Hb to achieve a plasma [Hb] of ∼2 g/dl during MCAO produced significant reductions in infarct volume, whereas transfusion with a 3% solution to achieve a plasma [Hb] of ∼1 g/dl did not produce significant reductions (17). Thus the lack of a statistically significant reduction with topload transfusion of 10 ml/kg of either the 6% non-CO ZL-Hb solution or the 4% non-CO PEG-Hb solution to achieve [Hb] of <1 g/dl was not unexpected. Higher concentrations of plasma [Hb] likely are necessary to optimize the benefit of plasma [Hb] to O2 delivery in the ischemic state. At low plasma [Hb], the benefit of unloading CO from the infused Hb to salvaging the ischemic border region can be more readily revealed. However, this argument does not exclude the possibility that, once CO is unloaded, the plasma oxyhemoglobin could still be assisting in oxygenating the ischemic border region. It should also be noted that the use of a simple topload transfusion would be easier to implement than the large volume exchange transfusion in a clinical trial.
One limitation of using cell-free Hb is that it can scavenge nitric oxide and produce vasoconstriction. In the present experiments, topload transfusion with the PEG-Hb and PEG-CO Hb solutions produced small increases in MABP. Because the increases were comparable to that obtained with the albumin solution, the increases likely were due largely to blood volume expansion. In the absence of MCAO, we observed no significant differences in pial arteriole diameter responses with PEG-Hb or PEG-COHb transfusion compared with albumin transfusion. Thus neither solution appears to exert strong vasoactive effects under normal conditions.
Endogenous CO produced by heme oxygenase dilates pial arteries of newborn piglets in response to a variety of stimuli, such as glutamate and hypoxia (15). Thus an increase in pial arteriole diameter might have been anticipated in response to exogenous administration of PEG-COHb in the absence of ischemia. However, systemic increases in CO may produce different responses than localized increases in endogenous CO. For example, ventilation with CO gas produces percent increases in cerebral blood flow that are inversely related to the percent COHb (13). Thus the systemic partial pressure of CO at a level of 3% COHb produced by transfusion of PEG-COHb in the present experiments would be expected to produce only minor increases in blood flow and pial arteriole diameter. In addition, the sensitivity of pial arteries to exogenous CO is less in adult rat than in newborn piglets (9).
In agreement with previous work (4), pial arterioles in the ischemic border region initially dilated after the onset of MCAO. During the subsequent 2 h of MCAO, however, the dilation subsided. Transfusion of the albumin or the PEG-Hb solution at 20 min of MCAO attenuated, but did not completely prevent, this loss of dilation. The simplest explanation for this moderate attenuation is that the topload transfusion produced a blood volume expansion that helped maintain the dilated state. In support of this possibility, our laboratory previously found no such attenuation in the loss of dilation after isovolumetric exchange transfusion with bovine ZL-Hb (4).
With transfusion of PEG-COHb, pial arteriole dilation was fully sustained throughout the 2 h of MCAO and was significantly greater than that obtained with either albumin or PEG-Hb solution. Thus CO released from PEG-COHb helps to maintain pial arterioles in the dilated state during ischemia, despite not producing dilation in the nonischemic state. Because previous work showed that the loss of dilation during MCAO could be prevented by administration of an endothelin A receptor antagonist (4), one possibility is that CO inhibits the release of endothelin during ischemia. CO is also capable of activating KCa channels, which would produce vasodilation (11).
A clinical trial of transfusion with a cross-linked tetrameric Hb solution in acute ischemic stroke failed to show a benefit (22). Exchange transfusion with a different cross-linked tetramer did not show an improvement in intraischemic blood flow within 2 h of MCAO in cats (21). Here, we show that adipyl-cross-linked Hb given as a topload did not prevent the loss of pial arteriole dilation. Thus PEG-COHb may be superior to the earlier generation of acellular Hb in sustaining vasodilation in the ischemic border region.
Although transfusion with PEG-COHb at 90 min of MCAO did not significantly increase perfusion in the ischemic core, it did increase perfusion that was measured over the ischemic border region where the reduction in LDF during MCAO was less severe than in the core. Presumably, this regional increase in LDF after 90 min of MCAO is the result of collateral arteriolar dilation at a time when dilation had subsided from that which initially occurred after the onset of MCAO. Because the development of infarction is associated with greater than 50% reductions in CBF (10), the observed increase in LDF to 82% of the preischemic value has the potential to salvage tissue in this region. Therefore, vasodilation is likely one of the mechanisms by which transfusion with PEG-COHb reduces infarct volume.
CO is also known to reduce oxidative stress, inflammation, and apoptosis in a variety of pathophysiological models in different organs (18). Ventilation with CO during reperfusion after MCAO can reduce infarct volume in the brain (25). In addition, ventilation with CO can be protective in permanent MCAO via a mechanism that requires the antioxidant transcription factor Nrf-2 (24). Thus other mechanisms may also have contributed to the reduction in infarct volume produced by transfusion with cell-free COHb in the present experiments.
In summary, topload transfusion of rats with 10 ml/kg cell-free Hb in the carboxy state resulted in the rapid exchange of CO with RBC-based Hb without causing a major negative impact on O2-carrying capacity. This fixed amount of CO delivery to the body helped to maintain pial arterioles in the ischemic border region in a vasodilated state as the duration of MCAO was prolonged to 2 h and was capable of improving perfusion in the border region to levels above that associated with infarct development. Vasodilation and possibly other factors contribute to a more robust reduction in infarct volume than that seen with non-CO-bound Hb. Therefore, maintaining the chemically modified Hb in the carboxy state not only prevents metHb formation during storage, but also provides greater protective properties in the setting of transient focal cerebral ischemia.
GRANTS
This study was supported by Grant NS38684 (R. C. Koehler) from the National Institute of Neurological Disorders and Stroke. PEGylated hemoglobin was produced under National Institutes of Health Fast-Track SBIR Contract HHSN268200800010C to Prolong Pharmaceuticals, Inc., and was provided for this study as a gift.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., S.C., H.K., and K.K.K. performed experiments; J.Z., S.C., D.C., K.K.K., and R.C.K. analyzed data; J.Z., S.C., H.K., D.C., K.K.K., and R.C.K. approved final version of manuscript; R.C.K. conception and design of research; R.C.K. interpreted results of experiments; R.C.K. prepared figures; R.C.K. drafted manuscript; R.C.K. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Claire F. Levine for editorial assistance.
REFERENCES
- 1. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke 29: 159–166, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Bobofchak KM, Mito T, Texel SJ, Bellelli A, Nemoto M, Traystman RJ, Koehler RC, Brinigar WS, Fronticelli C. A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am J Physiol Heart Circ Physiol 285: H549–H561, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bucci E, Gryczynski Z, Razynska A, Kwansa H. Entropy-driven intermediate steps of oxygenation may regulate the allosteric behavior of hemoglobin. Biophys J 74: 2638–2648, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao S, Wang LC, Kwansa H, Roman RJ, Harder DR, Koehler RC. Endothelin rather than 20-HETE contributes to loss of pial arteriolar dilation during focal cerebral ischemia with and without polymeric hemoglobin transfusion. Am J Physiol Regul Integr Comp Physiol 296: R1412–R1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole DJ, Drummond JC, Patel PM, Nary JC, Applegate RL. Effect of oncotic pressure of diaspirin cross-linked hemoglobin (DCLHb) on brain injury after temporary focal cerebral ischemia in rats. Anesth Analg 83: 342–347, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cole DJ, Drummond JC, Patel PM, Reynolds LR. Hypervolemic-hemodilution during cerebral ischemia in rats: effect of diaspirin cross-linked hemoglobin (DCLHb) on neurologic outcome and infarct volume. J Neurosurg Anesthesiol 9: 44–50, 1997 [PubMed] [Google Scholar]
- 7. Cole DJ, Schell RM, Drummond JC, Przybelski RJ, Marcantonio S. Focal cerebral ischemia in rats: effect of hemodilution with α-α cross-linked hemoglobin on brain injury and edema. Can J Neurol Sci 20: 30–36, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Cole DJ, Schell RM, Drummond JC, Reynolds L. Focal cerebral ischemia in rats. Effects of hypervolemic hemodilution with diaspirin cross-linked hemoglobin versus albumin on brain injury and edema. Anesthesiology 78: 335–342, 1993 [PubMed] [Google Scholar]
- 9. Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exp Biol Med (Maywood) 232: 1465–1469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacewicz M, Tanabe J, Pulsinelli WA. The CBF threshold and dynamics for focal cerebral infarction in spontaneously hypertensive rats. J Cereb Blood Flow Metab 12: 359–370, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97: 805–812, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klaus JA, Kibler KK, Abuchowski A, Koehler RC. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif Cells Blood Substit Immobil Biotechnol 38: 223–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koehler RC, Traystman RJ, Zeger S, Rogers MC, Jones MD., Jr Comparison of cerebrovascular response to hypoxic and carbon monoxide hypoxia in newborn and adult sheep. J Cereb Blood Flow Metab 4: 115–122, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Kwansa HE, Young AD, Arosio D, Razynska A, Bucci E. Adipyl crosslinked bovine hemoglobins as new models of allosteric systems. Proteins 39: 166–169, 2000 [PubMed] [Google Scholar]
- 15. Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol 301: H1–H11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol 93: 1479–1486, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Mito T, Nemoto M, Kwansa H, Sampei K, Habeeb M, Murphy SJ, Bucci E, Koehler RC. Decreased damage from transient focal cerebral ischemia by transfusion of zero-link hemoglobin polymers in mouse. Stroke 40: 278–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motterlini R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans 35: 1142–1146, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Nemoto M, Mito T, Brinigar WS, Fronticelli C, Koehler RC. Salvage of focal cerebral ischemic damage by transfusion of high O2-affinity recombinant hemoglobin polymers in mouse. J Appl Physiol 100: 1688–1691, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nho K, Glower D, Bredehoeft S, Shankar H, Shorr R, Abuchowski A. PEG-bovine hemoglobin: safety in a canine dehydrated hypovolemic-hemorrhagic shock model. Biomater Artif Cells Immobilization Biotechnol 20: 511–524, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Rebel A, Ulatowski JA, Joung K, Bucci E, Traystman RJ, Koehler RC. Regional cerebral blood flow in cats with cross-linked hemoglobin transfusion during focal cerebral ischemia. Am J Physiol Heart Circ Physiol 282: H832–H841, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, Stern KN, Koudstaal PJ. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 30: 993–996, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, Winslow RM. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol 154: 1649–1661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 42: 2605–2610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res 15: 33–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]