Abstract
We examined the impact of arousal state, sex, and obstructive sleep apnea (OSA) on the magnitude of progressive augmentation of the hypoxic ventilatory response and ventilatory long-term facilitation (vLTF). We also examined whether exposure to intermittent hypoxia during sleep has an impact on the apnea-hypopnea index (AHI) in individuals with OSA. Ten men and seven women with OSA, along with ten healthy men and ten healthy women, were exposed to twelve 2-min episodes of hypoxia (end-tidal Po2: 50 Torr) in the presence of sustained hypercapnia (end-tidal Pco2: 3 Torr above baseline), followed by a 30-min recovery period during wakefulness and sleep. The OSA participants completed an additional sham study during sleep. The AHI during the first hour of sleep following the intermittent hypoxia and sham protocols were compared. Progressive augmentation was only evident during wakefulness and was enhanced in the OSA participants. vLTF was evident during wakefulness and sleep. When standardized to baseline, vLTF was greater during wakefulness and was enhanced in the OSA group (men: wakefulness 1.39 ± 0.08 vs. sleep 1.14 ± 0.03; women: wakefulness 1.35 ± 0.03 vs. sleep 1.16 ± 0.05 fraction of baseline; P ≤ 0.001) compared with control (men: wakefulness 1.19 ± 0.03 vs. sleep 1.09 ± 0.03; women: wakefulness 1.26 ± 0.05 vs. sleep 1.08 ± 0.04 fraction of baseline; P ≤ 0.001). The AHI following exposure to intermittent hypoxia was increased (intermittent hypoxia 72.8 ± 7.3 vs. sham 56.5 ± 7.0 events/h; P ≤ 0.01). Sex-related differences were not observed for the primary measures. We conclude that progressive augmentation is not evident, and the magnitude of vLTF is diminished during sleep compared with wakefulness in men and women. However, when present, the phenomena are enhanced in individuals with OSA. The AHI data indicate that, under the prevailing experimental conditions, vLTF did not serve to mitigate apnea severity.
Keywords: men, women, wake, sleep, apnea-hypopnea index, intermittent hypoxia, carbon dioxide
exposure to acute intermittent hypoxia elicits two forms of respiratory motor plasticity: progressive augmentation of the hypoxic ventilatory response (HVR) and ventilatory long-term facilitation (vLTF) (11, 34, 42, 49, 54, 63, 77, 78, 80). The HVR is characterized by an increase in minute ventilation that occurs during exposure to hypoxia. During an intermittent hypoxia protocol, which is typically composed of a series of hypoxic episodes of short duration, minute ventilation gradually increases from the initial to the final hypoxic episode (21, 34, 63, 80). This enhancement of the HVR is characteristic of progressive augmentation. On the other hand, vLTF is characterized by a sustained proliferation of respiratory motor activity following exposure to intermittent hypoxia for up to 90 min after the final hypoxic stimulus is removed (39, 41, 42, 48). Both phenomena have been recorded in numerous animal models, including humans. However, variability with respect to the manifestation and magnitude of each phenomenon persists between and within species. Several factors, including sex, arousal state, and prior exposure to hypoxia, may lead to differences in the magnitude of progressive augmentation and vLTF in humans and other animals (see reviews, Refs. 41, 42). However, these possibilities have not been fully explored in humans. Variation in the magnitude of progressive augmentation and vLTF might be important because of the potential link between these phenomena and breathing stability or lack thereof in humans (41, 42). More specifically, enhancement of the HVR has been linked to breathing instability in individuals residing at high altitude (16) and in individuals suffering from sleep apnea (16). In contrast, it has been hypothesized that initiation of vLTF might promote breathing stability (41) (see discussion, Physiological Significance: Respiratory Plasticity and Breathing Events, for further elaboration).
Mitchell et al. suggested initially that LTF may be more robust in anesthetized or decerebrate animals compared with awake animals (48). This led to the hypothesis that the manifestation of vLTF is impacted by differences in arousal state (39). The rate of discharge of serotonergic neurons located in the raphe nucleus is considerably diminished during sleep compared with wakefulness, where the rate of discharge is close to maximal (25, 28, 29, 79). Since the release of serotonin from raphe neurons has been shown to be necessary in initiating vLTF (3, 36, 45, 47) and may have a role in the expression of progressive augmentation (5, 60), we speculated that arousal state-dependent differences in the dynamic range of activation of these neurons may impact on the manifestation of both phenomena in humans. The predominant finding in rat models is that the magnitude of vLTF is greater during sleep compared with wakefulness (51, 76). Studies investigating the effect of arousal state on progressive augmentation in rats or mice have not been completed to our knowledge. Similarly, no study has systematically examined the impact of arousal state on the magnitude of progressive augmentation and vLTF in humans. Nonetheless, a comparison of published results obtained from studies completed in humans during wakefulness or sleep (42) indicates that the magnitude of these responses is seemingly greater during wakefulness. However, because the studies differed on many levels (i.e., type of population, level of sustained carbon dioxide, number and duration of hypoxic episodes), it remains unclear if differences noted are related to arousal state or other factors related to the experimental design. Thus the present investigation was designed in part to investigate whether or not arousal level impacts on the magnitude of vLTF.
In addition to arousal state, sex may also have an impact on the manifestation of respiratory plasticity. Normal testosterone levels are required for LTF of phrenic and hypoglossal nerve activity in male rats, since decreased testosterone levels and gonadectomy diminish LTF (84). Likewise, LTF of both phrenic and hypoglossal nerve activity is reinstated following testosterone replacement in gonadectomized male rats (85). In females, LTF is impacted by the estrous cycle, and the magnitude is inversely related to the ratio of progesterone to estrogen (86). In humans, the magnitude of vLTF is not different in young healthy men and women during wakefulness (80). However, studies designed to explore sex differences in the manifestation of progressive augmentation and vLTF during sleep in healthy humans have not been previously completed. More importantly, differences between men and women with sleep apnea have not been explored during wakefulness or sleep. Exploring the possible differences within this population may be of interest, since sex-related variability in the initiation and maintenance of both progressive augmentation and vLTF could provide a plausible explanation for differences in apnea severity that exist between men and women with similar anthropometric characteristics (9). Examining differences between the sexes in both healthy individuals and individuals with sleep apnea will also allow us to determine whether the enhanced progressive augmentation and vLTF we observed in men with OSA during wakefulness (34) and attributed to chronic exposure to intermittent hypoxia is also evident in women with OSA during wakefulness and sleep.
Therefore, the purpose of our investigation was to determine the impact of arousal state, sex, and sleep-disordered breathing on the magnitude of progressive augmentation of the HVR and vLTF in humans. Based on previous studies in animals, we hypothesized that the magnitude of progressive augmentation of the HVR and vLTF would be reduced during wakefulness compared with sleep, but would be greater in OSA compared with healthy participants and in men compared with women.
METHODS
Protocol
The Institutional Review Boards of Wayne State University and John D. Dingell Veterans Affairs Medical Center approved the experimental protocol. Seventeen participants with obstructive sleep apnea (OSA) (10 men and 7 women), who were newly diagnosed, naive to treatment with continuous positive airway pressure, and otherwise healthy (i.e., absence of hypertension, diabetes, obesity, and abnormal lung function), completed the protocol. The protocol was also completed by 20 healthy participants (10 men and 10 women) who served as controls. In addition, three other participants were enrolled in the study, but did not complete the protocol. The data from these participants were not included in the analysis. The men and women within the OSA group were matched to control men and women on the basis of body mass index, age, and race. The control participants visited the laboratory on four occasions, whereas the OSA participants completed two additional visits (Fig. 1A).
Fig. 1.
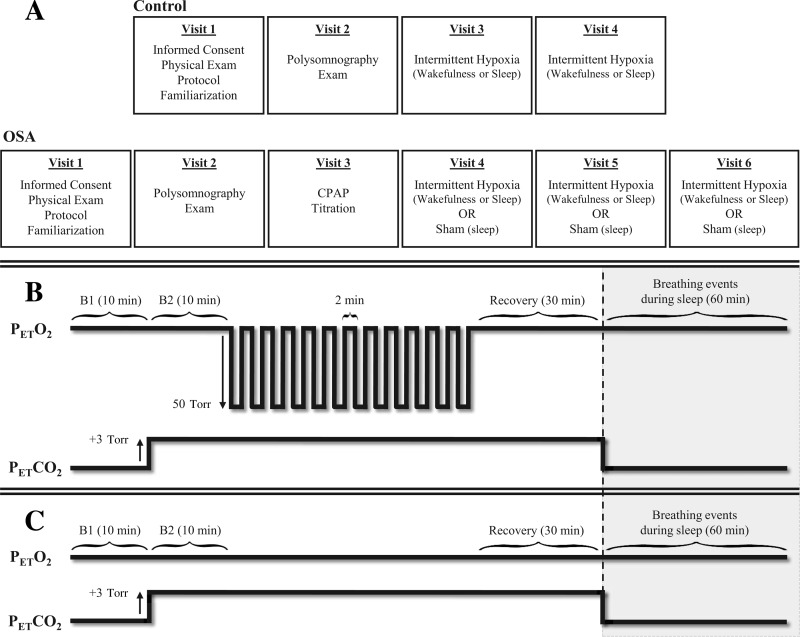
Experimental design and intermittent hypoxia and sham protocols. A schematic diagram shows the experimental design (A), and the intermittent hypoxia (B) and sham (C) protocols. Breathing events were recorded for 1 h following the intermittent hypoxia and sham protocol during sleep in the obstructive sleep apnea (OSA) participants. CPAP, continuous positive airway pressure; PetCO2, partial pressure of end-tidal carbon dioxide; PetO2, partial pressure of end-tidal oxygen; B1, initial baseline period measured under normoxic conditions; B2, second baseline period measured under hypercapnic conditions.
During the preliminary visit, the participants were screened to ensure that they were healthy, or in the case of the OSA participants, did not present other comorbid conditions. Once informed consent was obtained, blood pressure was measured, a 12-lead electrocardiogram (ECG) was completed, and a pulmonary function test was performed. All female participants were given a pregnancy test and were excluded from the study if the test revealed a positive result. Likewise, each participant completed health and lifestyle questionnaires and underwent a physical exam. Participants who met all inclusion criteria were then exposed to two 2-min episodes of hypoxia to achieve familiarization of the experimental protocol, as well as the experimental apparatus. On successful completion of the preliminary visit, the participants returned for a second visit to complete a baseline polysomnogram. The study was completed to establish the presence of sleep apnea or the lack thereof in the OSA and control participants, respectively. On an ensuing visit, the OSA participants were treated with continuous positive airway pressure (CPAP) during sleep to determine the therapeutic pressure required to maintain airway patency. Thereafter, the healthy and OSA participants were exposed to intermittent hypoxia on two occasions: once during wakefulness, and the other during sleep. The OSA participants completed an additional sham protocol during sleep. The hypoxic and sham studies were randomized, and each study was separated by at least 7 days to avoid any carry-over effect from previous studies. To remove the potential impact of circadian rhythms, all studies completed during wakefulness and sleep were conducted at the same time of day (6–7 AM). When studies were completed during sleep, the participants arrived at the laboratory at ∼4:30 AM. Participants were instructed not to sleep in the late evening/early morning hours before arrival, to ensure that sleep would be consolidated during the studies. A supine position was maintained by all participants throughout the studies. Participants were instructed to restrict food intake and consumption of caffeinated beverages 4–6 h before each study. In addition, a quiet environment was maintained during each study to eliminate the impact of environmental noise (e.g., talking, phone calls) on measures of ventilation during wakefulness and to prevent disruption of sleep during sleep studies. All female participants completed the studies during the follicular phase of the menstrual cycle to eliminate the potential impact of hormone fluctuations and basal body temperature on measures of minute ventilation. The follicular phase was designated as the initial 10 days immediately following menses. If the study was not completed in its entirety during this period, an approximate 2-wk delay was imposed to reestablish the follicular phase and the remaining visits were completed.
The intermittent hypoxia protocol (Fig. 1B) consisted of an initial 10-min period, where baseline measures of room air minute ventilation, tidal volume, breathing frequency, and the partial pressure of end-tidal oxygen (PetO2) and carbon dioxide (PetCO2) were obtained. Subsequently, PetCO2 was elevated 3 Torr above baseline levels, established during wake or sleep, for 10 min and was sustained at that level throughout the protocol, since we and others previously showed that vLTF was not elicited in humans under conditions of hypo- or isocapnia (24, 30, 31, 50). Thereafter, twelve 2-min episodes of hypoxia were administered where PetO2 was maintained at 50 Torr. Each hypoxic episode was followed by a 2-min normoxic recovery period, with the exception of the last recovery period, which was 30 min in duration. During the sham protocol (Fig. 1C), PetO2 was maintained at room air during the twelve 2-min episodes. The PetO2 was accompanied by PetCO2 values that were elevated 3 Torr above baseline. For the OSA participants, the intermittent hypoxia and sham protocols were administered in conjunction with CPAP treatment maintained at the therapeutic pressure determined for each participant. In addition, breathing events during sleep were recorded for 1 h in the OSA participants immediately following the intermittent hypoxia and sham protocols. During this period, PetCO2 was uncontrolled, and CPAP treatment was removed.
Instrumentation
Nocturnal polysomnography.
During completion of the sleep studies (i.e., screening polysomnogram and administration of intermittent hypoxia and sham protocols during sleep), the sleep-monitoring montage included an electroencephalogram (C3/A2, C4/A1, O1/A2, and O2/A1), electrooculogram, submental electromyogram, and an ECG. Abdominal and chest wall movements were monitored using a piezoelectric band, and upper airway pressure was measured using a pressure transducer (model MPC-500; Millar Instruments, Houston, TX). Airflow, breathing frequency, and inspiratory/expiratory volumes were recorded via a pneumotachograph (model RSS-100HR; Hans-Rudolph, Kansas City, MO) that was attached to a face mask. Measures of end-tidal oxygen (model 17625; Vacumed, Ventura, CA) and end-tidal carbon dioxide (model 17515; Vacumed) were obtained from air expired into sampling tubes that were attached to ports on the face mask. Oxygen saturation (SaO2) was measured via pulse oximetry (model 3900P; Datex-Ohmeda, Boulder, CO). All physiological variables were analog-to-digitally converted at a sampling frequency of 100 Hz/channel and input into a microcomputer using a commercially available software package (Gamma version 4.0; Astro-Med, West Warwick, RI).
Intermittent hypoxia protocol: control participants.
Control participants breathed through a face mask during exposure to intermittent hypoxia throughout wakefulness or sleep. End-tidal oxygen (model no. 17625; Vacumed) and carbon dioxide (model no. 17515; Vacumed) were sampled from two separate mask ports. The face mask was connected to a pneumotachograph (model RSS100-HR; Hans Rudolph) that monitored breath-by-breath changes in minute ventilation and airflow. The pneumotachograph was attached to a two-way valve. The inspiratory port of the valve was connected to a five-way stopcock. Participants inspired either room air or the contents from one of two bags attached to the stopcock that contained either 8% oxygen-balance nitrogen or 100% oxygen. Additionally, the output from a flowmeter was attached to the inspiratory port of the valve. Gas from two cylinders containing 100% oxygen and 100% carbon dioxide was connected to the flowmeter. Thus supplemental oxygen and carbon dioxide could be added to the 8% oxygen-balance nitrogen that was inspired during a given 2-min hypoxic episode to maintain desired levels of PetO2 (50 Torr) and PetCO2 (3 Torr above baseline), respectively. Participants breathed from the bag containing 8% oxygen-balance nitrogen during each 2-min hypoxic episode, while hypoxia was abruptly terminated with a single breath from the bag containing 100% oxygen. An ECG was continuously monitored, and SaO2 was measured with a pulse oximeter (model 3900P; Datex-Ohmeda, Boulder, CO). A 16-bit analog-to-digital converter (AT-MIO-16XE-50; National Instruments, Austin, TX) digitized the analog signals for online computer analysis using software specifically designed for this purpose. The software calculated minute ventilation, PetO2, and PetCO2 on a breath-by-breath basis.
Intermittent hypoxia and sham protocol: OSA participants.
The instrumentation employed during exposure to the intermittent hypoxia protocol throughout wakefulness was identical to that described for the control participants (see Intermittent hypoxia protocol: control participants). The instrumentation employed during sleep was similar, with the following exceptions. To carry out the intermittent hypoxia and sham protocols, a patent airway was maintained using CPAP. The breathing circuit consisted of a face mask with an airtight seal connected to a pneumotachograph (model RSS100-HR; Hans Rudolph). The pneumotachograph was attached to a plateau exhalation valve (Respironics, Pittsburgh, PA). The exhalation valve was attached to the CPAP machine. The exhalation valve functioned as an exhaust vent generating a continuous leak path in the breathing circuit. The output from a flowmeter, as well as a cylinder containing 8% oxygen-balance nitrogen, was attached to two ports in the inspiratory line of the CPAP machine. The flowmeter had inputs from two gas cylinders containing 100% oxygen and 100% carbon dioxide, which allowed for supplemental oxygen and carbon dioxide to be added to the 8% oxygen-balance nitrogen that was inspired during a given 2-min hypoxic episode to maintain desired levels of PetO2 and PetCO2, respectively.
Data Analysis
Nocturnal polysomnography.
Sleep stages, arousals, apneas, and hypopneas were scored according to standard criteria (27). An event characterized by the absence of inspiratory airflow for a minimum of 10 s was identified as an apnea. A hypopnea was defined as an event accompanied by a >50% reduction in airflow that lasted for a minimum of 10 s and was also accompanied by either an arousal or a ≥3% reduction in SaO2 in the absence of an arousal. The apnea-hypopnea index was defined as the total number of events per hour of sleep. Segments that were accompanied by arousal and wakefulness were not included in the analysis of data recorded during the intermittent hypoxia or sham trials completed during sleep.
Intermittent hypoxia and sham protocol.
During both wakefulness and sleep trials, average values for minute ventilation, tidal volume, breathing frequency, PetCO2, PetO2, and SaO2, henceforth referred to as the physiological variables, were determined for the last 5 min of the initial baseline period, during which participants breathed room air. Similarly, average values for these measures were obtained from the last 5 min of the second baseline period, where PetCO2 was elevated by 3 Torr. For each 2-min hypoxic episode and the subsequent 2-min normoxic recovery periods, average values of the physiological variables were obtained from the last 30 s of the 2-min periods. The 30-min end-recovery period was divided into six 5-min intervals, and the physiological variables were averaged from each interval. The data analysis for the sham protocol was identical to the intermittent hypoxia protocol. The sham protocol was completed to ensure that the vLTF observed during sleep was a consequence of exposure to intermittent hypoxia rather than other potentially confounding variables. To determine the magnitude of vLTF, the average values of minute ventilation, tidal volume, and breathing frequency from the 30-min end recovery period were compared with the average values from the second 10-min baseline period. The physiological variables measured during the end-recovery period were also standardized to baseline to account for differences in baseline measures that existed between the sexes and arousal states. These measures were also standardized to baseline in the sham protocol.
To calculate the HVR during each episode, the change in the average minute ventilation from the second baseline period to each individual hypoxic episode was divided by the change in average SaO2 between the two periods. The presence of progressive augmentation was determined by averaging the HVR from the initial two episodes and comparing it to the average HVR of the final two episodes. To determine the magnitude of vLTF, the average values of minute ventilation, tidal volume, and breathing frequency from the 30 min end-recovery period were compared with the average values from the second 10-min baseline period. The physiological variables measured during the end-recovery period were also standardized to baseline to account for differences in baseline measures that existed between the sexes and arousal states. These measures were also standardized to baseline in the sham protocol.
For 33 out of the 37 participants exposed to the intermittent hypoxia protocol during sleep, the physiological variables were recorded during stable N2 or N3 of non-rapid eye movement sleep during the baseline and end-recovery periods. Two participants were in N1 during the baseline and end-recovery periods, whereas, in the remaining two participants, there was a stage change from N2/N3 to N1 sleep from the baseline to the end-recovery period. The data for these latter four participants was included in the analysis, because eliminating the data did not alter the overall findings and statistical significance.
For the 17 OSA participants exposed to the sham protocol, the magnitude of minute ventilation during end-recovery, standardized to baseline, was compared with the intermittent hypoxia trial. In 15 of the 17 participants, the sleep stage between the sham and intermittent hypoxia trials was similar. In two participants, the sleep stage was lighter during the sham compared with the intermittent hypoxia protocol. The data from these latter participants was included in the analysis, because eliminating the data did not alter the overall findings.
Postintermittent hypoxia and sham protocol polysomnography.
For the OSA participants, the apnea-hypopnea index was determined for the first hour of sleep immediately following the intermittent hypoxia and sham protocols. During this time interval, participants were not administered CPAP, and carbon dioxide levels were not controlled. In other words, the participants were not subject to any intervention. The sleep stages during the 1-h postprotocol period were analyzed carefully and compared between protocols, because of the potential impact that sleep stage has on breathing events (65, 66). The data from two participants was not included in the analysis because the participants were awake during the 1-h period. For 12 of 15 participants analyzed, the sleep stage during at least 50% of the 1-h postprotocol period was similar between the protocols. In the remaining three participants, the sleep stage during at least 50% of the 1-h postprotocol period was deeper following the sham protocol compared with the sleep stage during the period following the intermittent hypoxia protocol.
Statistical Analysis
A two-way ANOVA was used to determine whether age, height, weight, body mass index, apnea-hypopnea index, blood pressure, and scores derived from the Epworth and Stanford sleepiness scales were different between the groups (healthy vs. OSA) and sexes (men vs. women). A repeated-measures ANOVA with two within-factors (arousal state: wakefulness vs. sleep, and period: initial episodes vs. final episodes) and two between-factors (group: OSA vs. control, and sex: men vs. women) was used to compare the HVR measured during the intermittent hypoxia protocol. The absolute measures of minute ventilation, tidal volume, breathing frequency, PetCO2, and PetO2 during the initial baseline were compared between the arousal states, sexes, and groups using a repeated-measure ANOVA. The between-factor in the ANOVA were group (OSA vs. control) and sex (men vs. women), and the within-factor was arousal state (wake and sleep). An ANOVA also was used to compare the same physiological measures during the second baseline and end-recovery period of the intermittent hypoxia protocol. The two within-factors were arousal state (wakefulness vs. sleep) and period (second baseline vs. end-recovery). The two between-factors were group (OSA vs. control) and sex (men vs. women). For men and women of the OSA group, a two-way repeated-measures ANOVA, in conjunction with a Student-Newman-Keuls post hoc test, was used to compare 1) minute ventilation, tidal volume, and breathing frequency during the end-recovery period of the intermittent hypoxia and sham protocols, expressed as a fraction of the second baseline; and 2) absolute measures of the apnea-hypopnea index following the intermittent hypoxia and sham protocols between and within the men and women with OSA. All data are presented as means ± SE. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Anthropometric Variables and Baseline Sleep Study Measures
Table 1 shows the anthropometric variables obtained for each group. The groups were matched for age, race, and body mass index; thus these measures were similar between the groups. Within a given sex, participants were matched for height and weight. Consequently, no differences in these variables existed between the healthy men and men with OSA, or between the healthy women and women with OSA. The results show that the participants were relatively young and were not obese, as indicated by the body mass index (Table 1). In addition, systolic and diastolic blood pressure measures were within normal limits (Table 1). As expected, the apnea-hypopnea index was greater in the OSA participants compared with the healthy participants (P ≤ 0.001). The apnea-hypopnea index in the women with OSA was significantly less than the index measured for the men with OSA (P ≤ 0.001), even though the groups were matched for age, race, and body mass index. The Epworth sleepiness scale indicated a history of mild sleepiness in the OSA groups and normal sleep function in the control groups (P ≤ 0.03) (Table 1). However, scores from the Stanford sleepiness scale indicated normal alertness for both groups on the day of the screening visit (Table 1). The average therapeutic pressure required to eliminate apnea during sleep in the OSA participants was 12.8 ± 0.7 cmH2O. When studies were completed during sleep, arousals and wakefulness accounted for 4.9 ± 0.6 min of the 48-min period of hypoxic administration (i.e., twelve 2-min episodes interspersed with 2-min recovery periods) and 2.5 ± 0.6 min of the 30-min recovery period.
Table 1.
Anthropometric and sleep measures from baseline sleep study
| Control Men | Control Women | OSA Men | OSA Women | |
|---|---|---|---|---|
| Age, yr | 24.7 ± 1.2 | 24.8 ± 1.3 | 24.9 ± 1.0 | 26.6 ± 2.2 |
| Height, cm | 179.7 ± 2.6 | 168.9 ± 2.5† | 177.3 ± 2.9 | 166.9 ± 1.9† |
| Weight, kg | 81.1 ± 1.9 | 72.8 ± 4.2† | 80.7 ± 3.1 | 70.8 ± 2.1† |
| Body mass index, kg/m2 | 25.1 ± 0.7 | 25.3 ± 0.8 | 25.4 ± 0.6 | 25.7 ± 0.9 |
| Systolic pressure, Torr | 118.2 ± 3.0 | 118.7 ± 1.5 | 123.9 ± 2.2 | 118.4 ± 2.2 |
| Diastolic pressure, Torr | 78.6 ± 2.7 | 75.9 ± 1.5 | 75.6 ± 2.2 | 78.6 ± 3.0 |
| Epworth sleepiness scale | 6.1 ± 1.2 | 5.8 ± 0.7 | 9.0 ± 1.2* | 8.1 ± 1.2* |
| Stanford sleepiness scale | 1.7 ± 0.2 | 2.0 ± 0.3 | 2.2 ± 0.4 | 2.0 ± 0.4 |
| Apnea/hypopnea index, events/h | 2.8 ± 0.8 | 1.7 ± 0.4 | 48.7 ± 6.0* | 21.7 ± 5.1*† |
| Race | 5 African American, 5 Caucasian | 5 African American, 5 Caucasian | 5 African American, 5 Caucasian | 5 African American, 2 Caucasian |
Values are means ± SE. OSA, obstructive sleep apnea. Significantly different from
control and
men: P ≤ 0.05.
Intermittent Hypoxia Protocol: Initial Baseline Period
Absolute measures of the respiratory parameters obtained during the initial baseline (i.e., B1) are shown in Table 2. Minute ventilation during the initial baseline period was significantly greater during wakefulness compared with sleep within each group (P ≤ 0.0001). Minute ventilation was reduced during sleep primarily because of a reduction in tidal volume (P ≤ 0.0001) (Table 2). The PetCO2 during the initial baseline period was similar during wakefulness and sleep (P ≤ 0.10) in both the OSA and control groups. However, PetCO2 in men was greater compared with that in women (P ≤ 0.001) (Table 2). The PetO2 was similar during wakefulness and sleep within a given group or sex (Table 2).
Table 2.
Measures of respiratory parameters before and after exposure to intermittent hypoxia
| Control Men |
Control Women |
OSA Men |
OSA Women |
|||||
|---|---|---|---|---|---|---|---|---|
| Wake | Sleep | Wake | Sleep | Wake | Sleep | Wake | Sleep | |
| V̇e, l/min | ||||||||
| B1 | 13.8 ± 1.1†‡ | 11.8 ± 0.9‡ | 10.5 ± 0.4† | 9.6 ± 0.5 | 11.9 ± 0.4†‡ | 9.5 ± 0.7‡ | 10.7 ± 0.6† | 7.6 ± 0.5 |
| B2 | 17.2 ± 1.1†‡ | 14.1 ± 1.0‡ | 13.7 ± 0.5† | 12.0 ± 0.6 | 14.7 ± 0.4†‡ | 11.7 ± 0.9‡ | 13.9 ± 0.8† | 10.4 ± 0.8 |
| ER | 20.3 ± 1.1*†‡ | 15.3 ± 1.1*‡ | 17.5 ± 1.3*† | 12.8 ± 0.5* | 20.4 ± 1.1*†‡ | 13.3 ± 0.9*‡ | 18.6 ± 0.7*† | 12.0 ± 1.2* |
| Vt, ml | ||||||||
| B1 | 941.6 ± 60.4†‡ | 747.8 ± 70.4‡ | 643.8 ± 24.2† | 611.1 ± 27.7 | 880.4 ± 42.6†‡ | 710.9 ± 59.5‡ | 717.6 ± 76.3† | 526.4 ± 39. |
| B2 | 1,073.3 ± 67.5†‡ | 886.3 ± 85.0‡ | 823.0 ± 41.8† | 734.7 ± 37.8 | 1,027.3 ± 48.9†‡ | 862.7 ± 64.7‡ | 864.3 ± 78.2† | 685.4 ± 4 |
| ER | 1,206.6 ± 74.5*†‡ | 954.9 ± 86.8*‡ | 935.6 ± 44.8*† | 776.0 ± 38.5* | 1,298.0 ± 87.1*†‡ | 956.1 ± 75.5*‡ | 1,030.9 ± 43.8*† | 761.2 ± 53.8* |
| Bf, breaths/min | ||||||||
| B1 | 14.8 ± 0.9 | 16.2 ± 0.9 | 16.7 ± 1.1 | 16.3 ± 1.2 | 14.0 ± 0.8 | 13.5 ± 0.4 | 15.5 ± 1.0 | 14.6 ± 0.6 |
| B2 | 16.3 ± 0.9 | 16.5 ± 0.9 | 17.2 ± 1.2 | 16.7 ± 1.1 | 14.8 ± 1.0 | 13.7 ± 0.5 | 16.6 ± 1.1 | 15.1 ± 0.6 |
| ER | 17.2 ± 0.8*† | 16.5 ± 0.9 | 18.9 ± 1.3*† | 17.0 ± 1.1 | 16.2 ± 0.8*† | 14.1 ± 0.4 | 18.3 ± 0.8*† | 15.8 ± 0.6 |
| PetCO2, Torr | ||||||||
| B1 | 41.1 ± 0.7‡ | 41.0 ± 1.0‡ | 37.0 ± 0.7 | 38.4 ± 1.1 | 39.3 ± 1.0‡ | 40.4 ± 1.0‡ | 37.3 ± 0.6 | 37.3 ± 0.6 |
| B2 | 43.9 ± 0.7‡ | 43.9 ± 0.7‡ | 40.1 ± 0.8 | 41.6 ± 1.2 | 42.6 ± 1.0‡ | 43.3 ± 0.9‡ | 40.2 ± 0.6 | 39.9 ± 0.5 |
| ER | 43.9 ± 0.7‡ | 44.3 ± 0.8‡ | 40.1 ± 0.7 | 41.5 ± 1.1 | 42.3 ± 0.9‡ | 43.3 ± 0.8‡ | 39.9 ± 0.6 | 40.4 ± 0.6 |
| PetO2, Torr | ||||||||
| B1 | 96.4 ± 1.3 | 95.8 ± 1.7 | 98.8 ± 0.9 | 94.2 ± 1.9 | 96.2 ± 1.6 | 95.1 ± 2.6 | 101.4 ± 1.1§ | 101.0 ± 1.9§ |
| B2 | 106.7 ± 1.2 | 105.3 ± 1.3 | 110.2 ± 1.4 | 105.3 ± 1.2 | 106.9 ± 1.2 | 105.1 ± 1.7 | 112.9 ± 1.0§ | 111.7 ± 2.9§ |
| ER | 111.6 ± 1.4*† | 107.6 ± 1.0* | 115.5 ± 1.9*† | 108.3 ± 1.0* | 115.8 ± 1.5*† | 109.5 ± 1.1* | 120.5 ± 0.8*†§ | 114.9 ± 2.0*§ |
Values are means ± SE. V̇e, minute ventilation; Vt, tidal volume; Bf, breathing frequency; PetCO2, partial pressure of end-tidal carbon dioxide; PetCO2, partial pressure of end-tidal oxygen; B1, initial 10-min baseline period measured under normoxic conditions; B2, second 10-min baseline period measured under hypercapnic conditions (i.e., 3 Torr above B1 measures); ER, 30-min end-recovery period. Significantly different from
sleep,
women,
baseline, and
all other groups: P ≤ 0.05.
Intermittent Hypoxia Protocol: HVR and Progressive Augmentation
The HVR during the initial and final two episodes of the intermittent hypoxia protocol was significantly greater during wakefulness compared with sleep in both the OSA and control groups (Fig. 2, P ≤ 0.0001). Moreover, the HVR during the final two episodes of the intermittent hypoxia protocol was significantly greater than the initial two episodes during wakefulness, but not during sleep (Fig. 2, P ≤ 0.0001). The progressive increase in the HVR from the initial two episodes to the final two episodes during wakefulness was significantly enhanced in the OSA group compared with control (P ≤ 0.03). The differences observed between arousal states were similar within the men and women and was not due to variations in stimulus intensity. The average SaO2 measured for the initial episodes between groups and across arousal states were similar in men (OSA: wakefulness 86.9 ± 0.4 vs. sleep 86.8 ± 0.7; control: wakefulness 86.0 ± 0.6 vs. sleep 85.8 ± 0.8%) and women (OSA: wakefulness 87.9 ± 0.4 vs. sleep 89.1 ± 0.7; controls: wakefulness 87.9 ± 0.6 vs. sleep 87.6 ± 0.7%). Likewise, the SaO2 was also similar during the final hypoxic episodes in each group and arousal state in men (OSA: wakefulness 87.7 ± 0.4 vs. sleep 86.4 ± 0.6; control: wakefulness 86.6 ± 0.7 vs. 86.1 ± 0.6%) and women (OSA: wakefulness 88.3 ± 0.3 vs. sleep 87.7 ± 0.7; control: wakefulness 87.2 ± 0.6 vs. sleep 87.8 ± 0.7%). To summarize, the results indicate that the HVR was greater during wakefulness compared with sleep, and that the response was progressively augmented over the time course of the intermittent hypoxia protocol. The progressive augmentation of the HVR was evident solely during wakefulness and was enhanced in the OSA group compared with control.
Fig. 2.

Measures of the hypoxic ventilatory response (HVR) during the intermittent hypoxia protocol completed during wakefulness and sleep. Average values of the HVR determined for the initial two episodes and the final two episodes of the intermittent hypoxia protocol in men and women of the OSA and control groups are shown. Note that the HVR was greater during wakefulness compared with sleep. Moreover, note that there was a progressive increase in the HVR from the initial two episodes to the final two episodes during wakefulness but not sleep. Furthermore, note that progressive augmentation of the HVR was greater in the OSA group compared with control in both men and women. SaO2, Arterial oxygen saturation. Values are means ± SE. ‡Significantly different from initial episodes. †Significantly different from sleep.
Intermittent Hypoxia Protocol: vLTF
Baseline vs. end-recovery.
Figure 3 shows a raw record obtained from one participant exposed to the intermittent hypoxia protocol during wakefulness and sleep. Note that minute ventilation during the 30-min recovery period was significantly greater than baseline during both arousal states, which is indicative of vLTF. However, the magnitude of the increase, relative to baseline, was greater during wakefulness compared with sleep. The response shown in the raw record is similar to the average results. Absolute measures of minute ventilation were greater during recovery compared with baseline (i.e., baseline 2, Table 2) for each group within a given arousal state (P ≤ 0.0001). Since baseline measures of minute ventilation and tidal volume were impacted by arousal state and sex, the data were expressed as a fraction of baseline to make comparisons between groups. The standardized data show that the magnitude of vLTF was significantly greater during wakefulness compared with sleep (Fig. 4, P ≤ 0.0001). In addition, the magnitude of vLTF was greater in the OSA group compared with control during both wakefulness and sleep (Fig. 4, P ≤ 0.001). In contrast, the magnitude of vLTF was similar between the sexes for a given group (i.e., healthy or OSA) and arousal state (i.e., wakefulness or sleep).
Fig. 3.
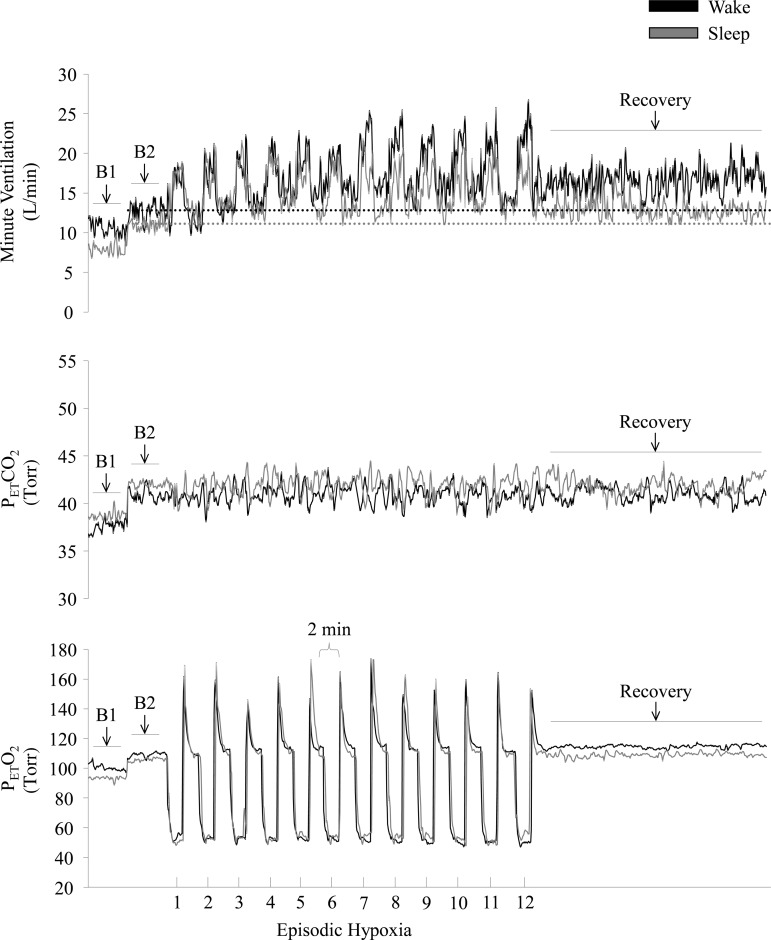
Measures of minute ventilation (top), PetCO2 (middle), and PetO2 (bottom) during completion of the intermittent hypoxia protocol during wakefulness and sleep. A raw record of breath-by-breath minute ventilation recorded from one participant exposed to intermittent hypoxia is shown. The dotted lines represent baseline values specific to each state. Note that minute ventilation during the end-recovery period was greater than baseline during wakefulness as well as during sleep. B1, last 5 min of the initial normocapnic 10 min baseline period; B2, last 5 min of the second baseline period during which PetCO2 was elevated 3 Torr above B1 measures.
Fig. 4.
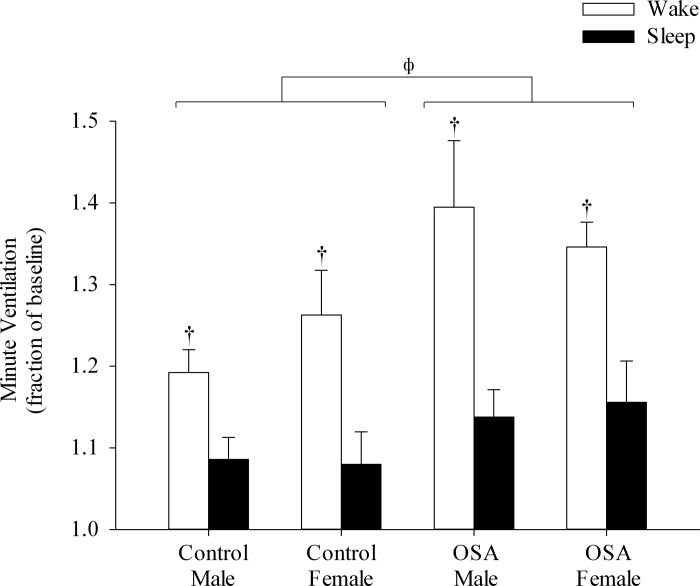
Minute ventilation during the end-recovery period expressed as a fraction of baseline measured during wakefulness and sleep. Note that ventilatory long-term facilitation (vLTF) was elicited during both wakefulness and sleep in both OSA and control groups; however, the magnitude of vLTF was greater during wakefulness compared with sleep. Also note that the magnitude of vLTF was more enhanced in the OSA group compared with control during both wakefulness and sleep. Values are means ± SE. †Significantly different from sleep. ΦSignificantly different from control.
Contribution of tidal volume and breathing frequency to vLTF.
The respective contribution of tidal volume and breathing frequency towards vLTF is shown in Table 2 and Fig. 5. During the end-recovery period, tidal volume was elevated above baseline values during both wakefulness and sleep in all groups (Table 2, P ≤ 0.0001). However, the magnitude of this increase was greater during wakefulness compared with sleep (Fig. 5, P ≤ 0.002). In addition, tidal volume was significantly greater in the OSA group compared with control (Fig. 5, P ≤ 0.02). On the other hand, breathing frequency during the end-recovery period was significantly greater than baseline only during wakefulness in all groups (Table 2, P ≤ 0.001). Consequently, long-term facilitation of breathing frequency was greater during wakefulness compared with sleep (Fig. 5, P ≤ 0.001). No differences in the breathing frequency response were observed between the OSA and control groups or between the sexes. To summarize, tidal volume was the primary component responsible for vLTF during sleep, while both tidal volume and breathing frequency contributed to vLTF during wakefulness, independent of sex or health status.
Fig. 5.

The relative contribution of tidal volume (top) and breathing frequency (bottom) toward minute ventilation during the end-recovery period expressed as a fraction of baseline. Note that tidal volume during the end-recovery period contributed to vLTF during both wakefulness and sleep, whereas the contributions of breathing frequency to vLTF were significant during wakefulness only. Values are means ± SE. †Significantly different from sleep. ΦSignificantly different from control.
Intermittent Hypoxia Protocol vs. Sham Protocol
Minute ventilation was significantly greater during the end-recovery period following exposure to intermittent hypoxia compared with sham exposure (Fig. 6, P ≤ 0.002). As mentioned previously (see Contribution of tidal volume and breathing frequency to vLTF), this increase was primarily due to an elevation in tidal volume (Fig. 7, P ≤ 0.002), with no contribution from breathing frequency. The lack of an increase in vLTF following sham exposure was also reflected in the unchanging tidal volume and breathing frequency response during the end-recovery period (Fig. 7).
Fig. 6.

Minute ventilation during the end-recovery period expressed as a fraction of baseline following exposure to the intermittent hypoxia and sham protocols during sleep in the OSA group. Note that minute ventilation was significantly greater than baseline following exposure to intermittent hypoxia compared with sham exposure. Values are means ± SE. δSignificantly different from sham.
Fig. 7.
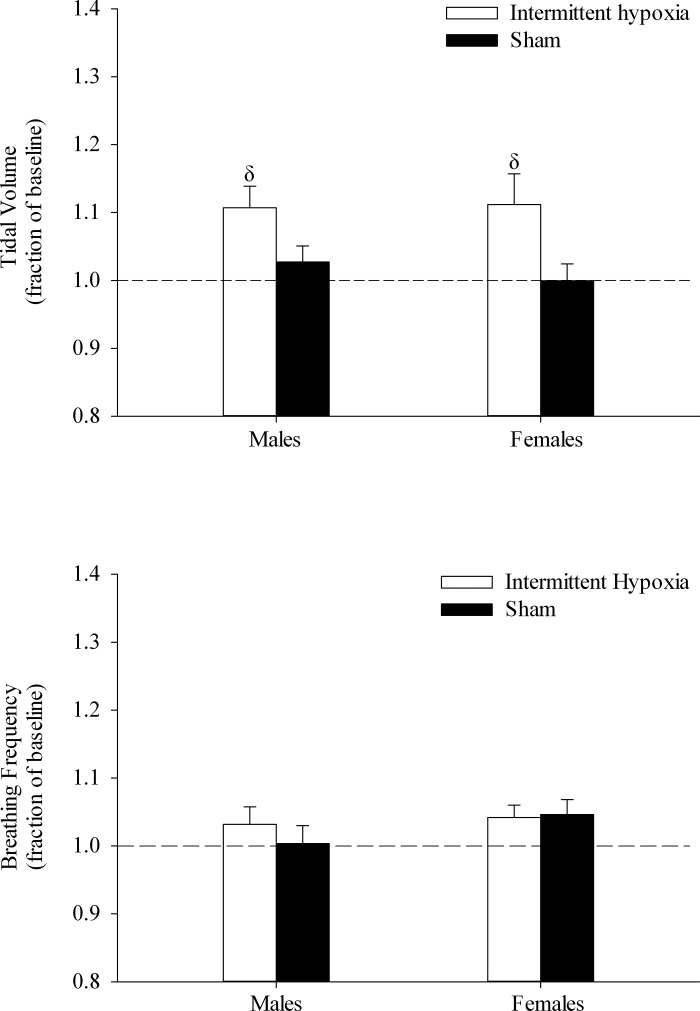
Measures of tidal volume (top) and breathing frequency (bottom) during the end-recovery period expressed as a fraction of baseline in the OSA group following exposure to the intermittent hypoxia and sham protocols during sleep. Note that tidal volume is significantly greater following exposure to intermittent hypoxia compared with sham exposure, while breathing frequency is similar following exposure to both protocols. Values are means ± SE. δSignificantly different from sham.
Respiratory Plasticity and Breathing Events
The apnea-hypopnea index measured in the men and women with OSA 1 h after exposure to the intermittent hypoxia or sham protocol during sleep is shown in Fig. 8. The index was significantly greater following exposure to intermittent hypoxia compared with measures obtained after exposure to the sham protocol in both men and women (P ≤ 0.02).
Fig. 8.
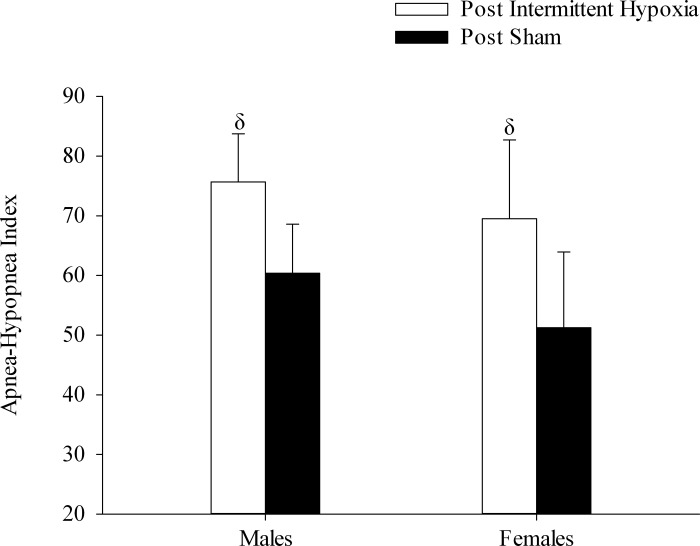
Measures of the apnea-hypopnea index obtained from 1 h following exposure to the intermittent hypoxia and sham protocols during sleep in the OSA participants. Note that the number of respiratory events increased following exposure to intermittent hypoxia compared with sham exposure. Values are means ± SE. δSignificantly different from sham.
DISCUSSION
We showed that the HVR was greater during wakefulness compared with sleep, and that this response was progressively augmented over the time course of the intermittent hypoxia protocol during wakefulness but not during sleep. We also demonstrated that vLTF was elicited during wakefulness and sleep, but the magnitude of vLTF was greater during wakefulness. We also confirmed that the magnitude of progressive augmentation and vLTF was enhanced in the OSA group compared with control. Our results established that sex did not impact on the manifestation or magnitude of progressive augmentation and vLTF during wakefulness or sleep both in healthy individuals, as well as in individuals with sleep apnea. Lastly, exposure to intermittent hypoxia during sleep led to a corresponding increase in respiratory events compared with sham exposure, irrespective of sex.
Methodological Considerations
Participants recruited for our investigation were relatively young, did not suffer from other comorbid conditions (i.e., diabetes, cardiovascular disease, and obesity), and were typically exposed to mild hypoxemia at night. Thus the participants recruited for our investigation were not typical of many patients seen in the sleep clinic, who are older, obese, and often suffer from other comorbid conditions. Consequently, findings from our investigation may be solely applicable to a specific phenotype within the sleep apnea population.
Impact of carbon dioxide on progressive augmentation and vLTF.
The confounding effect of carbon dioxide on the manifestation of progressive augmentation and vLTF has been well established in animals as well as humans (41). Long-term facilitation is not fully expressed in unanesthetized rats during wakefulness when accompanied by hypocapnia, likely due to disfacilitation of the peripheral or central chemoreflex (55). In humans, progressive augmentation and vLTF are not detectable under poikilocapnic or normocapnic conditions (30, 40, 44, 50); however, both progressive augmentation and vLTF are evident when carbon dioxide levels are sustained slightly above normocapnic values during hypoxic exposure (21, 24, 34, 80). Based on these findings, we elevated the carbon dioxide levels 3 Torr above resting values during and following exposure to intermittent hypoxia in the present study. It may be argued that the increase in minute ventilation that we observed during the end-recovery period following exposure to intermittent hypoxia was caused by the sustained hypercapnia employed in our protocol. However, we have previously demonstrated that prolonged exposure to sustained hypercapnia did not cause a significant ventilatory drift in healthy men and women and elicited a drift in minute ventilation in men with OSA during wakefulness (24) that was significantly less than the increase observed following exposure to intermittent hypoxia combined with sustained hypercapnia (21). In the present investigation, we also demonstrated that vLTF observed during sleep in OSA men and women was not a consequence of sustained carbon dioxide levels, since minute ventilation was significantly greater following the intermittent hypoxia compared with the sham protocol.
Impact of diurnal variations on progressive augmentation and vLTF.
The presence of diurnal variations in the HVR and vLTF has been previously demonstrated in animals as well as humans. Seifert and Mortola (68) reported that the HVR in adult rats is greater in the light compared with the dark phase. In addition, despite some variability in the findings, there is a strong indication that the HVR in humans varies in a diurnal fashion. Likewise, studies conducted on healthy participants (64, 73) as well as OSA participants (20, 21) have shown that the magnitude of the HVR and vLTF is different in the morning compared with the evening. More specifically, we found that the HVR was greater and vLTF was reduced in the morning compared with the evening (21). Consequently, in the present investigation, studies during wakefulness and sleep were conducted in the morning in an attempt to eliminate the impact of diurnal rhythms on the manifestation and magnitude of progressive augmentation and vLTF. For the sleep studies, participants were asked to arrive at the lab 2 h before the initiation of the study and were instructed not to sleep in the late evening/early morning. Therefore, in some cases, participants may have been sleep deprived at the onset of the study. However, if this were the case, we believe that the degree of sleep deprivation was mild, since the participants' normal bedtime on average was in the early morning (i.e., 1 AM) and some participants reported napping during the day of the study. Moreover, the magnitude of vLTF was similar to that reported in previous studies that were completed during sleep in the late evening/early morning (15, 62, 71). This similarity supports the notion that the reduction in vLTF during sleep was a consequence of arousal state and not sleep deprivation.
HVR
Exposure to hypoxia led to a corresponding increase in minute ventilation, which is characteristic of the HVR. Our results showed that, within a given state (i.e., wake vs. sleep), the HVR was similar between men and women, independent of health status. This finding is in contrast to the results of Douglas and colleagues (17), which indicated that the HVR was greater in men compared with women during wakefulness, but is similar to the findings of White et al. (81) and Tarbichi et al. (75), who reported that the HVR was similar in men and women during sleep. In the present study, we also showed that the HVR was greater during wakefulness compared with sleep, irrespective of sex and health status. This difference was due to a greater change in minute ventilation during the hypoxic exposure and not due to variations in the intensity of the stimulus, which was similar between arousal states. Our finding replicates the results of Douglas and colleagues (17), as well as Berthon-Jones and Sullivan (6), who showed that the HVR was reduced during sleep compared with wakefulness in healthy men (6, 17) and women (17). However, our findings are in contrast to a study completed by the same group, which reported that the HVR was similar during wake and non-rapid eye movement sleep in healthy premenopausal women (81). Lastly, our results showed that the HVR was not significantly different between the healthy and OSA participants, independent of arousal state. There are equivocal results in the published findings with respect to the magnitude of HVR in OSA participants compared with healthy controls. One study reported that the HVR compared with control participants is increased in the OSA participants (53), while others have shown that it is decreased in OSA participants compared with control (56, 74). Our finding is surprising, given that individuals with sleep apnea are chronically exposed to intermittent hypoxia, which is thought to enhance the ventilatory sensitivity to hypoxia (48, 52, 61). Under such circumstances, one would expect the HVR to be greater in the OSA population. However, it is possible that the difference only manifests after a more intense or prolonged exposure to hypoxia. Indeed, our laboratory has shown previously that the ventilatory response to hypercapnia/hypoxia during the completion of a modified rebreathing protocol was greater in OSA individuals compared with healthy controls following exposure to intermittent hypoxia (34). This was also the case in the present study (see OSA vs. Control: Progressive Augmentation and vLTF).
Arousal State
Progressive augmentation and vLTF.
In the present study, progressive augmentation of the HVR was evident during wakefulness, but not during sleep, in both the OSA and control groups, irrespective of sex. In addition, vLTF was evident during both wakefulness and sleep in both healthy men and women, as well as in men and women with OSA. However, the magnitude of the response was greater during wakefulness compared with sleep, independent of sex. These results confirm previous findings, which showed that progressive augmentation and vLTF can be initiated during wakefulness in healthy men and women (24, 80) and in OSA men (21, 34). We have added to the published findings by showing that progressive augmentation and vLTF can be initiated in women with OSA during wakefulness as well as sleep.
The absence of progressive augmentation in healthy individuals during sleep was reported previously by Chowdhuri and colleagues (14). Moreover, our analysis of published results (42) suggested that the magnitude of vLTF could be less during sleep compared with wakefulness, although the initiation of vLTF during sleep has been variable and dependent on experimental conditions and/or populations (see OSA vs. Control for further discussion). However, whether differences in the initiation of progressive augmentation or manifestation of vLTF during wake and sleep were a consequence of arousal state or were due to differences in experimental design could not be deciphered. The present investigation has established that, despite the initiation of progressive augmentation during wakefulness, exposure to an identical stimulus during sleep does not initiate this phenomenon in healthy men and women, and in men and women with sleep apnea. Likewise, in these populations, we have established that vLTF can be elicited during sleep, but that the magnitude is significantly less compared with the amplitude of the phenomenon during wakefulness. Moreover, our results show that both breathing frequency and tidal volume contribute to vLTF during wakefulness, while tidal volume alone is responsible for the vLTF observed during sleep. Thus the pattern generator may have a greater role in the manifestation of vLTF during wakefulness in humans.
Mechanisms (arousal state).
It is possible that serotonin could, in part, be responsible for the magnitude differences in progressive augmentation and vLTF that was evident during wakefulness and sleep, since intermittent application of serotonin on ex vivo carotid bodies elicits a progressive increase in carotid sensory nerve activity (60), and the application of methysergide eliminates the enhanced phrenic and recurrent laryngeal response to hypoxia following intermittent hypoxia (5). In addition, a number of studies have provided strong evidence that serotonin plays an important role in the initiation of vLTF or phrenic nerve long-term facilitation (3, 36, 45, 47, 54). Most importantly, the rate of discharge of serotonergic neurons located in the raphe nucleus is considerably diminished during sleep compared with wakefulness, where the rate of discharge is close to maximal (25, 28, 29, 79). If the basal discharge rates of serotonergic neurons are close to maximal, there is likely a limited capacity to increase activity following administration of intermittent hypoxia. Consequently, the magnitude of progressive augmentation or vLTF could be marginal under these circumstances. Conversely, if the basal rate of discharge is diminished, as it is during sleep, there might be room for a greater range of activity after administration of intermittent hypoxia, before maximum rates are achieved. Thus the magnitude of progressive augmentation or vLTF could be greater compared with wakefulness. Indeed, a few studies have supported this hypothesis, since the magnitude of vLTF (51, 77) as well as diaphragm LTF (76) was greater during sleep compared with wakefulness in unanesthetized rats. However, contrary to the findings in rats, our investigation showed that progressive augmentation was not elicited, and the magnitude of vLTF was significantly diminished during sleep compared with wakefulness. Thus, if serotonin does impact on the manifestation of these phenomena, it may not be principally dependent on a greater dynamic range of activation of serotonergic neurons in humans.
Alternatively, it is possible that the mechanism responsible for the manifestation of progressive augmentation and vLTF may be independent or only partially dependent on serotonin. Indeed, in a few studies, administration of a 5-HT receptor antagonist did not alter the magnitude of progressive augmentation in anesthetized rats and cats (19, 54), indicating that other neuromodulators might impact on the phenomenon, depending on the conditions. With respect to vLTF, the serotonin-dependent mechanism was thought to be the sole pathway responsible for intermittent hypoxia-induced vLTF until a study completed by Golder et al. (22) showed that LTF is also elicited by the activation of A2A receptors in anesthetized rats. More recently, Nichols et al. (54) demonstrated that the A2A and 5-HT2 receptor-mediated cellular pathways that initiate LTF, which were designated the Gs and Gq pathways, respectively, are distinct from each other. Nichol and colleagues (54) further demonstrated that these two pathways influence each other via cross-talk inhibition, since activation of the Gs pathway diminished the magnitude of LTF generated initially by the Gq pathway. Extracellular adenosine, a metabolic end product, transiently increases during wakefulness, and the corresponding accumulation promotes the induction of sleep through the actions of A1 and A2A receptors (26, 43). It could be that the increase in adenosine levels that occurs with prolonged wakefulness may promote the Gs pathway and via cross-talk inhibition diminish the influence of the Gq pathway. Conversely, since the wakefulness studies were completed early in the morning shortly after the subjects had woken up, the levels of adenosine were most likely at their lowest as they gradually decline during sleep. This reduction in adenosine may have caused the cross-talk inhibition of the Q pathway to be weakened and, therefore, may have resulted in a more enhanced vLTF during wakefulness.
The difference in the magnitude of vLTF that we observed between the arousal states could also have been influenced by orexin, since these neurons innervate serotonergic raphe neurons (7) that impact on the manifestation of vLTF. Indeed, Terada et al. (77) demonstrated that orexin is necessary in eliciting LTF, as it was absent in the prepro-orexin knockout mice. Based on these findings and given that our intermittent hypoxia protocol consisted of mild hypercapnia, we speculate that orexin may have also contributed to the enhanced magnitude of vLTF that was observed during wakefulness in the present study. Hence, progressive augmentation and vLTF are likely impacted by the interaction of various neuromodulators that include, but are not limited to, serotonin, adenosine, and orexin.
The absence of progressive augmentation and the diminution of vLTF during sleep relative to wakefulness do not preclude these phenomena from being altered in the sleep state. Indeed, in anesthetized rats, progressive augmentation is generally not observed following exposure to an intermittent hypoxia protocol of moderate severity (54, 63), but has been observed following exposure to a more severe intermittent hypoxia protocol (54). Likewise, the magnitude of vLTF has also been shown to be enhanced after exposure to severe compared with moderate intermittent hypoxia. Since the intermittent hypoxia protocol used in our study was of moderate severity, it can be speculated that a more intense protocol may result in the manifestation of progressive augmentation during sleep as it did in anesthetized rats (54), while a less severe stimulus is required for the initiation of these phenomenon during wakefulness.
OSA vs. Control
Progressive augmentation and vLTF.
Progressive augmentation of the HVR and vLTF was enhanced in men and women with sleep apnea compared with healthy participants during wakefulness in the present investigation. These results confirm our previous findings in men and have shown for the first time that this difference also exists between healthy women and women with OSA. We also showed that vLTF was evident during sleep in both healthy men and women, and men and women with sleep apnea, and that the magnitude of the phenomenon during sleep was greater in individuals with OSA.
Our finding that vLTF was elicited during sleep in both healthy participants and participants with OSA is an important finding, since this phenomenon has not been consistently observed. Babcock and colleagues (2) reported initially that vLTF could only be initiated during sleep in participants with inspiratory flow limitation. Alternatively, vLTF was abolished following the elimination of inspiratory flow limitation with CPAP in individuals with sleep-disordered breathing and was not evident in healthy individuals (2). In contrast, we have shown that the vLTF is evident in men and women with sleep apnea after reducing flow limitation with CPAP and in healthy individuals. The reason for the discrepant findings remains to be determined. However, our laboratory has previously shown during wakefulness that vLTF is not evident when carbon dioxide is sustained at or below normocapnic levels (40, 50), but is apparent when carbon dioxide is sustained above baseline levels (24, 34). It is possible that the level of sustained carbon dioxide is responsible for the differences observed during sleep in the present study compared with the previous studies (1, 2), as the carbon dioxide levels in these studies were maintained at (1) or below normocapnic levels (2), in contrast to the present investigation. Indeed, finer control of carbon dioxide levels was also considered to be a viable reason for the manifestation of vLTF that was eventually reported during sleep in healthy individuals (62). Thus we have established that vLTF can be elicited during sleep in both healthy individuals and individuals with OSA, and that the magnitude of the response is greater in individuals with OSA.
Mechanisms (healthy vs. OSA).
Enhancement of progressive augmentation and vLTF in men and women with OSA could be due to previous chronic exposure to intermittent hypoxia, a hallmark of sleep apnea. Support for this hypothesis comes from a number of studies that have reported that chronic exposure to intermittent hypoxia leads to accumulation of reactive oxygen species in animals, which contributes to the initiation and enhancement of long-term facilitation (57–59). Likewise, work completed in healthy humans has shown that chronic exposure to intermittent hypoxia leads to progressive augmentation of the HVR (32, 38, 69). In addition, our laboratory has shown previously that daily exposure to intermittent hypoxia enhances the HVR and vLTF in humans with OSA (21). We and others have also demonstrated that biomarkers of oxidative stress are elevated in individuals with OSA compared with healthy individuals (12, 33, 34) under baseline conditions, and that exposure to intermittent hypoxia increases the level of oxidative stress in OSA but not control participants (34). Lastly, administration of an antioxidant cocktail reduces the magnitude of progressive augmentation and vLTF in OSA participants compared with the responses following placebo administration (34). Collectively, the published evidence suggests that chronic exposure to intermittent hypoxia and its link to the accumulation of reactive oxygen species may be responsible for the enhancement of the HVR and vLTF that was evident in the OSA participants during wake and sleep in the present investigation.
Sex
Progressive augmentation, vLTF, and mechanisms.
As outlined above (see Arousal State, Progressive augmentation and vLTF), the manifestation and magnitude of progressive augmentation and vLTF were similar between the sexes, independent of health status (i.e., healthy vs. OSA) and arousal state. This finding is similar to our previous results, which showed that the magnitude of progressive augmentation and vLTF were similar in healthy men and women during wakefulness. The absence of any sex differences is somewhat surprising considering that differences, with respect to these forms of plasticity, have been observed in other animals. Long-term facilitation has been shown to be greater in young male rats compared with young female rats, while the magnitude of LTF is greater in middle-aged female rats compared with males of a similar age (83–85). The magnitude of LTF is thought to be linked to levels of testosterone in males. Gonadectomy in male rats abolishes LTF, while LTF is reinstated upon testosterone replacement (85). Interestingly, it has been shown that a reduction in 5-HT synthesis also occurs following gonadectomy in adult male rats (37). Thus it is possible that the reduced testosterone levels impact the magnitude of LTF by influencing serotonin production. Similarly, the magnitude in female rats is thought to be linked to serotonergic activity, which varies with the estrous cycle. Estrogen has been shown to cause a two- to threefold increase in 5HT2A receptor mRNA in rats (46) and has been shown to increase the expression of tryptophan hydroxylase in primates (8). Since ventilation is stimulated by high levels of circulating progesterone (72), it also leads to an enhanced HVR in the presence of high levels of this hormone (35). Furthermore, a combination of both estrogen and progesterone enhances the HVR more than progesterone alone (23, 67). Hence, the effects of the various sex hormones on ventilation as well as the synthesis and function of serotonin provide strong support for the notion that sex-specific hormones and events impact the manifestation and magnitude of progressive augmentation and vLTF differently.
Since young men and women were recruited to participate in the present study, we hypothesized that the magnitude of progressive augmentation and vLTF would be greater in men compared with women. However, this was not the case. It is possible that the similarities observed between the sexes occurred because the women completed the studies during the follicular phase of the menstrual cycle. This may have removed the influence of certain hormones, particularly estrogen and progesterone, which resulted in the similarities that were observed. However, Zabka et al. (83–85) reported that the magnitude of LTF in young male rats was greater than that in female rats, irrespective of the estrous cycle. Nonetheless, an investigation tightly controlling for hormone levels during the luteal phase of the menstrual cycle may reveal a significant sex difference in the manifestation and magnitude of progressive augmentation and vLTF. Conversely, the absence of a sex difference could be simply due to a lack of power, which prevented us from detecting small differences between men and women in the follicular phase of the menstrual cycle. To address this issue, a much larger sample size may be required.
Physiological Significance: Respiratory Plasticity and Breathing Events
In our investigation, we measured breathing events in men and women with sleep apnea for 1 h after exposure to the intermittent hypoxia and sham protocols. During this time period, events were measured without any intervention (i.e., CPAP was removed and carbon dioxide levels were not controlled). Our results revealed that the number of breathing events were increased following exposure to intermittent hypoxia compared with the sham protocol. It might be argued that the time period was too short to reflect accurately the severity of sleep-disordered breathing. Nonetheless, our findings are similar to our laboratory's previous results (82), which were measured over a longer time period (i.e., 3 h) and showed that the apnea-hypopnea index was increased after exposure to intermittent hypoxia. We have added to the previous findings in that we have shown that this increase occurs in both men and women with sleep apnea. Moreover, we showed that the increase in severity was similar between men and women. We did not expect this latter result since sleep apnea is more prevalent in men compared with women (9) with similar anthropometric measurements. Thus we hypothesized that the increase in apnea severity might be greater in men compared with women. However, even though baseline measures indicated that the number of breathing events were greater in men, the increase in apnea severity following exposure to intermittent hypoxia compared with the sham study was similar in both men and women with OSA.
Given that our laboratory proposed previously (41) that vLTF and LTF of upper airway muscle activity might have a role in mitigating apnea, our result may be unexpected on initial consideration. However, given that the manifestation of vLTF and long-term facilitation of upper airway muscle activity are dependent on the maintenance of carbon dioxide levels (24), it is possible that these phenomena did not manifest themselves, because carbon dioxide levels were uncontrolled while breathing events were measured. Based on this possibility, it might be argued that long-term facilitation typically does not manifest itself throughout the night in individuals who are naturally exposed to intermittent hypoxia because of the accompanying hypocapnia that is often induced by hyperventilation upon arousal from an event. This may be the case, since clinical studies have reported that apnea severity increases throughout the night (10, 13, 18, 70), indicating the absence of factors that might mitigate apnea. Nonetheless, even though our model of elevating the carbon dioxide levels by 3 Torr does not accurately simulate the characteristic fluctuations in carbon dioxide that are experienced by individuals with sleep apnea, our findings might have implications for the treatment of sleep apnea. The maintenance of carbon dioxide during sleep could promote the manifestation of respiratory plasticity and ultimately be used as a tool to mitigate apnea in some individuals with sleep apnea.
The increase in apnea severity following exposure to intermittent hypoxia indicates that detrimental forms of plasticity might be responsible for our findings (41). Indeed, progressive augmentation of the ventilatory sensitivity to hypoxia and hypercapnia during and following exposure to intermittent hypoxia (31, 40) has been linked to reductions in the carbon dioxide reserve (15). The accompanying reduction in the carbon dioxide reserve could lead to a profound hypocapnia induced by the ventilatory overshoot that accompanies respiratory arousal. The induced hypocapnia could promote breathing events (4). Indeed, increased sensitivity of the HVR has been linked to increases in apnea severity (82). In the present investigation, progressive augmentation of the HVR did manifest itself during wakefulness, providing support for our model that is predicated on the role that the ventilatory response to hypoxia and/or hypercapnia during wakefulness has on the induction of hypocapnia before reestablishing sleep.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S., H.-S.L., and J.H.M. performed experiments; Z.S. and J.H.M. analyzed data; Z.S. and J.H.M. interpreted results of experiments; Z.S. and J.H.M. prepared figures; Z.S. and J.H.M. drafted manuscript; Z.S., H.-S.L., and J.H.M. edited and revised manuscript; Z.S., H.-S.L., and J.H.M. approved final version of manuscript; J.H.M. conception and design of research.
ACKNOWLEDGMENTS
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and a National Heart, Lung, and Blood Institute Grant (R01-HL-085537).
REFERENCES
- 1. Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol 91: 2751–2757, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol 94: 53–59, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 78: 1806–1815, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bautista TG, Xing T, Fong AY, Pilowsky PM. Recurrent laryngeal nerve activity exhibits a 5-HT-mediated long-term facilitation and enhanced response to hypoxia following acute intermittent hypoxia in rat. J Appl Physiol 112: 1144–1156, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypoxia in sleeping humans. Am Rev Respir Dis 125: 632–639, 1982 [DOI] [PubMed] [Google Scholar]
- 7. Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol 123: 147–156, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobiol 18: 87–123, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163: 608–613, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Cala SJ, Sliwinski P, Cosio MG, Kimoff RJ. Effect of topical upper airway anesthesia on apnea duration through the night in obstructive sleep apnea. J Appl Physiol 81: 2618–2626, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol 73: 2083–2088, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 124: 1386–1392, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest 106: 1695–1701, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160: 65–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol 108: 369–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douglas NJ, White DP, Weil JV, Pickett CK, Martin RJ, Hudgel DW, Zwillich CW. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis 125: 286–289, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Fanfulla F, Patruno V, Bruschi C, Rampulla C. Obstructive sleep apnoea syndrome: is the “half-night polysomnography” an adequate method for evaluating sleep profile and respiratory events? Eur Respir J 10: 1725–1729, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuse K, Satoh M, Yokota T, Ohdaira T, Muramatsu Y, Suzuki E, Arakawa M. Regulation of ventilation before and after sleep in patients with obstructive sleep apnoea. Respirology 4: 125–130, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Gerst DG, 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol 110: 15–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hannhart B, Pickett CK, Moore LG. Effects of estrogen and progesterone on carotid body neural output responsiveness to hypoxia. J Appl Physiol 68: 1909–1916, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res 251: 259–276, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem 11: 1047–1057, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Iber CAIS, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (1st Ed.). Westchester, IL: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 28. Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol 7: 820–825, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol 93: 2129–2136, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Khodadadeh B, Badr MS, Mateika JH. The ventilatory response to carbon dioxide and sustained hypoxia is enhanced after episodic hypoxia in OSA patients. Respir Physiol Neurobiol 150: 122–134, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Koehle MS, Sheel AW, Milsom WK, McKenzie DC. Two patterns of daily hypoxic exposure and their effects on measures of chemosensitivity in humans. J Appl Physiol 103: 1973–1978, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27: 123–128, 2004 [PubMed] [Google Scholar]
- 34. Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol 587: 5451–5467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lefter R, Morency CE, Joseph V. Progesterone increases hypoxic ventilatory response and reduces apneas in newborn rats. Respir Physiol Neurobiol 156: 9–16, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol 164: 233–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Long JB, Youngblood WW, Kizer JS. Effects of castration and adrenalectomy on in vitro rates of tryptophan hydroxylation and levels of serotonin in microdissected brain nuclei of adult male rats. Brain Res 277: 289–297, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol 575: 961–970, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol 96: 1197–1205; discussion 1196, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol 176: 1–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med 8: 302–330, 2007 [DOI] [PubMed] [Google Scholar]
- 44. McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol 81: 866–875, 1996 [DOI] [PubMed] [Google Scholar]
- 45. McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334–R341, 2004 [DOI] [PubMed] [Google Scholar]
- 46. McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res Mol Brain Res 45: 13–23, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980 [DOI] [PubMed] [Google Scholar]
- 48. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol 97: 1673–1680, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Nakamura A, Olson EB, Jr, Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. J Appl Physiol 109: 323–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177: 385–390, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol 112: 1678–1688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol 91: 709–716, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Osanai S, Akiba Y, Fujiuchi S, Nakano H, Matsumoto H, Ohsaki Y, Kikuchi K. Depression of peripheral chemosensitivity by a dopaminergic mechanism in patients with obstructive sleep apnoea syndrome. Eur Respir J 13: 418–423, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242; discussion 1196, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol 94: 2342–2349, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576: 289–295, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 180: 1002–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Raschke F, Moller KH. [The diurnal rhythm of chemosensitivity and its contribution to nocturnal disorders of respiratory control]. Pneumologie 43, Suppl 1: 568–571, 1989 [PubMed] [Google Scholar]
- 65. Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 5: 519–524, 2009 [PMC free article] [PubMed] [Google Scholar]
- 66. Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, Catcheside PG. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax 65: 107–112, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Pickett CK, Bender PR, Moore LG. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol 66: 808–813, 1989 [DOI] [PubMed] [Google Scholar]
- 68. Seifert EL, Mortola JP. Circadian pattern of ventilation during prolonged hypoxia in conscious rats. Respir Physiol Neurobiol 133: 23–34, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Serebrovskaya TV, Swanson RJ, Karaban IN, Serebrovskaya ZA, Kolesnikova EE. Intermittent hypoxia alters hypoxic ventilatory responses. Fiziol Zh 45: 9–18, 1999 [PubMed] [Google Scholar]
- 70. Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur Respir J 12: 1257–1263, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol 92: 2565–2570, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Skatrud JB, Dempsey JA, Kaiser DG. Ventilatory response to medroxyprogesterone acetate in normal subjects: time course and mechanism. J Appl Physiol 44: 393–344, 1978 [DOI] [PubMed] [Google Scholar]
- 73. Stephenson R, Mohan RM, Duffin J, Jarsky TM. Circadian rhythms in the chemoreflex control of breathing. Am J Physiol Regul Integr Comp Physiol 278: R282–R286, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Tafil-Klawe M, Thiele AE, Raschke F, Mayer J, Peter JH, von Wichert W. Peripheral chemoreceptor reflex in obstructive sleep apnea patients; a relationship between ventilatory response to hypoxia and nocturnal bradycardia during apnea events. Pneumologie 45, Suppl 1: 309–311, 1991 [PubMed] [Google Scholar]
- 75. Tarbichi AG, Rowley JA, Shkoukani MA, Mahadevan K, Badr MS. Lack of gender difference in ventilatory chemoresponsiveness and post-hypoxic ventilatory decline. Respir Physiol Neurobiol 137: 41–50, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol 110: 1299–1310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol 104: 499–507, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499: 543–550, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol 104: 1625–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis 126: 530–533, 1982 [DOI] [PubMed] [Google Scholar]
- 82. Yokhana SS, Gerst DG, 3rd, Lee DS, Badr MS, Qureshi T, Mateika JH. Impact of repeated daily exposure to intermittent hypoxia and mild sustained hypercapnia on apnea severity. J Appl Physiol 112: 367–377, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol 531: 509–514, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol 563: 557–568, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol 576: 903–912, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zabka AG, Mitchell GS, Olson EB, Jr, Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol 95: 2614–2623; discussion 2604, 2003 [DOI] [PubMed] [Google Scholar]


