Abstract
Following right pneumonectomy (PNX), the remaining lung expands and its perfusion doubles. Tissue and microvascular mechanical stresses are putative stimuli for initiating compensatory lung growth and remodeling, but their relative contributions to overall compensation remain uncertain. To temporally isolate the stimuli related to post-PNX lung expansion (parenchyma deformation) from those related to the sustained increase in perfusion (microvascular distention and shear), we replaced the right lung of adult dogs with a custom-shaped inflated prosthesis. Following stabilization of perfusion and wound healing 4 mo later, the prosthesis was either acutely deflated (DEF group) or kept inflated (INF group). Physiological studies were performed pre-PNX, 4 mo post-PNX (inflated prosthesis, INF1), and again 4 mo postdeflation (DEF) compared with controls with simultaneous INF prosthesis (INF2). Perfusion to the remaining lung increased ∼76–113% post-PNX (INF1 and INF2) and did not change postdeflation. Post-PNX (INF prosthesis) end-expiratory lung volume (EELV) and lung and membrane diffusing capacities (DlCO and DmCO) at a given perfusion were 25–40% below pre-PNX baseline. In the INF group EELV, DlCO and DmCO remained stable or declined slightly with time. In contrast, all of these parameters increased significantly after deflation and were 157%, 26%, and 47%, respectively, above the corresponding control values (INF2). Following delayed deflation, lung expansion accounted for 44%-48% of total post-PNX compensatory increase in exercise DlCO and peak O2 uptake; the remainder fraction is likely attributable to the increase in perfusion. Results suggest that expansion-related parenchyma mechanical stress and perfusion-related microvascular stress contribute in equal proportions to post-PNX alveolar growth and remodeling.
Keywords: mechanical deformation, pulmonary perfusion, mechanical signals, lung resection, lung diffusing capacity
the adult canine lung responds vigorously to pneumonectomy (PNX). The remaining lobes expand asymmetrically to fill the thorax, with a simultaneous increase in perfusion by a factor of 1/(fraction of lung removed). The mechanical stresses deform the bronchovasculature, alveolar tissue, and capillaries and stimulate multiple regulatory pathways involved in tissue growth and remodeling (15, 51, 52), leading to partial restoration of alveolar-capillary tissue volumes, surface areas, lung diffusing capacity (DlCO) as well as exercise capacity (17, 22, 23). The intensity and sources of compensation depend on the intensity of the resection stimuli. Following loss of 42–45% of lung units, recruitment of the remaining microvascular reserves and remodeling of the remaining alveolar septa are the principal mechanisms of functional compensation with a notable absence of new growth of gas exchange tissue (21). Following 55–58% resection, recruitment and remodeling are accompanied by generation of new gas exchange tissue and surface area, resulting in greater compensation per unit of remaining lung (22). Following 65–70% resection, recruitment, remodeling, and growth of the parenchyma are even more vigorous but overall compensation begins to be limited by the need for greater septal structural support and by dysanaptic growth of the conducting bronchovasculature (16, 48). Our group has shown a significant correlation between regional mechanical deformation and long-term compensatory lung growth and remodeling following major lung resection (49). Combined with ample literature that diminished mechanical parenchyma stress impairs lung development (34, 44) whereas excessive mechanical stress causes lung injury and cell death (12), the data suggest the existence of an “optimal range” of tissue stress and strain that is conducive to beneficial remodeling, new alveolar septal growth, and functional compensation without causing tissue damage.
Our goal is to understand the sources and the extent of putative in vivo stimuli for post-PNX compensation as an essential step toward designing rational approaches for inducing or amplifying compensatory lung growth. In earlier studies, we implanted at the time of PNX a space filling, inflatable, silicone prosthesis in the size and shape of the removed right lung (46). The prosthesis was either kept inflated (INF) to prevent mediastinal shift and lateral lung expansion or deflated (DEF) to allow mediastinal shift and lateral expansion. Long-term compensatory responses were 45–80% lower in animals with INF than DEF prosthesis depending on the parameter measured (19, 24, 46), supporting the interpretation that lung expansion was the principal stimulus triggering post-PNX compensation. However, in the presence of INF prosthesis, the remaining lung still enlarged ∼20% in air and tissue volumes mainly via caudal elongation and depression of the hemidiaphragm (46), suggesting that growth did not occur just to fill an empty space but that additional stimuli, e.g., increased perfusion, also compelled lung growth even when space was not readily available. Because (1) the INF prosthesis did not prevent a 2.2-fold post-PNX increase in perfusion to the remaining lung, (2) expansion- and perfusion-related signals were not temporally separated, and (3) the previous results were compared with historical controls rather than to each animal as its own longitudinal control, we could not rule out the possibility that perfusion-related stimuli may have contributed to the observed compensation in the DEF group. In one instance, a leak in the INF prosthesis developed 9 mo post-PNX; the subsequent mediastinal shift and lung expansion was associated with a rising DlCO over several months (46). This observation indicated the feasibility of temporally separating the induction of lung expansion from the immediate post-PNX increase in perfusion. In a subsequent study, delayed prosthesis deflation (3 wk post-PNX) was associated with rapid upregulation of a major signaling network involving hypoxia-inducible factor-1α, erythropoietin receptor, and VEGF (51) that has been implicated in tissue-capillary growth and remodeling. However, long-term structural and functional effects of delayed lung expansion have not been examined.
Here, we hypothesized that both lung expansion and increased perfusion provide in vivo mechanical stimuli for post-PNX compensation. We used the inflatable prosthesis to institute delayed lung expansion long after the immediate post-PNX perfusion changes have stabilized, using each animal as its own longitudinal control.
METHODS
Animals.
The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center approved all protocols. Adult male mixed breed foxhounds (n = 9, 9–12 mo of age, Marshall BioResources, North Rose, NY) were trained to exercise on a treadmill; cardiopulmonary function was measured pre-PNX, followed by right PNX and the implantation of an inflated prosthesis manufactured in the shape and resting expiratory volume of the right lung. Physiological measurements were repeated 4 mo post-PNX with the prosthesis inflated in all animals (INF1). Then the prosthesis was deflated in five animals but remained inflated in four animals, and the physiological studies were repeated 6–8 mo later (DEF and INF2 groups). In one animal a leak in the INF prosthesis was detected 8 mo post-PNX. Physiological data from this animal were excluded, resulting in a total of n = 5 in DEF and n = 3 in INF groups. The timeline of studies is shown in Fig. 1.
Fig. 1.
Time line of the studies is shown for pre- and post- right pneumonectomy (PNX) with inflated prosthesis at 2 time points (INF1 and INF2) and following delayed deflation (DEF) of the prosthesis.
PNX and prosthesis implantation.
These procedures have been described previously (46). The inflatable silicone prosthesis (McGhan Medical, Santa Barbara, CA) was fabricated from magnetic resonance imaging and 3D reconstruction of a normal adult canine right lung at rest. A reinforced patch on the dorsal surface of the prosthesis was connected to a silicone filling tube and a subcutaneous injection port. The animal was fasted overnight, premedicated (acepromazine glycopyrrolate and buprenorphine), anesthetized (isofluorane), intubated, and ventilated. A peripheral intravenous catheter was started, and the chest was shaved and prepared. In a sterile manner the right lung was exposed via lateral thoracotomy in the 5th intercostal space. Lobar blood vessels were ligated and cut. The right main stem bronchus was stapled and cut and the stump was oversewn with loose hilar tissue. The prosthesis was placed in the empty hemithorax, and the filling tube was tunneled through the 4th intercostal space to the injection port buried at the nape of the neck. The prosthesis was inflated with a 50%/50% mixture of sulfur-hexafluoride (SF6) and air to ∼20% above supine end-expiratory lung volume (EELV). This volume maintained the mediastinum at midline and was well tolerated. Topical lidocaine was applied to intercostal nerves and the chest wall closed in five layers. Analgesics were administered regularly for 48 h and then as needed. The animal typically was ambulatory and eating and drinking by the next day. Skin sutures were removed after 10–14 days.
Refilling the prosthesis.
Gas volume was measured weekly for the first month and then monthly by helium dilution using a mass spectrometer (MGA 1100, Perkin Elmer). The desired volume was refilled with the SF6/air mixture. Mediastinal position was verified by chest X-ray. The deflated prosthesis contained a minimal volume of gas (<50 ml) to prevent pleating. Representative imaging of the lung showing the position of mediastinum is shown in Fig. 2.
Fig. 2.
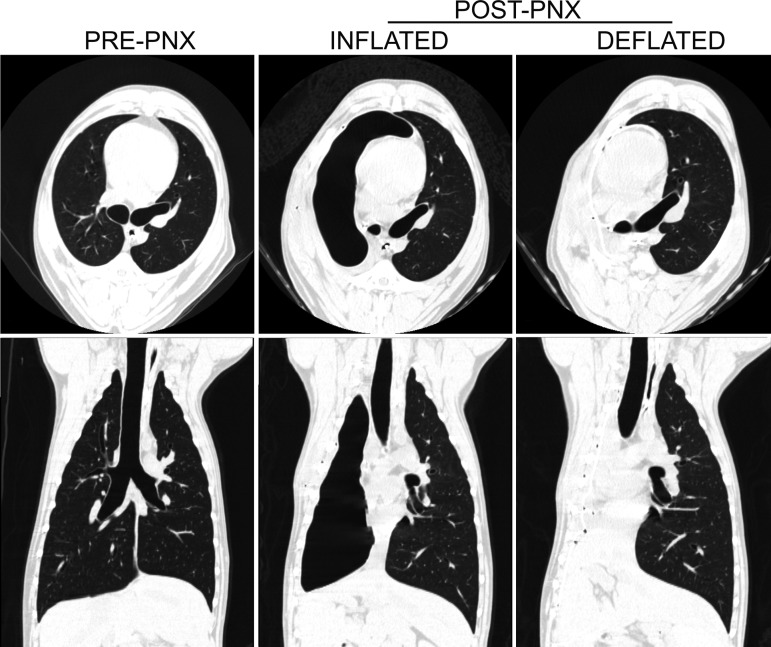
Representative high resolution computed tomography images from 1 animal at baseline (pre-PNX), following right PNX (post-PNX) with inflated prosthesis, and following delayed prosthesis deflation, demonstrating the change in the position of the mediastinum. Images are shown at a transpulmonary pressure of 30 cmH2O.
Exercise training and peak O2 uptake.
The training program (23) began pre-PNX, resumed 3 wk post-PNX, and continued throughout the study, consisting of running on a treadmill 30 min a day, 5 days a week at a workload equivalent to 60–80% of maximal O2 uptake. Animals were considered “trained” when O2 uptake at a given speed/incline was reproducible and a maximum speed and incline was established at which exercise could not be sustained beyond 5 min.
Exercise lung function.
Via a custom respiratory mask (1) and a two-way nonrebreathing valve, inspired and expired ventilation was measured using separate screen pneumotachographs. Expired gas concentrations were monitored by a mass spectrometer distal to a mixing chamber. Breath-by-breath ventilatory parameters were averaged every 10 breaths. ECG and rectal temperature were continuously recorded. Following established methodology (17, 41), an anesthetic bag was prefilled (200 ml ATPD + average tidal volume) with a test gas mixture (0.6% acetylene, 0.3% C18O, 9% helium, and 30% O2 in balance of N2 or 90% O2). At end expiration, the animal inspired and rebreathed from the bag for ∼8 s while gas concentrations at the mouth were monitored. Lung volume (BTPS) was measured from helium dilution, pulmonary blood flow from the logarithmic disappearance of end-tidal acetylene with respect to helium corrected for the intercept of CO disappearance, and DlCO from the logarithmic disappearance of end-tidal CO with respect to helium. End-tidal points were selected from the log-linear portion of the disappearance curves. From DlCO measured at two alveolar O2 tensions (PAO2, mmHg) membrane diffusing capacity (DmCO) and pulmonary capillary blood volume (Vc) were estimated (37):
| (1) |
where θCO is empirical CO uptake by canine whole blood at 37°C [ml·CO·(min·mmHg·ml blood)−1] calculated from mean PAO2 during rebreathing and hemoglobin concentration ([Hb] in g/dl):
| (2) |
and β is a function of rectal temperature (RT; in K):
| (3) |
DlCO was expressed under standard conditions (PAO2 = 120 mmHg and [Hb] = 14.6 g/dl). Gas exchange tissue volume was estimated from the extrapolated intercept of acetylene disappearance; replicate measurements were averaged.
Lung function at rest.
Following established methods (7, 8) the animal was fasted overnight, premedicated, anesthetized, intubated, and mechanically ventilated in the supine position. Esophageal and mouth pressures, rectal temperature, heart rate, and transcutaneous O2 saturation were monitored. Static transpulmonary pressure-lung volume curves were measured using a calibrated syringe to inflate the lung with air (15, 30, 45, and 60 ml/kg above EELV) in incremental and then decremental order. Lung volume, pulmonary blood flow, DlCO, and tissue volume were measured by an established rebreathing technique (8, 36) at two inspired O2 concentrations (21% and 99%) and two lung volumes (30 and 45 ml/kg above EELV) in random order. Duplicate measurements under each condition were averaged. A venous blood sample was drawn before and immediately after the experiment to measure hemoglobin and carboxyhemoglobin concentrations (OSM3, Radiometer, Copenhagen, Denmark).
Data analysis.
Results (means ± SD) were normalized by body weight and compared among groups by one-way ANOVA. Pressure-volume curves and temporal changes were compared by repeated measures ANOVA with post hoc test by Fisher's protected least significant difference. Exercise DlCO was also plotted with respect to pulmonary blood flow and compared at a constant blood flow of 400 ml·(min·kg)−1 by interpolation. Pre-PNX whole lung physiological parameters were multiplied by a factor of 0.42 to derive the expected values for a normal left lung. A P value ≤0.05 was considered significant.
RESULTS
Lung function data are shown at rest (Table 1) and at the highest sustained workload (Table 2) at 3 time points: pre-PNX, ∼4 mo post-PNX (INF1), and 8–12 mo post-PNX (INF2 or DEF) (Table 2). Body weight and resting hemoglobin concentration did not differ with respect to time or intervention.
Table 1.
Resting cardiopulmonary function under anesthesia
| PrePNX | INF1 | PostPNX INF2 | DEF | |
|---|---|---|---|---|
| Body weight, kg | 22.2 ± 3.0 | 21.8 ± 3.4 | 22.6 ± 2.1 | 22.9 ± 3.78 |
| Hemoglobin, g/dl | 12.8 ± 0.6 | 11.7 ± 0.6* | 11.0 ± 1.0* | 11.8 ± 0.92 |
| Heart rate, beats/min | 166 ± 26 | 151 ± 22 | 140 ± 24 | 157 ± 19* |
| EELV, ml/kg | 35 ± 5 | 18 ± 6* | 16 ± 5* | 41 ± 9†‡ |
| EILV, ml/kg | 72 ± 5 | 56 ± 7* | 57 ± 8* | 78 ± 9†‡ |
| Pulmonary blood flow, ml/kg | 122 ± 27 | 90 ± 20* | 92 ± 18* | 133 ± 18*†‡ |
| DlCO, ml·(min·mmHg·kg)−1 | 0.62 ± 0.06 | 0.32 ± 0.07* | 0.32 ± 0.05* | 0.54 ± 0.05*†‡ |
| DmCO, ml·(min·mmHg·kg)−1 | 1.04 ± 0.14 | 0.60 ± 0.24* | 0.57 ± 0.12* | 1.06 ± 0.33†‡ |
| Vc, ml/kg | 2.4 ± 0.6 | 1.2 ± 0.4* | 1.1 ± 0.1* | 1.9 ± 0.3*†‡ |
| Septal (tissue and blood) volume, ml/kg | 7.8 ± 2.6 | 4.3 ± 1.6* | 4.0 ± 1.4* | 5.7 ± 2.0 |
| Extravascular septal tissue volume, ml/kg | 5.8 ± 2.4 | 3.3 ± 1.4* | 3.2 ± 1.4* | 4.2 ± 2.1 |
| Specific lung compliance, ml·(cmH2O·l)−1 | 22.0 ± 3.3 | 32.8 ± 10.9* | 39.6 ± 13.8* | 22.8 ± 7.1‡ |
Values are means ± SD. Inflation volume 30 ml/kg.
P < 0.05 vs. PRE,
vs. INF1,
vs. INF2 by factorial ANOVA.
Table 2.
Cardiopulmonary function at heavy exercise
| PrePNX | INF1 | PostPNX INF2 | DEF | |
|---|---|---|---|---|
| Hemoglobin, g/dl | 13.7 ± 0.9 | 16.5 ± 1.5* | 15.4 ± 1.3 | 15.4 ± 1.4* |
| Respiratory rate, breaths/min | 149 ± 27 | 134 ± 17 | 120 ± 21 | 107 ± 16* |
| Heart rate, beats/min | 247 ± 25§ | 265 ± 15 | 250 ± 11 | 281 ± 18*‡ |
| Ventilation, l·(min·kg)−1 | 7.09 ± 1.37 | 5.36 ± 0.83* | 5.48 ± 1.0* | 5.98 ± 1.19 |
| O2 uptake, ml·(min·kg)−1 | 117 ± 23 | 93 ± 18* | 89 ± 25* | 119 ± 14†‡ |
| CO2 output, ml·(min·kg)−1 | 95 ± 20 | 90 ± 18* | 94 ± 28 | 110 ± 18 |
| EELV, ml/kg | 81 ± 23 | 51 ± 7* | 38 ± 5* | 66 ± 10‡ |
| EILV, ml/kg | 147 ± 22 | 109 ± 12* | 102 ± 12* | 137 ± 14†‡ |
| Pulmonary blood flow, ml·(min·kg)−1 | 491 ± 85 | 438 ± 61 | 459 ± 24 | 467 ± 52 |
| DlCO, ml·(min·mmHg·kg)−1 | 1.31 ± 0.25 | 0.81 ± 0.24* | 0.69 ± 0.2* | 0.87 ± 0.09*‡ |
| DmCO, ml·(min·mmHg·kg)−1 | 1.53 ± 0.22 | 0.94 ± 0.15* | 0.77 ± 0.1* | 1.13 ± 0.05*†‡ |
| Vc, ml/kg | 6.9 ± 2.0 | 4.6 ± 1.7 | 6.5 ± 3.0 | 4.8 ± 2.4 |
| Septal (tissue and blood) volume, ml/kg | 20.7 ± 7.4 | 9.8 ± 4.7* | 9.5 ± 1.0* | 8.9 ± 6.6* |
Values are means ± SD. Pre-PNX peak O2 uptake = 124 ml·(min·kg)−1. Rebreathing measurements were obtained at 80-90% of peak O2 uptake.
P ≤ 0.05 vs. PrePNX,
P ≤ 0.05 versus INF1,
P ≤ 0.05 versus INF2 by factorial ANOVA.
Pre-PNX heart rate data were from a previous age- and sex-matched unoperated cohort (33) measured at the same exercise intensity.
Lung volume.
In the INF group, resting parameters remained relatively stable between INF1 and INF2. Compared with pre-PNX (both lungs), post-PNX resting EELV with inflated prosthesis (INF1) was 50% lower, representing a small (19%) increase in the volume of the initial left lung. Following deflation, EELV normalized with respect to pre-PNX (both lungs), representing a 157% increase compared with the normal left lung or with INF controls (INF2). EILV was reduced 25% post-PNX (INF1) and then remained stable between INF1 and INF2. Following deflation, EILV normalized with respect to pre-PNX, i.e., 35% higher than that in INF controls (INF2). Thus the INF prosthesis largely prevented expansion of the left lung at rest, but allowed expansion to occur upon exercise (Table 2).
Pulmonary blood flow.
At rest, post-PNX blood flow with INF prosthesis (INF1) was 25% lower than pre-PNX (through both lungs) (Table 1), whereas peak exercise blood flow was similar to that pre-PNX (Table 2). These changes represent a sustained perfusion increase of 76–113% above that in the normal left lung. Following deflation, resting blood flow increased to pre-PNX level, whereas exercise blood flow remained unchanged compared with pre-PNX or INF2. Thus the INF prosthesis had mild to no effect on the expected post-PNX increase in perfusion to the remaining lung.
Static lung mechanics.
Post-PNX (INF1 and INF2) pressure-volume relationship was reduced compared with pre-PNX (both lungs), but partially normalized following deflation (Fig. 3, top left). Post-PNX volume of the left lung at a given Ptp (INF1 and INF2) was modestly elevated compared with the pre-PNX left lung. Following deflation, alveolar volume further increased significantly at any given Ptp (Fig. 3, top right). In all groups, post-PNX compliance (CL) of the left lung was higher than that pre-PNX (Fig. 3, bottom left). Specific compliance (Cs) of the left lung increased post-PNX (INF1 and INF2) compared with pre-PNX then normalized following deflation (Fig. 3, bottom right).
Fig. 3.
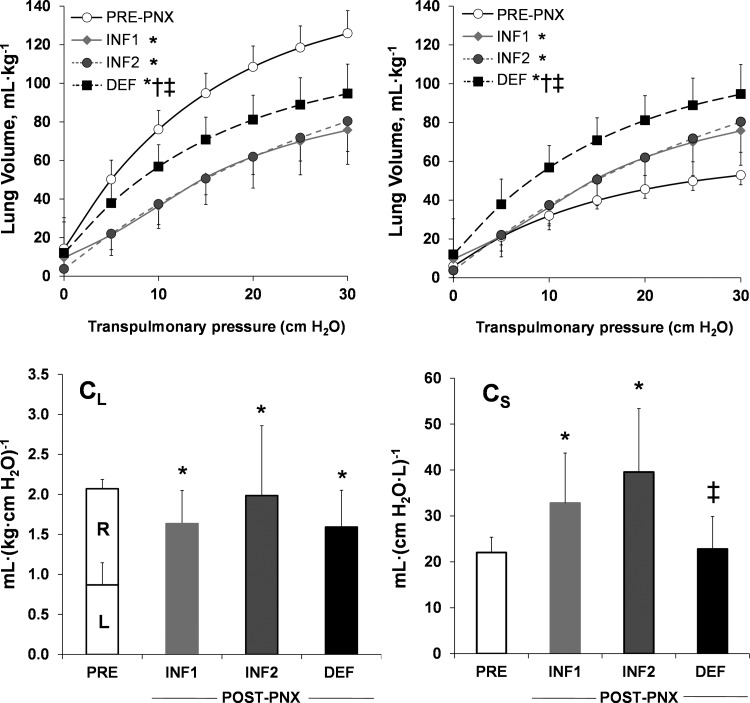
Lung mechanics. Top: static lung volume-transpulmonary pressure relationships are shown pre-PNX, 4 mo post-PNX with INF prosthesis (INF1), and 4 mo following prosthesis deflation (DEF) compared with the simultaneous INF controls (INF2). Pre-PNX curves are shown for the whole lung (top left) or the left lung only (top right). Bottom: whole lung compliance (CL; bottom left) and specific lung compliance (Cs; bottom right) were calculated between 15 and 30 cmH2O of transpulmonary pressure. Partition between the normal left (L) and right (R) lungs is indicated. Means ± SD. P < 0.05 vs. *Pre-PNX, vs. †INF1 by repeated-measures ANOVA, ‡P < 0.05 DEF vs. INF2 by factorial ANOVA.
Exercise lung function.
Post-PNX, exercise hemoglobin concentration (INF1, 16.5 g/dl) was modestly higher than pre-PNX (13.7 g/dl) but not different between INF2 and DEF groups (15.4 g/dl) (Table 2). Compared with pre-PNX, peak exercise minute ventilation, O2 uptake, and CO2 output were modestly (20–25%) lower post-PNX (INF1 and INF2) than pre-PNX. Following deflation exercise O2 uptake and CO2 output normalized compared with INF2, whereas ventilation remained reduced. Post-PNX DlCO, DmCO, Vc, and gas exchange tissue volume at rest were reduced 50% or more (INF1, INF2) compared with pre-PNX, then completely or partially normalized following deflation. Due to technical problems, pre-PNX heart rate data were erratic. Therefore, we used heart rate data measured at the same exercise intensity from a previous cohort of age- and sex-matched unoperated animals (33).
Lung diffusing capacity.
Compared with pre-PNX baseline, post-PNX resting DlCO was similarly reduced by ∼40% at INF1 and INF2 and then normalized following deflation (Fig. 4, left). When compared at a constant exercise blood flow (400 ml·min−1·kg−1), DlCO decreased 36% from pre- to post-PNX (INF1) (Fig. 4, middle and right). In the animals with inflated prosthesis, DlCO further declined slightly (12%) with time (INF1 to INF2) (Fig. 4, middle). In contrast, DlCO increased ∼25% in all animals following deflation (INF1 to DEF) (Fig. 4, right). By 8–12 mo post-PNX, peak exercise DlCO was 26% higher in DEF group compared with INF2 group. Similarly, resting DmCO decreased 40% pre- to post-PNX (INF1) in all animals, remaining stable in the those with inflated prosthesis (INF1 to INF2) while increasing ∼77% in those following deflation (INF1 to DEF), and ultimately was 86% higher in DEF than in INF2 controls (Table 1). Exercise DmCO was reduced ∼40% pre- to post-PNX (INF1) and another 18% (INF1 to INF2) in the inflated prosthesis group. In contrast, DmCO increased ∼20% after deflation (INF1 to DEF), resulting in a value 47% higher compared with INF2 (Table 2). Similarly, resting Vc was reduced (50%) post-PNX (INF1), then either remained stable (INF2) or increased 55% after deflation (Table 1). Exercise Vc was variable and not significantly different among groups (Table 2).
Fig. 4.
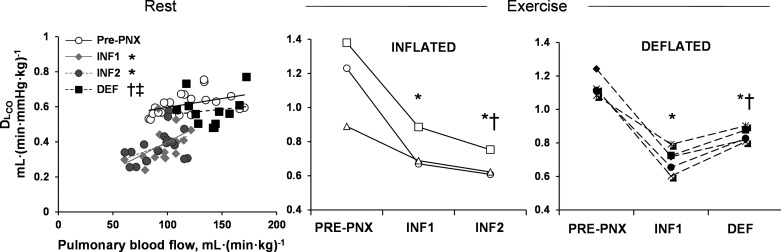
Lung diffusing capacity (DlCO). Left: changes in resting DlCO and pulmonary blood flow are shown pre-PNX and post-PNX with inflated prosthesis (INF1 and INF2) and after prosthesis deflation (DEF). Middle and right: changes in exercise DlCO at a constant pulmonary blood flow [400 ml·(min·kg)−1] were compared between individual animals in INF and DEF groups. P ≤0.05 vs. *Pre-PNX, vs. †INF1 by repeated-measures ANOVA. ‡P < 0.05 DEF vs. INF2 by factorial ANOVA.
Relative compensation.
Post-PNX responses were expressed as ratios to that expected in the normal left lung: (0.42 × pre-PNX value). The contribution of lung expansion to overall compensation was estimated as:
| (4) |
At a constant pulmonary blood flow of 400 ml·min−1·kg−1, delayed lung expansion accounted for 88% of the total post-PNX increase in EELV of the left lung. Delayed expansion also accounted to 47%, 48%, and 44% of the total post-PNX increases in EILV, DlCO, and O2 uptake, respectively, of the left lung (Fig. 5).
Fig. 5.
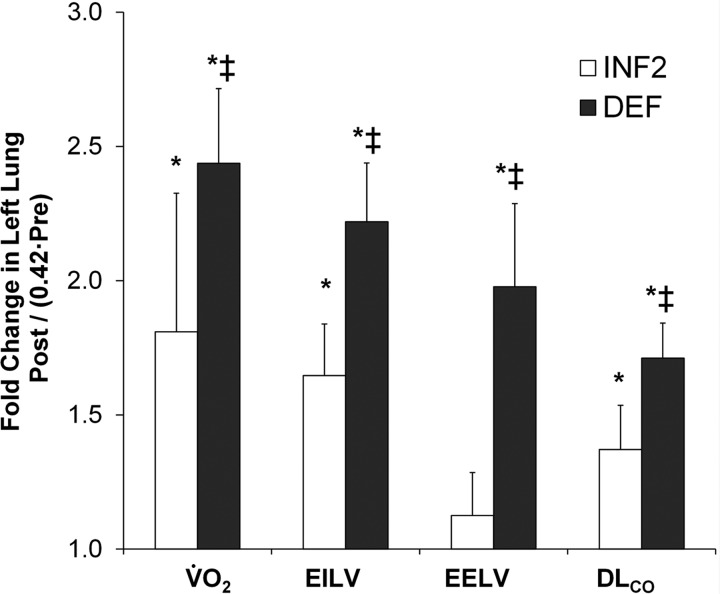
Post-PNX physiological compensation in the remaining left lung were estimated from peak O2 uptake (V̇o2), end-inspiratory and end-expiratory lung volumes (EILV and EELV), and DlCO [at pulmonary blood flow = 400 ml·(min·kg)−1], expressed as a ratio to that of the normal left lung (0.42% to pre-PNX value for 2 lungs). The differences between inflated (INF2) and deflated (DEF) groups reflect the contribution from delayed lung expansion. *P ≤ 0.05 vs. normal left lung (1.0) by repeated measures ANOVA, ‡DEF vs. INF2 by factorial ANOVA.
DISCUSSION
Summary of results.
This study temporally separated post-PNX expansion-related responses from those related to the increase in perfusion in adult canine lung and estimated their respective contribution to overall functional compensation. The inflated prosthesis prevented expansion of the remaining left lung at rest while allowing expansion to occur upon exercise. The prosthesis did not prevent the post-PNX increase in pulmonary blood flow at rest or exercise (∼76% and ∼112%, respectively). Following delayed deflation of prosthesis, EELV increased markedly (by 157% and 76% at rest and exercise, respectively) above that in controls at the same time point (INF2). The vigorous post-deflation expansion was associated with a further 68% increase in resting DlCO due to roughly proportional increases in both DmCO and Vc, reflecting preserved alveolar microvascular recruitment. Overall, delayed expansion of the left lung accounted for 44–48% of the total long-term physiological compensation. Because the remainder is most likely related to the sustained post-PNX increase in perfusion per unit of the initial left lung, these results support the interpretation that tissue and microvascular mechanical stresses contribute equally to post-PNX compensation.
Critique of methodology.
A longitudinal experimental design with each animal as its own control was an important feature that enabled us to obtain consistent results with conservative use of animals. Our earlier studies (19, 24, 46) established the feasibility of using the space-filling inflatable silicone prosthesis to limit post-PNX mediastinal shift and lung expansion. Wound healing was complete by 2–3 wk post-PNX. The interval volume change in the prosthesis between fillings was no more than ∼20%. The relatively compliant prosthesis conformed to the chest cavity to minimize the risk of mediastinal compression especially during exercise. The animals were able to sustain heavy exercise post-PNX without clinical adverse effects for more than 1 yr. Although post-PNX resting pulmonary blood flow under anesthesia was ∼25% lower in the presence of inflated prosthesis, upon exercise pulmonary blood flow increased to the same level as pre-PNX. Physiological parameters remained relatively stable between 4 and 8 mo post-PNX (INF1 to INF2) with a small (15–18%) reduction in exercise DlCO and DmCO. Following deflation, lung expansion was not impeded, with lung volumes returning to 80–100% of that in two normal lungs. At postmortem, the pleural surfaces in contact with the prosthesis were thickened without any adhesion.
Effects of lung expansion.
Stretching lung cells in vitro induces wide-ranging changes, including ion flux (29), gene expression of surfactant-associated proteins (38) and growth factors (26, 45), signal transduction molecules (5, 27), apoptosis (39), cell proliferation (5, 28), and turnover of matrix (2, 47) and cytoskeletal proteins (40). Lung distention in vivo is associated with activation or upregulation of major homeostatic pathways (6, 38, 51, 52), the generation of protein and DNA (34, 53), and new alveolar tissue; the latter occurs preferentially at the lung periphery (11, 31) where mechanical stresses are borne mainly by the septa. Diminished mechanical lung stress is associated with impaired lung development, whereas excessive stress induces cell injury and death (10). Tissue deformation quickly alters permeability, leading to fluxes of ions, fluid, growth factors, and cells. Mechanical stresses transduce nuclear transcriptional factors via the cytoskeleton to directly or indirectly activate cell proliferation, gene transcription, and growth-related regulatory pathways (35). By using high-resolution computed tomography and nonrigid registration of anatomical features, we recently mapped the heterogeneous distribution of regional parenchyma deformation during passive positive pressure inflation in the canine lung before and after removal of 70% of lung units (18, 49), directly illustrating an in vivo correlation between lung expansion and mechanical strain.
In addition to the above short-term events, post-PNX mechanical stresses likely modulate progressive remodeling of acinar and alveolar septal components that determine complex architectural features such as the volume proportions among respiratory bronchioles; alveolar ducts and alveolar sacs; the distribution of epithelial surface folds; the proportion and arrangement of interstitial cells, matrix, and fibers within the septum, as well as between the thin and thick sides of the gas exchange barrier; the matching between alveolar and capillary gas exchange surfaces; and the distribution of tissue surfaces with respect to red blood cell flow. Remodeling events occur over weeks to months, regardless of whether new alveolar tissue is generated, but can be difficult to quantify. Our recent canine studies suggest that post-PNX remodeling slowly improves the efficiency of gas exchange across the diffusion barrier over many months (36). Following 70% lung resection, an initial period of vigorous tissue regrowth is followed by ongoing heterogeneous changes in the distribution of regional deformation of the remaining parenchyma during inflation, ultimately leading to a reduction in lobar mechanical strain and an improvement in the mechanical function of the remaining lung (49).
We previously found that the expected post-PNX changes in lung volume, exercise lung function, and septal tissue volumes were 20–70% higher in animals with continuously deflated prosthesis compared with those with continuously inflated prosthesis (19, 46). Ventilation-perfusion matching was not altered by the presence of inflated or deflated prosthesis (24). However, because the prosthesis remained either inflated or deflated from the time of lung resection, the expansion- and perfusion-related stimuli were not temporally separated. When lateral expansion was prevented post-PNX, the remaining lung expanded ∼20% in the caudal direction by displacing the diaphragm (46), suggesting a response to stimuli other than expansion. Longitudinal pre-PNX controls were not available in the previous cohort, so we could not quantify the residual compensation in the animals with deflated prosthesis that were attributable to perfusion changes. The present study design directly addressed these issues. Our estimate of expansion-related contribution to post-PNX compensation (∼50%) represents a conservative lower limit.
Nonexpansion related post-PNX stimuli.
The ∼50% of total compensation not explained by lung expansion must be attributed to other stimuli; a principal candidate is the perfusion increase to the left lung, which imposes cyclic microvascular distention (circumferential stress) and fluid shear stress. Following 58% resection, pulmonary perfusion and capillary blood volume per unit of the remaining lung would theoretically increase by a factor of 1/0.42 = 2.38 at a given cardiac output. The measured increase in left lung perfusion (a factor of 2.13 at exercise) was 89% of the theoretical estimate. Following PNX, right heart systolic and pulmonary arterial pressure are normal at rest but increase at an accelerated rate upon exercise (3, 20, 42). Both the distending and shear stress synergistically induce morphological changes in endothelial cells (54) with distinct patterns of gene expression (9) that include pathways of angiogenesis (4) and arteriogenesis (14). Increased perfusion could stimulate neocapillarization by the process of intussusception—transluminal pillar formation (30) with subsequent division and lengthening of the capillary segment and morphological transition from double- to single-capillary profiles—resulting in increases in volume and capillary surface area.
Like expansion, perfusion is not a simple factor. Microvascular distention and distortion may act directly or via alterations in local capillary-tissue O2 tension gradients to transduce a complex network of endothelial cell and smooth muscle responses (9, 50, 54). There is likely a threshold of perfusion stress that must be exceeded before local cellular responses are stimulated. Lung expansion may accentuate the effects of a given change in perfusion. Depending on its nature, magnitude, and distribution, mechanical stress on endothelial cells may be beneficial or detrimental. There is likely an optimal range of microvascular distending or shear stress within which beneficial adaptation occurs without evidence of cell damage; this range remains to be defined in vivo. In newborn pig, ligation of one pulmonary artery increases the perfusion to and augments the alveolar growth in the contralateral lung (13). In contrast, pulmonary capillary congestion induced by chronic heart failure causes alveolar septal thickening (25, 43), which impedes O2 diffusion. Only one study has attempted to directly alter blood flow following PNX—in ferrets banding one lobar pulmonary artery had no effect on post-PNX DNA and protein content of the banded or unbanded lobe (32)—however, lobar blood flow was not assessed and the signaling events, alveolar structure, and function were not measured. Despite the possible synergistic interactions between perfusion- and expansion-related signals, future studies that directly manipulate in vivo lobar perfusion could yield more specific information about its role on growth-related events.
Conclusion.
Post-PNX expansion of the remaining lung, temporally isolated from the early increase in perfusion, explains approximately half of the long-term compensatory response assessed by physiological methods at rest and exercise. This estimate is slightly (up to ∼20%) lower than that estimated indirectly in animals with continually inflated prosthesis. The residual half of the compensatory response is most likely stimulated by a sustained increase in perfusion to the remaining lung. Although a role for nonmechanical stimuli, e.g., exercise-induced hypoxemia, cannot be ruled out, such contribution is likely to be minor. These results underscore the additive importance of different types of in vivo mechanical signals in the stimulation and maintenance of physiological recruitment and compensation in the adult lung. Understanding these signals has important practical implications for the design and testing of potential therapeutic interventions aimed at amplifying specific mechanosensitive pathways to enhance the innate potential for regrowth and adaptation of the remaining lung units in destructive lung disease.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants RO1-HL40070 and UO1-HL111146.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.M.D., C.Y., A.S.E., and C.C.H. performed experiments; D.M.D., C.Y., and C.C.H. analyzed data; D.M.D., C.Y., and C.C.H. interpreted results of experiments; D.M.D., C.Y., and C.C.H. prepared figures; D.M.D., C.Y., A.S.E., and C.C.H. approved final version of manuscript; C.C.H. conception and design of research; C.C.H. and D.M.D. drafted manuscript; D.M.D., C.Y., and C.C.H. edited and revised manuscript.
ACKNOWLEDGMENTS
We express our gratitude to our mentor, the late Dr. Robert L. Johnson, Jr. We appreciate the technical assistance of Jennifer Fehmel, Myresa Hurst, Corie Thorson, the veterinary assistance by the staff of the Animal Resources Center, and the imaging support by Greg Horton and the Dept. of Radiology.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or of the National Institutes of Health.
REFERENCES
- 1. Ampil J, Carlin JI, Johnson RL., Jr A mouthpiece face mask for the exercising dog. J Appl Physiol 64: 2240–2244, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol 88: 203–209, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Burrows B, Harrison RW, Adams WE, Humphreys EM, Long ET. The postpneumonectomy state. Clinical and physiologic observations in thirty-six cases. Am J Med 28: 281–297, 1960 [DOI] [PubMed] [Google Scholar]
- 4. Chang H, Shyu KG, Wang BW, Kuan P. Regulation of hypoxia-inducible factor-1alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 105: 447–456, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chess PR, Toia L, Finkelstein JN. Mechanical strain-induced proliferation and signaling in pulmonary epithelial H441 cells. Am J Physiol Lung Cell Mol Physiol 279: L43–L51, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Cloutier M, Maltais F, Piedboeuf B. Increased distension stimulates distal capillary growth as well as expression of specific angiogenesis genes in fetal mouse lungs. Exp Lung Res 34: 101–113, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Dane DM, Johnson RL, Jr, Hsia CCW. Dysanaptic growth of conducting airways after pneumonectomy assessed by CT scan. J Appl Physiol 93: 1235–1242, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Dane DM, Yan X, Tamhane RM, Johnson RL, Jr, Estrera AS, Hogg DC, Hogg RT, Hsia CCW. Retinoic acid-induced alveolar cellular growth does not improve function after right pneumonectomy. J Appl Physiol 96: 1090–1096, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Felder E, Siebenbrunner M, Busch T, Fois G, Miklavc P, Walther P, Dietl P. Mechanical strain of alveolar type II cells in culture: changes in the transcellular cytokeratin network and adaptations. Am J Physiol Lung Cell Mol Physiol 295: L849–L857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foster DJ, Yan X, Bellotto DJ, Moe OW, Hagler HK, Estrera AS, Hsia CCW. Expression of epidermal growth factor and surfactant proteins during postnatal and compensatory lung growth. Am J Physiol Lung Cell Mol Physiol 283: L981–L990, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med 38: S539–548, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Haworth SG, McKenzie SA, Fitzpatrick ML. Alveolar development after ligation of left pulmonary artery in newborn pig: clinical relevance to unilateral pulmonary artery. Thorax 36: 938–943, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10: 45–55, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol 97: 1992–1998, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hsia CC, Dane DM, Estrera AS, Wagner HE, Wagner PD, Johnson RL., Jr Shifting sources of functional limitation following extensive (70%) lung resection. J Appl Physiol 104: 1069–1079, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hsia CC, Herazo LF, Ramanathan M, Johnson RL., Jr Cardiopulmonary adaptations to pneumonectomy in dogs. IV Membrane diffusing capacity and capillary blood volume. J Appl Physiol 77: 998–1005, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Hsia CC, Tawhai MH. What can imaging tell us about physiology? Lung growth and regional mechanical strain. J Appl Physiol 113: 937–946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsia CC, Wu EY, Wagner E, Weibel ER. Preventing mediastinal shift after pneumonectomy impairs regenerative alveolar tissue growth. Am J Physiol Lung Cell Mol Physiol 281: L1279–L1287, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hsia CCW, Carlin JI, Cassidy SS, Ramanathan M, Johnson RL., Jr Hemodynamic changes after pneumonectomy in the exercising foxhound. J Appl Physiol 69: 51–57, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Hsia CCW, Fryder-Doffey F, Stalder-Navarro V, Johnson RL, Jr, Weibel ER. Structural changes underlying compensatory increase of diffusing capacity after left pneumonectomy in adult dogs [Published erratum in J Clin Invest 93: 913, 1994]. J Clin Invest 92: 758–764, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsia CCW, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest 94: 405–412, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsia CCW, Herazo LF, Johnson RL., Jr Cardiopulmonary adaptations to pneumonectomy in dogs. I Maximal exercise performance. J Appl Physiol 73: 362–367, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Hsia CCW, Johnson RL, Jr, Wu EY, Estrera AS, Wagner H, Wagner PD. Reducing lung strain after pneumonectomy impairs diffusing capacity but not ventilation-perfusion matching. J Appl Physiol 95: 1370–1378, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Huang W, Kingsbury MP, Turner MA, Donnelly JL, Flores NA, Sheridan DJ. Capillary filtration is reduced in lungs adapted to chronic heart failure: morphological and haemodynamic correlates. Cardiovasc Res 49: 207–217, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Liu M, Liu J, Buch S, Tanswell AK, Post M. Antisense oligonucleotides for PDGF-B and its receptor inhibit mechanical strain-induced fetal lung cell growth. Am J Physiol Lung Cell Mol Physiol 269: L178–L184, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Liu M, Qin Y, Liu J, Tanswell AK, Post M. Mechanical strain induces pp60src activation and translocation to cytoskeleton in fetal rat lung cells. J Biol Chem 271: 7066–7071, 1996 [PubMed] [Google Scholar]
- 28. Liu M, Skinner SJ, Xu J, Han RN, Tanswell AK, Post M. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol Lung Cell Mol Physiol 263: L376–L383, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Liu M, Xu J, Tanswell AK, Post M. Inhibition of mechanical strain-induced fetal rat lung cell proliferation by gadolinium, a stretch-activated channel blocker. J Cell Physiol 161: 501–507, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Makanya AN, Hlushchuk R, Baum O, Velinov N, Ochs M, Djonov V. Microvascular endowment in the developing chicken embryo lung. Am J Physiol Lung Cell Mol Physiol 292: L1136–L1146, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Massaro GD, Massaro D. Postnatal lung growth: evidence that the gas-exchange region grows fastest at the periphery. Am J Physiol Lung Cell Mol Physiol 265: L319–L322, 1993 [DOI] [PubMed] [Google Scholar]
- 32. McBride JT, Kirchner KK, Russ G, Finkelstein J. Role of pulmonary blood flow in postpneumonectomy lung growth. J Appl Physiol 73: 2448–2451, 1992 [DOI] [PubMed] [Google Scholar]
- 33. McDonough P, Dane DM, Hsia CC, Yilmaz C, Johnson RL., Jr Long-term enhancement of pulmonary gas exchange after high-altitude residence during maturation. J Appl Physiol 100: 474–481, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Nobuhara KK, Fauza DO, DiFiore JW, Hines MH, Fackler JC, Slavin R, Hirschl R, Wilson JM. Continuous intrapulmonary distension with perfluorocarbon accelerates neonatal (but not adult) lung growth. J Pediatr Surg 33: 292–298, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Papaiahgari S, Yerrapureddy A, Hassoun PM, Garcia JG, Birukov KG, Reddy SP. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol 36: 304–312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravikumar P, Dane DM, McDonough P, Yilmaz C, Estrera AS, Hsia CC. Long-term post-pneumonectomy pulmonary adaptation following all-trans-retinoic acid supplementation. J Appl Physiol 110: 764–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roughton FJW, Forster RE, Cander L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol 11: 269–276, 1957 [DOI] [PubMed] [Google Scholar]
- 38. Sanchez-Esteban J, Tsai SW, Sang J, Qin J, Torday JS, Rubin LP. Effects of mechanical forces on lung-specific gene expression. Am J Med Sci 316: 200–204, 1998 [PubMed] [Google Scholar]
- 39. Sanchez-Esteban J, Wang Y, Cicchiello LA, Rubin LP. Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 282: L448–L456, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Smith PG, Moreno R, Ikebe M. Strain increases airway smooth muscle contractile and cytoskeletal proteins in vitro. Am J Physiol Lung Cell Mol Physiol 272: L20–L27, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Takeda S, Hsia CCW, Wagner E, Ramanathan M, Estrera AS, Weibel ER. Compensatory alveolar growth normalizes gas exchange function in immature dogs after pneumonectomy. J Appl Physiol 86: 1301–1310, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Takeda S, Ramanathan M, Estrera AS, Hsia CCW. Postpneumonectomy alveolar growth does not normalize hemodynamic and mechanical function. J Appl Physiol 87: 491–497, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability. Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circ Res 77: 317–325, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Walker GM, Kasem KF, O'Toole SJ, Watt A, Skeoch CH, Davis CF. Early perfluorodecalin lung distension in infants with congenital diaphragmatic hernia. J Pediatr Surg 38: 17–20, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Waters CM, Chang JY, Glucksberg MR, DePaola N, Grotberg JB. Mechanical forces alter growth factor release by pleural mesothelial cells. Am J Physiol Lung Cell Mol Physiol 272: L552–L557, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Wu EY, Hsia CC, Estrera AS, Epstein RH, Ramanathan M, Johnson RL., Jr Preventing mediastinal shift after pneumonectomy does not abolish physiologic compensation. J Appl Physiol 89: 182–191, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Xu J, Liu M, Liu J, Caniggia I, Post M. Mechanical strain induces constitutive and regulated secretion of glycosaminoglycans and proteoglycans in fetal lung cells. J Cell Sci 109: 1605–1613, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Yilmaz C, Ravikumar P, Dane DM, Bellotto DJ, Johnson RL, Jr, Hsia CC. Noninvasive quantification of heterogeneous lung growth following extensive lung resection by high-resolution computed tomography. J Appl Physiol 107: 1569–1578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yilmaz C, Tustison NJ, Dane DM, Ravikumar P, Takahashi M, Gee JC, Hsia CC. Progressive adaptation in regional parenchyma mechanics following extensive lung resection assessed by functional computed tomography. J Appl Physiol 111: 1150–1158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoshizumi M, Abe J, Tsuchiya K, Berk BC, Tamaki T. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharm Sci 91: 172–176, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Q, Bellotto DJ, Ravikumar P, Moe OW, Hogg RT, Hogg DC, Estrera AS, Johnson RL, Jr, Hsia CC. Postpneumonectomy lung expansion elicits hypoxia-inducible factor-1alpha signaling. Am J Physiol Lung Cell Mol Physiol 293: L497–L504, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Zhang Q, Moe OW, Garcia JA, Hsia CC. Regulated expression of hypoxia-inducible factors during postnatal and postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 290: L880–L889, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol 81: 1471–1476, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Zhao S, Suciu A, Ziegler T, Moore JE, Jr, Burki E, Meister JJ, Brunner HR. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol 15: 1781–1786, 1995 [DOI] [PubMed] [Google Scholar]


