Abstract
We examined the effects of mild therapeutic exercise during a period of inactivity on size and contractile functions of myosin heavy chain (MHC) type I (n = 204) and type II (n = 419) single fibers from the medial gastrocnemius in three age groups. Young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) F344BNF1 rats were assigned to one of three groups: weight-bearing control, non-weight bearing (NWB), and NWB plus exercise (NWBX). Fourteen days of hindlimb suspension were applied in NWB rats. The NWBX rats exercised on the treadmill for 15 min, four times a day, during the period of NWB. The NWBX did not improve peak power, but increased normalized power of MHC type I fibers in young adult rats. In MHC type II fibers, NWBX did not change peak power, isometric maximal force, Vmax, and fiber size from young adult and middle-aged rats. NWBX did not improve peak power and isometric maximal force and showed a dramatic decline in Vmax and normalized power in the old rats. Collectively, mild treadmill exercise during a period of inactivity does not improve peak power of MHC type I or type II fiber from the gastrocnemius in young, middle-aged, and old rats. However, NWBX is beneficial in enhancing normalized power of MHC type I fibers in young adult rats, most likely due to the stimulus intensity and the ability of the individual fibers to adapt to the stimulus. In contrast, several factors, such as impaired adaptation potential, inappropriate exercise intensity, or increased susceptibility to muscle damage, may contribute to the lack of improvement in the older rats.
Keywords: aging, exercise, inactivity, single-fiber physiology
muscle atrophy and contractile dysfunction in skeletal muscle are known to be serious problems during periods of inactivity, such as bed rest and immobilization (9, 12). The deleterious effects of inactivity on contractile function in skeletal muscle are more markedly elevated in the elderly compared with young individuals due to the presence of sarcopenia, which is age-related loss of muscle mass and function (3, 16). The combined effects of inactivity and age, therefore, may result in further muscle deterioration and poor rehabilitation potential.
The extent of decline in contractile function with inactivity and age is muscle and fiber-type specific (35). For example, periods of inactivity [i.e., spaceflight in humans and hindlimb suspension (HS) in rats] result in a significant reduction in muscle size and strength, with the greatest change observed in the soleus muscle, which is composed predominantly of myosin heavy chain (MHC) type I fibers, whereas less change is observed in the gastrocnemius muscle, a muscle composed predominantly of MHC type II fibers (35). The greater decline in fiber size and contractile function with inactivity of the soleus muscle compared with the gastrocnemius muscle is attributed to its load-bearing role during standing and the subsequent reduced activation of the muscle with unloading (35). In contrast, age-associated decline in fiber size and function are most prominent in MHC type II fibers, whereas MHC type I fibers are resistant to age-related atrophy until very old age (36 mo old in Fischer 344 BNF1 rats) (5, 35). Given that inactivity and age-induced detrimental effects are muscle and fiber-type dependent, it is imperative to design therapeutic exercise treatments that improve contractile properties of both MHC type I and MHC type II fibers.
Muscle power is a key predictor of contractile performance and represents work output per unit time. The loss of muscle power is a common characteristic of aged individuals and correlates with a reduction of strength and/or contraction speed. An impairment of muscle power generation precipitates serious complications, such as fall injury, decreased gait speed, morbidity, and poor quality of life in the older population (6, 10, 23, 30). Despite the many investigations focused on age-associated changes in power generation of whole muscle, very little is known about the combined effects of inactivity and aging on power generation in single-muscle fibers. Because of the fiber-type-specific responses to age and inactivity, investigating power generation at the single-fiber level will provide important information requisite for developing rehabilitation exercise protocols.
Mild exercise, in the form of isometric (i.e., standing) or isotonic (i.e., walking), is suggested as one of the effective countermeasures to attenuate inactivity- and age-induced muscle deterioration. Isometric weight-bearing exercise during a period of inactivity is beneficial in improving contractile properties in MHC type I fibers in adult and old-aged rats. For example, isometric weight-bearing exercise attenuated the inactivity-induced decline in fiber size, force, and peak power generation in MHC type I soleus of adult rats (14). Moreover, the benefits of weight-bearing exercise are reported for the MHC type I fibers in both soleus and gastrocnemius muscles of old rats (1, 31). However, this exercise protocol (isometric contractions) is not effective in improving contractile function in MHC type II fibers from the gastrocnemius muscle of old rats (1, 31).
In contrast to isometric weight-bearing exercises, mild treadmill therapeutic exercise (isotonic contractions) is reported to attenuate the inactivity-induced decline in power generation in MHC type I fibers from the soleus in young adult and middle-aged rats (20). The mild treadmill exercise is suggested to recruit the gastrocnemius muscle composed of a mixture of MHC type I and MHC type II fibers (3). Specifically, during walking (i.e., mild treadmill exercise), the gastrocnemius muscle is recruited at the end of the swing phase, whereas the soleus muscle is activated in the stance phase (8). Hence, it is reasonable to predict that this form of exercise may be effective in improving contractile function in both MHC type I and MHC type II fibers of the gastrocnemius muscle.
Therefore, the primary purpose of this study was to investigate the effects of a mild therapeutic exercise during a period of inactivity on fiber size and contractile parameters (e.g., peak power, normalized power) in MHC type I and MHC type II fibers of the gastrocnemius muscle with age. We hypothesized that mild treadmill exercise would attenuate inactivity-induced decline in fiber size and function. Because sarcopenia is associated with impaired adaptive responses of muscle growth and function to exercise, we further postulated that the benefits of the therapeutic exercise would be age specific (15, 18, 27, 34).
METHODS
Animals.
The animal protocol for this study was approved by Animal Care and Use Committee at the University of Minnesota. Male Fischer 344 Brown Norway F1 Hybrid (F344BNF1) rats were purchased from National Institute on Aging colony (Harlan, IN) and housed individually in 40 in. × 40 in. × 7 in. cage. Rats were divided into young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) groups. Each group was determined by average survival rates from large populations of the F344BNF1 species representing survival rates of 95, 80–50, and <50%, respectively (40). It has been noted that this rodent strain has a very long lifespan. All animals were housed in a temperature-controlled room (20 ± 1°C) with a 12:12-h light-dark cycle. Purina Rodent Chow and tap water were provided ad libitum. Rats were acclimatized in the animal facility at least 1 wk and were randomly assigned to one of three groups: 14 days of normal weight bearing for the control group (Con; total n = 21), 14 days of HS for the non-weight-bearing group (NWB; total n = 14), and 14 days of HS plus mild treadmill exercise group (NWBX; total n = 13).
HS for non-weight bearing.
To examine the effect of inactivity on fiber size and contractile properties with age and fiber type, we used the HS model. HS is the preferred animal model to mimic the clinical condition of bed rest and inactivity (1). Animals in the NWB and NWBX groups were hindlimb suspended for a 2-wk period. The procedure of HS was followed, as previously described (1, 43). Briefly, the hindlimbs were elevated to a spinal orientation of 40–45° above horizontal using a harness with orthopedic traction tape wrapped around the proximal two-thirds of the tail. The height of the suspension was vertically adjusted so that the hindlimbs could not contact the cage floor, while forelimbs were in contact with the floor, allowing movement and access to food and water.
Therapeutic treadmill exercise.
To determine the effect of therapeutic exercise during a period of inactivity on fiber size and contractile properties with age and fiber type, we used the mild treadmill exercise protocol as described previously (20). Briefly, the animals in NWBX group were taken down from suspension, exercised on a motor-driven treadmill four times a day, ∼15 min each session, and subsequently resuspended during the 2 wk of HS. Each exercise protocol started in the morning (8–10 AM) and repeated every 2 h over a period of 8 h daily. The average speed of the treadmill exercise was 342 cm/min at a 0° incline and was tolerated by all three different age groups.
Bundle preparation and single-fiber isolation.
Following the experimental period, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (35 mg/kg body wt). Rats in the NWB group were anesthetized while still suspended to avoid risk of reloading-induced muscle injury. The gastrocnemius muscles were rapidly isolated and trimmed clean of excess fat and connective tissue. The muscles were weighed and prepared for single-muscle fiber contractile measures, as previously described (44). Briefly, immediately after dissection, the muscle was placed in ice-cold relaxing solution composed of the following: 20 mM imidazole (pH = 7.0), 7.0 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5.4 mM MgCl2, 14.5 mM creatine phosphate, 4.7 mM ATP, and CaCl2 to achieve pCa 9.0. The relaxing solution included KCl to reach an ionic strength of 180 mM. The medial parts of each gastrocnemius muscle were divided into small bundles composed of ∼50–100 fibers under microscope. To maintain optimal length of the muscle fibers, each end of the bundles was tied with surgical suture (4–0) to glass capillary tubes. Bundles then were stored in skinning solution containing 20 mM imidazole (pH = 7.0), 125 mM potassium-propionate, 2 mM EGTA, 4 mM ATP, 1 mM MgCl2, and 50% glycerol (vol/vol) at −20°C. At the time of the experiment, single-muscle fibers were isolated from a fiber bundle under a dissecting microscope and transferred immediately to an experimental bath filled with relaxing solution.
Determination of fiber length and fiber diameter.
The single-fiber segment (2–3 mm) was mounted between a force transducer (Cambridge Model 400A, sensitivity 2 mV/mg) and a lever controller (Cambridge Model 300H) using microtweezers. The sarcomere length was adjusted to 2.5 μm by moving the microscope stage with a micrometer so that the fiber segment moved across the visual field of the eyepiece under the inverted microscope (×600) (43). Then fiber length (Lo) was determined from the micrometer displacement. Fiber diameter was measured as the average width of three places along the length of the fiber segment, and the fiber cross-sectional area (CSA) was calculated from the mean width, assuming the fiber forms a circular cross section (3).
Determination of isometric maximal force and specific tension (isometric maximal force/CSA).
To determine whether single-fiber contractile function, including isometric maximal force (Po) and specific tension, is influenced by inactivity, aging, and therapeutic exercise, we performed single-muscle fiber physiology experiments. After measuring Lo and fiber diameter, the fiber was then transferred from relaxing solution (pCa = 9.0) to activating solution (pCa = 4.5) containing 20 mM imidazole, 7.0 mM EGTA, 5.4 mM MgCl2, 14.5 mM creatine phosphate, 4.7 mM ATP, and CaCl2. The activating solution included KCl to reach an ionic strength of 180 mM. Output information of force during activation periods was recorded on a digital storage oscilloscope (Nicolet 310) and custom software. Po (μN) was determined when the force output reached a plateau. Specific tension (Po/CSA; kN/m2) was calculated by the normalization of Po by CSA. Throughout the single-muscle fiber contractility tests, the temperature of relaxing and activating solutions was set at 15°C.
Determination of power and maximal shortening velocity.
To determine the effect of exercise during a period of inactivity on single-muscle fiber power and maximal shortening velocity (Vmax), we used the isotonic load-clamping test, as previously described (41). Briefly, when each fiber was fully activated in activating solution, the lever controller then rapidly stepped the fiber to three successive submaximal isotonic loads. The duration of each isotonic load step was 100 ms, and shortening velocity and force were measured over the last 30 ms in each step. After the third isotonic load step, the fiber was moved back to relaxing solution and extended to Lo. The entire procedure was repeated five to six times at different loads until each single fiber was subjected to a total of 15–18 isotonic contractions. The output data obtained from the isotonic contractions were fit with the hyperbolic Hill equation: (P + a)(V + b) = (Po + a)b, where P is the force during isotonic load clamping, V is velocity, Po is developed before the submaximal isotonic load clamps, and a and b are constants of force and velocity, respectively. Vmax was determined from the extrapolated intercept of the force-velocity relationship with the velocity axis. Only individual fibers that had r2 > 0.98, showed no visual damage, and did not decline >20% in maximal force-generating capacity during the experimental protocol were included in the database. Absolute peak power [μN·fiber length (FL)·s−1] was determined in terms of the fitted force-velocity parameters (Po, Vmax, and a/Po) when the product of force and shortening velocity is maximized. Normalized power (kN·m−2·FL·s−1) was determined from the product of normalized force (force per CSA; kN/m2) and shortening velocity (FL/s).
Determination of MHC compositions.
Following the single-muscle fiber physiology experiments, the MHC compositions of each fiber were determined by SDS-PAGE gel electrophoresis and silver staining method, as described previously (44). Briefly, the fiber segment was carefully removed from the experimental apparatus and solubilized in 50 μl of sample buffer (24 mM EDTA, 60 mM Tris, 1% SDS, 5% β-mercaptoethanol, 15% glycerol, 2 mg/ml bromophenol blue, pH 6.8). The samples were heated at 95°C for 4 min and centrifuged at 8,000 rpm for 2 min. Ten microliters of sample were loaded on an SDS-PAGE electrophoresis system (Hoefer SE 600) that consisted of a 4% (wt/vol) acrylamide stacking gel and a 8% (wt/vol) separating gel. Gels were then silver-stained and scanned using molecular multi-imaging system (GS-800, Bio-Rad). MHC compositions (type I, IIa, IIx, IIb) of each fiber were determined by comparing the migration of sample proteins with standard proteins from rat tibialis anterior muscle sample.
Statistical analysis.
MHC type II fibers, including MHC type IIa, MHC type IIx, and hybrids (i.e., MHC type IIa/IIx, IIa/IIb, IIb/IIx, and I/IIa) from medial gastrocnemius muscle were pooled for MHC type II data analysis. MHC type IIb fibers were not included in the data analysis because there was only one fiber with this MHC isoform. MHC type I fibers contained only MHC type I isoform. The sample size of each experimental group was based on the number of individual fibers rather than the number of rats investigated (Table 1).
Table 1.
Myosin heavy chain composition of single-muscle fiber from the medial gastrocnemius studied for physiological experiments from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats
| Young Adult |
Middle Aged |
Old |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fiber Types | Con (99) | NWB (59) | NWBX (48) | Con (49) | NWB (27) | NWBX (52) | Con (134) | NWB (91) | NWBX (64) |
| MHC type I | 40 | 4 | 9 | 8 | 10 | 20 | 54 | 30 | 29 |
| MHC type II | 59 | 55 | 39 | 41 | 17 | 32 | 80 | 61 | 35 |
| Type IIa | 33 | 20 | 12 | 26 | 8 | 20 | 30 | 13 | 13 |
| Type IIx | 5 | 10 | 9 | 1 | 1 | — | 5 | 11 | 1 |
| Hybrids | 21 | 25 | 18 | 14 | 8 | 12 | 45 | 37 | 21 |
Fiber nos. are in parentheses. Con, control group; NWB, non-weight-bearing group; NWBX, NWB plus exercise group. Myosin heavy chain (MHC) type II fibers were divided into the MHC type IIa, IIx, IIb, and hybrid fibers. Hybrid fibers were mostly composed of MHC type IIa/IIx, IIa/IIb, IIb/IIx, and I/IIa fibers.
To determine the effect of age (young adult, middle aged, and old) and condition (control, inactivity, and therapeutic exercise) on fiber size and contractile parameters (power, Po, Po/CSA, Vmax), we used two-way ANOVA. Because the two-way ANOVA analyses revealed no statistical interaction between age and condition for absolute power and normalized power, the following statistical analyses were performed. To determine age-induced changes by fiber type, we performed one-way ANOVA using the Con groups (young adult, middle aged, old) followed by Fisher's least significant difference post hoc test. To determine the benefits of a mild therapeutic exercise protocol by fiber type, we performed one-way ANOVA within each age group (e.g., Con young adult, NWB young adult, NWBX young adult) followed by Fisher's least significant difference post hoc test.
All statistical analyses from SPSS software program (version 18.0) were expressed as means ± SE, and significance was set at <0.05. Statistical analyses, as noted above, were also performed by MHC type IIa, MHC type IIx, and MHC hybrid (data not shown).
RESULTS
To determine the impact of a mild treadmill exercise as a therapeutic intervention during a 2-wk period of inactivity or non-weight bearing (hindlimb-suspended rats), the contractile properties (absolute peak power, normalized power, Po, specific tension, and Vmax) and fiber size of single MHC type I and MHC type II fibers were measured.
Absolute peak power (μN·FL·s−1).
Absolute peak power with age, inactivity, and therapeutic exercise in MHC type I and MHC type II fibers is shown in Fig. 1. In MHC type I fibers, absolute peak power declined with age, such that a 32% (P < 0.001) and a 33% (P = 0.016) reduction was observed in the old Con group compared with the young adult and middle-aged Con groups, respectively (Fig. 1A).
Fig. 1.
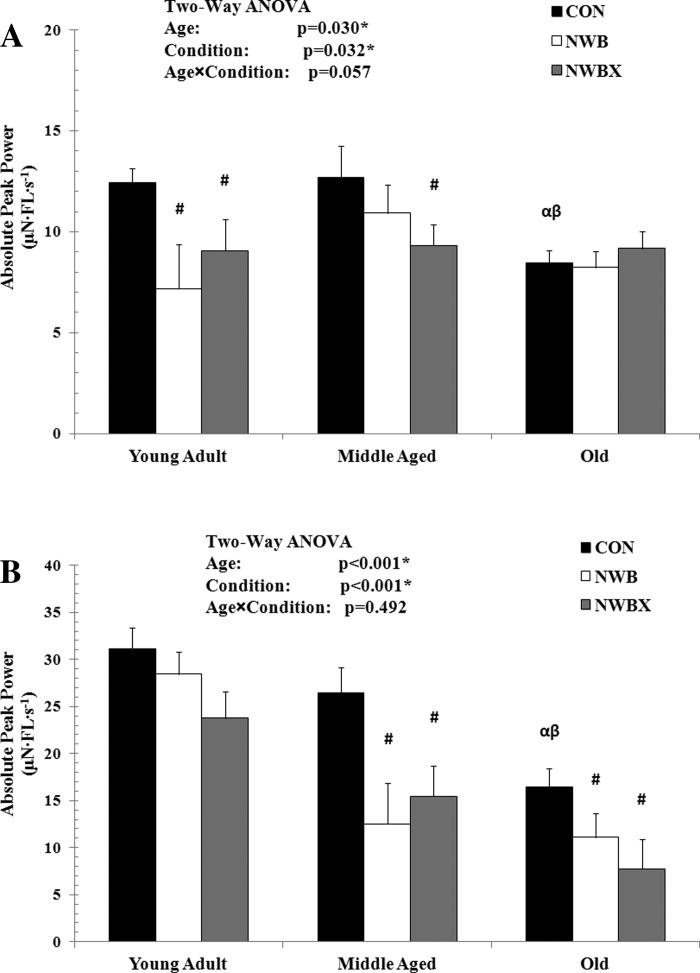
Absolute peak power of myosin heavy chain (MHC) type I (A) and MHC type II fibers (B) from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats. Con, control group; NWB, non-weight-bearing group; NWBX, NWB plus exercise group. The unit for absolute peak power is expressed in μN·fiber length (FL)·s−1, calculated from the force-velocity relationship. Values are means ± SE. #Significantly different from age-matched Con group. α Significantly different from young adult Con group. β Significantly different from middle-aged Con group. *#αβ Significance was set at P < 0.05.
In the young adult group, NWB resulted in a 42% reduction in absolute peak power of MHC type I fibers (P = 0.019) and remained reduced in the therapeutic treadmill exercise group, NWBX (27%, P = 0.04). In the middle-aged group, absolute peak power was not reduced with NWB, but the therapeutic exercise resulted in a 26% decline compared with Con group (P = 0.045). However, absolute peak power generation was not different between the NWB and the NWBX groups. In the old group, absolute peak power did not change with NWB, nor did it change with therapeutic treadmill exercise.
In MHC type II fibers, absolute peak power declined with age. Absolute peak power was reduced by 47% in the old Con group compared with the young adult (P < 0.001) and 38% lower in the old Con compared with the middle-aged Con group (P = 0.009) (Fig. 1B).
In the young adult group, absolute peak power of the MHC type II fibers did not change with NWB, nor did it change with mild treadmill exercise. NWB resulted in a 53% (P < 0.001) and a 33% (P = 0.039) decline in absolute peak power in the middle-aged and old group, respectively. Treadmill exercise did not attenuate the NWB-induced reduction in absolute peak power in these two age groups (Fig. 1B).
Taken together, the results suggest that this form of therapeutic exercise does not prevent nor attenuate the inactivity-induced decline in absolute peak power in MHC type II fibers.
Normalized power (kN·m−2·FL·s−1) and atrophy (diameter, μM).
Absolute peak power is expressed relative to the fiber's CSA under conditions that induce atrophy; this is defined as normalized power. Normalized power did not change with age in the MHC type I fibers (Fig. 2A). This finding is consistent with no change in fiber size (diameter) with age (Table 2).
Fig. 2.
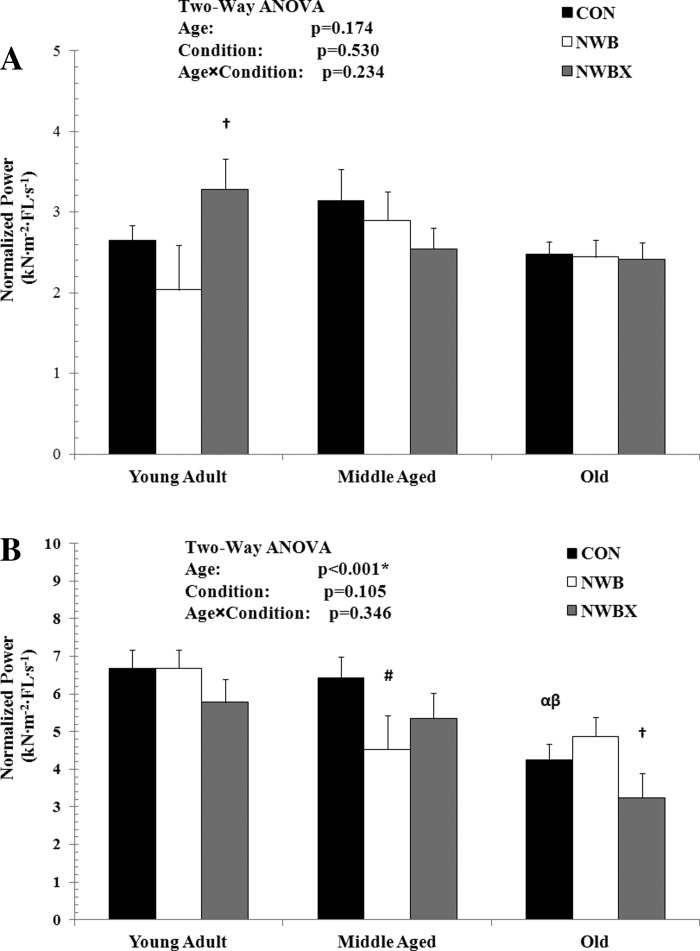
Normalized power of MHC type I (A) and MHC type II fibers (B) from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats. The unit for normalized power is kN·m−2·FL·s−1. Values are means ± SE. #Significantly different from age-matched Con group. †Significantly different from age-matched NWB group. α Significantly different from young adult Con group. β Significantly different from middle-aged Con group. *,#,†,α,βSignificance was set at P < 0.05.
Table 2.
Diameter, isometric maximal force, and specific tension of MHC type I and MHC type II fibers from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats
| Young Adult |
Middle Aged |
Old |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group Variable | CON | NWB | NWBX | CON | NWB | NWBX | CON | NWB | NWBX |
| MHC Type I | |||||||||
| Diameter, μm | 73.53 ± 1.95 (40) | 67.60 ± 6.15 (4) | 62.76 ± 4.10# (9) | 71.90 ± 4.35 (8) | 68.88 ± 3.89 (10) | 68.15 ± 2.75 (20) | 69.02 ± 1.68 (54) | 62.88 ± 2.25# (30) | 71.90 ± 2.29#† (29) |
| Po, μN | 461.37 ± 21.08 (40) | 284.70 ± 66.66# (4) | 285.17 ± 44.44# (9) | 358.19 ± 47.14α (8) | 339.97 ± 42.16 (10) | 340.20 ± 29.81 (20) | 348.81 ± 18.14α (54) | 249.38 ± 24.34# (30) | 344.08 ± 24.76† (29) |
| Po/CSA, kN/m2 | 113.32 ± 4.99 (40) | 81.63 ± 15.79 (4) | 93.59 ± 10.53 (9) | 91.19 ± 11.17α (8) | 94.67 ± 9.99 (10) | 93.06 ± 7.06 (20) | 95.78 ± 4.30α (54) | 79.17 ± 5.77# (30) | 80.85 ± 5.87 (29) |
| MHC Type II | |||||||||
| Diameter, μm | 71.07 ± 1.65 (59) | 68.65 ± 1.71 (55) | 68.82 ± 2.04 (39) | 73.04 ± 1.99 (41) | 57.80 ± 3.08# (17) | 63.36 ± 2.25# (32) | 65.37 ± 1.42α,β (80) | 61.33 ± 1.63 (61) | 65.40 ± 2.15 (35) |
| Po, μN | 385.84 ± 16.65 (59) | 322.65 ± 17.25# (55) | 334.17 ± 20.49# (39) | 367.45 ± 19.98 (41) | 219.05 ± 31.03# (17) | 276.73 ± 22.62# (32) | 275.63 ± 14.30α,β (80) | 233.92 ± 16.80 (58) | 219.10 ± 21.62# (35) |
| Po/CSA, kN/m2 | 100.03 ± 4.47 (59) | 89.64 ± 4.63 (55) | 92.53 ± 5.50 (39) | 90.70 ± 5.37 (41) | 82.77 ± 8.33 (17) | 91.13 ± 6.07 (32) | 85.63 ± 3.84α (80) | 77.11 ± 4.51 (58) | 64.39 ± 5.81# (35) |
Values are means ± SE; nos. in parentheses represents the fiber no. Po, isometric maximal force; CSA, cross-sectional area; Po/CSA, specific tension. #Significantly different from age-matched Con group.
Significantly different from age-matched NWB group. α Significantly different from young adult Con group. β Significantly different from middle-aged Con group. #,†,α,βSignificance was set at P < 0.05.
In MHC type I fibers, NWB did not induce a decline in normalized power. Interestingly, normalized power was increased by 61% with therapeutic exercise compared with NWB in the young adult group (P = 0.024); however, there was no difference in fiber diameter. In the middle-aged and old groups, normalized power did not change with NWB or mild treadmill exercise. The diameter of the fibers from the middle-aged rats did not change with NWB or exercise. There was 9% atrophy in the old rats that experienced inactivity (P = 0.037), and this atrophy was prevented with treadmill exercise (P = 0.008).
Normalized power is reduced with age in MHC type II fibers (Fig. 2B). These age-induced changes in normalized power were similar to the age-induced single-fiber atrophy data (Table 2). Although there was no statistically significant interaction between age and conditions of inactivity and therapeutic exercise for normalized power in MHC type II fibers, there was a significant interaction present for single-fiber diameter (F = 3.014, P = 0.018) (Table 2).
In MHC type II fibers, NWB induced a 29% decline in normalized power and a 21% decrease in fiber diameter in the middle-aged rats only (P = 0.024, Fig. 2B; P < 0.001, Table 2). In this age group, the benefits of the mild treadmill exercise were inconclusive, because the normalized power for the animals receiving exercise was not significantly different compared with the Con group and not different compared with the NWB group. Fiber diameter remained reduced in the mild treadmill exercise group.
Importantly, the mild treadmill exercise decreased normalized power generation by 33% (P = 0.05) in the old-age group, indicating that this form of therapeutic exercise was detrimental for the MHC type II fibers in these old rats. Atrophy was not present with NWB or with exercise in these old rats.
Po (peak force) and specific tension (Po/CSA).
To determine possible mechanisms for alterations in absolute peak power output with age, inactivity, and therapeutic exercise, peak force (Po) was evaluated. There was a significant interaction between age, inactivity, and therapeutic exercise for Po in MHC type I fibers (F = 2.845, P = 0.025) (Table 2), but no interaction in the MHC type II fibers (Table 2). Single-fiber specific tension (force generation normalized by fiber size) is summarized in Table 2. No significant interaction between age, inactivity, and therapeutic exercise occurred for specific tension in MHC type I fibers or MHC type II fibers.
In MHC type I fibers, Po was reduced in the two older age groups (Con) compared with the young adult Con group. The middle-aged Con group showed a 29% reduction (P = 0.048), and the old Con group showed a 32% reduction (P < 0.001) in Po (Table 2). Specific tension decreased 15% with age in MHC type I fibers (old Con group compared with young adult Con group, P = 0.012), indicating that atrophy may contribute, in part, to the reduced Po, but muscle intrinsic quality is diminished with age as well.
NWB resulted in a 38% decline in Po in the young adult (P = 0.007) and a 28% decline in the old group (P = 0.004) in MHC type I fibers. There was a 17% decrease in specific tension with NWB in the old group only (P = 0.028), suggesting intrinsic quality may be impaired with NWB. Interestingly, treadmill exercise showed a significant improvement in Po by 38% compared with the NWB group in the old rats only (P = 0.014), suggesting a positive benefit of this form of exercise for MHC type I fibers in this particular age group. However, if peak force is normalized to the size of the fibers, the benefits of therapeutic exercise are not conclusive, because the specific tension from the MHC type I fibers from the exercising animals (NWBX) was not different from that of the Con group and not different from that of the NWB group.
In MHC type II fibers, Po decreased with age such that the old Con group showed a 29 and a 25% reduction from the adult Con (P < 0.001) and middle-aged Con group (P < 0.001), respectively (Table 2). In MHC type II fibers, specific tension decreased by 14% (old Con group compared with young adult Con group, P = 0.011), showing age-related changes in muscle quality.
NWB decreased Po by 16% in young adult (P = 0.008) and by 40% in middle-aged group (P < 0.001). Specific tension showed a tendency to be reduced with NWB in the three age groups. Treadmill exercise did not improve Po in these two age groups. Interestingly, the MHC type II fibers showed a reduction in Po in the mild therapeutic exercise group (NWBX experimental condition) in the old-age group compared with the Con group (P = 0.05), suggesting this form of exercise may be detrimental for force generation of MHC type II fibers in the old rats. Consistent with Po, therapeutic exercise did not change the specific tension in young adult and middle-aged rats, whereas the therapeutic exercise further reduced specific tension in the old group by 25% (P = 0.004).
Taken together, the benefits of this therapeutic exercise protocol are not clear in the young and middle-aged rats; however, the exercise appears to have a negative impact for the contractile parameter of specific tension in the old rats.
Vmax.
Vmax, another important functional parameter in single-muscle fiber contraction, was determined in terms of extrapolating hyperbolic nonlinear force-velocity data. Vmax is a function of the force-velocity characteristics, which represents the cycling rate of myosin-actin interaction (4).
In MHC type I fibers, Vmax did not change with age or with NWB (Fig. 3A). However, treadmill exercise resulted in a significant decline in Vmax compared with Con (−43%, P < 0.001) and NWB (−42%, P = 0.001) in the old rats.
Fig. 3.
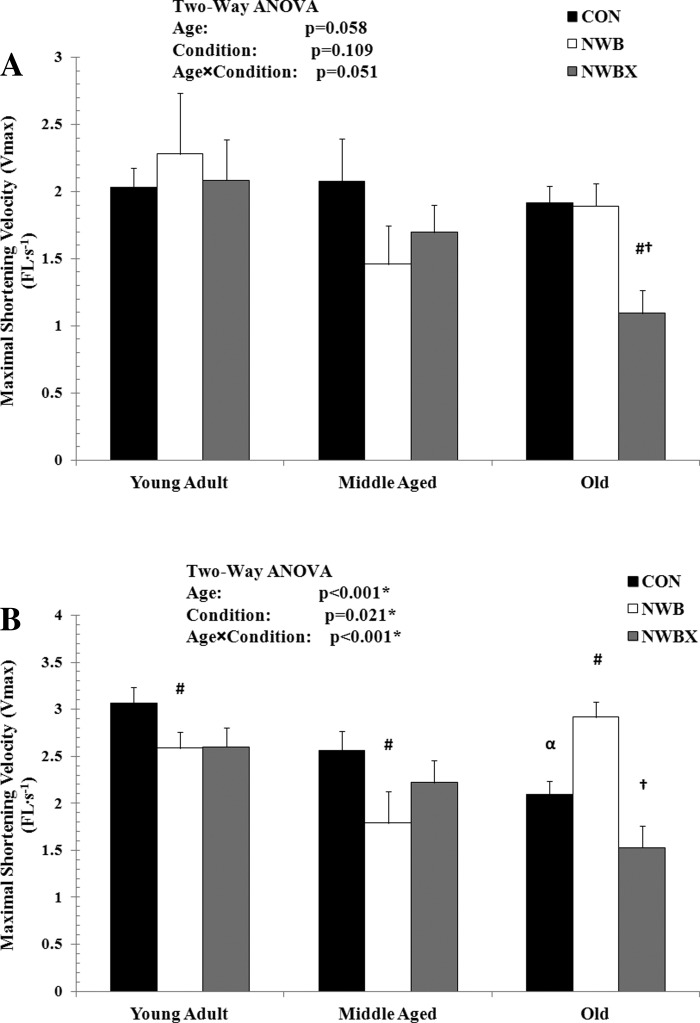
Maximal shortening velocity (Vmax), determined from the Hill-Plot of the force-velocity curve, of MHC type I (A) and MHC type II fibers (B) from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats. Vmax is expressed in FL/s. Values are means ± SE. #Significantly different from age-matched Con group. †Significantly different from age-matched NWB group. α Significantly different from young adult Con group. *#†α Significance was set at P < 0.05.
In MHC type II fibers, Vmax was altered with age (F = 8.475, P < 0.001) and condition of inactivity and therapeutic exercise (F = 3.894, P = 0.021) (Fig. 3B). Vmax was reduced by 32% in the old Con group compared with young adult Con group (P < 0.001), showing an age-related slowing of contraction speed. NWB resulted in a significant reduction in Vmax in both the young adult (−15%, P = 0.04) and middle-aged (−30%, P = 0.01) groups. In contrast to the young adult and middle-aged groups, Vmax increased by 39% in the old group with NWB (P = 0.001).
The benefits of the mild treadmill exercise in increasing Vmax are inconclusive because the Vmax of the exercising animals is not statistically different from the Con and NWB animals in the young adult and middle-aged groups. In contrast, treadmill exercise resulted in a 48% decline in Vmax in the old group (P < 0.001). Collectively, these data indicate that therapeutic treadmill exercise during inactivity periods deteriorates contraction speed of both MHC type I and MHC type II fibers in the old rats.
a/Po.
The a/Po is a mechanical constant describing the curvature of the force-velocity relationship. Our two-way ANOVA analysis indicated that there is a significant interaction between age, inactivity, and therapeutic exercise for a/Po in MHC type I fibers (F = 2.645, P = 0.035) and MHC type II fibers (F = 3.538, P = 0.007) (Fig. 4, A and B, respectively).
Fig. 4.
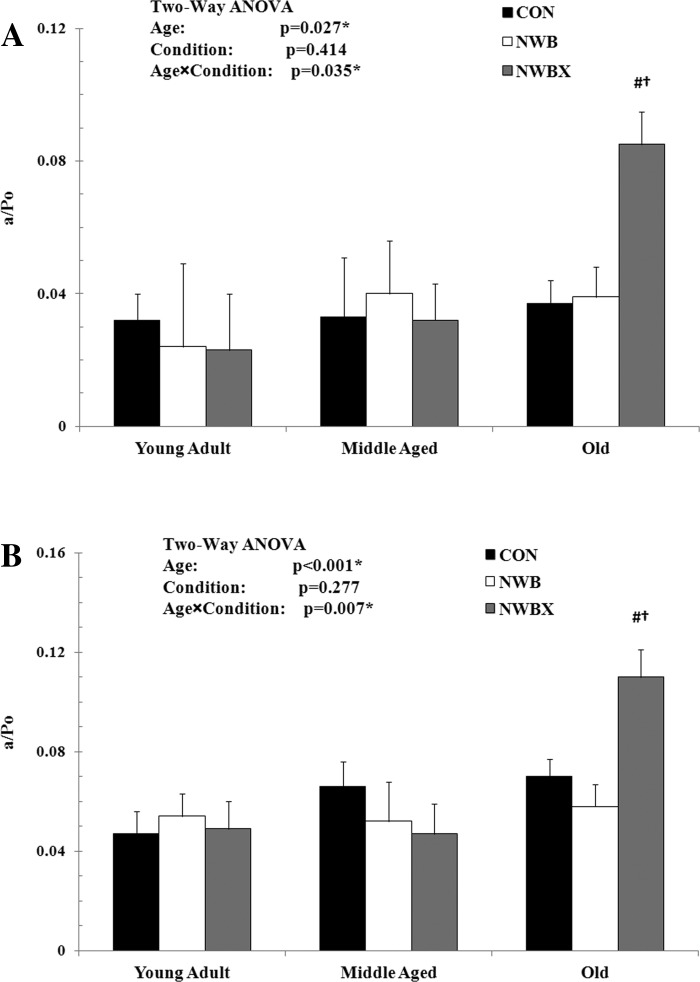
The a/isometric maximal force (Po) of MHC type I (A) and MHC type II fibers (B) from young adult (5–12 mo), middle-aged (24–31 mo), and old (32–37 mo) rats. The a/Po is an unitless constant. Values are means ± SE. #Significantly different from age-matched Con group. †Significantly different from age-matched NWB group. *#†Significance was set at P < 0.05.
In MHC type I fibers, the a/Po was not different in the two older age groups (Con) compared with the young adult (Con) group. NWB did not induce a change in a/Po (Fig. 4A). However, treadmill exercise resulted in a significant increase in a/Po compared with Con (P = 0.002) and NWB (P = 0.008) in the old rats.
In MHC type II fibers, a/Po did not change with age (Con) nor with NWB (Fig. 4B). In the old group, the a/Po was significantly higher in NWBX compared with Con (P = 0.023) and NWB (P = 0.006) groups. Taken together, these data indicate that treadmill exercise during inactivity periods results in less curvature of force-velocity relationship in MHC type I and type II fibers in the old rats.
DISCUSSION
The primary objective of this study was to investigate the effects of a mild therapeutic exercise during a period of inactivity on contractile parameters (e.g., peak power, normalized power) of MHC type I and MHC type II single fibers from the medial gastrocnemius muscle in young adult, middle-aged, and old rats. We hypothesized that therapeutic exercise would attenuate the detrimental changes in peak power and normalized power associated with inactivity, and the benefit of the mild treadmill exercise would be different with age. Foremost, our analyses did not report a statistical significance in the interaction between age, inactivity, and therapeutic exercise for two of the key contractile parameters determined in this study: peak power and normalized power. Therefore, our findings are discussed based on one-way ANOVA analyses, as described in methods.
The major findings from the present study are as follows. First, our mild treadmill exercise protocol does not improve absolute peak power of MHC type I and MHC type II fibers in adult, middle-aged, and old rats. Second, when absolute peak power is normalized to the size of the individual fibers, the mild therapeutic exercise improves in MHC type I fibers from young adult rats. However, the MHC type II single fibers from the old rats show a dramatic decline in normalized power with therapeutic exercise. Third, inactivity-induced decline in peak power generation is likely fiber-type dependent. A 2-wk period of inactivity (NWB) results in a significant decline in peak power in MHC type I fibers from young adult rats and in MHC type II fibers from middle-aged and old rats. In MHC type I fibers, the NWB-induced decline in peak power generation is primarily due to an impairment of Po. In MHC type II fibers, the detrimental effect of NWB is due to a significant decrease in both force and Vmax in the middle-aged rats.
The effect of mild treadmill exercise on contractile function.
Exercise training is suggested as a potential countermeasure to attenuate the negative effects of inactivity. The effectiveness of exercise training on muscle functions varies, depending on the exercise prescription (frequency, intensity, duration, and exercise type). The exercise prescription recommended to inactive older individuals requires careful consideration, because the muscles are highly vulnerable to damage and show impaired adaptive potentials during a period of inactivity (13, 18, 33, 39). Therefore, an exercise program consisting of low-intensity, moderate-duration, and increased frequency is a logical choice. Indeed, this type of exercise program is used in the practice of physical therapy.
Previously, the value of this mild treadmill exercise was assessed to prevent the inactivity-induced reduction in power output of MHC type I single fibers from the soleus muscles of young, middle-aged, and old rats (20). The study focused on power output, because this contractile property is fundamental to our understanding of human movement (the ability of muscle to move joints). In fact, the power output of a single fiber is dictated by both the force-generating capacity of the fiber (Po, which is related to maximal isometric force) and the properties of the fiber shortening against a load (contraction speed, Vmax). The results of the mild treadmill exercise program during a period of inactivity were age dependent for MHC type I fibers from the soleus muscle. In particular, for young adult rats, power output was partially restored, for the middle-aged group, the exercise fully restored the loss in power output, whereas, for the old rats, the treadmill exercise program was ineffective in attenuating or preventing inactivity-induced decline in power output. The improvement in power output in the young adult and middle-aged rats was attributed to combined increase in Vmax and Po. In contrast, the MHC type I fibers from the old rats receiving the therapeutic exercise showed a dramatic reduction in Vmax. Because force-generating capacity was impaired and contraction speed was reduced, power output in the old rats also remained reduced in MHC type I fibers from the soleus muscle. From the exercise prescription perspective, the extent or degree of effectiveness for the mild treadmill exercise is most likely related to the exercise stimulus intensity and the ability of the muscle to respond to the stimulus. Based on the fact that the MHC type I fibers from the young adult and middle-aged rats responded to the mild treadmill exercise with positive results, the exercise stimulus is likely reasonable in the soleus muscle. However, the lack of improvement in power output in the old rats suggests an age-related impaired adaptive potential or an inadequate exercise intensity stimulus in the soleus MHC type I fibers (15).
Similar to the soleus muscle, the gastrocnemius muscle is an ankle plantar flexor. The gastrocnemius is recruited during walking (24), and for this reason mild treadmill ambulation is a potential therapeutic exercise to prevent or attenuate a decline in power output associated with inactivity. Hence, in the present study, we examined whether the same mild-intensity, intermittent treadmill exercise was capable of maintaining power output during 14 days of inactivity in both MHC type I and MHC type II single fibers from the medial gastrocnemius in young adult (95% survival rate), middle-aged (80–50% survival rate), and old (<50% survival rate) rats. Our comprehensive evaluation shows that our mild treadmill exercise protocol does not improve absolute peak power of MHC type II fibers in the old rats. The data analyzed by MHC fiber types also support this finding, where mild therapeutic exercise does not improve peak power generation in MHC type IIa fibers, nor in hybrid fibers (data not shown).
Because power is a product of force generation and velocity, we are able to decipher the importance of each contractile parameter. In MHC type II fibers (MHC type IIa and hybrid fibers, data not shown), force and velocity do not increase in response to the exercise protocol in the old rats. Interestingly, although absolute peak power does not change with exercise in the MHC type I fibers of the old rats, both force and velocity are responding to the exercise. Specifically, force is improving with exercise, whereas velocity is decreasing. Although the isotonic mild treadmill exercise stimulates both MHC type I and MHC type II fibers present in the gastrocnemius muscle, the fiber-type responses (increase in force generation in the MHC type I and no response in the MHC type II fibers) suggest that the exercise stimulus may not be optimal for the MHC type II fibers. Moreover, there are two lines of evidence indicating that a low-intensity exercise protocol does not improve force generation of fast-twitch muscles (i.e., gastrocnemius, plantaris) or MHC type II fibers in old rats (2, 31).
In the young adult and middle-aged rats, peak power and force-generation deficits are not attenuated with mild treadmill exercise in both MHC type I and MHC type II fibers. The lack of attenuation is likely associated with the low exercise intensity as well. If the exercise intensity is adequate to elicit a physiological response, single-fiber force-generating capacity and single cell size increase. For instance, in studies designed to evaluate single-fiber contractile responses to a specific exercise protocol “isotonic resistance exercise (average maximal weight lift ∼155% of body mass)” increased Po and Vmax in both MHC type I and MHC type IIa fibers from the gastrocnemius muscle (25). Likewise, Widrick et al. (42) reported improvement in peak power generation in MHC type I and MHC type IIa fibers taken from the vastus lateralis muscle (young adult humans) following short-term resistance exercise (6–12 repetitions maximum, 3 sets, 3 times/wk). Nevertheless, in the present study, force-generating capacity, Vmax, and cell size remained reduced in MHC type I and MHC type II fibers, supporting the idea that the exercise stimulus was not adequate.
Although, in the present study, the exercise intensity does not appear to be adequate to prevent the inactivity-induced decline in power generation, the optimal therapeutic exercise for the MHC type I and MHC type II fibers within the muscle might require combinations of resistance exercise (38), as well as isotonic or isometric exercise (14). MHC type II fibers respond to a high-intensity exercise, as Trappe et al. (38) demonstrated amelioration of inactivity-induced decline in single-fiber size, Po, unloaded contraction speed, and peak power in MHC type II fibers from the vastus lateralis muscle. In contrast, Hurst and Fitts (14) reported the effectiveness of isometric exercise after a period of inactivity on peak power generation in MHC type I fibers from the soleus.
The effect of mild treadmill exercise on normalized power.
Normalized power (and specific tension) represents single-muscle fiber function, because this parameter takes into account fiber size. This parameter is used as an index of intrinsic muscle quality. Previous studies suggest that muscle atrophy does not solely explain inactivity-induced decline power and force generation (1, 11, 31). For example, prolonged bed rest (∼37 days) results in diminished normalized power and specific tension, and this deterioration is reported in both MHC type I and MHC type II fibers from vastus lateralis in adult humans (21, 38). Previously, our laboratory also found a significant decline in specific tension in both MHC type I and MHC type II fibers from the gastrocnemius muscle after 1 wk of HS in the old rats (37).
The potential cellular mechanisms responsible for the deterioration in muscle quality are a reduced number of cross bridges, including lower myosin concentration, alterations in calcium kinetics (i.e., calcium uptake and release, and calcium transient), and a decrease in force per cross bridge during the low- and strong-binding state (7, 19, 21, 28). Our laboratory has demonstrated that about one-third of the force loss after periods of inactivity is attributed to a decreased population of myosin heads in the strong-binding (force-generating) structural state during muscle contraction (43). Taken together, inactivity-induced deterioration in muscle quality is highly associated with structural changes in the myosin head and primarily plays a role in impaired muscle performance, including power generation.
A major finding of this study is the effect of mild therapeutic exercise on normalized power. Although absolute power is reduced in MHC type I fibers with NWB and mild treadmill exercise, normalized power improves. This finding suggests and is supported by a concomitant change in force and diameter in the type I fibers from adult rats. This strong relationship implies that the fibers have strong adaptation potential, and the quality of muscle tissue remains intact.
The MHC type II (MHC type IIa fibers and MHC hybrid fibers, data not shown) fibers from middle-aged rats and old rats show impaired adaptation potential and poor muscle quality, because changes in force generation do not follow changes in fiber size. Specifically, the MHC type II fibers from the old rats show significant declines in force with no atrophy, whereas the middle-aged rats show incongruent changes. There is evidence for single skeletal muscle fiber impaired adaptation potential following various forms of exercise in the older population (15, 27, 33, 34). For instance, Thomas and colleagues (34) reported that 5–7 mo of treadmill running exercise did not rescue the age-induced decline in force generation or atrophy of the gastrocnemius/plantaris muscles in very old rats. Slivka et al. (33) showed that 12 wk of resistance exercise did not improve normalized power generation of MHC type IIa fibers from the vastus lateralis muscles in old (>80 yr) men. Recently, normalized power of MHC type IIa fibers did not improve following a resistance exercise program (27).
Another possible factor besides inadequate exercise stimulus intensity or a failure of the muscle fibers to negligibly adapt to the exercise in the old population has been attributed to increased susceptibility of muscle to contraction-induced damage. Because the data from our laboratory (15) and others (32) suggest that aged muscles are susceptible to damage during inactivity periods, either too vigorous or inappropriate exercise type might increase the susceptibility to damage. If the muscle fibers are damaged, muscle function would be impaired or would not show any improvement, as reported in the present study and previous studies (13, 22, 26).
In light of this, the lack of positive responses to the therapeutic exercise in the older rats may be due to the inadequate exercise stimulus intensity, the lack of cellular adaptation potential, or increased susceptibility to muscle damage.
The effect of inactivity on contractile function with age and fiber type.
Many studies report periods of inactivity (i.e., HS, bed rest, and immobilization) result in diminished muscle contractility (17, 36, 38, 41, 43). These inactivity-induced changes occur in both MHC type I and MHC type II single skeletal muscle fibers (17, 36); however, the extent of deterioration between the MHC fiber types is variable. The variable response is due to the activation patterns of the muscles (8). Our data supports the inactivity-induced fiber-type responses. In the young adult rats, MHC type I fibers were more responsive to NWB than MHC type II fibers, with the extent of functional decrement in the MHC type I fibers ranging from 8 to 42% compared with the MHC type II fibers ranging from 1 to 16%. The MHC type II fibers show resistance to inactivity. However, there is a preponderance of data indicating that MHC type II fibers show age-associated deterioration at ages beyond the 80% survival (late middle age) in both humans and animals, whereas the MHC type I fibers are somewhat resistant to the aging process (29). Hence, the aging of skeletal muscle, specifically the MHC type II fibers, may have an impact during the period of inactivity. Indeed, the MHC type II fibers from the older rats show a greater decline with inactivity in functional parameters compared with the type I fibers. This finding is in agreement with the result of a previous study showing that MHC type II fibers from gastrocnemius elicited a greater decrease in contractile functions from inactivity in the aged rats (31).
Conclusions.
Our data suggest that mild treadmill exercise during a period of inactivity does not prevent inactivity-induced decline in absolute peak power generation of MHC type I and MHC type II fiber from the gastrocnemius muscles in young adult, middle-aged, and old rats. However, this exercise protocol is beneficial in enhancing normalized power generation of MHC type I fibers in young adult rats, most likely due to the stimulus intensity and the ability of the individual fibers to adapt to the stimulus (force and size). In contrast, several factors, such as impaired adaptation potential, inappropriate exercise stimulus intensity, or increased susceptibility to muscle damage, may contribute to the lack of improvement in normalized power in MHC type II fibers observed in the older rats.
GRANTS
This research was funded by National Institute on Aging Grant AG017768 (L. V. Thompson) and the Foundation for Physical Therapy (L. V Thompson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-H.K. and L.V.T. conception and design of research; J.-H.K. and L.V.T. performed experiments; J.-H.K. and L.V.T. analyzed data; J.-H.K. and L.V.T. interpreted results of experiments; J.-H.K. and L.V.T. prepared figures; J.-H.K. and L.V.T. drafted manuscript; J.-H.K. and L.V.T. edited and revised manuscript; J.-H.K. and L.V.T. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Sheng Zhong and Janice Shoeman for expert technical assistance.
REFERENCES
- 1. Alley KA, Thompson LV. Influence of simulated bed rest and intermittent weight bearing on single skeletal muscle fiber function in aged rats. Arch Phys Med Rehabil 78: 19–25, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Andersen NB, Andreassen TT, Orskov H, Oxlund H. Growth hormone and mild exercise in combination increases markedly muscle mass and tetanic tension in old rats. Eur J Endocrinol 143: 409–418, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Arora P, Husom AD, Ferrington DA, Thompson LV. Age-dependent effects of treadmill exercise during a period of inactivity. Exp Gerontol 43: 668–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bangart JJ, Widrick JJ, Fitts RH. Effect of intermittent weight bearing on soleus fiber force-velocity-power and force-pCa relationships. J Appl Physiol 82: 1905–1910, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Carter EE, Thomas MM, Murynka T, Rowan SL, Wright KJ, Huba E, Hepple RT. Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Exp Gerontol 45: 662–670, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci 59: 1200–1206, 2004 [DOI] [PubMed] [Google Scholar]
- 7. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duysens J, Tax AA, van der Doelen B, Trippel M, Dietz V. Selective activation of human soleus or gastrocnemius in reflex responses during walking and running. Exp Brain Res 87: 193–204, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am J Phys Med Rehabil 81: S127–S147, 2002 [DOI] [PubMed] [Google Scholar]
- 10. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gehrke AG, Krull MS, McDonald RS, Sparby T, Thoele J, Troje SW, ZumBerge J, Thompson LV. The effects of non-weight bearing on skeletal muscle in older rats: an interrupted bout versus an uninterrupted bout. Biol Res Nurs 5: 195–202, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Herbert ME, Roy RR, Edgerton VR. Influence of one-week hindlimb suspension and intermittent high load exercise on rat muscles. Exp Neurol 102: 190–198, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Hurst JE, Fitts RH. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J Appl Physiol 95: 1405–1417, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Husom AD, Ferrington DA, Thompson LV. Age-related differences in the adaptive potential of type I skeletal muscle fibers. Exp Gerontol 40: 227–235, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol 109: 1628–1634, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol 589: 4745–4757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci 64: 618–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol 87: 386–390, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kim JH, Thompson LV. Differential effects of mild therapeutic exercise during a period of inactivity on power generation in soleus type I single fibers with age. J Appl Physiol 112: 1752–1761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflügers Arch 432: 320–328, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Linderman JK, Gosselink KL, Booth FW, Mukku VR, Grindeland RE. Resistance exercise and growth hormone as countermeasures for skeletal muscle atrophy in hindlimb-suspended rats. Am J Physiol Regul Integr Comp Physiol 267: R365–R371, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91: 450–472, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Meinders M, Gitter A, Czerniecki JM. The role of ankle plantar flexor muscle work during walking. Scand J Rehabil Med 30: 39–46, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Norenberg KM, Fitts RH. Contractile responses of the rat gastrocnemius and soleus muscles to isotonic resistance exercise. J Appl Physiol 97: 2322–2332, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Pierotti DJ, Roy RR, Flores V, Edgerton VR. Influence of 7 days of hindlimb suspension and intermittent weight support on rat muscle mechanical properties. Aviat Space Environ Med 61: 205–210, 1990 [PubMed] [Google Scholar]
- 27. Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol 106: 1611–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol 69: 58–66, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Rowan SL, Purves-Smith FM, Solbak NM, Hepple RT. Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp Gerontol 46: 660–669, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging 24: 335–340, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Sandmann ME, Shoeman JA, Thompson LV. The fiber-type-specific effect of inactivity and intermittent weight-bearing on the gastrocnemius muscle of 30-month-old rats. Arch Phys Med Rehabil 79: 658–662, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol 105: 1695–1705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas MM, Khan W, Betik AC, Wright KJ, Hepple RT. Initiating exercise training in late middle age minimally protects muscle contractile function and increases myocyte oxidative damage in senescent rats. Exp Gerontol 45: 856–867, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther 32: 44–57, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Thompson LV, Johnson SA, Shoeman JA. Single soleus muscle fiber function after hindlimb unweighting in adult and aged rats. J Appl Physiol 84: 1937–1942, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Thompson LV, Shoeman JA. Contractile function of single muscle fibers after hindlimb unweighting in aged rats. J Appl Physiol 84: 229–235, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–B501, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Widrick JJ, Maddalozzo GF, Hu H, Herron JC, Iwaniec UT, Turner RT. Detrimental effects of reloading recovery on force, shortening velocity, and power of soleus muscles from hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 295: R1585–R1592, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283: R408–R416, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Zhong S, Lowe DA, Thompson LV. Effects of hindlimb unweighting and aging on rat semimembranosus muscle and myosin. J Appl Physiol 101: 873–880, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Zhong S, Thompson LV. The roles of myosin ATPase activity and myosin light chain relative content in the slowing of type IIB fibers with hindlimb unweighting in rats. Am J Physiol Cell Physiol 293: C723–C728, 2007 [DOI] [PubMed] [Google Scholar]


