Abstract
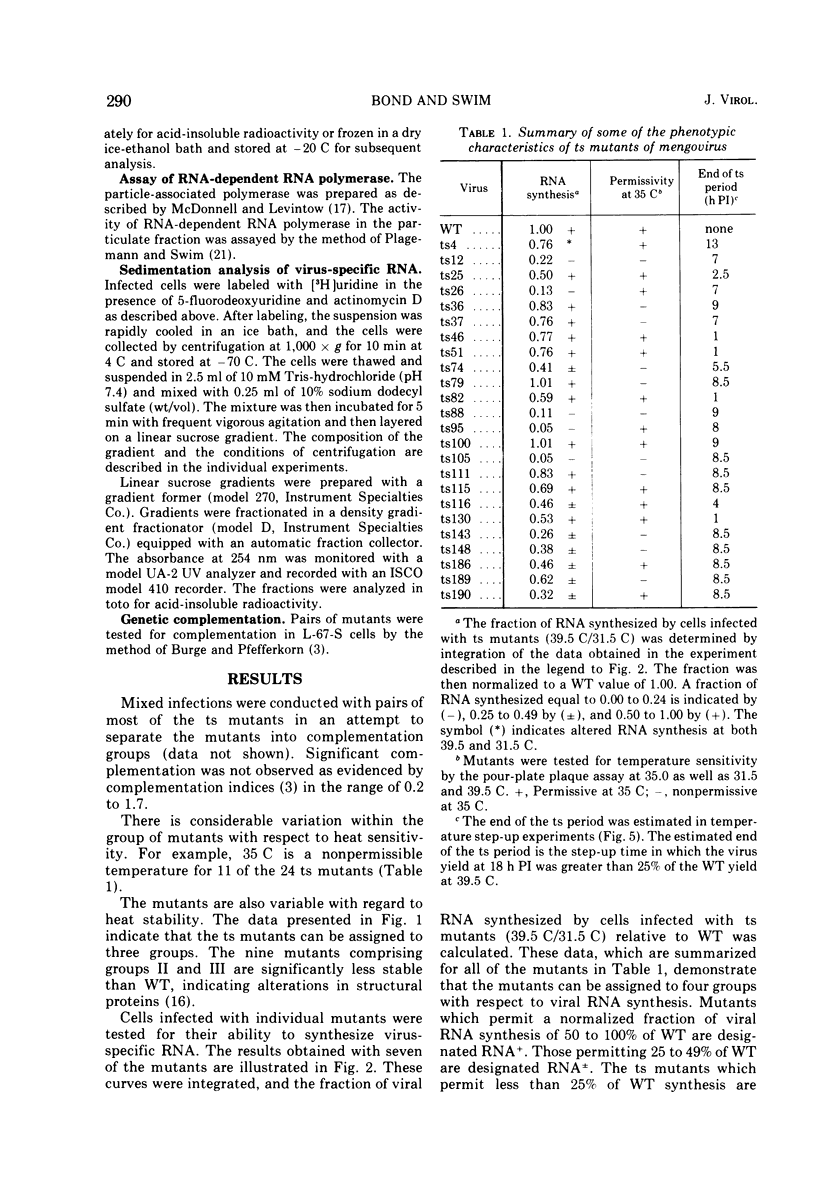
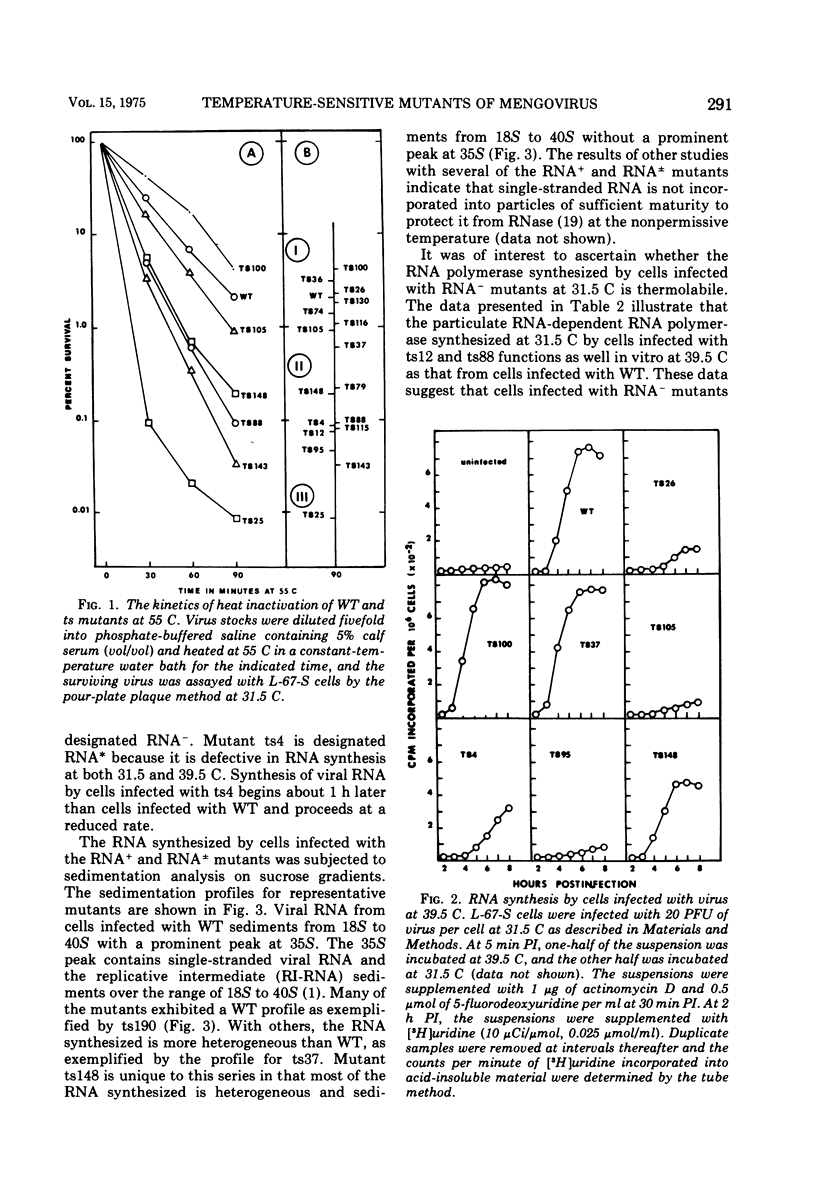
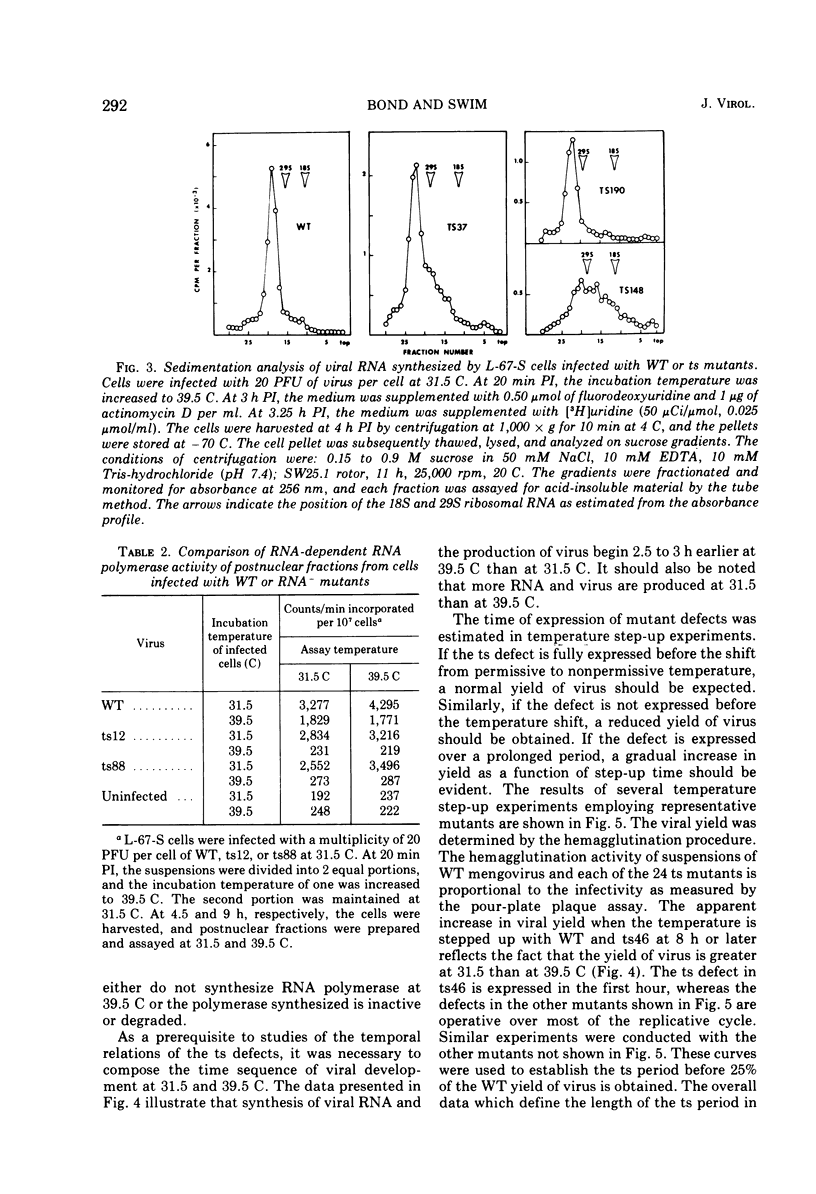
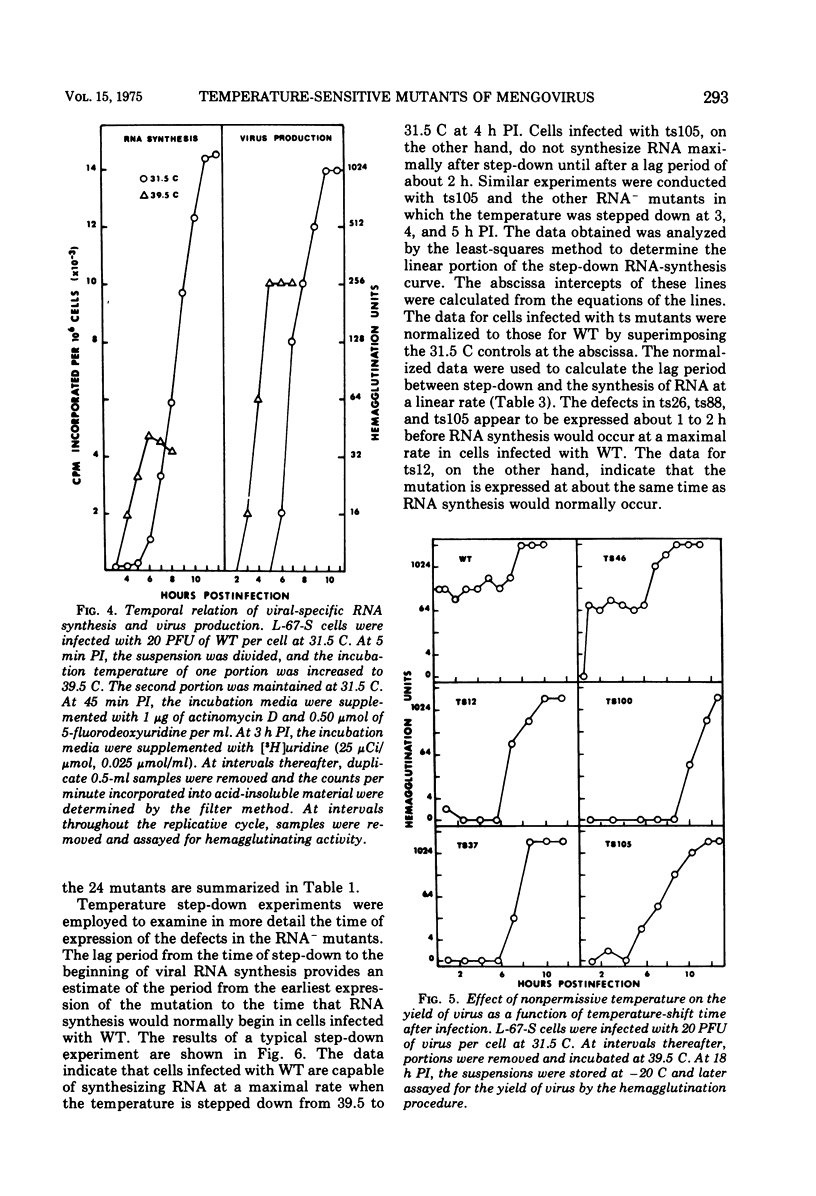
Twenty-four temperature-sensitive mutants of mengovirus were characterized physiologically with respect to phenotype. The mutants were separated into four classes on the basis of viral RNA synthesis. L-67-S cells infected with five of the mutants synthesized little viral RNA at 39.5 C. These mutants are designated RNA-. One mutant is designated RNA* since its RNA synthesis is altered at both 39.5 and 31.5 C. The other mutants were divided into two groups, RNA plus or minus (25 TO 49% of wild-type RNA synthesis) and RNA plus (50 to 100% of wild-type RNA synthesis). The time of expression of the mutation in the RNA- mutants was estimated from the results of reciprocal temperature-shift experiments. The mutatation in ts12 appears to be expressed at the time RNA synthesis normally begins. The defect in three of the mutants was expressed 1 to 2 h before RNA synthesis is normally detectable. Protein synthesis is required before RNA synthesis begins when the cells are shifted from 39.5 to 31.5 C. The RNA polymerase synthesized by cells infected with these RNA- mutants at 31.5 C was stable and fully active when assayed at 39.5 C in vitro. The sedimentation profiles of the viral RNA synthesized by cells infected with RNA plus and RNA plus or minus mutants are similar to wild-type profiles with the exception of ts148. Cells infected with this RNA plus or minus mutant synthesize RNA that sediments in a sucrose gradient like replicative-intermediate RNA, but little mature viral RNA is evident. The results of step-up experiments indicate that the temperature-sensitive period for the majority of the RNA plus and RNA plus and minus mutants extends through most of the replicative cycle. The temperature-sensitive defect of four of the mutants, however, was expressed in the first hour, suggesting that some undefined early function is required for the eventual maturation of mengovirus. The virions of three of the RNA- mutants were more thermolabile than wild-type virions. Five of the RNA plus and RNA plus or minus mutants were also thermolabile. Genetic complementation at a significant level was not detectable in mixed infections of the mutants described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. RESCUE OF ONE PHENOTYPE IN MIXED INFECTIONS WITH HEAT-DEFECTIVE MUTANTS OF TYPE 1 POLIOVIRUS. Virology. 1965 Mar;25:431–438. doi: 10.1016/0042-6822(65)90064-4. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Geissler E., Scotti P. D., Tannock G. A. Further characterization of the genetic map of poliovirus temperature-sensitive mutants. In: strategy of the viral genome. Ciba Found Symp. 1971:75–100. doi: 10.1002/9780470719824.ch5. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Johnson R. T., Garwes D. J. Physiological characterization of heat-defective (temperature-sensitive) poliovirus mutants: preliminary classification. Virology. 1966 Dec;30(4):638–649. doi: 10.1016/0042-6822(66)90169-3. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Stancek K., Summers D. F. Synthesis of double-stranded RNA by poliovirus temperature-sensitive mutants. Virology. 1970 Apr;40(4):971–977. doi: 10.1016/0042-6822(70)90143-1. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Wentworth B. B., McCahon D. Guanidine inhibition of poliovirus: a dependence of viral RNA synthesis on the configuration of structural protein. Virology. 1970 Mar;40(3):480–493. doi: 10.1016/0042-6822(70)90191-1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Conditional lethal mutants of animal viruses. Curr Top Microbiol Immunol. 1969;48:1–28. doi: 10.1007/978-3-642-46163-7_1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahon D., Cooper P. D. Identification of poliovirus temperature-sensitive mutants having defects in virus structural protein. J Gen Virol. 1970 Jan;6(1):51–62. doi: 10.1099/0022-1317-6-1-51. [DOI] [PubMed] [Google Scholar]
- McDonnell J. P., Levintow L. Kinetics of appearance of the products of poliovirus-induced RNA polymerase. Virology. 1970 Dec;42(4):999–1006. doi: 10.1016/0042-6822(70)90348-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Shea M. A. Transport as the rate-limiting step in the incorporation of uridine into mengovirus ribonucleic acid in Novikoff rat hepatoma cells. J Virol. 1971 Jan;7(1):137–143. doi: 10.1128/jvi.7.1.137-143.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. II. General properties of the viral-induced ribonucleic acid polymerase. J Bacteriol. 1966 Jun;91(6):2327–2332. doi: 10.1128/jb.91.6.2327-2332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Ward G. A., Plagemann P. G. Fluctuations of DNA-dependent RNA polymerase and synthesis of macromolecules during the growth cycle of Novikoff rat hepatoma cells in suspension culture. J Cell Physiol. 1969 Jun;73(3):213–231. doi: 10.1002/jcp.1040730307. [DOI] [PubMed] [Google Scholar]


