Abstract
Aim
We investigated association of maternal retinol binding protein 4 (RBP4) with risk of gestational diabetes (GDM).
Methods
GDM cases (N=173) and controls (N=187) were selected from among participants of a cohort study of risk factors of pregnancy complications. Early pregnancy (16 weeks on average) serum RBP4 concentration was measured using an ELISA-based immunoassay. Logistic regression was used to estimate unadjusted and adjusted odds ratios (ORs/aORs) and 95% confidence intervals (95%CI).
Results
Mean serum RBP4 was significantly higher among GDM cases compared with controls (47.1 vs. 41.1 μg/ml, respectively; p-value<0.05). Participants in the highest quartile for serum RBP4 had a 1.89-fold higher risk of GDM compared with participants in the lowest quartile (95%CI: 1.05-3.43). However, this relationship did not reach statistical significance after adjustment for confounders (aOR: 1.54; 95%CI: 0.82-2.90). Women who were ≥35 years old and who had high RBP4 (≥38.3 μg/ml, the median) had a 2.31-fold higher risk of GDM compared with women who were < 35 years old and had low RBP4 (<38.3 μg/ml) (aOR: 2.31; 95%CI: 1.26-4.23; p-value for interaction=0.021).
Conclusion
Overall, there is modest evidence of a positive association of early pregnancy elevated RBP4 concentration with increased GDM risk, particularly among women with advanced age.
Keywords: Retinol binding protein 4, gestational diabetes, pregnancy, maternal age
Introduction
Gestational diabetes (GDM), a common complication of pregnancy occurring in 5-10% of all pregnancies [1,2,3,4], is characterized by glucose intolerance that first appears during pregnancy [5,6,7,8]. GDM is associated with adverse maternal (e.g., C-sections and increased risk of type 2 diabetes [T2DM]) and offspring (e.g., fetal hyperinsulinism, macrosomia, and birth injuries) outcomes [2,4,8,9]. Underlying pathophysiological disturbances commonly identified in GDM include reduced insulin secretion and abnormal insulin resistance [10,11,12,13,14,15].
Retinol binding protein 4 (RBP4) is a recently identified adipokine that has been linked to obesity and related co-morbidities including insulin resistance and T2DM [10,16]. Hepatocytes are the main sources of RBP4; and, RBP4 is known to be highly expressed in adipose tissues [5,16,17,18,19,20]. An increase in adipose tissue mass is associated with an increase in RBP4, IL-6 and TNF-α production [17]. Using an animal model, Yang et al [21] have shown that RBP4 may serve as an adipocyte derived signal that plays a role in the development of insulin resistance. Decreased adiponectin and increased RBP4 concentration have been associated with decreased fatty acid oxidation, increased hepatic gluconeogenesis, and insulin resistance [17].
Several investigators have reported associations of RBP4 concentrations with insulin resistance and T2DM [5,21,22,23]. RBP4 is encoded by the RBP4 gene, which maps to chromosome 10q23-q24, the genomic region that has been linked to an increased risk of T2DM [5,17]. Further, advanced maternal age has previously been shown to influence the association of RBP4 concentrations with insulin sensitivity, percent fat mass and lipid profiles [24]. Despite previous reports of associations of serum concentrations of RBP4 with insulin resistance and risk of T2DM, the role of RBP4 in GDM risk is not well established. In a cross-sectional study, Lewandowski et al documented elevated serum RBP4 concentrations at 28 weeks of gestation among women with GDM as compared with controls [25]. However, Teper et al, in a prospective cohort study, reported that neither circulating RBP4 nor the RBP4:retinol molar ratio was significantly different between GDM cases and controls [18]. Given the scarcity of research on this topic and inconsistency of findings, we investigated whether early pregnancy serum RBP4 concentrations is associated with risk of GDM later in pregnancy. We also examined whether this relationship is modified by maternal age.
Methods
Overview
Study participants for this nested case-control study were drawn from participants of the Omega study, a prospective cohort study designed to examine maternal dietary and life style risk factors of pregnancy complication such as preeclampsia and gestational diabetes [26,27,28,29]. The study population was comprised of women attending prenatal care clinics affiliated with Swedish Medical Center (SMC) in Seattle, WA and Tacoma General Hospital (TGH) in Tacoma, WA. Women were eligible if they began prenatal care before 20 weeks of gestation, were older than 18 years, were able to speak and read English, planned to carry the pregnancy to term, and planned to deliver at either of the two research hospitals.
Study population
All study participants underwent a screening test, a 50 gram of 1-hour oral glucose challenge test, at 24-28 weeks of gestation. For participants who failed this test (blood glucose ≥ 140 mg/dl), a follow-up test consisting of a 100g, 3-hour oral glucose tolerance test (OGTT) was administered. Based on the American Diabetes Association (ADA) recommendations, women were diagnosed with GDM if two or more of results of the OGTT exceeded the ADA criteria [30] as follows: fasting ≥95 mg/dl; 1-hour ≥ 180 mg/dl; 2-hour ≥ 155mg/dl; 3-hour ≥ 140mg/dl [30]. We included all 173 GDM cases and 187 randomly selected controls in the current study. Study protocols were approved by the Institutional Review Boards of SMC and TGH. All participants provided written informed consent.
Data collection
Using standardized questionnaires administered by trained-interviewers at or near the time of enrollment (16 weeks on average), information was gathered on maternal characteristics including maternal age, pre-pregnancy weight, height, pre-pregnancy body mass index (BMI), medical and reproductive history, gestational age at glucola, maternal socio-demographic factors, and family history of medical illness in first degree family members. At the end of the pregnancy period, maternal and infant medical records were reviewed for information on the course and outcomes of pregnancy.
At or near the time of enrollment, blood samples were collected for biomarker measurement. Biological samples were either processed immediately or stored at −80°C until further processing. A commercial enzyme-linked immunoassay (Catalog number DRB400, Quantikine TM, R&D Systems, Minneapolis, MN, USA) was used to measure serum RBP4 concentrations according to the manufacturer’s instructions. All assays were performed by one technician who was blinded to the case control status of study participants.
Statistical analysis
We examined general characteristics of the study population using mean (standard deviation) for continuous variables and numbers (%) for categorical variables. We compared maternal characteristics between GDM cases and controls using Student’s T-test (for continuous variables) and chi square tests (for categorical variables). We used unadjusted and multivariable adjusted logistic regression models to compute unadjusted/adjusted odds ratios (ORs/aORs) and 95% confidence intervals (95% CI) comparing risk of GDM among the upper three quartiles for RBP4 concentrations with the referent (the lowest quartile). Besides a priori identified potential confounding variables (maternal age and race/ethnicity), variables that resulted in >10% difference in estimated ORs (comparing unadjusted and adjusted regression coefficients) were considered as confounders and were retained in final multivariable models. We explored possibility of a nonlinear relation between RBP4 concentrations and GDM odds, using generalized additive logistic regression modeling procedures (GAM) [31]. We also evaluated potential effect modification of the relationship between serum RBP4 concentrations and risk of GDM by maternal age using stratified models and interaction terms in multivariable logistic regression models. For these analyses, we defined advanced maternal age as age ≥35 years. We used the median RBP4 concentration (38.3ug/ml) as the cut-off to define high and low RBP4. Overweight was defined as BMI ≥ 25 kg/m2. For the interaction analyses, we defined three variables for age (<35 years or ≥35years), RBP4 concentrations (< 38.3ug/ml or ≥ 38.3ug/ml), and their interaction. The P-value for the interaction term was the interaction p-value.
All analyses were conducted using SPSS version 14 and S-Plus (version 6.1, release 2, Insightful Inc. Seattle, WA). Statistical significance was defined as two sided p<0.05.
Results
GDM cases were older compared with controls (34.2 vs. 33.0 years, respectively) (Table 1). Controls were more likely to be non-Hispanic White compared with GDM cases (86% vs. 71%). Mean (standard deviation, SD) pre-pregnancy BMI among cases and controls was 26.6(6.5) kg/m2 and 23.4(5.1) kg/m2, respectively (p-value<0.05). GDM cases were more likely to report a positive history of hypertension, family history of diabetes and family history of hypertension compared with controls (all p-values<0.05).
Table 1.
Selected clinical characteristics of the study population Seattle and Tacoma, Washington, USA 1996 - 2008
| GDM Cases | Controls | ||
|---|---|---|---|
| Characteristics | N (%) | N (%) | P-value |
| Numbers | 173 (48.1) | 187 (51.9) | |
| Maternal Age (years)* | 34.15±4.56 | 32.95±4.32 | |
| <35 | 89 (51.4) | 117 (62.6) | |
| ≥35 | 84 (48.6) | 70 (37.4) | |
| 0.011 | |||
| Race, non-Hispanic White | 122 (70.9) | 161 (86.1) | <0.001 |
| Education ≤ high school | 8 (5.0) | 5 (2.9%) | 0.316 |
| Unmarried | 29 (16.8) | 27 (14.4) | 0.543 |
| Multiparous | 82 (47.4) | 82 (43.9) | 0.499 |
| Pre-pregnancy BMI (kg/m2)* | 26.62±6.50 | 23.38±5.11 | |
| <18.5 | 4 (2.3) | 9 (4.8) | |
| 18.8-24.9 | 84 (48.6) | 130 (69.5) | |
| 25.0-29.9 | 48 (27.7) | 39 (20.9) | |
| ≥30.0 | 37 (21.4) | 9 (4.8) | |
| <0.001 | |||
| Gestational age at blood collection | 15.52±2.82 | 15.65±2.94 | 0.67 |
| Exercise during pregnancy | 137 (79.2) | 158 (84.5) | 0.191 |
| Worked during pregnancy | 140 (81.9) | 152 (84.0) | 0.599 |
| History of chronic hypertension | 18 (10.4) | 7 (3.7) | 0.013 |
| Family history of diabetes mellitus | 53 (30.6) | 28(15.0) | <0.001 |
| Family history of hypertension | 99 (57.2) | 87 (46.5) | 0.042 |
| smoking during pregnancy | 13 (7.5) | 9 (4.8) | 0.346 |
| Serum Retinol Binding Protein 4 | |||
| μg/ml* | 47.13±30.02 | 41.14±21.29 | 0.029 |
Mean (SD) otherwise N (%)
Mean (SD) serum RBP4 concentrations were significantly higher among GDM cases 47.1 (30.0) μg/ml compared with controls 41.1 (21.3) μg/ml (P<0.05). Participants in the highest quartile for serum RBP4 had a 1.89-fold higher risk of GDM compared with participants in the lowest quartile (95% CI: 1.05-3.43) (Table 2). However, this relationship was greatly attenuated and became statistically insignificant after adjustment for confounders including maternal age, race/ethnicity, family history of diabetes, and pre-pregnancy overweight status (aOR: 1.54, 95%CI: 0.82-2.90). In analyses employing GAM procedures, higher odds of GDM risk was observed with increasing concentrations of RBP4 (Figure 1).
Table 2.
Gestational diabetes mellitus (GDM) risk according to quartiles of maternal serum RBP4 concentrations in early pregnancy
| Serum RBP4 (μg/ml) |
GDM
N= 173 |
Controls
N= 187 |
Unadjusted
OR (95%CI) |
Adjusted
*
OR (95%CI) |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Quartile 1 (<31.2) | 31 (17.9) | 46 (24.6) | Referent | Referent |
| Quartile 2 (31.2 - 38.2) | 39 (22.5) | 46 (24.6) | 1.25 (0.67-2.34) | 0.99 (0.50-1.93) |
| Quartile 3 (38.3 - 46.3) | 43 (24.9) | 48 (25.7) | 1.32 (0.72-2.45) | 1.30 (0.67-2.51) |
| Quartile 4 (≥ 46.4) | 60 (34.7) | 47 (25.1) | 1.89 (1.04-3.43) | 1.53 (0.81-2.89) |
| P -value for the trend | 0.035 | 0.14 |
OR and 95%CI adjusted for maternal age, race/ethnicity, family history of diabetes and pre-pregnancy overweight status
Figure 1.
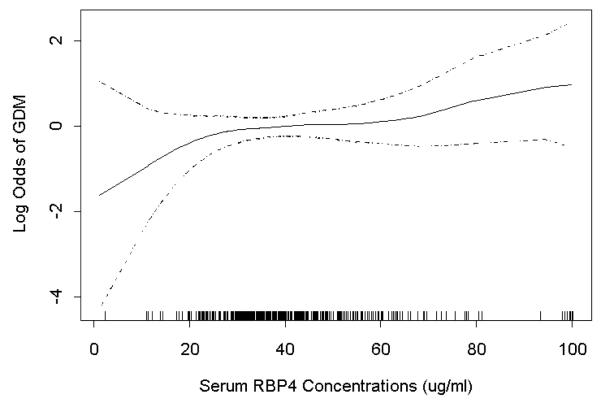
Relation between maternal serum RBP4 concentrations (X-axis) and adjusted relative odds of gestational diabetes (GDM) (Y-axis). Estimates are depicted by the solid line while 95% CIs are depicted by the dotted lines. The vertical bars along the X-axis indicate distribution of study subjects. The estimates were adjusted for maternal age, race/ethnicity, family history of diabetes and pre-pregnancy overweight status.
Among women who were ≥35 years of age, those in the highest quartile for RBP4 had a greater than 2-fold increase in risk of GDM compared with women in the lowest quartile (aOR: 2.39, 95%CI: 0.91-6.29) (Table 3). This relationship, however, was not observed among women < 35 years of age (trend p-value=0.94). We observed statistically significant interaction between higher RBP4 concentration and maternal age (p value for interaction = 0.021). Women who were ≥35 years of age and who had high RBP4 (≥38.3 μg/ml, the median) had a greater than 2-fold higher risk of GDM compared with women who were < 35 years of and who had low RBP4 (<38.3 μg/ml) (aOR: 2.31, 95%CI: 1.26-4.23) (Table 4).
Table 3.
Gestational diabetes mellitus (GDM) risk according to quartiles of early pregnancy maternal serum RBP4 concentrations stratified by advanced maternal age
| Serum RBP4 (μg/ml) |
GDM
N= 173 |
Controls
N= 187 |
Unadjusted
OR (95%CI) |
Adjusted
OR (95%CI) * |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Maternal Age ≥35 years |
||||
| Quartile 1 (<31.2) | 11 (13.1) | 16 (22.9) | Referent | Referent |
| Quartile 2 (31.2 - 38.2) | 14 (16.7) | 18 (25.7) | 1.13 (0.40-3.19) | 0.96 (0.32-2.89) |
| Quartile 3 (38.3 - 46.3) | 22 (26.2) | 16 (22.9) | 2.00 (0.73-5.44) | 1.92 (0.67-5.51) |
| Quartile 4 (≥ 46.4) | 37 (44.0) | 20 (28.6) | 2.69 (1.05-6.89) | 2.40 (0.89-6.45) |
| p-value for the trend | p=0.02 | p=0.03 | ||
| Maternal Age< 35 years |
||||
| Quartile 1 (<31.2) | 20 (22.5) | 30 (25.6) | Referent | Referent |
| Quartile 2 (31.2 - 38.2) | 25 (28.1) | 28 (23.9) | 1.33 (0.61-2.92) | 0.98 (0.41-2.35) |
| Quartile 3 (38.3 - 46.3) | 21 (23.6) | 32 (27.4) | 0.98 (0.44-2.16) | 1.03 (0.43-2.45) |
| Quartile 4 (≥ 46.4) | 23 (25.8) | 27 (23.1) | 1.27 (0.57-2.82) | 1.02 (0.42-2.45) |
| p-value for the trend | 0.8 | 0.93 |
OR and 95%CI adjusted for maternal race/ethnicity, family history of diabetes, and pre-pregnancy overweight status.
Number (%)
Table 4.
Higher early pregnancy serum maternal RBP4 concentrations, advanced maternal age, and gestational diabetes mellitus (GDM) risk
| Higher RBP4 and Age ≥35 years |
GDM
(N=173) |
Controls
(N= 187 ) |
Unadjusted
OR (95%CI) |
Adjusted
OR (95%CI) * |
|---|---|---|---|---|
| N (%) | N (%) | |||
| RBP4 < 38.3 and <35years | 45 (26.0) | 60 (32.1) | Referent | Referent |
| RBP4 ≥ 38.3 and <35years | 44 (25.4) | 57 (30.5) | 1.02 (0.59-1.78) | 1.08 (0.60-1.96) |
| RBP4 < 38.3 and ≥35years | 25 (14.5) | 34 (18.2) | 0.98 (0.51-1.86) | 1.01 (0.50-2.01) |
| RBP4 ≥ 38.3 and ≥35years | 59 (34.1) | 36 (19.3) | 2.18 (1.24-3.85) | 2.31 (1.26-4.23) |
| p-value for interaction | 0.018 | 0.021 |
OR and 95%CI adjusted for maternal race/ethnicity, family history of diabetes, and pre-pregnancy overweight status.
Discussion
Overall, there is modest evidence to support association of serum RBP4 concentrations in early pregnancy with an increased risk of GDM. Maternal age may be a potential effect modifier of the association between serum RBP4 and GDM. The relationship between RBP4 and GDM was observed only among women ≥ 35 years of age.
Our findings are similar to some previous reports of associations of RBP4 with insulin resistance, obesity and diabetes in non-pregnant and pregnant populations [2,5]. An increase in RBP4 in serum was observed among T2DM patients in a cross-sectional study by Takebayashi et al [32]. They reported a significant elevation of RBP4 concentrations in diabetic patients compared with healthy controls (mean and SD, 23.8±8.8 vs. 21.1±4.3 μg/ml, respectively). T2DM shares a number of risk factors with GDM; and some investigators consider GDM as a precursor of T2DM [4,5,7,22]. RBP4, like other adipokines, may participate in GDM pathogenesis [8]. A correlation between RBP4 concentrations and insulin resistance has been demonstrated among obese or T2DM study participants [5,22,23]. Chan et al reported that serum concentrations of RBP4 measured in samples collected between 24-28 weeks of gestation were significantly higher among women with GDM pregnancies compared with serum RBP4 concentrations among women with pregnancies uncomplicated by GDM (mean and SD, 42.4 ± 13.8ng/ml vs. 32.0 ± 8.7ng/ml; p=0.007)[5].
Our findings are similar to those reported by Su et al. In a cross-sectional study, Su et al measured RBP4 concentrations among pregnant women with and without GDM (at median gestational age of 26 weeks), as well as non-pregnant women. They found significantly increased mean RBP4 concentrations in GDM cases (mean and SD, 41.64 ± 12.21 mg/l) compared to concentrations among non-GDM pregnant women (mean and SD, 34.50 ± 9.80 mg/L) and healthy non-pregnant women (mean and SD, 30.64 ± 9.46 mg/l) [23]. In contrast, Krzyzanowska et al found that women with GDM had lower RBP4 concentrations as compared to controls in a study that involved enzyme immunometric assay (EIA) (median 6.8; inter-quartile range, 3.9-14.3; vs. median 11.3; inter-quartile range 7.8-19.9 ug/ml, for GDM cases and controls respectively; p< 0.001) and western blot (median 25.1; inter-quartile range 21.7-29.6 vs. median 26.6, inter-quartile range 23.5-32.2 ug/ml, for GDM cases and controls respectively; p=0.026) based measurements [6]. However, they found that women with GDM had a higher RBP4 to retinol molar ratio than their control counterparts [6]. In the study conducted by Teper et al, neither circulating RBP4 nor RBP4 to retinol molar ratio was significantly different between GDM cases and corresponding controls [18]. Inconsistent findings in previous reports can result from differences in study population characteristics, including age and socio-economic status indicators. For instance, the study population investigated by Teper et al comprised of a cohort of borderline obese women who may have different underlying physiological and patho-physiological characteristics to our study population.
Advanced maternal age is one of the risk factors for GDM [33]. For instance, investigators have reported that women ≥40 years of age have greater than 10-fold increased risk of GDM as compared with women 20-40 years of age [33]. RBP4 concentrations were also higher among older study participants compared with younger participants [24,34]. Among characteristics that have been related to aging are changes in body fat distribution and renal function [24]. Both of these are direct or indirect risk factors for GDM. Possible hypotheses for observed relationships in our study could be that age related alteration in body fat distribution and/or changes in renal function may account for differences in association of serum RBP4 concentrations with GDM risk among younger or older pregnant women.
Several strengths and limitations should be considered when interpreting results from our study. Unlike previous studies which collected samples between 24 and 28 weeks of gestation, we collected serum samples early in pregnancy, and thus were able to determine maternal early pregnancy RBP4 concentrations. Therefore, we can safely identify temporal relationship between the exposure and outcome. We controlled for several variables that could be potential confounders. Finally, we examined potential effect modification by maternal age. Some limitations of our study deserve mention. Although we adjusted for several potential confounders, we cannot exclude the possibility of residual confounders or confounding by unmeasured variables. Generalizability of our findings may be limited because our study participants were predominantly non-Hispanic White and residents of the Pacific Northwestern region of the USA.
Conclusion
There is modest evidence of a positive association of early pregnancy elevated RBP4 concentration with increased risk of GDM, particularly among women with advanced maternal age. Additional research is warranted to further explore these relationships.
Acknowledgment
The authors are very thankful to the participants of the Omega study and staff of Center for Perinatal Studies, Swedish Medical Center, Seattle, WA, USA for their technical support with this research. This research was supported by awards from the National Institutes of Health (R01HD-055566 and R01HD-32562). Dr. Enquobahrie was supported by a K01 award (K01HL-103174).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus. Obstet Gynecol. 2004;103:526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 2.Klein K, Bancher-Todesca D, Leipold H, Knöfler M, Haslinger P, Handisurya A, Kautzky-Willer A, Worda C. Retinol-binding protein 4 in patients with gestational diabetes mellitus. J Womens Health (Larchmt) 2010;19:517–521. doi: 10.1089/jwh.2009.1615. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 4.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Kim SK, Mittal P, Dong Z, Pacora P, Yeo L, Hassan SS. Retinol-binding protein 4: a novel adipokine implicated in the genesis of LGA in the absence of gestational diabetes mellitus. J Perinat Med. 2010;38:147–155. doi: 10.1515/JPM.2010.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan TF, Chen HS, Chen YC, Lee CH, Chou FH, Chen IJ, Chen SY, Jong SB, Tsai EM. Increased serum retinol-binding protein 4 concentrations in women with gestational diabetes mellitus. Reprod Sci. 2007;14:169–174. doi: 10.1177/1933719106298407. [DOI] [PubMed] [Google Scholar]
- 6.Krzyzanowska K, Zemany L, Krugluger W, Schernthaner GH, Mittermayer F, Schnack C, Rahman R, Brix J, Kahn BB, Schernthaner G. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. 2008;51:1115–1122. doi: 10.1007/s00125-008-1009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maghbooli Z, Hossein-Nezhad A, Mirzaei K, Karimi F, Besharati A, Omidfar K, Larijani B. Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus and the risk of developing metabolic syndrome after pregnancy. Reprod Sci. 2010;17:196–201. doi: 10.1177/1933719109351097. [DOI] [PubMed] [Google Scholar]
- 8.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf) 2012;76:2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 9.Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol. 2003;102:850–856. doi: 10.1016/s0029-7844(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 10.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Kwak SH, Youn BS, Lim S, Park YJ, Lee H, Lee N, Cho YM, Lee HK, Kim YB, Park KS, Jang HC. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2008;93:3142–3148. doi: 10.1210/jc.2007-1755. [DOI] [PubMed] [Google Scholar]
- 12.Masuyama H, Inoue S, Hiramatsu Y. Retinol-binding protein 4 and insulin resistance in preeclampsia. Endocrine Journal. 2011;58:47–53. doi: 10.1507/endocrj.k10e-288. [DOI] [PubMed] [Google Scholar]
- 13.Ost A, Danielsson A, Lidén M, Eriksson U, Nystrom FH, Strålfors P. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696–3704. doi: 10.1096/fj.07-8173com. [DOI] [PubMed] [Google Scholar]
- 14.Shangguan X, Liu F, Wang H, He J, Dong M. Alterations in serum adipocyte fatty acid binding protein and retinol binding protein-4 in normal pregnancy and preeclampsia. Clin Chim Acta. 2009;407:58–61. doi: 10.1016/j.cca.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, Humpert PM. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 16.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- 17.Esteve E, Ricart W, Fernandez-Real JM. Adipocytokines and insulin resistance. Diabetes care. 2009;32:S362–S367. doi: 10.2337/dc09-S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tepper BJ, Kim YK, Shete V, Shabrova E, Quadro L. Serum retinol-binding protein 4 (RBP4) and retinol in a cohort of borderline obese women with and without gestational diabetes. Clin Biochem. 2010;43:320–323. doi: 10.1016/j.clinbiochem.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiworapongsa T, Gotsch F, Than NG, Dong Z, Pacora P, Lamont R, Yeo L, Hassan SS, Kusanovic JP. Retinol binding protein 4-a novel association with early-onset preeclampsia. J Perinat Med. 2010;38:129–139. doi: 10.1515/JPM.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf G. Serum retinol-binding protein: a link between obesity, insulin resistance, and type 2 diabetes. Nutr Rev. 2007;65:251–256. doi: 10.1111/j.1753-4887.2007.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 22.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 23.Su YX, Hong J, Yan Q, Xu C, Gu WQ, Zhang YF, Shen CF, Chi ZN, Dai M, Xu M, Zhang YW, Liu QR, Li XY, Ning G, Wang WQ. Increased serum retinol-binding protein-4 levels in pregnant women with and without gestational diabetes mellitus. Diabetes Metab. 2010;36:470–475. doi: 10.1016/j.diabet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-Binding Protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 25.Lewandowski KC, Stojanovic N, Bienkiewicz M, Tan BK, Prelevic GM, Press M, Tuck S, O’Hare PJ, Randeva HS. Elevated concentrations of retinol-binding protein-4 (RBP-4) in gestational diabetes mellitus: negative correlation with soluble vascular cell adhesion molecule-1 (sVCAM-1) Gynecol Endocrinol. 2008;24:300–305. doi: 10.1080/09513590802141052. [DOI] [PubMed] [Google Scholar]
- 26.Abetew DF, Enquobahrie DA, Dishi M, Rudra CB, Miller RS, Williams MA. Age at menarche, menstrual characteristics, and risk of preeclampsia. ISRN Obstet Gynecol. 2011 doi: 10.5402/2011/472083. Epub 2011. doi:10.5402/2011/472083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dishi M, Enquobahrie DA, Abetew DF, Qiu C, Rudra CB, Williams MA. Age at menarche, menstrual cycle characteristics and risk of Gestational diabetes. Diabetes Res Clin Pract. 2011;93:437–442. doi: 10.1016/j.diabres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Enquobahrie DA, Qiu CF, Hevner K, Abetew D, Williams MA. Maternal plasma protein profiles in response to oral 50-gram glucose load in mid-pregnancy: a pilot study. Int J Mol Epidemiol Genet. 2011;30:292–299. [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C, Hevner K, Abetew D, Enquobahrie DA, Williams MA. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus. Clin Biochem. 2011;44:804–808. doi: 10.1016/j.clinbiochem.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ADA Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie TJ, Tibshirani RJ. Generalized Additive Models. Chapman-Hall; London: 1990. [Google Scholar]
- 32.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–2719. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 33.Virjee S, Robinson S, Johnston DG. Screening for diabetes in pregnancy. J R Soc Med. 2001;94:502–509. doi: 10.1177/014107680109401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, Mcnurlan MA, Gelato MC. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity. 2008;16:893–895. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]


