Abstract
Nowadays, functional magnetic resonance imaging (fMRI) has become one of the most important ways to explore the central mechanism of acupuncture. Among these studies, activations around the somatosensory-related brain network had the most robust blood oxygen level-dependent (BOLD) responses. However, due to the insufficient control of the subjective sensations during acupuncture stimulation, whether these robust activations reflected the pattern of de-qi, sharp pain, or mixed (de-qi + sharp pain) sensations was largely unknown. The current study recruited 50 subjects and grouped them into two groups according to whether he/she experienced sharp pain during acupuncture stimulation to give a definite answer to the aforesaid question. Our results indicated that BOLD responses associated with de-qi during acupuncture stimulation at ST36 were activation dominated. Furthermore, both the quantitative and qualitative differences of BOLD responses between de-qi and mixed sensations evoked by acupuncture stimulation were significant. The pattern of BOLD responses of sharp pain might be partly separated from that of de-qi in the spatial distribution. Therefore, we proposed that in order to explore the specific central mechanism of acupuncture, subjects with sharp pain should be excluded from those with only de-qi.
1. Introduction
With the aid of functional magnetic resonance imaging (fMRI) techniques, over one hundred studies have been published to explore the neurobiological mechanisms of acupuncture in the past few decades [1–8]. Among these studies, activations around the somatosensory-related brain network had the most robust blood oxygen level-dependent (BOLD) responses in acupuncture [9–11]. Although the majority of early researchers considered that the specific central regulation of acupuncture may be engaged in these activations, recent studies suggested that these results may reflect only an ordinary brain process of the somatosensory stimulation [9] and that acupuncture may turn out to be a kind of deep pain [12]. Therefore, sophisticated studies which form the perspective that acupuncture is a stimulation of the body with multidimensional sensations, focused on exploring the central responses associated with these multi-dimensional sensations evoked by acupuncture stimulation, were urgently needed.
During acupuncture stimulation, multidimensional and intense needling sensations, such as soreness, numbness, distention, heaviness, dull pain, and sharp pain, are experienced by subjects. In general, studies focused on qualification and quantification of needling sensations reached a consensus that de-qi, which is traditionally believed to be very important for the possible therapeutic effects of acupuncture, and sharp pain, which is considered to be irrelevant to the acupuncture effect and what acupuncturists try to avoid during needle manipulation, should be quantified separately [13, 14]. However, most previous acupuncture studies in fMRI did not quantify and explicitly distinguish subjects into de-qi and sharp pain based on needle sensations, which made striking discrepancies between results of different studies.
Only several studies excluded subjects who experienced sharp pain for fMRI data analysis based on a quantitative needling sensation questionnaire [15–17]. Fewer studies divided subjects into two groups according to whether subjects experienced sharp pain during acupuncture manipulation and compared the BOLD responses between the two groups [6, 18–20]. To make matters worse, significant incompatibilities were shown across the results of these studies. On the one hand, the pattern of BOLD responses of de-qi (without sharp pain) evoked by acupuncture stimulation was declared to be activationdominant by some studies but deactivationdominant by others. On the other hand, the pattern of the BOLD responses of mixed sensations (de-qi with sharp pain) evoked by acupuncture stimulation was considered to be definite activation by some studies, although these may be two possibilities according to the proportion of different sensations by others. In brief, two core issues of the fMRI-based acupuncture studies are still far from clear. First, what are the de-qi related BOLD responses, that is, are they dominated by activation or deactivation? Second, what is the relationship between the de-qi related and the sharp pain related BOLD responses?
The current study aims to give a definite answer to these questions. Subjects were grouped into the de-qi group or the mixedgroup (de-qi + sharp pain) according to whether he/she experienced sharp pain during acupuncture stimulation. The level of the de-qi score was compared between groups. The group results of each group and different results/regions between groups were presented. Particularly, the different regions were defined as regions of interest (ROIs) and correlated with the scores from the needling sensations.
2. Materials and Methods
2.1. Subjects
Participants were recruited from a group of 50 college students (25 males and 25 females; ages 23.3 ± 2.1 years). All subjects were right handed with normal or corrected-to-normal vision. Subjects were acupuncture naïve, had no history of major medical illnesses, head trauma, neuropsychiatric disorders, or any prescription medications one month preceding the experiment, and did not have any contraindications to exposure to a high magnetic field. All subjects gave written and informed consent after the experimental procedures were fully explained. All research procedures were approved by the West China Hospital Subcommittee on Human Studies and were conducted in accordance with the Declaration of Helsinki.
2.2. Experimental Procedures
For each subject, a functional scanning of the acupuncture stimulation was done. During the scanning, all subjects were instructed to keep their eyes closed to prevent them from actually observing the procedures. Acupuncture stimulation was performed at acupoint ST36 on the right leg (Zusanli, located in the tibialis anterior muscle four fingerbreadths below the lower margin of the patella and one fingerbreadth lateral from the anterior crest of the tibia). The fMRI paradigm for the acupuncture stimulation run lasted 8 minutes and consisted of three one-minute acupuncture manipulations (Figure 1). The needle was inserted perpendicularly to a depth of 2-3 cm before the scan started. A one-minute baseline period was held preceding the first acupuncture stimulation. The interval between the first two acupuncture manipulations was two minutes, while the second and third acupuncture manipulations were separated by an interval of one minute. Scanning was then continued for another minute after the third manipulation. During the acupuncture procedure, the needle was rotated manually clockwise and counterclockwise for one minute at a rate of 60 times per minute. The stimulation was administered by a balanced “tonifying and reducing” technique using a sterile disposable 38 gauge stainless steel acupuncture needle (0.3 mm × 40 mm). After the scan ended, the needle was extracted. In the end, the subjects were facilitated by the acupuncturist to quantify their sensations using a 10-point visual analogue scale (VAS) to rate their de-qi experience felt during the acupuncture run, including soreness, numbness, fullness/distention, heaviness, spread, dull pain, and sharp pain. The VAS was scaled as follows: 0, no sensation; 1–3, mild; 4–6, moderate; 7-8, strong; 9, severe; 10, unbearable sensation. Based on the score of sharp pain, 22 subjects were assigned to the de-qi group (the score of sharp pain equals zero). The other 28 subjects, with the score of sharp pain being above zero, were assigned to the mixed group.
Figure 1.
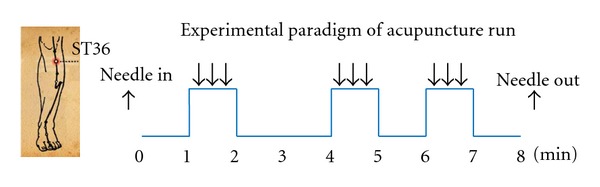
Experimental paradigm. It lasted 8 min and consisted of three one-minute acupuncture stimulations.
2.3. fMRI Scanning Procedure
Imaging data were collected from a 3T Siemens scanner (Allegra, Siemens Medical System) at the Huaxi MR Research Center, West China Hospital of Sichuan University, Chengdu, China. A standard birdcage head coil was used, along with restraining foam pads to minimize head motion and to diminish scanner noise. Thirty axial slices (FOV = 240 mm × 240 mm, matrix = 64 × 64, thickness = 5 mm) parallel to the AC-PC plane covering the whole brain were obtained using a T2*-weighted single-shot, gradient-recalled echo planar imaging (EPI) sequence (TR = 2,000 ms, TE = 30 ms, flip angle = 90°). The scan covered the entire brain including the cerebellum and brainstem. After the functional run, high-resolution structural information on each subject was acquired using 3D MRI sequences with a voxel size of 1 mm3 for anatomical localization (TR = 2.7 s, TE = 3.39 ms, matrix = 256 × 256, FOV = 256 mm × 256 mm, flip angle = 7°, in-plane resolution = 1 mm × 1 mm, slice thickness = 1 mm).
2.4. fMRI Data Analysis
Preprocessing and statistical analyses at both the individual level and group level were performed using the Statistical Parametric Mapping software (SPM5, http://www.fil.ion.ucl.ac.uk/spm). Initially, the first 5 time points were discarded in order to avoid the instability of the initial MRI signal. The remaining images were realigned to the first volume. Three subjects in the de-qi group and five subjects in the mixed group exceeded our rigorous motion threshold of less than 1 mm spatial displacement in any direction. Another four subjects in the mixed group were deserted randomly in order to balance the number of groups. Ultimately, 19 subjects (8 males) in the de-qi group and 19 subjects (9 males) in the mixed group remained. Subsequently, the images were normalized to the standard EPI template, resampled to a voxel size of 3 × 3 × 3 mm3, and then smoothed spatially using a 6 mm full-width-at-half maximum (FWHM) isotropic Gaussian kernel to decrease spatial noise. Global normalization by proportional scaling was not applied. Then, the time-series from each voxel was high-pass filtered (1/235-Hz cutoff) to remove low-frequency noise and signal drift. For each subject, the preprocessed fMRI data were then submitted for fixed-effects model analyses using the general linear model (GLM) performed at each voxel across the whole brain. After acquiring the contrast images, individual level analyses were accomplished and statistical parametric maps for the t statistics (spmT) were then generated for each contrast image. At the group level, the random-effects model analysis was performed based on inference images (i.e., t test for contrast images) from the individual level analysis. For exploring the de-qi related and mixed related BOLD response evoked by acupuncture stimulation, the group results of the one sample t-test for each group were mapped and listed at P < 0.0001, uncorrected (|t | >4.65), and a minimum cluster size of 5 voxels. The different BOLD responses between the de-qi and the mixed group were explored based on a two sample t-test at P < 0.0001, uncorrected (|t | >4.14), and a minimum cluster size of 5 voxels. Then, the significant regions of the two sample t-test were defined as the ROI. In each ROI, the BOLD responses of each subject in the mixed group were extracted and correlated with the sharp pain score.
3. Results
3.1. Psychophysical Results
The average score of sharp pain in the mixed group was 1.4 ± 0.7. The sensation of spread was significantly stronger in the mixed group (2.2 ± 2.3 versus 4.1 ± 3.0, P = 0.03). Other sensations of de-qi were comparable between groups (P > 0.05; see Table 1 for details). In the mixed group, the score of sharp pain and the sum score of de-qi were uncorrelated (r = 0.25, P = 0.30).
Table 1.
Scores of needling sensations between groups.
| Sensations | VAS scores (mean ± SD) | Significance level | |
|---|---|---|---|
|
de-qi group (n = 19) |
Mixed group (n = 19) |
||
| Soreness | 2.7 ± 2.3 | 2.7 ± 2.5 | P > 0.05 |
| Numbness | 1.7 ± 2.0 | 2.9 ± 2.3 | P > 0.05 |
| Fullness | 5.0 ± 1.6 | 6.3 ± 2.5 | P > 0.05 |
| Heaviness | 2.4 ± 2.9 | 4.1 ± 2.6 | P > 0.05 |
| Spread | 2.2 ± 2.3 | 4.1 ± 3.0 | P = 0.03 |
| Dull pain | 0.8 ± 1.4 | 1.4 ± 1.4 | P > 0.05 |
| Sharp pain | 0 | 1.4 ± 0.7 | |
VAS: visual analogue scale; SD: standard deviation.
3.2. Group Results of the de-qi Group
Figure 2(a) shows group activations and deactivations of the de-qi group evoked by acupuncture stimulation at ST36. The primary somatosensory cortex (SI), the secondary somatosensory cortex (SII), the inferior parietal lobule (IPL), the insula, the thalamus, the cingulate gyrus (Brodmann area (BA) 24/32), the precentral gyrus (BA4/6/44), the inferior frontal gyrus, the putamen, the claustrum, the superior temporal gyrus (STG), and the transverse temporal gyrus and the cerebellum (VI, VIIb, VIII, CrusI, and CrusII) were significantly activated. Most of these activations were bilateral and showed a lateralization to the hemisphere contralateral to the stimulation. Only the ipsilateral parahippocampal gyrus was significantly deactivated.
Figure 2.
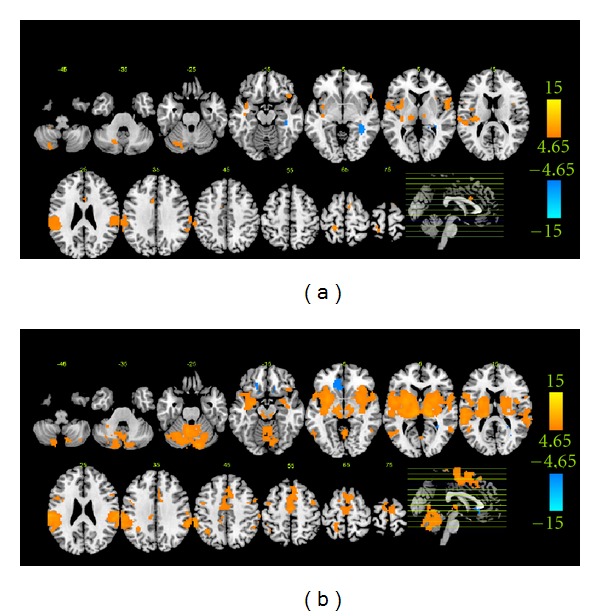
Group-level BOLD responses for each group. Panels (a) and (b) show the groups results of de-qi and mixed group evoked by acupuncture stimulation at P < 0.00001, uncorrected with 5 contiguous voxels, respectively.
3.3. Group Results of the Mixed Group
Figure 2(b) shows group activations and deactivations of the mixed group evoked by acupuncture stimulation at ST36. Significant activations were presented in the SI, the SII, the IPL, the supramarginal gyrus (SMG), the insula, the thalamus, the putamen, the caudate, the cingulate gyrus (BA24/32), the claustrum, the precentral gyrus (BA4/6/44), the inferior frontal gyrus, the medial frontal gyrus, the middle frontal gyrus, the superior frontal gyrus, the lingual gyrus, the fusiform gyrus, the parahippocampal gyrus, the middle temporal gyrus, the inferior temporal gyrus, the STG, the transverse temporal gyrus, and the cerebellum (see Table 2 for details). Most of these activations were bilateral. The contralateral perigenual anterior cingulate and the contralateral medial frontal gyrus were significantly deactivated.
Table 2.
Significant activations of each group.
| Regions | BA or A/P | de-qi group | Mixed group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Talairach | t value | Voxels | Talairach | t value | Voxels | |||||||
| x | y | z | x | y | z | |||||||
| Cerebrum | ||||||||||||
| Inferior frontal gyrus | 6/9/13/44/45/47 | L | −36 | 8 | −11 | 5.04 | 8 | −33 | 17 | −3 | 7.18 | 81 |
| R | 42 | 20 | −14 | 6.08 | 16 | 36 | 17 | −3 | 6.17 | 32 | ||
| Medial frontal gyrus | 6/32 | L | −3 | −9 | 53 | 7.40 | 69 | |||||
| R | 3 | −9 | 50 | 7.51 | 68 | |||||||
| Middle frontal gyrus | 6/9 | L | −42 | 0 | 50 | 6.89 | 18 | |||||
| R | 42 | 2 | 50 | 7.32 | 33 | |||||||
| Superior frontal gyrus | 6 | L | −3 | 5 | 49 | 6.21 | 31 | |||||
| R | 3 | 11 | 55 | 8.09 | 70 | |||||||
| Precentral gyrus | 6/44 | L | −15 | −32 | 65 | 5.93 | 18 | −48 | 0 | 8 | 9.50 | 43 |
| R | 50 | −2 | 8 | 6.16 | 8 | 48 | 0 | 8 | 6.56 | 23 | ||
| Postcentral gyrus | 1/2/3/5/7/40/43 | L | −18 | −35 | 65 | 8.20 | 65 | −62 | −22 | 20 | 11.04 | 98 |
| R | 65 | −22 | 31 | 5.49 | 17 | 56 | −28 | 21 | 9.16 | 48 | ||
| Inferior parietal lobule | 40 | L | −65 | −28 | 32 | 7.43 | 62 | −65 | −25 | 23 | 9.52 | 151 |
| R | 59 | −28 | 26 | 6.41 | 38 | 65 | −36 | 29 | 9.33 | 85 | ||
| Supramarginal gyrus | 40 | L | −53 | −46 | 22 | 7.30 | 24 | |||||
| R | 59 | −39 | 32 | 9.33 | 15 | |||||||
| Thalamus | L | −12 | −20 | 7 | 5.90 | 44 | −15 | −23 | 9 | 10.55 | 238 | |
| R | 9 | −17 | 4 | 6.06 | 22 | 15 | −23 | 7 | 11.02 | 189 | ||
| Insula | 13/40/41/47 | L | −33 | −23 | 12 | 7.26 | 58 | −56 | −34 | 18 | 7.89 | 153 |
| R | 39 | 6 | 11 | 5.15 | 6 | 42 | 1 | 11 | 7.21 | 98 | ||
| Claustrum | L | −33 | −20 | 9 | 7.62 | 24 | −30 | 9 | −3 | 9.19 | 55 | |
| R | 30 | 11 | −3 | 8.28 | 52 | |||||||
| Cingulate gyrus | 24/32 | L | −6 | 13 | 32 | 6.01 | 14 | −3 | −1 | 47 | 6.99 | 56 |
| R | 6 | 22 | 27 | 6.02 | 5 | 6 | 20 | 40 | 6.54 | 56 | ||
| Caudate | L | −15 | 4 | 14 | 5.97 | 25 | ||||||
| R | 36 | −15 | −7 | 6.82 | 26 | |||||||
| Putamen | L | −30 | −17 | 9 | 6.02 | 6 | −27 | 0 | 8 | 9.88 | 284 | |
| R | 24 | 3 | 5 | 8.16 | 255 | |||||||
| Amygdala | L | −27 | −4 | −12 | 7.73 | 17 | ||||||
| Lingual gyrus | 18 | L | −15 | −82 | −11 | 5.54 | 7 | |||||
| R | 18 | −79 | −14 | 7.25 | 11 | |||||||
| Fusiform gyrus | 19 | L | −21 | −79 | −14 | 5.41 | 8 | |||||
| R | 21 | −79 | −14 | 6.03 | 7 | |||||||
| Middle temporal gyrus | 21/22/37/39 | L | −56 | −64 | 3 | 6.85 | 20 | |||||
| R | 56 | −52 | 11 | 5.93 | 4 | |||||||
| Inferior temporal gyrus | 19/37 | L | −53 | −64 | 1 | 5.94 | 7 | |||||
| Superior temporal gyrus | 13/22/38/41/42 | L | −59 | 3 | 3 | 6.14 | 38 | −59 | −34 | 18 | 9.13 | 150 |
| R | 53 | 6 | 2 | 6.10 | 8 | 56 | 0 | 3 | 8.01 | 90 | ||
| Transverse temporal gyrus | 41/42 | L | −39 | −23 | 12 | 5.51 | 6 | −45 | −23 | 12 | 7.79 | 22 |
| R | 62 | −17 | 12 | 5.38 | 14 | |||||||
|
| ||||||||||||
| Cerebellum | ||||||||||||
| Cerebellum_4_5 | A | L | −6 | −53 | −15 | 7.93 | 31 | |||||
| R | 9 | −50 | −13 | 5.95 | 22 | |||||||
| Cerebellum_6 | A | L | −27 | −56 | −20 | 6.89 | 60 | |||||
| R | 9 | −59 | −10 | 5.47 | 25 | |||||||
| P | L | −18 | −62 | −22 | 5.98 | 24 | −27 | −59 | −20 | 9.07 | 124 | |
| R | 24 | −62 | −20 | 7.00 | 113 | |||||||
| Cerebellum_7b | P | L | −24 | −72 | −34 | 5.55 | 6 | −15 | −75 | −37 | 6.83 | 27 |
| R | 9 | −75 | −34 | 8.11 | 13 | |||||||
| Cerebellum_8 | A | L | −6 | −62 | −25 | 6.48 | 12 | |||||
| P | L | −18 | −65 | −27 | 6.36 | 8 | −9 | −62 | −22 | 7.33 | 32 | |
| R | 9 | −72 | −37 | 6.89 | 14 | |||||||
| Cerebellum_Crus1 | A | L | −36 | −56 | −22 | 5.89 | 14 | |||||
| R | 36 | −51 | −25 | 4.89 | 5 | |||||||
| P | L | −18 | −68 | −24 | 6.37 | 54 | −15 | −68 | −19 | 8.02 | 173 | |
| R | 12 | −83 | −19 | 9.34 | 88 | |||||||
| Cerebellum_Crus2 | P | L | −24 | −75 | −34 | 5.65 | 11 | −12 | −77 | −21 | 7.96 | 148 |
| R | 12 | −85 | −18 | 10.23 | 94 | |||||||
| Vermis_3 | A | R | 3 | −44 | −15 | 5.21 | 7 | |||||
| Vermis_4_5 | A | L | 0 | −58 | −2 | 7.27 | 46 | |||||
| R | 3 | −61 | −2 | 6.22 | 55 | |||||||
| Vermis_6 | A | L | −3 | −56 | −15 | 6.51 | 18 | |||||
| R | 0 | −56 | −15 | 6.24 | 20 | |||||||
| P | L | −3 | −71 | −12 | 5.27 | 18 | ||||||
| R | 3 | −59 | −17 | 6.86 | 25 | |||||||
| Vermis_7 | P | L | −3 | −62 | −17 | 6.96 | 13 | |||||
| R | 0 | −62 | −17 | 6.69 | 26 | |||||||
| Vermis_8 | A | L | −3 | −62 | −25 | 6.45 | 10 | |||||
| R | 3 | −56 | −20 | 6.72 | 14 | |||||||
| P | L | 0 | −65 | −27 | 6.31 | 19 | ||||||
| R | 3 | −68 | −29 | 6.76 | 26 | |||||||
| Vermis_9 | A | L | 0 | −57 | −25 | 6.15 | 6 | |||||
| R | 3 | −56 | −22 | 6.61 | 8 | |||||||
| Vermis_10 | A | L | −3 | −51 | −20 | 6.81 | 5 | |||||
| R | 0 | −51 | −20 | 6.35 | 8 | |||||||
The coordination of voxel with the maximal t within each region is listed. The regions are thresholded at P < 0.0001, uncorrected.
BA: Brodmann area; L: left; R: right; A: anterior lobe; P: posterior lobe.
3.4. Different Results between the de-qi Group and the Mixed Group
Figure 3 shows the different results between groups. Significantly stronger activations of the mixed group were presented in the bilateral putamen, the bilateral thalamus, and the bilateral cerebellum (CrusI and CrusII). No regions showed stronger activations of the de-qi group (see details in Table 3). In these regions, BOLD signals of the de-qi group were barely changed, while significant BOLD responses were shown in the mixed group. The level of the BOLD responses and the intensity of subjective sharp pain was uncorrelated (all P > 0.1 uncorrected) in these regions in the mixed group (figure not shown).
Figure 3.
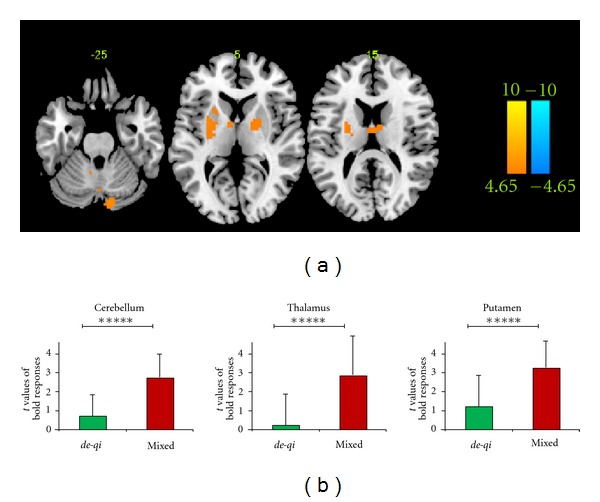
Different BOLD responses between groups. Panel (a) shows the between-group results of “mixed > de-qi” at P < 0.0001, uncorrected with 5 contiguous voxels. Panel (b) shows the mean t values of the BOLD responses in each ROI for both groups (*****is equivalent to P < 0.00001).
Table 3.
Significant difference between groups (mixed > de-qi).
| Regions | Talairach | t value | Voxels | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cerebrum | |||||||
| Thalamus | L | −6 | −8 | 11 | 5.11 | 19 | |
| R | 6 | −5 | 11 | 4.93 | 9 | ||
| Putamen | L | −27 | −2 | 11 | 5.10 | 53 | |
| R | 21 | 0 | 6 | 4.78 | 32 | ||
|
| |||||||
| Cerebellum | |||||||
| Cerebellum_Crus1 | A | L | |||||
| R | |||||||
| P | L | ||||||
| R | 12 | −83 | −19 | 5.75 | 20 | ||
| Cerebellum_Crus2 | A | L | |||||
| R | |||||||
| P | L | −9 | −86 | −23 | 4.95 | 6 | |
| R | 9 | −83 | −19 | 6.11 | 12 | ||
The coordination of voxel with the maximal t within each region is listed. The regions are thresholded at P < 0.0001, uncorrected.
BA: Brodmann area; L: left; R: right; A: anterior lobe; P: posterior lobe.
3.5. Different Results between the Weak de-qi Group and the High de-qi Group
For eliminating the possibility that the three discrepant regions in Section 3.4 were due to the different global de-qi intensity between groups, we divided the pure de-qi group into two subgroups based on the sum score of de-qi (weak de-qi group: 10 subjects, averaged sum score of de-qi was 9.48 ± 3.92; strong de-qi group: 9 subjects, averaged sum score of de-qi was 20.63 ± 5.35). Although the difference of the de-qi intensity between these two subgroups (weak de-qi group and strong de-qi group) was much greater than that between the two groups (de-qi group and mixed group), no regions were shown in the intersubgroup results (P > 0.01, uncorrected for all voxels, figure not shown).
4. Discussion
The present study focused on two core issues of the fMRI-based acupuncture studies. Firstly, our results indicated that the de-qi related BOLD responses were dominated by activations, mainly around the somatosensory-related brain network. Secondly, our results showed that the de-qi + sharp pain evoked by acupuncture stimulation were associated with more extensive and strong activations. Particularly, specific activations of pain-related regions at the part of the bilateral putamen, thalamus, and cerebellum were shown only in the mixed group, which presumed that the BOLD response patterns of de-qi and sharp pain were partly separated in the spatial distribution.
4.1. Activation-Dominated BOLD Responses Associated with de-qi during Acupuncture Stimulation
Few studies explored the BOLD responses associated with pure de-qi (i.e., without sharp pain). In Na et al., 2009, with pure de-qi, patterns of BOLD responses to acupuncturing at GB34 were predominately activated [21]. In Bai et al., 2010, with pure de-qi, predominantly positive signal changes were seen during acupuncturing at GB37 [22]. In accordance with these previous studies, our results clearly indicated that significant activations mainly in the SI, the SII, the IPL, the insula, the thalamus, the cingulate cortex, and the cerebellum were associated with pure de-qi during acupuncture stimulation at ST36 (Figure 2(a) and Table 2). However, our results also conflicted with other previous studies. Hui and colleagues proposed a brain network called the limbic-paralimbic-neocortical network to name the predominant deactivated brain regions associated with pure de-qi during acupuncture stimulation [6, 19, 20, 23]. We inferred that several possible reasons might contribute to the deactivation-dominated BOLD response patterns. Firstly, the average of the repeated runs for each subject was applied. Repetition may itself alter the distribution of the activated regions due to the influence of memory and expectation [24]. Secondly, global normalization, a questionable data processing step adopted in several fMRI-based acupuncture studies, can introduce an artificially negative relationship with the task [25–29]. Particularly, our recent study clarified that for the fMRI-based acupuncture's data, global normalization significantly changed the activation-dominated results to deactivation-dominated [29]. Therefore, we suggested that BOLD responses associated with de-qi during acupuncture stimulation at ST36 should be activation dominated.
These de-qi related activations were mainly around the somatosensory-related brain network. Previous acupuncture studies tended to believe that these results reflected the specific central regulation of acupuncture [5, 21–23]. However, several recent studies argued that acupuncture stimulation was similar to a special deep pain stimulation [12]. Therefore, the BOLD responses of acupuncture stimulation were considered to mainly reflect the general central processing of a special deep pain stimulation [30]. In contrast to superficial (cutaneous) pain, deep pain (originating from muscle, joints or viscera) is dull, diffuse, and difficult to localize [30, 31]. In Henderson et al., 2006, the researchers used intramuscular injections of hypertonic saline to construct a deep pain model [30]. The subjective sensations under this deep pain mainly included tenderness, heaviness, aching, cramping, throbbing, and gnawing, which were similar to that of de-qi evoked by acupuncture stimulation. Activations of deep pain based on intramuscular injections of hypertonic saline were located in the middle cingulate gyrus, insula, SI, SII, motor regions, and cerebellum [24, 30]. Other studies explored the central processing of deep pain based on intramuscular electrical stimulation (IMES) [31] or mechanical pressure on muscle [32]. During IMES, the SI, SII, insula, IPL, precuneus, superior temporal gyrus, cingulate gyrus (anterior, middle, and posterior), claustrum, thalamus, precentral gyrus, and frontal gyrus (inferior, medial, and middle) were activated [31]. Activations of the ACC, insula, SII, IPL, thalamus, putamen, claustrum, and caudate were correlated with the mechanical-pressure-based muscle pain [32]. Most activations of de-qi in the present study were reported in the aforementioned deep pain studies, which might indicate similar central processing of the same origin of stimulation and similar subjective sensations. Due to individual variability of brain morphology and differences in experimental design, the central patterns of activation during deep pain and acupuncture stimulation were difficult to compare between studies. Therefore, we suggested that a specific central effect of de-qi during acupuncture stimulation might be illustrated after comparing it directly to deep pain stimulation.
4.2. Relationship between de-qi Related and Sharp Pain Related Bold Responses
In the domain of pain studies, it is generally known that differences of the quality and origin between sharp pain and deep pain were remarkable [33]. Although one early positron emission tomography (PET) study argued that skin pain and muscle pain had a common representation [34], abundant evidence from animal investigations [35, 36], clinical data [37–39], and human neuroimage studies [24, 30, 31, 40–43] indicated different brain processing of acute superficial pain (sharp pain) and deep pain. For acupuncture, both the deep-pain-like de-qi sensations and sharp pain were evoked by identical acupuncture processing. The sharp pain in acupuncture could be due to stimulation of the superficial cutaneous pain fibers or the pain fibers in deep tissues. Therefore, the differences between de-qi and sharp pain in acupuncture could possibly be attributed to only the quality of sensation or both the quality and origin of sensation. In either case, certain differences of central responses should be detected. Since subjects with pure sharp pain during acupuncture stimulation were hardly obtained, the difference between de-qi and sharp pain evoked by acupuncture stimulation could not be directly presented. Therefore, we had to infer their differences between the de-qi and mixed groups. When considering all the needling sensations as a whole, activations of the SII, insula, SI, cerebellum, and thalamus were the most robust BOLD responses evoked by acupuncture stimulation in previous acupuncture fMRI studies [9–11]. Consistent results were shown in our study so that extensive activations of cortical areas were relevant to the processing of somatosensory, motor, and pain signals which were associated with de-qi + sharp pain evoked by acupuncture stimulation (Figure 2(b) and Table 2).
Based on the visual comparison of the de-qi and mixed groups' group results, the activated regions of the mixed group mainly covered that of the de-qi group and had a larger extent and stronger intensity. Moreover, activations of the frontal gyrus (medial, middle, and superior), temporal gyrus (inferior and middle), SMG, caudate, amygdala, parahippocampal gyrus, lingual gyrus, fusiform gyrus, and numerous subregions in the cerebellum were also activated in the mixed group. Most of these visual differences failed to survive in the two-sample t test, thus the interpretation of these differences should be taken with caution. We argued that they might reflect the slightly stronger subjective sensations of de-qi in the mixed group or the common pattern of both de-qi and sharp pain, rather than the specific pattern of sharp pain, such as detection of a painful stimulus and/or encoding of pain localization, intensity, and duration [30]. In addition, deactivations of the parahippocampal gyrus and the subgenual cingulate/medial frontal gyrus were unique to the de-qi and mixed groups, respectively. A previous study showed that the BOLD responses in the hippocampus/parahippocampus were altered depending on the type of tissue stimulated, that is, deactivated during deep pain and activated during superficial pain [24], which accorded with our results and might indicate different emotional and cognitive processing of these two kinds of pain [24, 44, 45]. As part of the default mode network (DMN), the deactivation of the subgenual cingulate/medial frontal gyrus was usually reported in previous acupuncture fMRI studies associated with the mixed group [5, 10, 29], indicating a function to enter a mode of preparedness and alertness for possible changes in the internal or external milieu [5]. However, these two deactivated regions also failed to survive in the two-sample t test. The deactivation of the hippocampus or parahippocampus was also found in previous acupuncture fMRI studies associated with the mixed group [5, 29]. Therefore, these differences should also be interpreted with caution.
Based on the two-sample t test between groups, parts of the putamen, thalamus, and cerebellum were significantly different (Figure 3 and Table 3). Specifically, in these regions, BOLD signals were barely changed in the de-qi group, but they were significantly increased in the mixed groups. We suggested that this might reflect the specific brain processing of sharp pain, rather than the different global de-qi intensity between groups (see Section 3.5 for details) or the common pattern but with a different degree for the de-qi and mixed group. The thalamus, as a structure that receives projections from multiple ascending pain pathways, is involved in the sensory discriminative and affective motivational components of pain [46–48]. Particularly, the activations of the thalamus would most be expected in the acute pain state [49], which was consistent with the current study. Since de-qi might originate from deep tissues and sharp pain might originate from superficial cutaneous pain fibers, the difference in the thalamus might reflect the different originating structures of pain [30, 31]. As a brain region of the salience network, the putamen was important in the integration of cognitive information [50, 51]. For pain, the putamen might be able to utilize the cognitive context to influence the selection of appropriate brain networks, which were engaged during the inflow of afferent nociceptive information [52]. In the current study, the difference in the putamen might reflect the specific processing of sharp pain. The difference in the cerebellum was located in lobules CrusI and CrusII, which are involved in cognitive processing of pain [53] and might reflect the specific central processing of sharp pain. However, the activities of these discrepant regions were not correlated with the subjective sensations, indicating that they did not encode subjective pain intensity (figure not shown). In short, we suggested that these discrepant regions might be associated with specific perceptual and emotional qualities of sharp pain.
In particular, another interpretation for these quantitative and qualitative differences that should be discussed is the different salience of de-qi and sharp pain. Previous deep pain studies found that the cortices of the temporoparietal junction (BA22/39/40), which included both bilateral STG and SMG, were significantly activated [31]. The temporoparietal junction was considered to respond preferentially to behaviorally relevant stimuli and play a general role in detecting salient stimuli [54–56]. Recently, the “salience network” was dissociated, which enables the integration of highly processed sensory data with visceral, autonomic, and hedonic markers so that the organism can decide what to do (or not to do) next [57–59]. The salience network includes the anterior insula, the anterior cingulate cortex, as well as more limbic and paralimbic structures (such as the amygdala and putamen) and the cerebellum (VI) [52, 57, 59, 60]. Most of these regions were activated in both the de-qi and mixed groups, which might indicate that both de-qi and sharp pain sensations evoked by acupuncture stimulation were salient external stimuli. Furthermore, because sharp pain is often escapable and generally evokes active emotional coping behaviors, while deep pain is usually inescapable and evokes often a passive emotional coping behavior [24, 30], the stronger and more extensive activation in these regions in the mixed group, as well as the regions that were activated only in the mixed group, might reflect that sharp pain is more salient than deep-pain-like de-qi sensations.
In conclusion, both the quantitative and qualitative differences of BOLD responses between de-qi and mixed sensations evoked by acupuncture stimulation were distinct. We inferred that the pattern of BOLD responses of sharp pain might be partly separated from that of de-qi in the spatial distribution. The subjects with sharp pain should be excluded from those with only de-qi, when exploring the central BOLD responses during acupuncture stimulation.
4.3. Limitations
This paper still has space for improvement in upcoming studies. Firstly, the scoring of the subjective sensations during acupuncture stimulation was based on recollection, so the reference vector was strictly based on the “ON-OFF” pattern. Because the sensations elicited by acupuncture stimulation might fail to disappear immediately when the manipulation was stopped, the BOLD responses of the acupuncture stimulation partly remained in the intervals between acupuncture manipulations [4, 61]. Therefore, the interaction effect among manipulations might exist. The real-time scoring in further studies would provide more precise information. Even so, using a relatively conservative threshold, authentic activations (or deactivations) under the “ON-OFF” pattern could be detected. Particularly, the results of this paper mainly reflected the BOLD responses of de-qi or de-qi mixed with sharp pain evoked by acupuncture stimulation, rather than the effect of acupuncture. Secondly, the control group with pure sharp pain sensation was absent. Since subjects with pure sharp pain were difficult to obtain during acupuncture stimulation, further studies would collect these subjects from a large sample size. Finally, the quantity of the stimulus during the BLOCK-designed manual acupuncture was hardly equivalent. Further studies should quantify the stimulus of acupuncture with assistive devices.
5. Conclusions
The current study focused on two core issues of the fMRI-based acupuncture studies. For the first question about what are the de-qi related BOLD responses dominated by, our answer was that BOLD responses associated with de-qi during acupuncture stimulation at ST36 were activation dominated. These de-qi related activations were mainly around the somatosensory-related brain network and were similar to those in previous deep pain studies. Therefore, we suggested that these activations might indicate similar central processing to the same origin of stimulation and similar subjective sensations. For the second question about what is the relationship between de-qi related and sharp pain related BOLD responses, our answer was that the pattern of BOLD responses for sharp pain might be partly separated from that of de-qi in the spatial distribution. Furthermore, we proposed that subjects with sharp pain should be excluded from those with only de-qi when exploring the central BOLD responses during acupuncture stimulation, because both the quantitative and qualitative differences of BOLD responses between de-qi and mixed sensations evoked by acupuncture stimulation were significant.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
This study was supported by the Project for the National Key Basic Research and Development Program (973) under Grant nos. 2012CB518501 and 2011CB707702, the National Natural Science Foundation of China under Grant nos. 30930112, 30970774, 81000640, 81000641, 81030027, 81101036, 81101108, 31150110171, 30901900, 81271644, and 31200837, and the Fundamental Research Funds for the Central Universities.
References
- 1.Bai L, Qin W, Tian J, et al. Acupuncture modulates spontaneous activities in the anticorrelated resting brain networks. Brain Research. 2009;1279:37–49. doi: 10.1016/j.brainres.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 2.Bai L, Tian J, Zhong C, et al. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Molecular Pain. 2010;6, article 73 doi: 10.1186/1744-8069-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin W, Tian J, Bai L, et al. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Molecular Pain. 2008;4, article 55 doi: 10.1186/1744-8069-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang J, Jin Z, Wang Y, et al. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Human Brain Mapping. 2009;30(4):1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui KKS, Liu J, Marina O, et al. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage. 2005;27(3):479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KKS. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Human Brain Mapping. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo SS, Teh EK, Blinder RA, Jolesz FA. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. NeuroImage. 2004;22(2):932–940. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Beissner F. Functional magnetic resonance imaging studies of acupuncture mechanisms: a critique. Focus on Alternative and Complementary Therapies. 2011;16(1):3–11. [Google Scholar]
- 10.Kong J, Gollub RL, Webb JM, Kong JT, Vangel MG, Kwong K. Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. NeuroImage. 2007;34(3):1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Qin W, Dong M, et al. Evaluation of group homogeneity during acupuncture stimulation in fMRI studies. Journal of Magnetic Resonance Imaging. 2010;32(2):298–305. doi: 10.1002/jmri.22238. [DOI] [PubMed] [Google Scholar]
- 12.Beissner F, Deichmann R, Henke C, Bär KJ. Acupuncture—deep pain with an autonomic dimension? NeuroImage. 2012;60:653–660. doi: 10.1016/j.neuroimage.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 13.MacPherson H, Asghar A. Acupuncture needle sensations associated with De Qi: a classification based on experts’ ratings. Journal of Alternative and Complementary Medicine. 2006;12(7):633–637. doi: 10.1089/acm.2006.12.633. [DOI] [PubMed] [Google Scholar]
- 14.Kong J, Gollub R, Huang T, et al. Acupuncture De Qi, from qualitative history to quantitative measurement. Journal of Alternative and Complementary Medicine. 2007;13(10):1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- 15.Vincent CA, Richardson PH, Black JJ, Pither CE. The significance of needle placement site in acupuncture. Journal of Psychosomatic Research. 1989;33(4):489–496. doi: 10.1016/0022-3999(89)90010-x. [DOI] [PubMed] [Google Scholar]
- 16.Park J, White A, Stevinson C, Ernst E, James M. Validating a new non-penetrating sham acupuncture device: two randomised controlled trials. Acupuncture in Medicine. 2002;20(4):168–174. doi: 10.1136/aim.20.4.168. [DOI] [PubMed] [Google Scholar]
- 17.White P, Bishop F, Hardy H, et al. Southampton needle sensation questionnaire: development and validation of a measure to gauge acupuncture needle sensation. Journal of Alternative and Complementary Medicine. 2008;14(4):373–379. doi: 10.1089/acm.2007.0714. [DOI] [PubMed] [Google Scholar]
- 18.Asghar AU, Green G, Lythgoe MF, Lewith G, MacPherson H. Acupuncture needling sensation: the neural correlates of deqi using fMRI. Brain Research. 2010;1315:111–118. doi: 10.1016/j.brainres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Hui KK, Liu J, Makris N, et al. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Human Brain Mapping. 2000;9(1):13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui KKS, Marina O, Claunch JD, et al. Acupuncture mobilizes the brain’s default mode and its anti-correlated network in healthy subjects. Brain Research. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na BJ, Jahng GH, Park SU, et al. An fMRI study of neuronal specificity of an acupoint: electroacupuncture stimulation of Yanglingquan (GB34) and its sham point. Neuroscience Letters. 2009;464(1):1–5. doi: 10.1016/j.neulet.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Bai L, Yan H, Li N, et al. Neural specificity of acupuncture stimulation at pericardium 6: evidence from an fMRI study. Journal of Magnetic Resonance Imaging. 2010;31(1):71–77. doi: 10.1002/jmri.22006. [DOI] [PubMed] [Google Scholar]
- 23.Hui KKS, Marina O, Liu J, Rosen BR, Kwong KK. Acupuncture, the limbic system, and the anticorrelated networks of the brain. Autonomic Neuroscience: Basic and Clinical. 2010;157(1-2):81–90. doi: 10.1016/j.autneu.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. NeuroImage. 2008;39(4):1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 25.Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage. 1998;8(3):302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- 26.Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. NeuroImage. 2001;13(4):751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilescu M, Shaw ME, Stuart GW, Eckersley P, Svalbe ID, Egan GF. Simulation of the effects of global normalization procedures in functional MRI. NeuroImage. 2002;17(2):532–542. [PubMed] [Google Scholar]
- 28.Junghöfer M, Schupp HT, Stark R, Vaitl D. Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. NeuroImage. 2005;25(2):520–526. doi: 10.1016/j.neuroimage.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Qin W, Jin L, et al. Impact of global normalization in fMRI acupuncture studies. doi: 10.1155/2012/467061. Evidence-Based Complementary and Alternative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson LA, Bandler R, Gandevia SC, MacEfield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2006;120(3):286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Niddam DM, Yeh TC, Wu YT, et al. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. NeuroImage. 2002;17(3):1437–1450. doi: 10.1006/nimg.2002.1270. [DOI] [PubMed] [Google Scholar]
- 32.Maeda L, Ono M, Koyama T, et al. Human brain activity associated with painful mechanical stimulation to muscle and bone. Journal of Anesthesia. 2011;25(4):523–530. doi: 10.1007/s00540-011-1173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis T. Pain. New York, NY, USA: McMillan; 1942. [Google Scholar]
- 34.Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. Journal of Neurophysiology. 1997;78(1):450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- 35.Bandler R, Price JL, Keay KA. Brain mediation of active and passive emotional coping. Progress in Brain Research. 2000;122:333–349. doi: 10.1016/s0079-6123(08)62149-4. [DOI] [PubMed] [Google Scholar]
- 36.Lumb BM. Hypothalamic and midbrain circuitry that distinguishes between escapable and inescapable pain. News in Physiological Sciences. 2004;19(1):22–26. doi: 10.1152/nips.01467.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nathan PW, Smith MC, Cook AW. Sensory effects in man of lesions of the posterior columns and of some other afferent pathways. Brain. 1986;109(5):1003–1041. doi: 10.1093/brain/109.5.1003. [DOI] [PubMed] [Google Scholar]
- 38.Nauta HJW, Soukup VM, Fabian RH, et al. Punctate midline myelotomy for the relief of visceral cancer pain. Journal of Neurosurgery. 2000;92(2):125–130. doi: 10.3171/spi.2000.92.2.0125. [DOI] [PubMed] [Google Scholar]
- 39.Noordenbos W, Wall PD. Diverse sensory functions with an almost totally divided spinal cord. A case of spinal cord transection with preservation of part of one anterolateral quadrant. Pain. 1976;2(2):185–195. doi: 10.1016/0304-3959(76)90114-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen ACN, Shimojo M, Svensson P, Arendt-Nielsen L. Brain dynamics of scalp evoked potentials and current source densities to repetitive (5-pulse train) painful stimulation of skin and muscle: central correlate of temporal summation. Brain Topography. 2000;13(1):59–72. doi: 10.1023/a:1007886303206. [DOI] [PubMed] [Google Scholar]
- 41.Niddam DM, Graven-Nielsen T, Arendt-Nielsen L, Chen ACN. Non-painful and painful surface and intramuscular electrical stimulation at the thenar and hypothenar sites: differential cerebral dynamics of early to late latency SEPs. Brain Topography. 2001;13(4):283–292. doi: 10.1023/a:1011180713285. [DOI] [PubMed] [Google Scholar]
- 42.Shimojo M, Svensson P, Arendt-Nielsen L, Chen ACN. Dynamic brain topography of somatosensory evoked potentials and equivalent dipoles in response to graded painful skin and muscle stimulation. Brain Topography. 2000;13(1):43–58. doi: 10.1023/a:1007834319135. [DOI] [PubMed] [Google Scholar]
- 43.Svensson P, Beydoun A, Morrow TJ, Casey KL. Non-painful and painful stimulation of human skin and muscle: analysis of cerebral evoked potentials. Electroencephalography and Clinical Neurophysiology/Evoked Potentials. 1997;104(4):343–350. doi: 10.1016/s0168-5597(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 44.Coen SJ, Kano M, Farmer AD, et al. Neuroticism influences brain activity during the experience of visceral pain. Gastroenterology. 2011;141(3):909–917. doi: 10.1053/j.gastro.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Dostrovsky JO. Role of thalamus in pain. Progress in Brain Research. 2000;129:245–257. doi: 10.1016/S0079-6123(00)29018-3. [DOI] [PubMed] [Google Scholar]
- 47.Duncan GH, Bushnell MC, Talbot JD, et al. Pain and activation in the thalamus. Trends in Neurosciences. 1992;15(7):252–253. doi: 10.1016/0166-2236(92)90062-d. [DOI] [PubMed] [Google Scholar]
- 48.Lenz FA, Weiss N, Ohara S, Lawson C, Greenspan JD. The role of the thalamus in pain. Supplements to Clinical Neurophysiology. 2004;57:50–61. doi: 10.1016/s1567-424x(09)70342-3. [DOI] [PubMed] [Google Scholar]
- 49.May A. Neuroimaging: visualising the brain in pain. Neurological Sciences. 2007;28(2):S101–S107. doi: 10.1007/s10072-007-0760-x. [DOI] [PubMed] [Google Scholar]
- 50.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89(4):1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 52.Starr CJ, Sawaki L, Wittenberg GF, et al. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134(7):1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Research Reviews. 2010;65(1):14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 55.Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. NeuroImage. 2001;14(6):1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- 56.Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- 57.García-García I, Jurado MA, Garolera M, et al. Alterations of the salience network in obesity: a resting-state fMRI study. doi: 10.1002/hbm.22104. Human Brain Mapping. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan Z, Qin W, Wang D, Jiang T, Zhang Y, Yu C. The salience network contributes to an individual’s fluid reasoning capacity. Behavioural Brain Research. 2012;229(2):384–390. doi: 10.1016/j.bbr.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 61.Bai L, Qin W, Tian J, et al. Time-varied characteristics of acupuncture effects in fMRI studies. Human Brain Mapping. 2009;30(11):3445–3460. doi: 10.1002/hbm.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]


