Abstract
Purpose
Using the horizontal ladder task, we examined some issues that need to be resolved before task-specific rehabilitative training can be employed clinically for the frequent contusive spinal cord injury (SCI). We hypothesized that improving recovery in task performance after contusive thoracic SCI requires frequent re-training and initiating the re-training early during spontaneous recovery.
Methods
Contusive SCI was produced at the adult female Sprague Dawley rat T10 vertebra. Task re-training was initiated one week later when occasional weight-supported plantar steps were taken overground (n=8). It consisted of 2 repetitions each day, 5 days each week, for 3 weeks. Task performance and overground locomotion were assessed weekly. Neurotransmission through the SCI ventrolateral funiculus was examined. SCI morphometry was determined.
Results
Re-training did not improve task performance recovery compared to untrained Controls (n=7). Untrained overground locomotion and neurotransmission through the SCI did not change. Lesion area at the injury epicenter as a percentage of the total spinal cord area as well as total tissue, lesion, and spared tissue, white matter, or gray matter volumes did not differ.
Conclusions
For the horizontal ladder task after contusive thoracic SCI, earlier re-training sessions with more repetitions and critical neural circuitry may be necessary to engender a rehabilitation effect.
Keywords: spinal cord injury, rehabilitation, re-training, horizontal ladder, locomotion, transcranial magnetic motor evoked potentials
1. Introduction
The life expectancy of traumatic spinal cord injury (SCI) survivors can be similar to able-bodied individuals. Therefore, the long-term motor, sensory, and autonomic dysfunctions that occur and the physical, psychosocial, and financial hardships that they lead to could be devastating. Early administration of high doses of the synthetic glucocorticoid methylprednisolone, while controversial (Rozet, 2008), surgical spinal cord decompression (Furlan, 2010), and emergency medicine are the only treatments for SCI persons. Given this, studies of a variety of approaches, especially for chronic SCI, are ongoing (Xu & Onifer, 2009). In particular, rehabilitative training or re-training after SCI to improve locomotion and sensorimotor function is being evaluated and used clinically (Barbeau et al., 2006; Behrman et al., 2007; Mehrholz et al., 2008; Dobkin, 2009; Fong et al., 2009; Sadowsky & McDonald, 2009; van Hedel & Dietz, 2010).
Similar to bipedal humans, spontaneous locomotor and sensorimotor recovery occur in quadrupedal adult rats with various types of incomplete thoracic SCI (Majczyński & Slawińska, 2007). Treadmill training (Fouad et al., 2000; Multon et al., 2003; Stevens et al., 2006; Liu et al., 2008; Heng & de Leon, 2009), wheel running (Beaumont et al., 2008; Siegenthaler et al., 2008; van Meeteren et al., 2003), and hindlimb activity on a motor-driven stationary bicycle (Liu et al., 2008) have been ineffective to modestly effective for improving performance beyond spontaneous recovery. Moreover, the results of experiments where enriched environments were used have been equivocal (Lankhorst et al., 2001; Erschbamer et al., 2006; Berrocal et al., 2007; Burke et al., 2007; Fischer & Peduzzi, 2007).
As determined with a novel swimming rating scale and a novel plantar stepping index, swimming and shallow water stepping by adult rats exposed to a pool lane prior to incomplete, contusive thoracic SCI then trained 3-4 days weekly beginning 1-2 weeks after injury markedly improved compared to un-trained, injured rats (Smith et al., 2006b; Kuerzi et al., 2010). However, un-trained overground locomotion assessed with the Basso, Beattie, and Bresnahan (BBB) open field locomotor rating scale (Basso et al., 1995) did not improve. Furthermore, task-specific rehabilitative training in adult rats after incomplete cervical SCI led to significant improvements in recovery of pellet retrieval performance but did not improve un-trained horizontal ladder task performance (Girgis et al., 2007; Krajacic et al., 2009, 2010).
These results suggest that task-specific rehabilitative training could be used clinically for treating SCI. Before this happens, however, there are a number of issues that need to be resolved. These include the rehabilitative training tasks, initiation time, frequency, intensity, and duration as well as SCI types and severity. We address some of these issues in this study with the horizontal ladder task. This is commonly used to quantitatively assess locomotor and sensorimotor deficits and recovery in adult rats with paraplegia after SCI (van Meeteren et al., 2003; Klapka et al., 2005; Liebscher et al., 2005; Norrie et al., 2005; Ballermann & Fouad, 2006; Bolton et al., 2006; McEwen & Springer, 2006; Piantino et al., 2006; Vavrek et al., 2006; Kanagal & Muir, 2008; Mann et al., 2008; Arvanian et al., 2009; Pereira et al., 2009). Moreover, female rats trained prior to thoracic SCI to traverse a horizontal ladder with irregularly spaced rungs then re-trained 5 days weekly beginning 3-4 days afterward improved recovery of their performance compared to rats similarly re-trained beginning 3 months post-SCI (Norrie et al., 2005). Spontaneous performance recovery of un-trained, injured rats was not examined. A unilateral over-dorsal quadrant SCI was used in this study. However, contusion is the most frequent type of human SCI (Norenberg et al., 2004). When female rats trained prior to contusive thoracic SCI to traverse a horizontal ladder with evenly spaced rungs were assessed once weekly beginning 2 weeks afterwards, they did not improve recovery of their performance 4 weeks later compared to injured rats that were only assessed at that time (McEwen & Springer, 2006). Here, assessment was initiated when frequent to consistent weight-supported plantar steps were occurring. These results led us to test the hypothesis that improving recovery in performance of the horizontal ladder task after contusive thoracic SCI requires frequent re-training and initiating the re-training early during spontaneous recovery. It has been reported that 6-8 minutes of swimming re-training began 3 days after less severe contusive thoracic SCI than used in our study and which exhausted the rats led to extravasation at the injury epicenter and did not improve performance (Smith et al., 2009). Therefore, we employed less intensive re-training than Norrie and colleagues (Norrie et al., 2005), that is, 2 trials over an 81.3cm distance as was done during our baseline assessment versus 30 trials over a 1m distance. Otherwise, our 3 weeks of 5 days per week re-training was the same. We also began re-training at 7 days post-SCI versus at 14 days (McEwen & Springer, 2006). Clinically, less intensive rehabilitation is similarly used initially after SCI and the intensity is increased (P. Kitzman, personal communication). Like Norrie and colleagues (Norrie et al., 2005), we kept the number of trials each day constant throughout the experiment.
2. Material and Methods
All methods were approved by the Institutional Animal Care and Use Committee at the University of Kentucky. They were conducted to minimize the number of rats as well as their discomfort and pain in accordance with the United States of America Public Health Service Policy on Humane Care and Use of Laboratory Animals and with the Policies on the Use of Animals and Humans in Neuroscience Research. The 25 adult female Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) used in this study were housed in groups of 2 or 3, in standard housing with access to food and water ad libitum, and on a 12h-12h light-dark cycle. The behavior and electrophysiology methods were done at approximately the same time each day in a quiet room with the overhead fluorescent lights on. The rats underwent the behavior methods in a different order each session to eliminate bias that may result from a constant training and subsequent testing order.
2.1 Pre-spinal cord injury behavior task training and testing
After one week of acclimatization, 22 rats began training of the horizontal ladder task. The Foot Misplacement Apparatus (Columbus Instruments, Columbus, OH) was used (McEwen & Springer, 2006). It consists of an elevated (48.3cm) horizontal ladder with a black plexiglass goal box at one end. A clear plexiglass start platform was 81.3cm away at the opposite end. The 25mm distances between the twenty-eight, 7mm in diameter metal rungs of the ladder were equidistant throughout the experiment. A metal plate was 2.4cm below the 26 rungs leading up to the goal box to provide cutaneous information to the hindpaws (Muir & Steeves, 1995; Smith et al., 2006a,b). All rats were individually trained as described (McEwen & Springer, 2006) for 15 days prior to SCI. Rung walking with little or no plate walking occurred after day 3 for all rats. On days 14 and 15, 2 investigators were designated to assess either the left hindpaw or right hindpaw. They counted the number of hindlimb footfalls, that is, when the distal hindpaws made plantar contact with the underlying metal plate without touching the nearest 2 rungs (Fig. 1A), as each rat traversed the horizontal ladder. This procedure was repeated once. The left and right hindlimb footfalls of each rat on days 14 and 15 were averaged after determination of no side differences.
Figure 1.
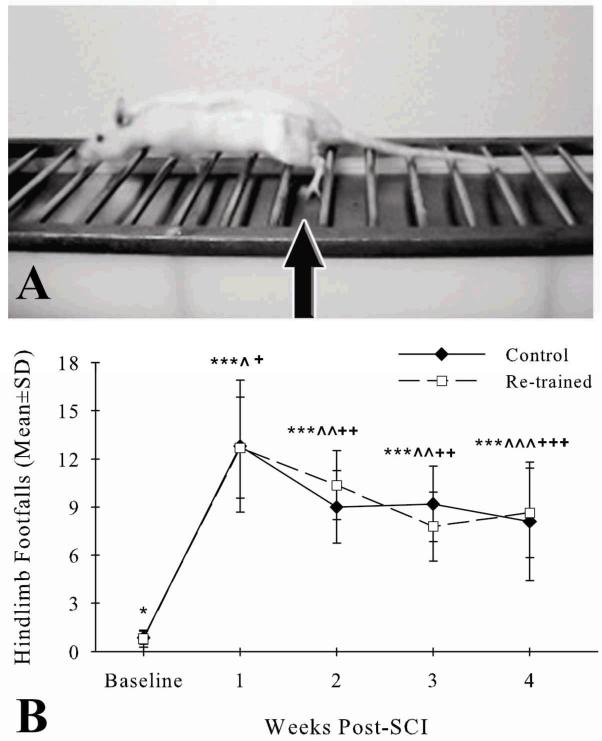
Representative photograph shows a rat at 4 weeks after contusive thoracic SCI traversing the horizontal ladder of the Foot Misplacement Apparatus (A). The arrow points to the left hindlimb of the injured rat making a footfall, that is, stepping between rungs, as it ends a step cycle and begins another. Both the Control (filled diamonds) and Re-trained (open squares) groups had more hindlimb footfalls when traversing the horizontal ladder at weeks 1-4 after contusive thoracic SCI (***p<.01) compared to baseline (*) (B). Fewer footfalls occurred in both groups at weeks 2-4 after SCI (Control: ^^^p<.01, ^^p<.05; Re-trained: +++p<.01, ++p<.05) compared to week 1 (^, +). There were no differences in footfalls between the groups at any time before or after SCI.
Beginning on day 9 after initial exposure to the horizontal ladder, the 22 rats were acclimated individually to an open field 5min each day for 5 days. The open field was a 100.3cm in diameter and 20.3cm deep plastic wading pool. One day after acclimatization, each rat was assessed by 2 trained raters with the BBB open field locomotor rating scale as described (Burke et al., 2007) before its horizontal ladder task test. The BBB scores for the left and right sides of each rat were averaged after determination of no side differences.
2.2 Spinal cord injury
After the baseline behavioral assessments, each of the 22 rats was anesthetized with a mixture of ketamine (80mg/kg) and xylazine (10 mg/kg) injected intraperitoneally (IP). Baytril antibiotic (10mg/kg) was injected subcutaneously. Using aseptic techniques, a laminectomy of the exposed dorsal T10 vertebra was performed. Afterwards, each rat underwent a contusive SCI using the Infinite Horizon Impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA) (Scheff et al., 2003; Onifer et al., 2007a, b). For the SCI, the rostral and caudal portions of the exposed T9 and T11 vertebral bodies were clamped with Adson forceps. A dorsal-to-ventral SCI, desired force of 200 kilodynes (Kdynes), was produced with a 2.5mm diameter tip. The dwell times for all injuries were zero seconds. The actual force, displacement, and velocity of each SCI were displayed by the Infinite Horizon Impactor software (Version 5.0). Afterwards, Lactated Ringers was injected subcutaneously. There were no differences in body temperatures between the Control and Re-trained groups 15 minutes after SCI (data not shown). The rats were cared for as described (Puckett et al., 2008). Both groups gained weight over the 4 weeks post-SCI compared to baseline. The body weights of rats in the groups did not differ from one another prior to euthanasia for histology (data not shown). While previously providing veterinary care for rats with contusive thoracic SCI, we observed that they spontaneously recover their ability to rear, that is, use plantar hindpaw placement and weight support to place the forepaws high up on the home cage. We recorded the day post-SCI each rat began rearing as an index of spontaneous recovery prior to the first day of assessments.
2.3 Post-spinal cord injury behavior task re-training and assessment
Open field locomotion followed by horizontal ladder task assessments were performed on day 7 post-SCI as described above. Seven of the 22 injured rats did not have BBB open field locomotor rating scale scores of 10 (occasional weight-supported plantar steps, no forelimb-hindlimb coordination) or greater. Their hindlimbs dragged across the rungs as they traversed the length of the horizontal ladder. Thus, they were removed from the study due to their inability to perform the horizontal ladder task (McEwen & Springer, 2006). The remaining 15 rats were assigned to Control (n=7) or Re-trained (n=8) groups so that both groups had animals with similar BBB scale scores (score range 10-12, Control: 11.4 ± 0.5, Re-trained: 11.0 ± 0.5). The Re-trained group then performed the horizontal ladder task 5 days each week with 2 repetitions each day. The Control group did not undergo re-training. However, they were brought into the testing room at this time and given a food reward, a rat chow pellet dipped in Regular JIF® Creamy Peanut Butter and sprinkled with crushed M&M'S® MINIS®, similar to the Re-trained group. The Re-trained and Control groups of rats’ open field locomotion and horizontal ladder performances were assessed weekly until day 28 post-SCI.
2.4 Electrophysiology assessment
During the fourth week post-SCI and after the last behavior assessments, each unanesthetized rat was restrained as described (Fishback et al., 1995; Linden et al., 1999). Stainless steel, 28-G needle electrodes (BIOPAC Systems, Inc. Goleta, CA) were inserted into the left and right gastrocnemius muscles, in the calcaneal tendon at each ankle, and subcutaneously at the posterior portion of the pelvis. Transcranial magnetic motor evoked potentials (tcMMEPs) were elicited twice at 60% maximum output using a 50mm circular stimulating coil (Magstim Co. US LLC, Jali Medical, Inc., Woburn, MA) positioned over the cranium, one Magstim 2002 (Magstim Co. US LLC), and a mono-stimulation protocol (Burke et al., 2007). Electromyographic (EMG) activity was recorded simultaneously from both gastrocnemius muscles with the electrodes connected to a MP150 System (BIOPAC Systems, Inc.) and AcqKnowledge (v. 3.8.1, BIOPAC Systems, Inc.) on a Dell computer. The gain was 1000mV and the bandpass filter settings were 10Hz and 3000Hz. The onset latencies of EMG activity were measured from each replicated waveform (Burke et al., 2007) using AcqKnowledge and averaged.
2.5 Histology
Each rat received a sodium pentobarbital overdose (150mg/kg, IP) after the electrophysiology assessment. A transcardiac perfusion was performed with oxygenated, calcium-free Tyrodes solution followed by 0.1M phosphate buffer, pH 7.4, (PB) containing 4% paraformaldehyde (Onifer et al., 2005). Twenty millimeter spinal cord pieces centered at the lesion were cryoprotected in PB containing 20-30% sucrose at 4°C. They were embedded in cryomolds containing a mixture of gum tragacanth and PB with 20% sucrose then frozen at -80°C (Rabchevsky et al., 1999). The spinal cord pieces were serially sectioned with a Microm HM 500M cryostat (Walldorf, Germany) at 20μm in the transverse plane. Sections 100μm apart were mounted onto microscope slides (Rabchevsky et al., 2001). They were stained to see myelin and cell bodies by incubation in mixtures with 0.2% eriochrome cyanine and 0.04% cresyl violet (Rabchevsky et al., 1999). Coverslipped sections 1mm apart along each rat's 20mm spinal cord piece were viewed using a Nikon ECLIPSE E400 microscope (Melville, NY) equipped with a DAGE-MTI CCD 100 camera (Michigan City, IN). The areas of damaged and necrotic tissue, gray matter, white matter, and spinal cord were measured as described (Rabchevsky et al., 2000, 2002) using Scion Image for Windows (Beta 4.0.2, Scion Corp., Frederick, MD). Lesion areas at the injury epicenter as well as lesion plus both gray and white matter volumes were determined from these measurements. The section containing the injury epicenter of each rat also was photographed using an Olympus Provis AX80 Microscope equipped with a DP70 digital camera (Center Valley, PA).
3. Results
3.1 Post-spinal cord injury function
The actual forces, displacements, velocities and dwell times of the Control (n=7) and Re-trained (n=8) groups’ SCIs were not different (Tables 1 & 2). Both groups of rats began rearing in their home cage during the first week post-SCI (Control: 4.7 ± 1.7 days, Re-trained: 5.5 ± 1.8 days). The number of days post-SCI that it took to rear did not differ between the groups (Table 1).
Table 1.
Summary of data analysis procedures and results are listed for all experimental measures. (ns=not significant)
| Measure | Comparison | Analysis |
|---|---|---|
| Injury | ||
| Force | Groups | Independent t-test t=1.5(13), ns |
| Displacement | Groups | Independent t-test t=1.0(13), ns |
| Velocity | Groups | Independent t-test t=.16(13), ns |
| Behavior Assessments | ||
| Rearing | Groups | Independent t-test* t=.87(13), ns |
| Repeated Measures ANOVA^ | ||
| Horizontal Ladder | Groups | F=.01(1,13), ns |
| Weeks | F=84.2(4,52),p<.001 | |
| GxW | F=.77(4,52),ns | |
| Repeated Measures ANOVA^ | ||
| BBB | Groups | F=.10(1,13), ns |
| Weeks | F=106.8(2,23)#,p<.001 | |
| GxW | F=1.0(2,23)#, ns | |
| Morphology | ||
| Lesion Area at Epicenter (% of total SC area) | Groups | Independent t-test t=.23(12), ns |
| Total Tissue Volume | Groups | Independent t-test t=.52(12), ns |
| Lesion Volume | Groups | Independent t-test t=.84(12), ns |
| Spared Tissue Volume | Groups | Independent t-test t=1.1(12), ns |
| Spared White Matter Volume | Groups | Independent t-test t=1.3(12), ns |
| Spared Gray Matter Volume | Groups | Independent t-test t=.32(12), ns |
Independent t-test (for means with ≠ variances)
All Repeated Measures ANOVA: followed by Tukey HSDpost hoc t-tests; see text for specific mean comparison p values
Greenhouse-Geisser df correction for unequal variance
Table 2.
SCI data (Mean ± SD) for each group
| Group | n | Actual Force (Kdynes) | Actual Displacement (μ) | Velocity (mm/s) |
|---|---|---|---|---|
| Control | 7 | 207.4±5.1 | 1188.7±165.8 | 120.6±4.0 |
| Re-trained | 8 | 204±4.0 | 1124.0±85.2 | 120.9±3.4 |
With training prior to SCI, all rats similarly had few hindlimb footfalls when traversing the horizontal ladder at baseline (Fig. 1B). Contusive thoracic SCI led to increases in footfalls for both groups at weeks 1-4 compared to baseline (Control: p<.01, Re-trained: p<.01) (Table 1). Decreases in footfalls occurred between week 1 and weeks 2-4 for both groups (Control: p<.05, p<.05, p<.01; Re-trained: p=.05, p<.01, p<.01, respectively) (Table 1). At each week after SCI, however, the number of footfalls was similar between the groups (Table 1).
Contusive SCI resulted in decreased BBB open field locomotor rating scale scores for both groups at weeks 1-4 compared to baseline (Control: p<.01, Re-trained: p<.01) (Fig. 2, Table 1). Increased BBB scale scores occurred between weeks 1 and 4 for the Re-trained group (p<.05) (Table 1). However, the scores were similar between the groups at these times and throughout the other post-SCI times (Table 1).
Figure 2.
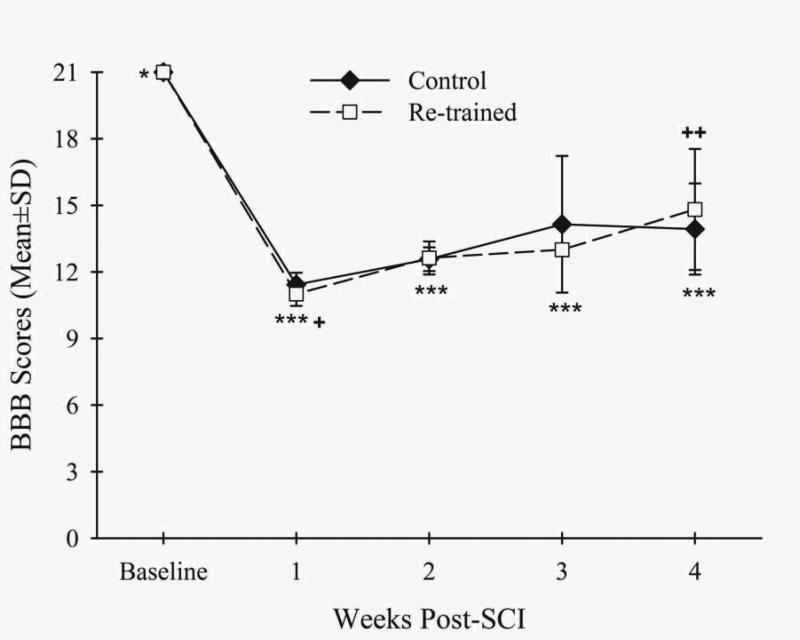
The BBB open field locomotor rating scale scores for both the Control (filled diamonds) and Re-trained (open squares) groups were decreased at weeks 1-4 after contusive thoracic SCI (***p<.01) compared to baseline (*). The scores of the Re-trained group increased at week 4 (++p<.05) compared to week 1 (+). There were no differences in scores between the groups at any time before or after SCI.
The onset latencies of EMG activity evoked by the tcMMEP procedure in 3 naïve rats (Fig. 3) were 5.4 ± 0.6ms. None of the rats in the Re-trained group had EMG activity evoked by the tcMMEP procedure with similar onset latencies. Only one rat in the Control group had EMG activity with an onset latency of 4.7 ± 0.2ms. However, the amplitudes of the waveforms were very small.
Figure 3.
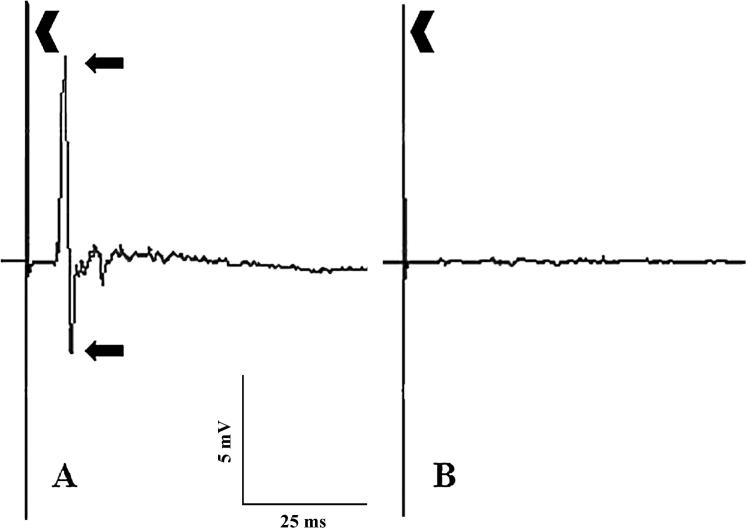
Representative examples are shown of EMG activity evoked in the gastrocnemius muscles of a normal rat (A) and of no EMG activity evoked in an injured rat of the Control group (B) by the tcMMEP procedure. A stimulus artifact (chevron) can be seen at the beginning of each waveform in both examples. The onset latency and peak-to-peak amplitude (arrows) of the EMG activity recorded in the normal rat (A) were 5ms and 11.2mV, respectively.
3.2 Post-spinal cord injury anatomy
Extensive white and gray matter damage occurred in the thoracic spinal cords of all rats after contusive SCI (Fig. 4) as previously described (Scheff et al., 2003). Importantly for locomotion (Magnuson et al., 1999; Loy et al., 2002a, b; Schucht et al., 2002; Cao et al., 2005), white matter damage was seen in the ventral, ventrolateral, dorsolateral, and dorsal funiculi at the epicenter as well as throughout the rostral and caudal extents of the injuries. There were no differences between the groups in the lesion area at the injury epicenter as a percentage of the total spinal cord area (Fig.5A), total tissue volume (Control: 41.6 ± 1.6mm3, Re-trained: 42.3 ± 3.2mm3), lesion volume (Fig. 5B), spared tissue volume (Fig. 5C), spared white matter volume (Fig. 5D) or spared gray matter volume (Control: 8.3 ± 0.5mm3, Re-trained: 8.4 ± 1.0mm3) (Table 1).
Figure 4.
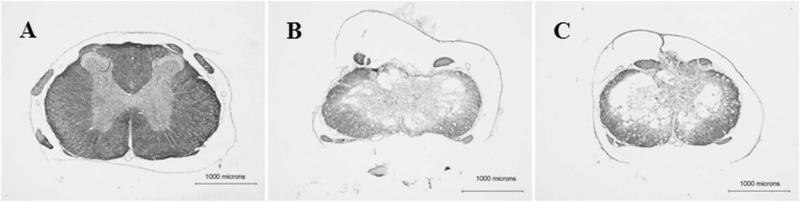
Shown are representative eriochrome cyanine and cresyl violet stained, transverse thoracic spinal cord sections of normal (A), Control group (B), and Re-trained group (C) rats. Contusive SCI produced extensive white and gray matter damage. Dorsal is up.
Figure 5.
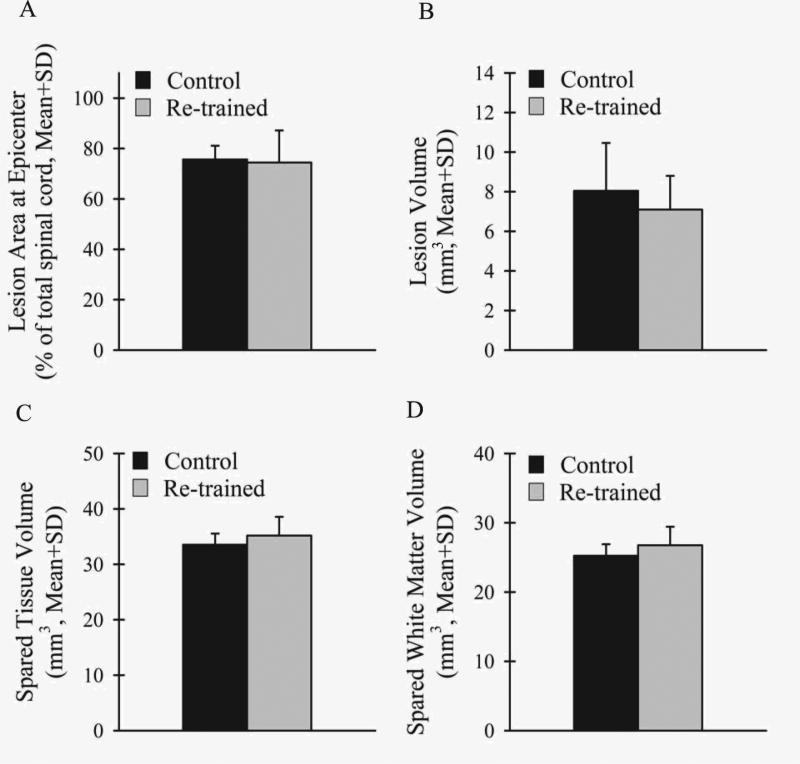
Similar lesions occurred in both the Control (black) and Re-trained (light gray) groups after contusive thoracic SCI. There were no differences between the groups in the lesion area at the injury epicenter as a percentage of the total spinal cord area (A), lesion volume (B), spared tissue volume (C), or spared white matter volume (D).
4. Discussion
The present study tested the hypothesis that improving recovery in performance of the horizontal ladder task after contusive thoracic SCI requires frequent re-training and initiating the re-training early during spontaneous recovery. All rats in the Control and Re-trained groups were able to take occasional weight-supported plantar steps overground (BBB scale score of 10) at one week post-SCI. Horizontal ladder task performance of rats in both groups at this time were similarly impaired.
As evidenced by a significant decrease in the number of hindlimb footfalls, there was an improvement in horizontal ladder task performance of the Control group between 7 and 14 days post-SCI. This spontaneous recovery reached a plateau as there were no significant changes over the next 14 days. It also was previously reported that weekly horizontal ladder task performances did not change between weeks 2 and 6 after contusive thoracic SCI (McEwen & Springer, 2006). The Foot Misplacement Apparatus and the BBB scale scores of the injured rats in that report at weeks 1 and 4 post-SCI were similar to those of the present study. A lack of further spontaneous recovery in horizontal ladder performances additionally was reported for rats with unilateral over-dorsal quadrant thoracic SCI between 3-4 days and 3 months post-injury (Norrie et al., 2005). That a plateau of spontaneous recovery was reached earlier than in the present study could be due to differences in the extent and regions of spinal cord damage.
Re-training began in the present study at one week post-SCI. It lasted for 3 weeks until 28 days post-SCI and sessions occurred 5 days each week. A significant improvement in the horizontal ladder task performance of the Re-trained group also occurred between 7 and 14 days post-SCI. However, there was no difference between the performances of the Re-trained and the Control groups at 2 weeks post-SCI. This indicates that the recovery was spontaneous and not improved by the first week of re-training. Similar to the Control group, the spontaneous recovery of the Re-trained group reached a plateau at this time as there were no further significant changes within and between the groups.
The similar, spontaneous improvement in horizontal ladder task performance of both groups between the first and second weeks post-SCI assessments, and not afterwards, could have been due to one or more plasticity mechanisms (Fouad & Tse, 2008). For example, alterations in the properties of spared neuronal circuitries (Barrière et al., 2008; Fouad et al., 2010; Murray et al., 2010), synaptic rearrangements (Rossignol, 2006), changes in polysynaptic flexor reflexes involved in locomotor circuits (Valero-Cabré et al., 2004; Lavrov et al., 2006), axon collateral sprouting (Bareye et al., 2004; Ballermann et al., 2006; Courtine et al., 2008), and/or rearrangements in cortex and brainstem somatotopic maps (Fouad et al., 2001; Kaas et al., 2008) may have occurred. Interestingly, there were no changes in BBB scores of either group during this time. By not affecting open field locomotion, this suggests that these spontaneous recovery mechanisms may have been task-specific. This also may have been seen after swimming and shallow water rehabilitative training (Smith et al., 2006b; Kuerzi et al., 2010). In these studies, task-specific, improved recovery mechanisms may not have affected open field locomotion. Alternatively, these mechanisms could have a detrimental effect on spontaneous, and improved, recovery of un-trained behaviors as has been observed for horizontal ladder task performance of rats undergoing task-specific rehabilitative training (Girgis et al., 2007; Krajacic et al., 2010). The lack of improvement in recovery of horizontal ladder task performance during re-training also may have been due to activity-based plasticity mechanisms (Fouad & Tetzlaff, 2011) not occurring or being ineffective. In addition to the mechanisms indicated above, these include increases in growth and plasticity-associated factors, such as neurotrophic factors and cAMP (Vaynman & Gomez-Pinilla, 2005; Ying et al., 2008; Krajacic et al., 2009; Côté et al., 2011).
Even though spontaneous recovery of un-trained, injured rats was not examined by Norrie and colleagues (Norrie et al., 2005), our finding of a lack of improvement in recovery of horizontal ladder task performance may contrast with extrapolated results from their report. They saw a 60% improvement in performance after 3 weeks of 5 days weekly re-training compared to when it began 3-4 days after unilateral over-dorsal quadrant thoracic SCI. The frequency and duration of re-training were similar to those of the present study. However, we waited 4 more days post-SCI to start re-training. This was due to the finding that re-training initiated 3 days after a contusive thoracic SCI, that was less severe than used in our study and which exhausted the rats, led to extravasation at the injury epicenter and did not improve swimming performance (Smith et al., 2009). Additionally, the injured rats were able to plantar step with weight support at this time making it possible for them to perform the horizontal ladder task. Each of our daily re-training sessions consisted of 2 trials. We observed that normal rats use 7-8 step cycles to traverse the 81.3cm length of the horizontal ladder. Therefore, at least 14-16 step cycles occurred during each session. Norrie and colleagues’ daily re-training sessions consisted of 30 trials traversing a 1m long horizontal ladder (Norrie et al., 2005), approximately 19cm longer than the one used in the present study. Collectively, these results suggest that starting re-training as soon as plantar stepping was observed may have been more optimal and that each re-training session may not have been intensive enough. Evidence that the latter may be more critical is that recovery of pellet retrieval performance improved when re-training began 4 days and 12 days after incomplete cervical SCI (Girgis et al., 2007; Krajacic et al., 2009). Similar to clinical rehabilitation, re-training that is less intensive could be used initially after SCI then the intensity could be increased to further improve strength, endurance, and performance.
The location and spacing of the horizontal ladder's rungs in our study were constant and equidistant. We also placed a metal plate underneath the horizontal ladder's rungs due to the report that cutaneous feedback to the hindpaws of rats with contusive thoracic SCI improved their swimming compared to rats that swam without cutaneous feedback (Smith et al., 2006a,b). We observed that normal rats step on the rungs to traverse the entire ladder within a few days of training even though the metal plate was underneath the rungs. Perhaps through a similar mechanism, both the Control and Re-trained groups had significantly fewer footfalls between 7 and 14 days post-SCI. However, they were not different between the groups. Both groups’ number of footfalls did not further change between 14 and 28 days post-SCI. In contrast, the rungs’ location and spacing were changed throughout the study where a re-training effect on recovery of horizontal ladder task performance was reported between rats where re-training began either 3-4 days or 3 months post-SCI (Norrie et al. 2005). It has been proposed that a constant location and equidistant spacing of the ladder's rungs facilitates learning their location (Metz et al., 2002). If the locations of the evenly spaced rungs or the plate between them were being learned during re-training, the Re-trained group should have stepped on more rungs or had more footfalls, respectively, than the Control group. Learning seems unlikely, however, since neither of these situations occurred between 14 to 28 days post-SCI.
There were no differences between the Re-trained and Control groups’ morphometric and electrophysiology measures in the present study. Importantly, similar observations have been reported in studies where significant functional changes occurred in adult rats that underwent rehabilitation after contusive thoracic SCI (Lankhorst et al., 2001; Norrie et al., 2005; Smith et al., 2006b; Kuerzi et al., 2010). It was recently shown in rats that underwent task-specific rehabilitation after cervical SCI that neural circuitry exist in spinal cord white matter that are critical for a therapeutic effect to occur (Krajacic et al., 2010). The lack of a task-specific rehabilitation effect on horizontal ladder performance in our study may have been due to similar damage of critical neural circuitry in both the Re-trained and Control groups. We and others showed that tcMMEPs are conducted by descending myelinated axons in the ventrolateral funiculi (VLF) of the adult rat spinal cord (Loy et al., 2002a; Cao et al., 2005; Nielsen et al., 2007). Neurotransmission along this pathway can be attenuated or persistently abolished following contusive thoracic SCI (Dimar et al., 1999; Magnuson et al., 1999; Cao et al., 2005). We observed EMG activity evoked by the tcMMEP procedure with an onset latency similar to that of normal in only one rat. This was in the Control group. Descending axons in the VLF may be critically necessary for a task-specific rehabilitation effect after contusive thoracic SCI. However, no correlation was found between improved horizontal ladder task performance following re-training and the extent of thoracic spinal cord VLF damage caused by unilateral over-dorsal quadrant lesion, even when it was as high as 55% (Norrie et al. 2005). Instead, they found a correlation between improved performance and the extent of damage to the dorsal corticospinal tracts and the ipsilesional rubrospinal tract.
Deficits in horizontal ladder task performance have been seen following bilateral dorsal funiculotomies, that is, lesions of the dorsal columns and the dorsal corticospinal tracts (Klapka et al., 2005; Bolton et al., 2006; Kanagal & Muir, 2008) or bilateral dorsal and dorsolateral funiculotomies combined with bilateral lesion of the ventral corticospinal tracts (Liebscher et al., 2005) at the thoracic spinal cord level. Therefore, the similar, extensive damage caused by contusive thoracic SCI to the dorsal funiculi in both the Re-trained and Control groups of our study may have eliminated sensory and motor axons in these locomotion-important white matter regions (Magnuson et al., 1999; Loy et al., 2002a, b; Schucht et al., 2002; Cao et al., 2005) that could be critical for improving performance of the horizontal ladder task. If spared or regenerated after contusive SCI, these axons may be used to engender a re-training effect on horizontal ladder task performance. In support of this contention, it has been reported that horizontal ladder task performance significantly improved in rats with bilateral dorsal funiculotomy (Klapka et al., 2005) or bilateral dorsal and dorsolateral funiculotomies (Piantino et al., 2006) of their thoracic spinal cords wherein treatments promoted corticospinal tract regeneration.
The present study shows for adult rats with damage of thoracic spinal cord white matter after contusive injury that horizontal ladder task re-training initiated one week after contusive injury when occasional weight-supported plantar steps are taken overground and consisting of 2 repetitions each day, 5 days each week, for 3 weeks did not improve performance recovery. This result and those of previous studies suggest that re-training sessions with more repetitions of the horizontal ladder task and critical neural circuitry may be necessary for a rehabilitation effect after contusive thoracic SCI.
Acknowledgements
This research was supported by a University of Kentucky Research Support Grant (Stephen M. Onifer), the Kentucky Spinal Cord and Head Injury Research Trust (Stephen M. Onifer), and by NIH/NINDS P30NS051220 (Edward D. Hall). Oliver Zhang was a participant in the Math Science and Technology Program at Paul Laurence Dunbar High School in Lexington, KY, U.S.A. The authors extend their appreciation to Linda Simmerman for assisting with the images. The funding source had no involvement in study design.
Footnotes
The authors do not have any actual or potential conflicts of interest.
References
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, Pearse DD, Robinson JK, Schwab ME, Fawcett JW, Mendell LM. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp Neurol. 2008;216:471–480. doi: 10.1016/j.expneurol.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma. 2006;23:571–585. doi: 10.1089/neu.2006.23.571. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barrière G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Kaloustian S, Rousseau G, Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci Res. 2008;62:147–154. doi: 10.1016/j.neures.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:183–202. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Berrocal Y, Pearse DD, Singh A, Andrade CM, McBroom JS, Puentes R, Eaton MJ. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma. 2007;24:1761–1772. doi: 10.1089/neu.2007.0327. [DOI] [PubMed] [Google Scholar]
- Bolton DA, Tse AD, Ballermann M, Misiaszek JE, Fouad K. Task specific adaptations in rat locomotion: runway versus horizontal ladder. Behav Brain Res. 2006;168:272–279. doi: 10.1016/j.bbr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Burke DA, Magnuson DS, Nunn CD, Fentress KG, Wilson ML, Shum-Siu AH, Moore MC, Turner LE, King WW, Onifer SM. Use of environmentally enriched housing for rats with spinal cord injury: the need for standardization. J Am Assoc Lab Anim Sci. 2007;46:34–41. [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005;191:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houlé JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimar JR, 2nd, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24:1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Motor rehabilitation after stroke, traumatic brain, and spinal cord injury: common denominators within recent clinical trials. Curr Opin Neurol. 2009;22:563–569. doi: 10.1097/WCO.0b013e3283314b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erschbamer MK, Pham TM, Zwart MC, Baumans V, Olson L. Neither environmental enrichment nor voluntary wheel running enhances recovery from incomplete spinal cord injury in rats. Exp Neurol. 2006;201:154–164. doi: 10.1016/j.expneurol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Fischer FR, Peduzzi JD. Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J Spinal Cord Med. 2007;30:147–155. doi: 10.1080/10790268.2007.11753926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishback AS, Shields CB, Linden RD, Zhang YP, Burke D. The effects of propofol on rat transcranial magnetic motor evoked potentials. Neurosurgery. 1995;37:969–974. doi: 10.1227/00006123-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Roy RR, Ichiyama RM, Lavrov I, Courtine G, Gerasimenko Y, Tai YC, Burdick J, Edgerton VR. Recovery of control of posture and locomotion after a spinal cord injury: solutions staring us in the face. Prog Brain Res. 2009;175:393–418. doi: 10.1016/S0079-6123(09)17526-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Fouad K, Rank MM, Vavrek R, Murray KC, Sanelli L, Bennett DJ. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J Neurophysiol. 2010;104:2975–2984. doi: 10.1152/jn.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.02.009. in press. [DOI] [PubMed] [Google Scholar]
- Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2010;27:1–29. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol. 2009;216:139–147. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD. The differential effects of cervical and thoracic dorsal funiculus lesions in rats. Behav Brain Res. 2008;187:379–386. doi: 10.1016/j.bbr.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP, Müller D, Zuschratter W, Müller HW. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- Krajacic A, Ghosh M, Puentes R, Pearse DD, Fouad K. Advantages of delaying the onset of rehabilitative reaching training in rats with incomplete spinal cord injury. Eur J Neurosci. 2009;29:641–651. doi: 10.1111/j.1460-9568.2008.06600.x. [DOI] [PubMed] [Google Scholar]
- Krajacic A, Weishaupt N, Girgis J, Tetzlaff W, Fouad K. Training-induced plasticity in rats with cervical spinal cord injury: Effects and side effects. Behav Brain Res. 2010;214:323–331. doi: 10.1016/j.bbr.2010.05.053. [DOI] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, Smith RR, Magnuson DS. Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010;224:178–187. doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankhorst AJ, ter Laak MP, van Laar TJ, van Meeteren NL, de Groot JC, Schrama LH, Hamers FP, Gispen WH. Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J Neurotrauma. 2001;18:203–215. doi: 10.1089/08977150150502622. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Linden RD, Zhang YP, Burke DA, Hunt MA, Harpring JE, Shields CB. Magnetic motor evoked potential monitoring in the rat. J Neurosurg. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- Liu M, Bose P, Walter GA, Thompson FJ, Vandenborne K. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord. 2008;46:488–493. doi: 10.1038/sj.sc.3102169. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002a;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002b;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Majczyński H, Sławińska U. Locomotor recovery after thoracic spinal cord lesions in cats, rats and humans. Acta Neurobiol Exp (Wars) 2007;67:235–257. doi: 10.55782/ane-2007-1651. [DOI] [PubMed] [Google Scholar]
- Mann C, Lee JH, Liu J, Stammers AM, Sohn HM, Tetzlaff W, Kwon BK. Delayed treatment of spinal cord injury with erythropoietin or darbepoetin--a lack of neuroprotective efficacy in a contusion model of cord injury. Exp Neurol. 2008;211:34–40. doi: 10.1016/j.expneurol.2007.12.013. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. Quantification of locomotor recovery following spinal cord contusion in adult rats. J Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Spine (Phila Pa 1976) 2008;33:E768–777. doi: 10.1097/BRS.0b013e3181849747. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Muir GD, Steeves JD. Phasic cutaneous input facilitates locomotor recovery after incomplete spinal injury in the chick. J Neurophysiol. 1995;74:358–368. doi: 10.1152/jn.1995.74.1.358. [DOI] [PubMed] [Google Scholar]
- Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci. 2007;25:805–814. doi: 10.1111/j.1460-9568.2007.05326.x. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophysiol. 2005;94:255–264. doi: 10.1152/jn.00970.2004. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol. 2007a;207:238–247. doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Rabchevsky AG, Scheff SW. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007b;48:385–395. doi: 10.1093/ilar.48.4.385. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG, Fornaro M, Di Scipio F, Geuna S, Maurício AC, Varejão AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71–81. doi: 10.1016/j.expneurol.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201:359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Puckett AH, Nunn CD, Onifer SM. Veterinary care methods for rats and mice in experimental spinal cord injury studies. In: Chen J, Xu Z, Xu X-M, Zhang J, editors. Animal Models of Acute Neurological Injuries. Contemporary Neuroscience. The Humana Press Inc.; Totowa NJ: 2008. pp. 47–60. [Google Scholar]
- Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–830. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW. Efficacy of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res. 2002;68:7–18. doi: 10.1002/jnr.10187. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–291. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361:1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozet I. Methylprednisolone in acute spinal cord injury: is there any other ethical choice? J Neurosurg Anesthesiol. 2008;20:137–139. doi: 10.1097/01.ana.0000314441.63823.b0. [DOI] [PubMed] [Google Scholar]
- Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev. 2009;15:112–116. doi: 10.1002/ddrr.61. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Siegenthaler MM, Berchtold NC, Cotman CW, Keirstead HS. Voluntary running attenuates age-related deficits following SCI. Exp Neurol. 2008;210:207–216. doi: 10.1016/j.expneurol.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Burke DA, Baldini AD, Shum-Siu A, Baltzley R, Bunger M, Magnuson DS. The Louisville Swim Scale: a novel assessment of hindlimb function following spinal cord injury in adult rats. J Neurotrauma. 2006a;23:1654–1670. doi: 10.1089/neu.2006.23.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Shum-Siu A, Baltzley R, Bunger M, Baldini A, Burke DA, Magnuson DS. Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J Neurotrauma. 2006b;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JE, Liu M, Bose P, O'Steen WA, Thompson FJ, Anderson DK, Vandenborne K. Changes in soleus muscle function and fiber morphology with one week of locomotor training in spinal cord contusion injured rats. J Neurotrauma. 2006;23:1671–1681. doi: 10.1089/neu.2006.23.1671. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Forés J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol. 2004;91:2838–2848. doi: 10.1152/jn.01177.2003. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Dietz V. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci. 2010;28:123–134. doi: 10.3233/RNN-2010-0508. [DOI] [PubMed] [Google Scholar]
- van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Xu XM, Onifer SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol. 2009;169:171–182. doi: 10.1016/j.resp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


