Abstract
Induced pluripotent stem cells (iPSCs) hold enormous promise for the treatment of complex tissue defects throughout the entire body. The ability for iPSCs to form all tissue types makes them an ideal autogenous cellular building block for tissue engineering strategies designed to replace any combination of skin, muscle, nerve, and bone deficiencies in the craniofacial region. Several obstacles to their use remain, however, chief among which include concerns over insertional mutagenesis and tumorigenicity. As studies continue to develop strategies minimizing these risks, the potential for development of patient-specific regenerative therapies has become tantalizingly close.
Keywords: iPS cells, regenerative medicine, craniofacial reconstruction, tissue engineering, adipose-derived stromal cells
Introduction
Reconstruction of craniofacial defects from both congenital and acquired etiologies can often present a daunting challenge to surgeons, as many involve complex tissue deficiencies of skin, muscle, nerve, and bone. Despite the significant progress made with free-tissue transfer of fasciocutaneous and functional muscle flaps and despite refinements in nerve, fat, and bone grafting techniques, limitations to all these contemporary strategies nonetheless exist. Donor site morbidity, as well as suboptimal restoration of form and function continues to drive the development of novel approaches. The field of regenerative medicine holds significant promise to address this need, employing cellular-based strategies to replace damaged or deficient tissues. While studies have evaluated the use of a variety of stem or progenitor cells for tissue engineering, it has become increasingly evident that pluripotent cells may be the optimal building block for reconstruction of complex defects such as those encountered in the craniofacial region.
Pluripotent stem cells possess enormous potential for regenerative medicine and the treatment of human disease. With the capacity to differentiate into tissues from each of the three embryonic germ layers (endoderm, mesoderm, and ectoderm), these cells can give rise to all adult cell types found in the craniofacial region. This property has allowed investigators to devise ground-breaking models for research and therapeutic testing and has led to the development of novel strategies for tissue engineering. The advent of much of this enthusiasm can be traced back to the first description by Thomson of embryonic stem cells (ESCs) derived from human blastocysts.1 Since that time, the field has continued to evolve at a breath-taking pace. In recent years, researchers have introduced the concept of induced pluripotent stem cells (iPSCs) which can be derived from a patient's own somatic cells.2-4 With this new addition to the pluripotent cell armamentarium, the ability for personalized regenerative medicine has become increasingly tangible.5
Evolution of Pluripotent Cells
Human ESCs were first characterized in the late 1990s, and in the ensuing decade, much of their promise was elaborated for use in understanding normal development and disease and for their potential application in cell-based therapies to treat currently incurable disorders.1, 6 Detailed protocols have emerged describing the reproducible in vitro generation of various differentiated cell lineages from ESCs including neurons, cardiomyocytes, osteoblasts, and hematopoietic cells.7-10 ESCs have also been employed in animal models of Parkinson's disease, showing ability for these cells to provide functional replacement of diseased tissue.11 And in other preliminary studies, investigators have begun to evaluate the use of ESCs in cellular-based therapies for spinal cord injury and macular degeneration.12 Despite this progress, however, wide-spread application of ESCs in clinical medicine has been hampered by several notable limitations, chief among which are the complex ethical debates rooted in human historical, cultural, and religious differences which have been waged.13 Their tumorigenic propensity and immunologic concerns represent other equally significant reservations which remain to be addressed.
To circumvent some of these issues, investigators have developed alternative sources for pluripotent cells. In particular, patient-specific ESC-like cells have been created through methods such as fusion of somatic cells with ESCs or transfer of nuclear contents into oocytes. These techniques, though, have still proven technically challenging and have not entirely eliminated many of the bioethical arguments raised with ESCs. In 2006, Takahashi and Yamanaka hypothesized that pluripotency could be induced through the recapitulation of early biomolecular events following somatic cell fusion.3 By introducing specific transcription factors known to be important in the regulation and maintenance of stem cell characteristics, newly generated iPSCs were observed to demonstrate morphology and genotype similar to ESCs.3 Working with a total of 24 separate genes, their group ultimately defined a “cocktail” consisting of Oct3/4, Sox2, c-Myc, and Klf4 which could induce pluripotency in adult mouse fibroblasts.3 Importantly, these cells were shown to form tissue from all three embryonic germ layers and were capable of generating viable chimeras when injected into mouse blastocysts.
Since this first description of iPSCs, subsequent studies have now derived these cells from human fetal fibroblasts, adult fibroblasts, and a variety of other cells (Figure 1).2, 14-16 Importantly, as iPSCs can be created from a patient's own somatic cells, many of the immunologic concerns surrounding use of ESCs are potentially obviated. And as they are generated from an individual patient's cells, iPSCs also offer investigators the opportunity to model disease on a patient-by-patient basis, enabling screening for individualized pharmacologic agents.17 The accession of iPSC technology has thus reinforced the possibility for therapeutic strategies to be designed “by the patient, for the patient, and given back to the patient” for the treatment of various human diseases and tissue deficiencies not only in the craniofacial region but throughout the entire body.
Figure 1.
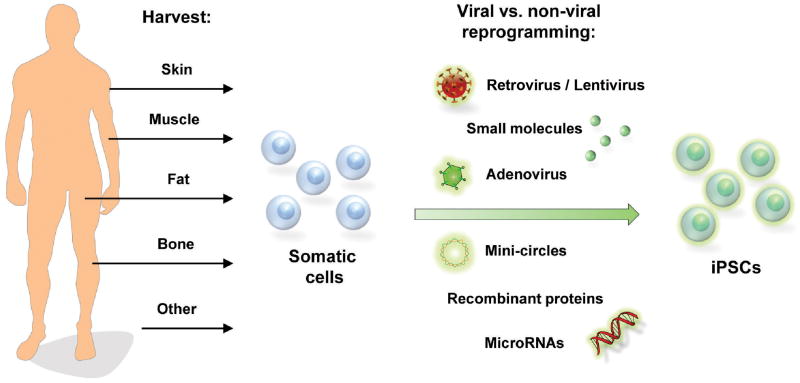
Schematic demonstrating different cell sources for creation of iPSCs. Reprogramming can be performed through viral and non-viral techniques including small molecules, mini-circle technology, and recombinant proteins.
Challenges to the Use of iPSCs
Despite potential advantages iPSCs offer over ESCs, however, significant barriers must still be addressed before these cells become relevant for clinical translation. iPSC generation remains a lengthy process with low reprogramming efficiency, often reported to be less than 0.01%.18-20 The use of viral vectors and incorporation of factors such as c-Myc and Klf4 have also raised concerns regarding oncogenicity. Finally, as iPSCs possess the capacity to form tissues from all three embryonic germ layers, the potential also exists for teratoma formation when these cells are implanted in vivo, thus limiting their clinical utility.
In the original description by Takahashi and Yamanaka, iPSCs were generated by retroviral infection of key transcription factors followed by selection of clones for two to three weeks.3 Similar studies employing the same four factors have described a duration of 15 to 20 days before iPSCs can be derived from mouse fibroblasts.21 And when adult human fibroblasts are used as the starting material, the efficiency of generating expandable iPSC colonies after transduction can be quite poor.18-19 These disadvantages have only been heightened by attempts to limit the use of oncogenic transcription factors such as c-Myc and Klf4 in the creation of iPSCs.22 To address this limitation, investigators have described use of ES cell-specific microRNA molecules (miR-291, miR-294, and miR-295) which have been found to act downstream of c-Myc.23 Exogenous application of such constructs normally active in the maintenance of ES cell pluripotency has been shown to dramatically increase the efficiency with which fibroblasts are transformed into iPSCs.23 Some success has also been noted with the use of histone deacetylase inhibitors which have been shown to enhance reprogramming while allowing for reduction of transcription factors to just Oct3/4 and Sox2.24 Alternatively, other parental cells for reprogramming have been evaluated, with adipose-derived stromal cells (ASCs) being found to yield iPSC colonies two-fold faster and 20-fold more efficiently than human fibroblasts.20 Such findings may be secondary to a baseline higher expression of both c-Myc and Klf4 in ASCs compared to fibroblasts.20
Given the concern for insertional mutagenesis with the use of viral vectors, researchers have also begun to develop novel methods for delivery of reprogramming factors. To minimize risk for viral integration, use of an episomal vector derived from the Epstein-Barr virus (oriP/EBNA) has recently been described.25 Such an approach has resulted in the introduction of transcription factors and generation of iPSCs with gradual loss of viral DNA as cells are cultured over time.25 However, the ability to reprogram adult donor cells using this technique has been questioned, and the expression of EBNA1 protein has been suggested to increase immune recognition of transfected cells.26 Another innovative strategy has been the creation of minicircle vectors with self-cleavage peptide 2A sequences.27 By incorporating reprogramming factors into a compact construct free of bacterial DNA, persistent high level of expression can be achieved and transgene-free iPSCs have been generated from ASCs with increased efficiency.27-28 Finally, investigators have demonstrated the ability to generate iPSCs without the use of genetic material through direct introduction of specific proteins. Fusing reprogramming transcription factor peptides with a polyarginine protein transduction domain, Zhou and colleagues demonstrated the capacity for cellular uptake and the induction of pluripotency from mouse embryonic fibroblasts.29 Similar protein-based strategies have since been demonstrated with human fetal and neonatal cells.27, 30
While work continues on devising more efficient means to generate iPSCs, the risk for teratoma formation following in vivo implantation still looms large as an obstacle which must be eliminated before pluripotent cells can used clinically. By definition, true pluripotency of human iPSCs is assessed through in vivo teratoma formation in immunocompromised mice. Ironically, this vast proliferation and differentiation potential of these cells must be curbed before iPSCs can be incorporated into tissue engineering strategies. Studies have already documented the formation of teratoma-like tumors in a mouse model for Parkinson's disease following injection of human ESCs.11 Similarly, Miura and colleagues generated neurospheres from iPSCs capable of tri-lineage differentiation into neurons, astrocytes, and oligodendrocytes, and following implantation into mice, abundant teratoma formation was noted.31 In nearly half of these animals, death or significant disability secondary to tumor formation was noted following transplantation.31 More worrisome, however, have been some reports which have suggested, in certain settings, a more aggressive nature for iPSC teratomas relative to ESC teratomas. Both a higher rate of tumor formation and shorter latency to detection have been described for iPSC-induced teratomas and it remains to be seen whether this may be attributed to alterations at the genome level during reprogramming and/or prolonged passage in vitro.13, 32
Recent investigations have evaluated risk factors for teratoma formation following iPSC implantation and have shown the development of these tumors to be relatively independent of oncogenic transcription factor use during reprogramming.31 Instead, rates of teratoma formation were found to directly correlate with the number of residual undifferentiated cells injected.31 As it remains quite difficult to generate pure populations of differentiated cells from iPSCs, elimination of persistent pluripotent cells prior to implantation remains a significant challenge. To address this, one recently described approach has employed fluorescence-activated cell sorting with antibodies against stage-specific embryonic antigen (SSEA)-5 which is highly and specifically expressed on both iPSCs and ESCs.33 Immunodepletion of in vitro differentiated cultures using SSEA-5 antibodies resulted in greatly reduced teratoma-formation relative to more heterogeneous and incompletely differentiated pluripotent cell cultures.33 Alternatively, suicide genes have been devised that are responsive to ganciclovir. This has allowed for subsequent selective ablation of teratomas as they arise.34 Finally, the possibility also exists to direct in vivo differentiation of iPSCs following implantation. Ongoing work has suggested that targeted manipulation of the surrounding niche may allow for select formation of tissue while simultaneously minimizing the risk for teratoma formation. Collectively, these studies have begun to shed light on potential avenues which may be employed to eliminate teratoma concerns, thus paving the way for development of clinical strategies exploiting the promise of pluripotent cells.
Clinical Applications for iPSCs
iPSCs hold great promise for reconstruction of composite tissue defects in the craniofacial region as they represent a powerful tool capable of regenerating skin, muscle, nerve, and bone deficiencies found in both congenital and acquired disorders (Figure 2). Importantly, these cells are not subject to the more restrictive lineage differentiation pathways noted with mesenchymal cells and still offer the same potential for development of patient-specific therapies. A number of studies have begun to evaluate the capacity of iPSCs for tissue engineering purposes, and as the safety profile improves, translation into the clinical realm has become increasingly possible.
Figure 2.
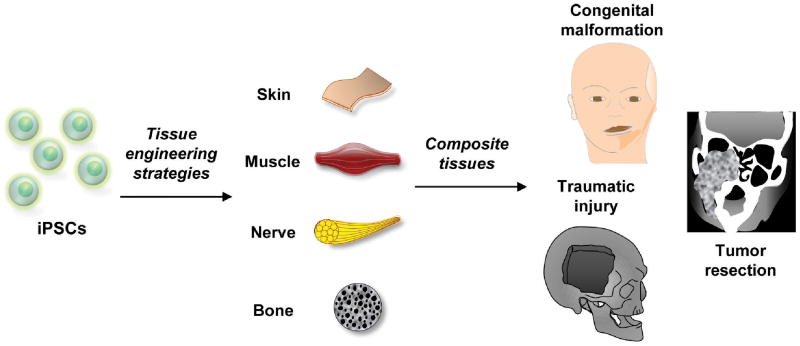
iPSCs can generate numerous tissues necessary for craniofacial reconstruction including skin, muscle, nerve, and bone. Complex tissue deficiencies from congenital malformations, traumatic defects, and tumor resection may be addressed through cellular-based tissue engineering strategies incorporating iPSCs.
The first iPSCs were generated using embryonic and adult fibroblasts and many studies have since demonstrated potential application for this technology in the treatment of skin disorders and wound healing. iPSCs have been shown to be capable of differentiation along multiple cutaneous lineages including both keratinocytes and melanocytes. Through sequential application of retinoic acid and bone morphogenetic protein (BMP)-4 to iPSCs cultured on collagen IV-coated plates, in vitro keratinocyte cultures can be established.35 Furthermore, by subsequently seeding iPSC-derived keratinocytes onto type I collagen matrices, three dimensional skin equivalents have been generated exhibiting a multilayered epidermis with an outer cornified surface.36 In mice, iPSCs have been shown to be capable of reconstituting normal skin with a fully differentiated epidermis, hair follicles, and sebaceous glands.35 Epidermal melanocytes have also been generated in vitro from iPSCs through the supplementation of culture medium with Wnt3a, stem cell factor, and endothelin-3. On gene analysis, multiple melanocyte markers could be readily detected after seven weeks, and by transmission electron microscopy, melanosomes could be observed in the pigmented cells.37 Together, these studies thus highlight the potential for iPSCs to generate functional, patient-specific skin equivalents that may be employed to treat both a variety of skin disorders and situations involving large skin deficits.
The diverse capacity of iPSCs to generate a variety of different tissues has also been underscored by multiple studies showing the ability for these cells to form skeletal muscle. Investigators have described the growth of iPSCs on low attachment culture plates in the presence of horse serum and β-mercaptoethanol to result in development of embryoid bodies which give rise to contractile spindle fibers.38-39 Interestingly, these fibers functioned spontaneously, stained positively for myosin heavy-chain, and on electron microscopy were found to demonstrate characteristic sarcomere features including Z-lines, I-bands, A-bands, and H-bands.38 More importantly, preliminary animal studies have shown iPSC-derived muscle cells to engraft and sustain their myogenic lineage differentiation following injection.39 And when these cells were implanted into a damaged tibialis anterior muscle in mice, significantly increased isometric tetanic force could be detected.40 Such studies suggest a role for iPSCs in the treatment of injured or deficient muscle. Whether from congenital or acquired etiologies, regenerative strategies using iPSCs may one day offer a treatment option for patients with insufficient functional skeletal muscle in the craniofacial region.
Relative to these potential applications for iPSCs however, neural differentiation represents one of the largest areas of interest for use of these pluripotent cells. In light of the limited available therapeutic modalities to manage damaged or degenerative neural conditions, this is one particular field where iPSCs hold significant promise. As already mentioned, protocols have been developed to generate iPSC-derived neurospheres capable of subsequent tri-lineage differentiation into neurons, astrocytes, and oligodendrocytes.31 Studies have reprogrammed human dermal fibroblasts to give rise to cells which demonstrate electrophysiological characteristics similar to functional neurons. Robust resting membrane potentials, large fast tetrodotoxin-sensitive action potentials, and voltage-gated sodium currents have all been described in these differentiated cells.41 The in vivo utility of iPSC-derived neurons has also been investigated in animal studies. In particular, Wernig and colleagues employed Sonic Hedgehog and FGF8 to further differentiate neurons into a midbrain dopamine-producing subtype.42 Subsequent implantation into a rat model of Parkinson's disease demonstrated successful synaptic integration. In addition, marked improvement was noted in the behavior of rats receiving iPSC treatment.42 Given these findings, future approaches employing iPSCs may hold substantial impact for various neurological disorders. Several phase I/II clinical trials utilizing ESCs are already underway, and as iPSCs offer the potential advantage for patient-specific therapy, one can imagine the development of novel, functional, and safer treatment options for patients with craniofacial nerve deficits or other debilitating neurologic diseases.
Finally, with craniofacial reconstruction, large skeletal defects can be among the most difficult for surgeons to address. Current strategies including autologous bone grafting or use of allogeneic / alloplastic materials are often associated with donor site morbidity and a variety of complications. In their stead, iPSCs represent a potent building block for regenerative strategies aimed at forming novel bone. In vitro differentiation of iPSCs along mesenchymal lineages has been thoroughly demonstrated, with standard osteogenic differentiation medium containing β-glycerophosphate and ascorbic acid being capable of promoting osteogenesis in these cells.43 Furthermore, Tashiro et al. found transduction of iPSCs with Runx2 to further accelerate this process. And when iPSCs have been implanted into various skeletal defects in mice, de novo bone formation has been reported. In combination with silk scaffolds and enamel matrix derivatives, iPSCs were found to promote enhanced alveolar bone regeneration, formation of both cementum and periodontal ligaments.44 Using Special AT-rich sequence-binding protein 2 transduced iPSCs, Ye and colleagues were also able to demonstrate significantly increased bone formation in critical-sized calvarial defects.45 Interestingly, no teratomas were noted in animals receiving this genetically manipulated iPSC. Therefore, these studies confirm the potential for iPSCs in bone regenerative strategies. Future studies will undoubtedly look to further promote this osteogenic capacity through guided differentiation while simultaneously enhancing their safety profile by limiting risk of teratoma formation.
Conclusions
iPSCs possess enormous potential for use in craniofacial reconstruction. Whether from congenital or acquired etiologies, defects in the craniofacial region are often complex with deficiencies in multiple tissue types. While research continues on alternative regenerative strategies, the ability for iPSCs to form all tissue types has positioned these cells to be an ideal cellular building block upon which novel approaches to tissue engineering may be based. Through different spatial and temporal signals, the potential thus exists to develop a patient-specific integrated approach to treat skin, muscle, nerve, and bone defects simultaneously. Though multiple barriers must still be overcome before this technology may be clinically translated, iPSCs have nonetheless emerged as a powerful tool to treat a variety of human diseases and tissue deficiencies throughout the entire body.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 7.Fathi A, et al. Comprehensive gene expression analysis of human embryonic stem cells during differentiation into neural cells. PLoS One. 2011;6:e22856. doi: 10.1371/journal.pone.0022856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou DB, et al. Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes. J Cell Biochem. 2011;112:3555–3562. doi: 10.1002/jcb.23283. [DOI] [PubMed] [Google Scholar]
- 9.Hwang YS, Randle WL, Bielby RC, Polak JM, Mantalaris A. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period:application to skeletal tissue engineering. Tissue Eng. 2006;12:1381–1392. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 10.Stankovich BL, Aguayo E, Barragan F, Sharma A, Pallavicini MG. Differential adhesion molecule expression during murine embryonic stem cell commitment to the hematopoietic and endothelial lineages. PLoS One. 2011;6:e23810. doi: 10.1371/journal.pone.0023810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorklund LM, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Puri MC, Nagy A. Concise review: embryonic stem cells versus induced pluripotent stem cells: the game is on. Stem Cells. 2012;30:10–14. doi: 10.1002/stem.788. [DOI] [PubMed] [Google Scholar]
- 14.Lowry WE, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 17.Ebben JD, Zorniak M, Clark PA, Kuo JS. Introduction to induced pluripotent stem cells: advancing the potential for personalized medicine. World Neurosurg. 2011;76:270–275. doi: 10.1016/j.wneu.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Sun N, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 23.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munz C, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia F, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narsinh KH, et al. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6:78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez-Aranda I, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang C, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao F, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 35.Bilousova G, Chen J, Roop DR. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J Invest Dermatol. 2011;131:857–864. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 36.Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2011;108:8797–8802. doi: 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta S, et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS One. 2011;6:e16182. doi: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawagoe S, et al. Generation of induced pluripotent stem (iPS) cells derived from a murine model of Pompe disease and differentiation of Pompe-iPS cells into skeletal muscle cells. Mol Genet Metab. 2011;104:123–128. doi: 10.1016/j.ymgme.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno Y, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. Faseb J. 2010;24:2245–2253. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- 40.Darabi R, et al. Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev. 2011;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song B, et al. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012;8:259–273. doi: 10.1016/j.scr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilousova G, et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011;29:206–216. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan X, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226:150–157. doi: 10.1002/jcp.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye JH, et al. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011;32:5065–5076. doi: 10.1016/j.biomaterials.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]


