Abstract
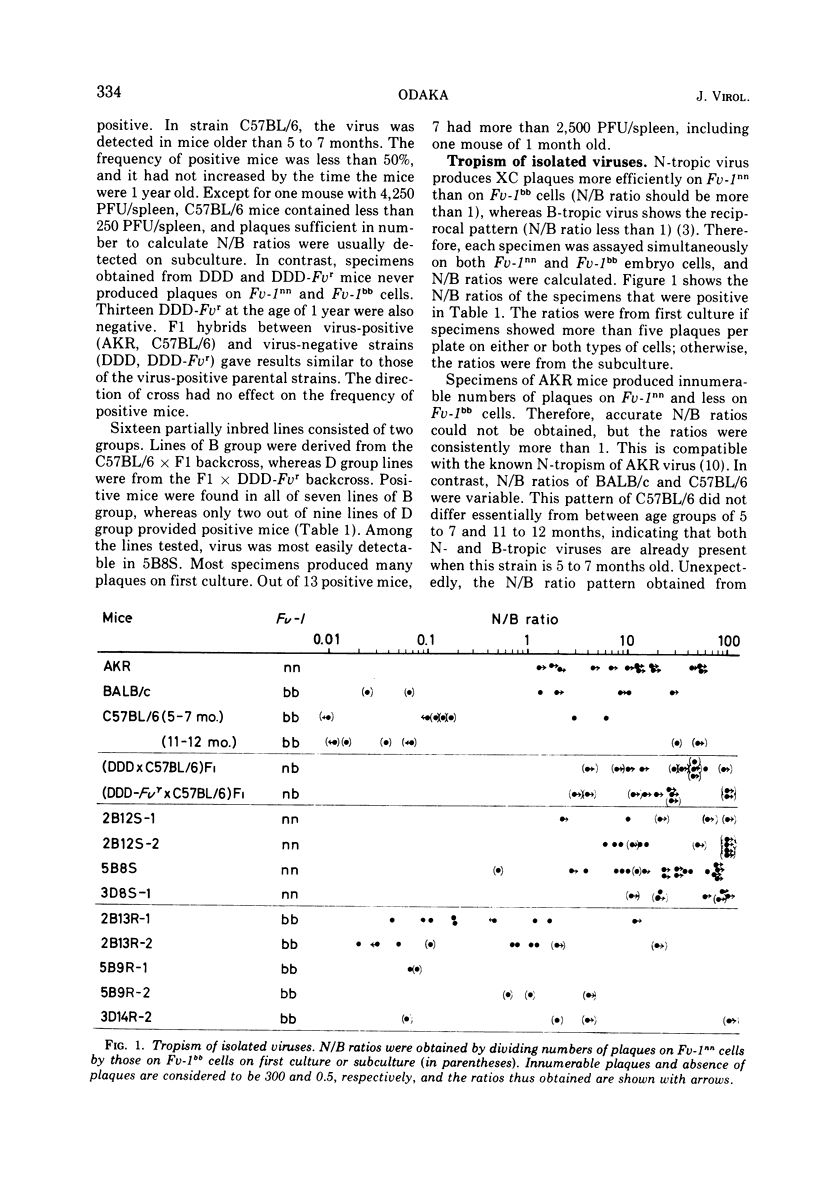
Spontaneous expression of endogenous N- and B-tropic murine leukemia viruses was stu1bb), DDD (Fuv-1nn), DDD-Fvr (fv-1nn), (DDD or DDD-Fvr times C57BL/6)F1, and 16 partially inbredlines with either the Fv-1nn or Fv-1bb genotype, which had been established from hybrids between C57BL/6 and DDD-Fvr. When tested at middle age, virus-positive mice were found in C57BL/6, F1 hybrids, and 9 out of 16 partially inbred lines. N-tropic viruses were isolated from Fv-1nn, Fv-1bb mice, whereas B-tropic viruses, except for one isolate, were from Fv-1bb mice only. C57BL/6 mice were positive for both N- and B-tropic viruses, whereas DDD-Fvr mice were negative. With respect to the Fv-1 genotype and the presence of endogenous murine leukemia viruses, the partially inbred lines were grouped into five types: (i) Fv-1bb, both N- and B-tropic virus positive, like C57BL/6; (ii) Fv-1nn, virus negative, like DDD-Fvr; (iii) Fv-1bb, virus negative; (iv) Fv-1nn, only N-tropic virus positive; and (v) less convincingly, Fv-1bb, only B-tropic virus positive. These findings indicate that the transmission of N- and B-tropic viruses in C57BL/6 is genetically controlled and that the expression of B-tropic virus, but not of N-tropic virus, is closely associated with the Fv-1 genotype.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. V. Introduction of a gene responsible for susceptibility in the genetic complement of resistant mice. J Virol. 1969 Jun;3(6):543–548. doi: 10.1128/jvi.3.6.543-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. VII. Establishment of a resistant strain. Int J Cancer. 1970 Jul 15;6(1):18–23. doi: 10.1002/ijc.2910060104. [DOI] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. XII. Effects of the Fv-1 locus. Int J Cancer. 1974 Aug 15;14(2):252–258. doi: 10.1002/ijc.2910140214. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Hartley J. W., Spahn G. J., Rabstein L. S., Whitmire C. E., Turner H. C., Huebner R. J. Prevalence of the group-specific (gs) antigen and infectious virus expressions of the murine C-type RNA viruses during the life span of BALB-cCr mice. Int J Cancer. 1972 Sep 15;10(2):283–289. doi: 10.1002/ijc.2910100208. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Genetic factors in the natural history of murine leukemia virus infection: G. H. A. Clowes Memorial Lecture. Cancer Res. 1973 Dec;33(12):3061–3068. [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Genetic factors influencing C-type RNA virus induction. J Exp Med. 1972 Jul 1;136(1):175–184. doi: 10.1084/jem.136.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Segregation of loci for C-type virus induction in strains of mice with high and low incidence of leukemia. Science. 1973 May 25;180(4088):865–866. doi: 10.1126/science.180.4088.865. [DOI] [PubMed] [Google Scholar]


