Abstract
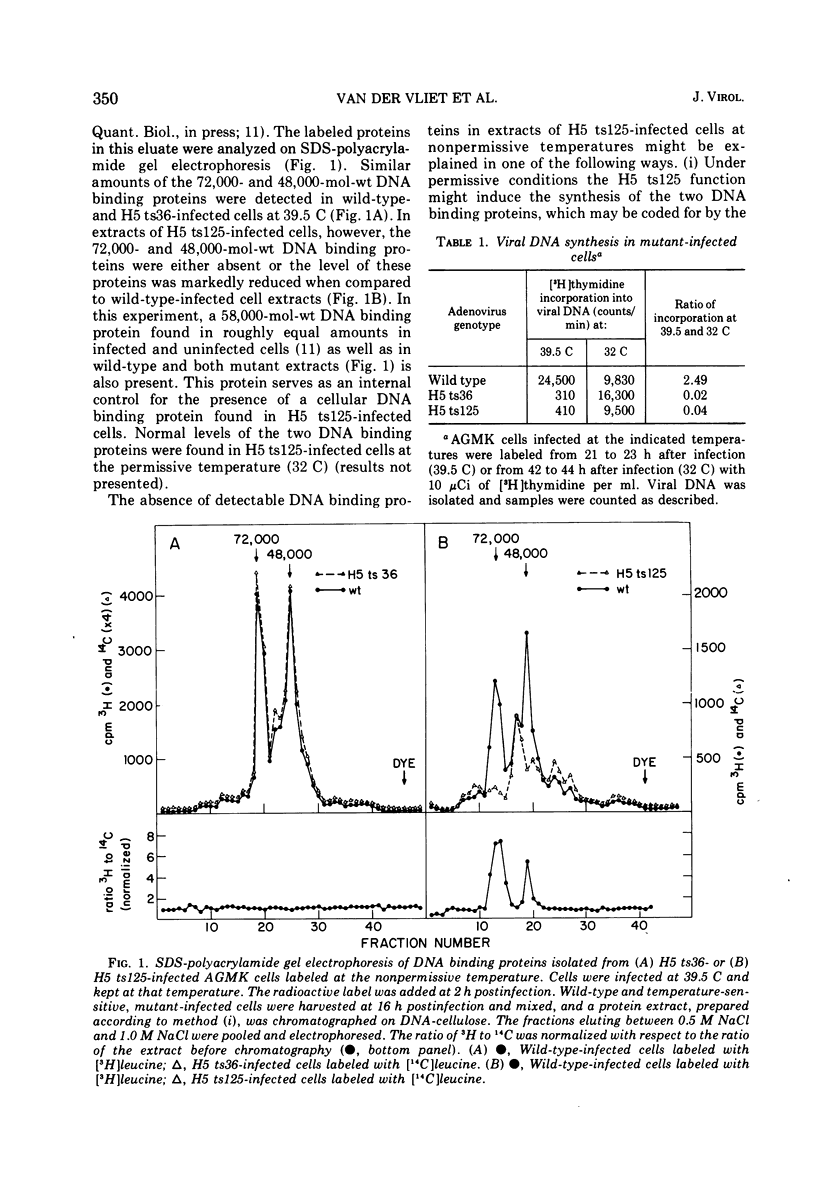
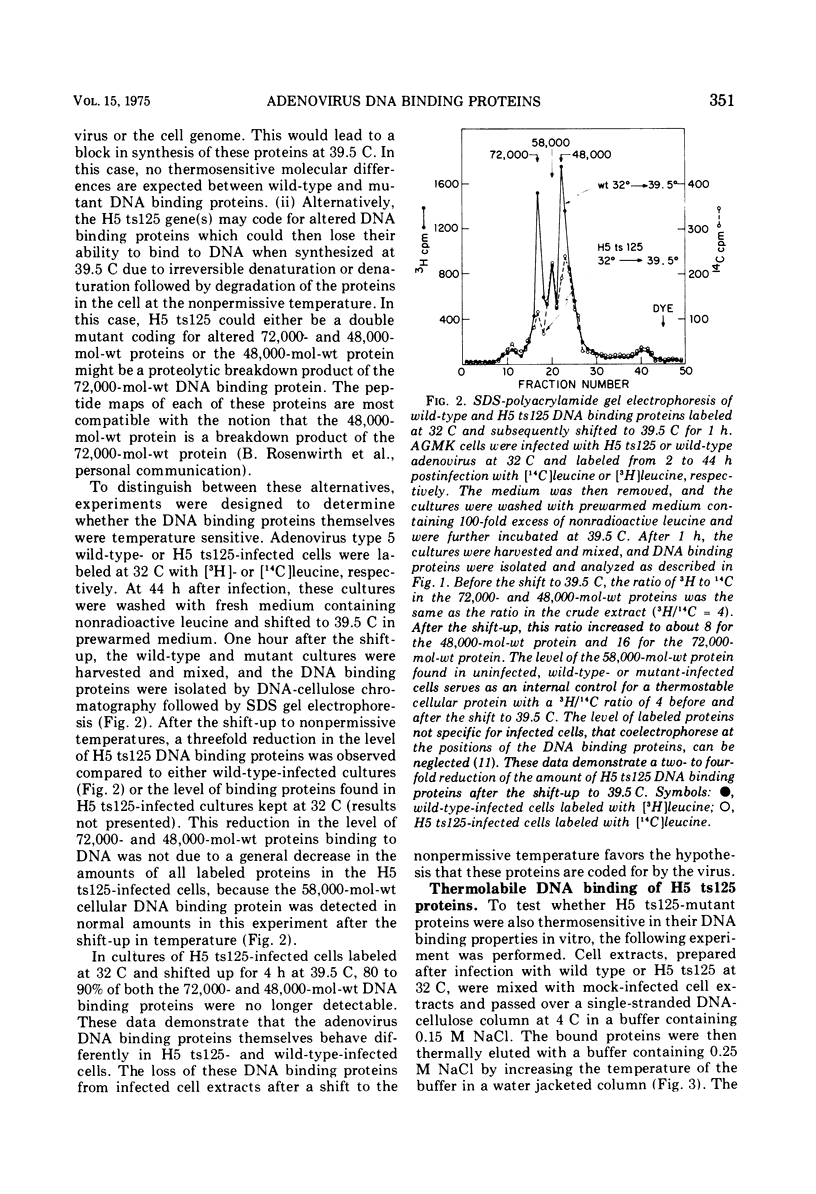
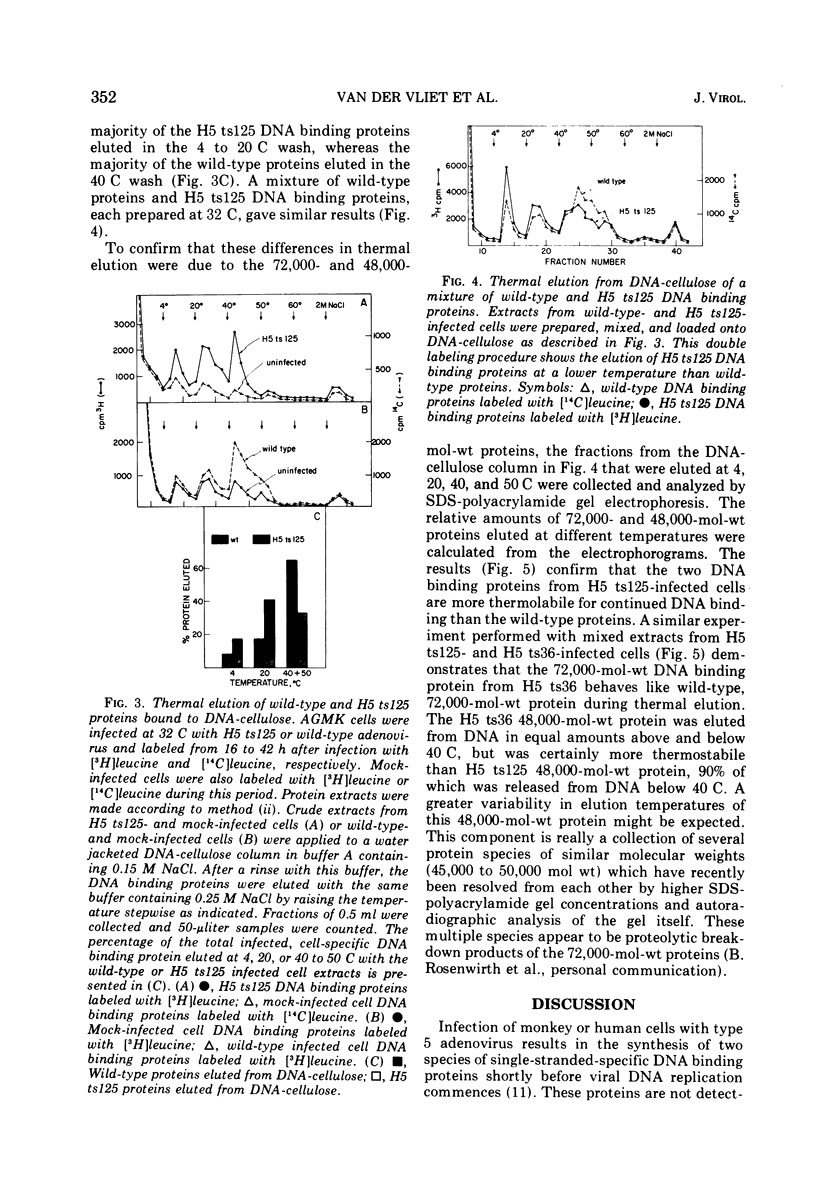
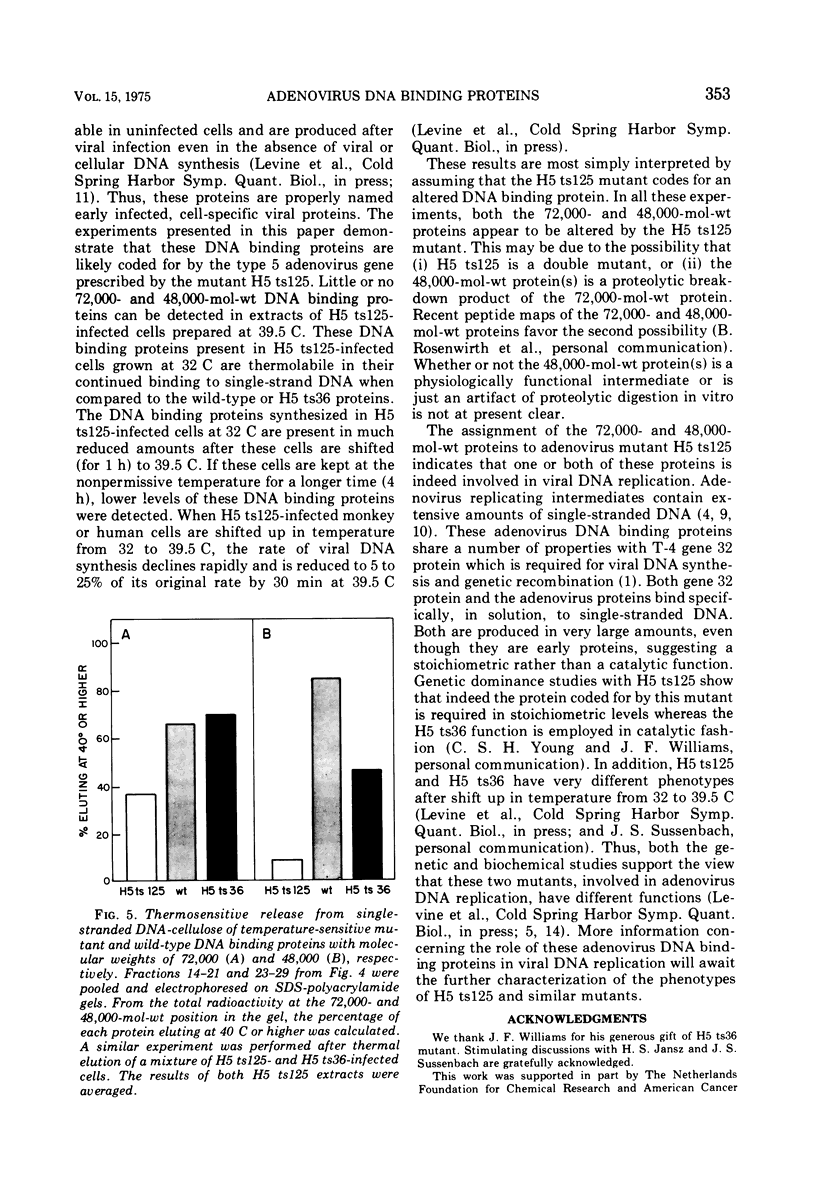
Infection of African green monkey kidney cells with type 5 adenovirus leads to the synthesis of two infected, cell-specific proteins with approximate molecular weights of 72,000 and 48,000, that bind specifically to single-stranded but not double-stranded DNA. The production of these two proteins was studied after infection with two DNA-negative adenovirus mutants belonging to different complementation groups (H5 ts36 and H5 ts 125). Both DNA binding proteins were detected in cells infected with either mutant at the permissive temperature (32 C) AND ALSO IN H5 ts36-infected cells at the nonpermissive temperature (39.5 C). In H5 ts125-infected cells at 39.5 C, however, less than 5% of the normal wild-type level of these DNA binding proteins was detectable. When H5 ts125-infected cells were labeled with radioactive leucine at 32 C and subsequently shifted to 39.5 C in the presence of unlabeled leucine (chase), the level of DNA binding proteins found in these infected cells was markedly reduced compared to cultures not shifted to 39.5 C. These data suggest that the DNA binding proteins themselves were temperature sensitive. This conclusion was confirmed by experiments in which the DNA binding proteins were eluted from DNA cellulose with buffers of increasing temperatures (thermal elution). The H5 ts 125 proteins were shown to elute at lower temperatures than either wild-type or H5 ts36 proteins. These results are taken to indicate that the H5 ts125 mutant codes for a DNA binding protein that is thermolabile for continued binding to single-stranded DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellett A. J., Younghusband H. B. Replication of the DNA of chick embryo lethal orphan virus. J Mol Biol. 1972 Dec 30;72(3):691–709. doi: 10.1016/0022-2836(72)90185-4. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J. The polypeptides of adenovirus-infected cells. J Gen Virol. 1972 Apr;15(1):45–57. doi: 10.1099/0022-1317-15-1-45. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Walter G., Maizel J. V., Jr The polypeptides of adenovirus. IV. Detection of early and late virus-induced polypeptides and their distribution in subcellular fractions. Virology. 1974 Feb;57(2):402–408. doi: 10.1016/0042-6822(74)90180-9. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Ustacelebi S., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: nucleic acid synthesis. Virology. 1973 Feb;51(2):499–503. doi: 10.1016/0042-6822(73)90450-9. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J. Intermediates in type 5 adenovirus DNA replication. Virology. 1973 Jan;51(1):11–23. doi: 10.1016/0042-6822(73)90361-9. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]


