Abstract
Background
Pseudomonas aeruginosa is the most common bacterial pathogen infecting the lungs of patients with cystic fibrosis (CF). The Liverpool Epidemic Strain (LES) is transmissible, capable of superseding other P. aeruginosa populations and is associated with increased morbidity. Previously, multiple inducible prophages have been found to coexist in the LES chromosome and to constitute a major component of the accessory genome not found in other sequenced P. aerugionosa strains. LES phages confer a competitive advantage in a rat model of chronic lung infection and may, therefore underpin LES prevalence. Here the infective properties of three LES phages were characterised.
Results
This study focuses on three of the five active prophages (LESφ2, LESφ3 and LESφ4) that are members of the Siphoviridae. All were induced from LESB58 by norfloxacin. Lytic production of LESφ2 was considerably higher than that of LESφ3 and LESφ4. Each phage was capable of both lytic and lysogenic infection of the susceptible P. aeruginosa host, PAO1, producing phage-specific plaque morphologies. In the PAO1 host background, the LESφ2 prophage conferred immunity against LESφ3 infection and reduced susceptibility to LESφ4 infection. Each prophage was less stable in the PAO1 chromosome with substantially higher rates of spontaneous phage production than when residing in the native LESB58 host. We show that LES phages are capable of horizontal gene transfer by infecting P. aeruginosa strains from different sources and that type IV pili are required for infection by all three phages.
Conclusions
Multiple inducible prophages with diverse infection properties have been maintained in the LES genome. Our data suggest that LESφ2 is more sensitive to induction into the lytic cycle or has a more efficient replicative cycle than the other LES phages.
Keywords: Pseudomonas aeruginosa, Prophage induction, Bacteriophage infection, Cystic fibrosis, Host range, Type IV pili
Background
Pseudomonas aeruginosa is a versatile Gram-negative bacterium, able to metabolise multiple carbon sources and exploit diverse ecological niches, e.g. soil, water, plants and animal hosts [1,2]. This opportunistic pathogen causes a range of human infections, including acute infections of severe wounds [3] and burns [4,5] and chronic lung infections in cystic fibrosis (CF) patients [6]. P. aeruginosa forms biofilms in the CF lung that are highly resistant to antibiotics and clearance by the immune system [7]. Once established, such biofilms cannot be eradicated and are associated with greatly increased morbidity and mortality [8].
Several CF-associated transmissible strains of P. aeruginosa, capable of between patient transmission, have been identified in the UK, Europe, Australia and North America [9]. The Liverpool Epidemic Strain (LES), a UK transmissible strain, was first isolated in 1996 at Alder Hey Children’s Hospital (AHCH), Liverpool [10]. This strain is capable of super-infection, supplanting pre-existing P. aeruginosa populations in the CF lung [11]. Chronic infection with LES is associated with increased morbidity and mortality compared to other P. aeruginosa strains [12]. The LES is highly prevalent within individual hospital CF units [13] and is the most abundant P. aeruginosa strain amongst CF patients in the UK [14]. It was also recently isolated from the sputa of CF patients in North America [15].
Sequencing of the earliest LES isolate, LESB58, demonstrated that the genome shares 95% similarity with the lab strain PAO1. However, its core genome is punctuated by multiple norfloxacin-inducible prophages [16]. Specifically, there are five inducible prophage genomes (LESφ2; LESφ3 LESφ4 LESφ5 and LESφ6) that are mosaic in nature. The gene organisation of LESφ2 and LESφ3 resembles that of lambdoid phages. These two phage genomes share 82.2% identity across a 13.6-kb region at their 3’ ends that makes up 32% of the phage genomes. The closest known relative to both these phages is the Pseudomonas phage F10 [17]. LESφ3 also contains a 7.5 kb region that shares 99.8% homology with LESφ5, which exhibits a considerable sequence similarity to the O-antigen converting phage D3 [18]. LESφ4 is a transposable Mu-like phage that closely resembles phage D3112 [19]. The LESφ6 sequence resembles a pf1-like filamentous phage [16].
Temperate phages have been shown to confer selective, beneficial traits to a range of P. aeruginosa hosts [20]. For example, phage D3 orchestrates O antigen conversion from O5 to O16 in PAO1, which may aid evasion of the immune system and resistance to phage superinfection [18,21]. Phage φCTX infection of PAS10 results in conversion to a toxigenic strain [22] and the filamentous phage, Pf1, has been associated with biofilm disruption and dispersal [23]. LES prophages have been suggested to contribute to the competitiveness of their bacterial host in vivo. LESB58 mutants, with disrupted prophage genes, exhibited 10 to 1000-fold decreased competitiveness in a rat model of chronic lung infection compared to wild type LESB58 [16]. The LES phages are induced by exposure to clinically relevant antibiotics, e.g. ciprofloxacin [24], and free LES phages and other tailed-phage virions have been detected in CF patient sputa [25,26].
Temperate phages are key vectors of horizontal gene transfer (HGT) [27]. Therefore, it is important to assess the ability of the LES phages to infect other bacterial hosts to which they may confer traits beneficial to life in the CF lung environment. Here we describe the infection characteristics of three of the five LES prophages LESφ2, LESφ3 and LESφ4, induced from the sequenced CF lung isolate LESB58.
Results
LES phage morphology
Three different Siphoviridae phages were induced from LESB58 cultures and visualised using electron microscopy. The phages possessed icosahedral heads (50–60 nm diameter) and long flexible tails (approximately 200 nm). Plaque assay of each phage on PAO1 resulted in the formation of small turbid plaques with different phage-specific morphologies. LESφ3 plaques were the largest (2–3 mm), with well-defined lysogen islands, whereas LESφ2 plaques were considerably smaller (0.5-1.5 mm). LESφ4 produced plaques with small, clear centres surrounded by a turbid halo. The identity of each LES phage responsible for the different plaque morphologies was confirmed using a multiplex PCR assay.
Differential induction of LES phages from LESB58
The sensitivity of the LES phages to induction into the lytic cycle was determined and compared. Real-time quantitative (Q)-PCR was used to measure relative increases in phage DNA copy number following induction by exposure of LESB58 to norfloxacin. After exposure to norfloxacin for 60 min and recovery for 2 h, LESφ2 was the most abundant free phage detected (6.2 x 107 copies μl-1), compared to LESφ3 (6.9 x 106 copies μl-1) and LESφ4 (1 x 107 copies μl-1) (Figure 1). Furthermore, the increase in LESφ2 production between 30 and 60 min exposure times was higher (3.67 fold increase) than that for LESφ3 (1.74 fold increase) and LESφ4 (2.06 fold increase). Thus while norfloxacin induction caused a significant increase in the replication of all three phages (LESφ2 - F1, 8 56.97, P 0.001; LESφ3 - F1, 8 14.02, P 0.006; LESφ4 - F1, 8 16.88, P 0.003), only LESφ2 showed significantly greater phage production after 60 min compared to 30 min norfloxacin exposure (induction*time interaction, F1, 8 20.90, P 0.002); by contrast, the duration of exposure had no effect on phage production in LESφ3 and LESφ4 (induction*time interaction, LESφ3 - F1, 8 1.05, P 0.336; LESφ4 - F1, 8 3.19, P 0.112). We suggest therefore that LESφ2 is either more sensitive to induction by norfloxacin or that it replicates more rapidly once induced.
Figure 1.
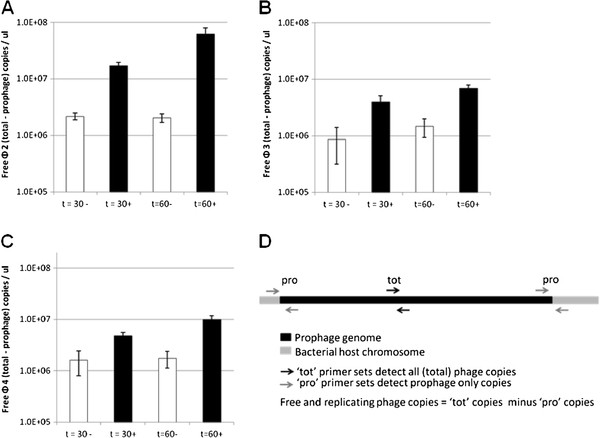
Exposure to sub-inhibitory concentrations of norfloxacin induces the lytic cycle of three LES phages. Mid-exponential phase LESB58 cultures (OD600 0.5) were exposed to sub-inhibitory norfloxacin (50 ug ml-1) for 30 and 60 min before recovery for 2 h and total DNA extraction. Total phage vs prophage numbers were quantified by Q-PCR with SYBR green and specific primers. Graphs show the production levels of each phage over time; A: LESφ2; B: LESφ3; C: LESφ4. ■ + norfloxacin; □ – norfloxacin. D: Quantities of free phage were calculated by deducting prophage numbers from total phage numbers. The average free phage numbers at each time interval were plotted and Standard error is shown. Three independent experimental repeats were performed, each with 3 technical repeats.
Lysogenic infection of a model PAO1 host
PAO1 LES phage lysogens (PLPLs) were created by infection of strain PAO1 with each LES phage and isolation of single colonies from turbid areas within plaques (Figure 2). Challenge of PLPLs with different LES phages, using plaque assays, revealed varying immunity profiles. Table 1 lists the efficiency of plating (eop) values of each LES phage on each PLPL lawn. Prophages 2 and 3 conferred immunity to super-infection by LESφ2 and LESφ3 respectively (eop < 1 x10-9). However, a few LESφ4 super-infection events were observed by detection of plaques following exposure of lysogens to 1 x 1010 p.f.u ml-1 of LESφ4 (eop = 3.33 x 10-9). LESφ2 was able to infect PLPLs harbouring prophages LESφ3 (eop 0.91) and LESφ4 (eop 1.09) at the same efficiency as non-lysogenic PAO1. However, lysogens harbouring the LESφ2 prophage were resistant to infection by LESφ3 (eop < 1x10-9) and showed considerably reduced susceptibility to LESφ4 (eop 0.017).
Figure 2.
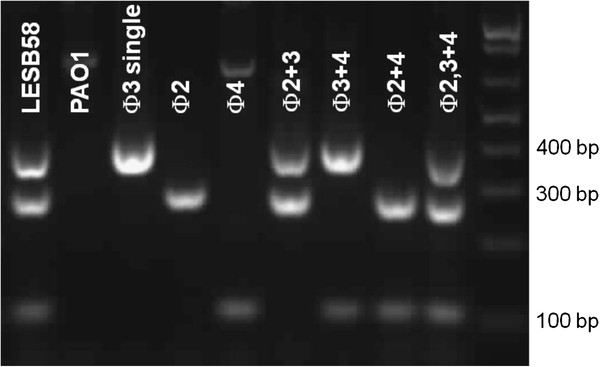
PCR confirmation of all PAO1 LES phage lysogens. Lysogens were isolated from turbid plaques following sequential infection of PAO1 with pure stocks of each LES phage. Lysogens were considered resistant if no plaques were observed following exposure to increasingly high titre phage suspensions (up to MOI 100). The presence of each prophage was confirmed using multiplex PCR with specific primer sets for each LES phage yielding differentially sized products: 325 bp (LESφ3); 250 bp (LESφ2); 100 bp (LES φ 4).
Table 1.
Differential Immunity profiles of each LES phage in PAO1
| Efficiency of plating values | φ2 | φ3 | φ4 |
|---|---|---|---|
| PAO1 naive host | 1.0 | 1.0 | 1.0 |
| Single φ2 lysogen | < 1x10 -9 | < 1x10 -9 | 0.017 |
| Single φ3 lysogen | 0.91 | < 1x10 -9 | 0.37 |
| Single φ4 lysogen | 1.09 | 0.94 | 3.3x10 -9 |
Immunity profiles of each LES phage were determined by plaque assay. Phage dilution series were spotted onto non-Lysogenic PAO1 and PLPL lawns. Efficiency of plating = the ratio of plaques observed (at the appropriate phage dilution) on the most permissive host (non-lysogenic PAO1)/plaques observed on assay host (PLPL harbouring LESφ2, LESφ3 or LESφ4 prophages). If no plaques were observed when neat phage suspensions of 1010 p.f.u ml-1 were used, an eop value < 1x10-9 was recorded.
Spontaneous phage production by all seven PLPLs was higher than that associated with LESB58, by 5–6 orders of magnitude (P < 0.05) (Figure 3). These data suggest that LES prophages are less stable in PAO1, with significantly higher rates of spontaneous lytic phage production than in LESB58. Little difference was observed in the levels of spontaneous phage production between single, double and triple PAO1 lysogens.
Figure 3.
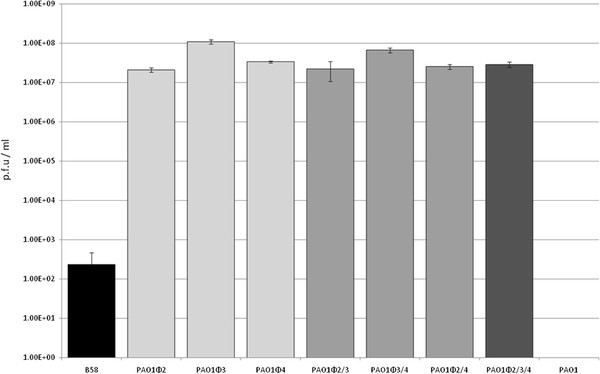
Spontaneous lysis exhibited by LES phages in PAO1 vs LESB58. Phage production was quantified from filtered culture supernatants of un-induced mid-exponential phase cultures using standard plaque assay. Standard deviation is shown (n = 3).
LES phages integrate at the same sites in different bacterial host strains
Southern blot analysis was used to demonstrate that lysogenic instability was not due to integration of the LES phages into unstable sites of the naive PAO1 chromosome, or from multiple integration events of the same phage (Figure 4). LESφ2 and LESφ3 integrated as single copies at identical locations in LESB58 and PAO1 chromosomes.
Figure 4.
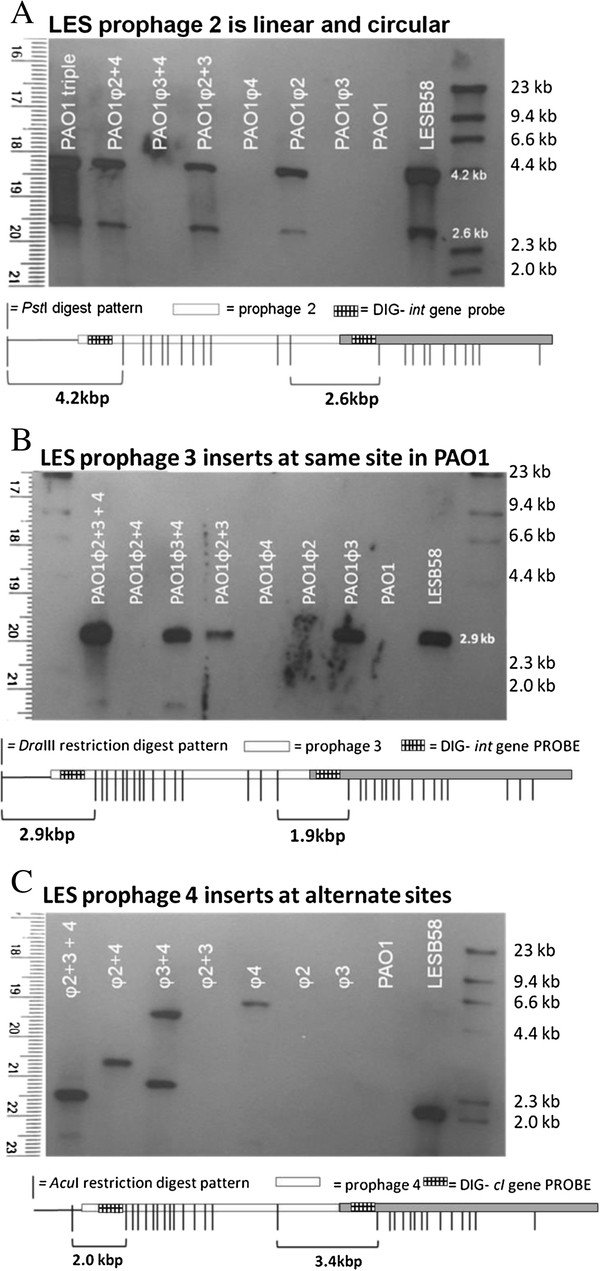
Southern analysis of LES phage integration sites in LESB58 and PAO1. Southern blot analysis to determine LES phage copies and integration sites in LESB58 and PLPL chromosomes: A) PstI digested LES phage lysogens hybridised to LESφ2 integrase (int) probe; B) DraIII digested LES phage lysogens hybridised to LESφ3 integrase probe; C) AcuI digested LES phage lysogens hybridised to LESφ4 cI probe. A diagrammatical representation of the restriction pattern is presented below each blot. This demonstrates the expected size of fragments that would hybridise each probe in the event of single phage integration (one band) or integration of two identical prophages in tandem (two bands). For clarity, the second phage copy has been shaded in grey. The 2-band pattern would also result if any additional phage copies were present in circular form.
The LESφ2 int probe hybridised to an additional DNA fragment in all lysogens containing LESφ2, including LESB58. The size of the additional hybridised fragment corresponds to one of two possibilities: 1) the integration of a second LESφ2 copy in to the chromosome directly downstream of the first; 2) an extra copy of LESφ2 in circular form (Figure 4). The published LESB58 genome sequence clearly shows a single LESφ2 copy in the chromosome. Since the hybridisation pattern of the PAO1 LESφ2 lysogen matches that of LESB58, a second chromosomal copy can be ruled out. This suggests that the extra copy is circular, which may represent phage replication resulting from spontaneous activation of the lytic life cycle. Alternatively, the extra copy may indicate pseudolysogeny, in which stable circular copies are maintained.
The LESφ4 cI probe indicated that LESφ4 is able to integrate in several chromosomal locations in PAO1.
LES phages infect a narrow host range in a type IV pilus-dependant manner
From a well-characterised panel of 32 clinical P. aeruginosa isolates, 6 were susceptible to LES phage infection. Of 25 environmental isolates, representing 17 different Pseudomonas species, only the P. aeruginosa strain was susceptible. In addition, PA14 was resistant to infection by LESφ2 and LESφ3, but susceptible to LESφ4. Plaques on PA14 appeared less turbid than those on PAO1 lawns. The host ranges of each LES phage were not identical and no correlation was found between bacterial clone-type [28] and susceptibility (data not shown). In addition, other common Gram-negative CF pathogens Burkholderia cenocepacia and B. multivorans strains were resistant to infection by all three LES phages (Table 2).
Table 2.
Susceptibility of a panel of Pseudomonas isolates to LES phages 2, 3 and 4
| Isolate source (#) | φ2 | φ3 | φ4 |
|---|---|---|---|
| Reference strains (2) | 50% (1/2) | 50% (1/2) | 100% (2/2) |
| Keratitis patient (12) | 8.3% (1/12) | 0% (0/12) | 33.3% (4/12) |
| Non-LES child (8) | 12.5% (1/8) | 0% (0/8) | 12.5% (1/8) |
| Non-LES adult (6) | 16.7% (1/6) | 0% (0/6) | 0% (0/6) |
| Anomalous LES (6) | 0% (0/6) | 0% (0/6) | 0% (0/6) |
| Environmental (25) | 0% (0/25) | 4% (1/25) | 0% (0/25) |
Percentage of LES phage-sensitive strains as determined by plaque assay. Actual numbers tested are shown in parentheses.
A non-piliated PAO1 mutant (pilA-) was resistant to infection by all 3 phages, suggesting that LESφ2, 3 and 4 all require type IV pili for infection. The hyper-piliated mutant (pilT-) was also resistant to the LES phages, whilst an alternative hyper-piliated mutant (pilU-) remained fully susceptible.
Discussion
Differential induction among co-infecting prophages
Induction experiments demonstrated that LESφ2 virions were produced from LESB58 in greater numbers than the other phages. These data suggest that LESφ2 replication is more efficient than the other phages and could out number and therefore out compete the other, co-infecting LES phages during the lytic cycle. Potentially supporting this hypothesis, we detected an extra copy of this phage in the LESφ2 lysogen genome. Southern analysis suggests the presence of either a pseudo-lysogenic plasmid form [29], or a highly active replicative form of LESφ2 during spontaneous phage production.
The implications of within-host competition between co-infecting prophages has been little studied, however Refardt et al.[30] observed hierarchical competition between multiple prophages in E. coli, which suggested that the sensitivity of the lytic switch can determine dominance of one prophage over another in a polylysogen. Carriage of phages that are very prone to activation of the lytic lifecycle may represent a significant cost to their host cells, and thus could be selected against in natural populations. However, while natural isolates of LES sampled from CF patient sputa often lack one or more of the LES prophages [25], there is no evidence that LESφ2 is more or less stably maintained than LESφ3 or LESφ4.
LES phages exhibit different immunity profiles
Each phage conferred inhibition of superinfection by the same phage, although the Mu-like phage, LESφ4 was observed to infect LESφ4 lysogens at a very low frequency. This may represent the development of rare mutations that affect immunity functions. There are several examples of such mutations in phage Mu [31]. Repressor/operator coevolution has been suggested to be the driving force for the evolution of superinfection immunity groups of lambdoid phages [32]. The same may hold true for Mu-like phages. For example, mutation of the operator region has been shown to affect binding of the repressor in Mu vir mutants [33].
Sequential infection of PAO1 with different LES phages revealed an interesting superinfection hierarchy. LESφ3 lysogens remained susceptible to LESφ2 and LESφ4; and LESφ4 lysogens were susceptible to LESφ2 and LESφ3. However, LESφ2 prevented infection by LESφ3 and greatly reduced susceptibility to LESφ4. Such uni-directional infection exclusion has been reported between other phages, and is commonly associated with super-infection exclusion genes such as the lambda rex genes [34] and sieA, sieB and a1 in the Salmonella phage, P22 [35-38].
It is likely that LESφ3 and LESφ4 prophages would have been acquired before LESφ2, because the infection hierarchy suggests that prior acquisition of LESφ2 would have prevented subsequent LESφ3 and LESφ4 infection.
LES prophages in PAO1 undergo spontaneous activation to the lytic cycle at a far higher rate than in LESB58
High levels of spontaneous induction were observed in PLPLs, suggesting that lysogeny is relatively unstable in the PAOl genetic background. We show that phage production remained high between PLPLs containing one, two or three LES prophages, suggesting that polylysogens were no more or less stable than any single lysogens. Southern analysis confirmed that LESφ2 and LESφ3 integrated into the same position in PLPLs as they did in LESB58. Therefore, the instability of PLPLs was not due to prophage integration into unstable sites. LESφ4 integrated in several alternative sites in PLPLs. The sequence of this phage shares a high level of genome synteny and homology with the transposable Mu-like phage D3112 [16], whose random integration has been demonstrated to create mutations within the host chromosome. LESφ4 may play a similar role in LES genome evolution.
The LES phages exhibit a narrow host-range
Our investigation of the LES phage host range revealed narrow, overlapping host specificity. No association between bacterial clone-type and phage susceptibility was observed, although testing more strains may have identified a pattern. Despite the high proportion of resistant clinical isolates, our data show that LES phages are capable of infecting some P. aeruginosa strains isolated from keratitis patients and non-LES infected CF patients. LES phages have been detected in CF patients’ sputa, and may therefore allow mobilisation of genes between P. aeruginosa strains [25,26]. By contrast, LES phages may allow LES to displace other P. aeruginosa strains during superinfection in the CF lung [11] by lysing susceptible resident strains [39].
LES phage infection is Type IV pilus-dependent
We demonstrate that LES phage infection is dependent on the type IV pilus, which is required by P. aeruginosa for adhesion, biofilm formation and twitching motility [40-42]. This important surface structure is commonly used as a receptor by diverse Pseudomonas phages [43]. Both non-piliated (pilA-) and hyper-piliated (pilT-) PAO1 mutants were resistant to infection by all three LES phages. However, a different hyper-piliated mutant (pilU-) remained susceptible. These findings mirror other pilin-dependent P. aeruginosa phage studies [43-45]. Hyper-piliated mutants are incapable of twitching motility due to abrogated pili retraction. These data suggest that retraction is involved in the infection process by LESφ2 LESφ3 and LESφ4.
Despite infecting via an important and common surface structure, all three LES phages exhibited narrow host ranges and each showed strain specificities. For example, LESφ4 was able to infect PA14 and several keratitis isolates that were resistant to infection by the other LES phages. It is likely that many clinical strains of P. aeruginosa harbour prophages that may belong to the same immunity group and therefore exclude super-infection by one or more of the LES phages [20]. Alternatively, resistance could be achieved by loss or modification of the type IV pili receptor [44,45].
Conclusion
In summary, we demonstrate that the LES phages exhibit differential sensitivities to induction, narrow host ranges and divergent infection behaviour in the model host PAO1 compared with the native LESB58 host background. Extensive genotypic and phenotypic variation has been observed in clinical LES populations [46], including changes in the number of resident LES prophages [25]. These phages may, therefore, be important contributors to diversity of the LES populations.
Methods
Bacterial strains and growth conditions
All bacterial strains used in this study and their sources are listed in Table 3. LES phages were induced from the sequenced CF P. aeruginosa isolate, LESB58 [16]. Strain PAO1 was susceptible to infection by all three LES phages and was therefore used as a model host to purify and study the characteristics of each phage. Successive infection of PAO1 with purified LES phages yielded single, double and triple PAO1 LES Phage Lysogens (PLPLs) each harbouring single copies of one, two or three LES phages simultaneously. All lysogens were confirmed by PCR amplification of specific prophage sequences and Southern blot analysis. Non-piliated (pilA-) or hyperpiliated (pilT- and pilU-) PAO1 mutants [47] were used to determine whether LES phages infect via the type IV pili. All bacterial strains and phages were grown and propagated in standard lysogeny broth (LB) at 37°C (clinical isolates) or 23°C (environmental isolates). Phage suspensions were stored in LB at 4°C.
Table 3.
Bacterial strains and sources
| Strain ( 1 Clone type) | Reference/source | |
|---|---|---|
| Laboratory P. aeruginosa strains: | ||
| PAO1(W) | [2] | |
| PAO1 pilA-; PAO1 pilU-; PAO1 pilT-2 | [47] | |
| PA14(A) | [48] | |
| Clinical LES isolates: | ||
| LESB 58 (T) - Sequenced isolate | [16] | |
| LES 431 (T) - Lacks LES prophage 2 | [49] | |
| Anomalous LES isolates3: | ||
| O69574 (T); 0521 (T); 43513 (T); 079444 (T); 0342 (T). | [50] | |
| P. aeruginosa isolates from keratitis patients4: | ||
| 39015 (B); 39115 (A); 39103 (A2); 39145 (A3); 39053 (A5); 39135 (C); 39016 (D); 39421 (F); 39061 (I); 39284 (L); 39376 (U); 39129 (V). | [51] | |
| P. aeruginosa isolates from non-LES infected CF patients: | ||
| CHILDREN: AH23 (B); AH4 (A); AH19 (A3); AH14 (C); AH1 (D); AH6 (L); AH9 (U); AH7 (A4); | AHCH5 | |
| ADULTS: NL28 (A); NL20 (C); NL25 (F); NL16 (U); NL21 (A4); NL14 (A7). | RLUH6 | |
| Environmental Pseudomonas spp : | Strain | |
| P. aeruginosa | 159 | RJ7 |
| P. fluorescens | WC5365; F113; ATCC 17400; pf5; pf01. | |
| P. syringae | ‘tomato’ DC300; B728a | |
| P. syringae pv. Coriandricola | Ccola | |
| P. syringae pv. maculocola | M4 | |
| P. syringae pv. antirrhini | 152E | |
| P. putida | KT2440; Paw340 | |
| P. cichori | 907 | |
| P. avellanae | 48 | |
| P. phaseiolicola | 1448A | |
| P. entomophila | L48 | |
| P. marginalis | 247 | |
| P. corrugata | 2445 | |
| P. tolaasii | 2192 T | |
| P. glycinea | 49a/90 | |
| P. lachrymans | 789 | |
| P. agarici | 2472 | |
| P. viridiflava | 2848 | |
| B. cenocepacia | K56-2; J2315. | [52] |
| B. multivorans | F-A1-1; LMG 13010. | |
1Clones typed using the Clondiag tube array system [51]; 2 PAO1 pil mutants acquired from Angus Buckling, University of Exeter. 3Isolates classified as anomalous following negative diagnostic PCR result for one of two specific target sequences, but identified as LES using the tube array system. These isolates were also missing one or more LES prophage. 4 Strains isolated from Keratitis patients from several hospitals across the UK. 5 AHCH: Isolates collected from child CF patients attending the Alder-Hey Children’s Hospital, Liverpool. 6 RLUH: Isolates collected from adult CF patients attending the Royal Liverpool University Hospital. 7 RJ -Environmental isolates of several Pseudomonas species donated by R Jackson, University of Reading.
Bacteriophage induction
P. aeruginosa LESB58 was grown to mid-exponential phase (OD600 0.5) and LES phages were induced into the lytic cycle by exposure to the minimum inhibitory concentration of norfloxacin (50 μg ml-1) for 1 h [24]. Induced cultures were sub-cultured (1:10) into fresh LB to enable recovery for 2 h before filtration (0.2 μm Millipore). Active phage particles in the induced supernatants were enumerated by standard plaque assay using PAO1 host cells.
Bacteriophage assays
LES phages were isolated from induced LESB58 cultures using plaque assays with PAO1 host cultures as described previously [24]. Phages were purified by picking individual plaques that were suspended in LB (1 ml), filter sterilized (0.2 μm Millipore) and used in a second plaque assay with PAO1. This process was repeated twice to ensure purity. Phage purity was confirmed using PCR assays. Amplification of phage stocks was achieved by modifying previous methods [53]. Briefly, mid-exponential phase PAO1 cultures (100 ml) were infected with purified LES phage (MOI = 0.1), at 37°C for 2 h. Lysed cultures were filter-sterilized.
Electron microscopy
Phage suspensions (1x109 – 1x1010 p.f.u. ml-1) were concentrated by centrifugation, negatively stained with 2% (w/v) uranyl acetate [54], and examined by transmission electron microscopy (magnification x 200,000).
Multiplex PCR to confirm pure phage stocks and lysogens
Three primer sets, LESnest1 F/R, Clust6nest F/R and 4tot1 F/R (Table 4), for the detection of LES phages 2, 3 and 4 respectively, were combined in a multiplex PCR assay for confirmation of each pure phage stock and each PLPL. Colony or filtered phage suspensions were used as templates in each reaction as described previously [25].
Table 4.
Primer sequences
| Primer | Sequence (5′-3′) | Amplicon (bp) | Cycling conditions | Reference |
|---|---|---|---|---|
| Multiplex PCR: | ||||
| LES1nestF | tttggtgatgatcggcttagc | 289 | 95°C, 4 min then 30 cycles: 95°C, 30 s; 58°C, 30 s; 72°C, 30 s; final extension step, 72°C, 7 min; | [25] |
| LES1nestR | tgtggaagcgatcagtct | |||
| Clust6nestF | ggatcgacgtggcataatctg | 410 | [25] | |
| Clust6nestR | acgattctccggcatgcagcg | |||
| 4tot1F | gctcatgagtggctgacaac | 105 | This study | |
| 4tot1R | tcttgggcagagaaccattc | |||
| Q-PCR: | ||||
| 2pro3F | caagccctgtctggattttc | 102 | 95°C, 10 min; then 40 cycles: 95°C, 10 s; 60°C, 15 s; 72°C s. | This study |
| 2pro3R | gagacaggttgggagggagt | |||
| 3tot1F | cgcaggtaccaccagacttt | 122 | This study | |
| 3tot1R | catgtccagcaggttcaaaa | |||
| 3pro3F | gcggatgttctcaaacgaat | 134 | This study | |
| 3pro3R | cgggagaagcaatgacctac | |||
| 4tot1F | gctcatgagtggctgacaac | 105 | This study | |
| 4tot1R | tcttgggcagagaaccattc | |||
| 4pro3F | tcgtgctgtgctgatctttt | 172 | This study | |
| 4pro3R | agcagtgccagttgatgttg | |||
| Preparation of DIG-labeled probes: | ||||
| φ2intDIGF | tgcctatctaacggggttca | 1097 | 95°C, 4 min. 30 cycles: 95°C, 30 s; 55°C, 30 s; 72°C, 1 min s; final extension step, 72°C, 7 min | This study |
| φ2intDIGR | gaagcaaccgagaagtggag | |||
| φ3intDIGF | ggatcatgtagcgggaaaga | 874 | This study | |
| φ3intDIGR | agaacctggcgaaagtctga | |||
| φ4cIDIGF | atcgttaattggcacggaat | 893 | This study | |
| φ4cIDIGR | acagcaacggatttccactc | |||
tot = to quantify total phage copies; pro = to quantify total phage copies.
Quantifying production of each LES phage from LESB58
Replication of each LES phage in response to induction of the lytic cycle was compared using Q-PCR to distinguish and enumerate each specific phage type. LESB58 induction experiments were performed on three separate occasions in the presence and absence of norfloxacin for 30 and 60 min exposure times before the 2 h recovery step. DNA was prepared from each replicate using the Bacterial and Virus DNA extraction kit (QIAGEN) and the automated QIAsymphony machine (QIAGEN; pathogen complex 200 protocol). Q-PCR was performed using six specific primer sets to differentiate between prophage and total copies of each phage.
Real-time Q-PCR
Q-PCR was used to quantify LES phages by comparing the number of specific amplicon copies in extracted DNA from induction experiments to a concentration gradient of known standards. Primer sets with the prefixes, “tot” (total) and “pro” (prophage) were designed to amplify unique regions within, and flanking, each LES phage genome (Figure 1D). All primer sequences and amplification details are listed in Table 4. Amplicon copy number μl-1 was calculated using the formula [(6.023 x 1023 x [DNA] g/ml)/(molecular weight of product)]/1,000 [55]. Molecular weight was calculated as number of base pairs x 6.58 x 102 g. A 10-fold dilution series of each DNA standard was prepared for quantification of phage numbers in each sample.
Q-PCR reactions (25 ul) contained 1 uM each primer pair and 1X Rotorgene-SYBR green supermix (QIAGEN). Phage numbers were quantified from DNA samples (1 μl) in triplicate using a Rotorgene cycler (QIAGEN). Q-PCR data were analyzed using Rotorgene Q series software 1.7 (QIAGEN). Total phage and prophage numbers from each sample were quantified in separate reactions using “tot” and “pro” primer sets for each phage and comparing fluorescent signals to those from standard concentration gradients. The level of free phage in a given sample was calculated by subtracting prophage numbers from total phage numbers.
Statistical analysis
Specific phage sequences were quantified in triplicate from each of 3 experimental replicates using Q-PCR, and technical replicates were averaged prior to analyses. Differences in phage numbers, with and without norfloxacin and between time-points were analysed using separate ANOVAs for each phage, fitting induction (2 levels), time (2 levels) and their interaction as fixed factors.
Isolation of PAO1 lysogens
PAO1 LES phage lysogens (PLPLs) were isolated from turbid islands in the centre of well-separated plaques using a sterile toothpick and streaked on to Columbia agar (Oxoid) to obtain single colonies. Individual lysogen colonies were analysed by multiplex PCR assays to confirm the presence of LES prophages.
Immunity assays
Lawns of PAO1 and each PLPL were created by adding mid-exponential phase (OD600 0.5) cultures (100 ul) to molten 0.4% (v/v) agar and pouring onto Columbia agar plates to set. A 10-fold dilution series of each purified phage suspension (1010 – 103 p.f.u ml-1) was spotted (20 ul) onto lawns of each host. Countable plaques were observed at varying dilutions depending on the phage-host combination. The efficiency of plating (eop) value was calculated as the ratio of assay titre/most permissive titre. The most permissive titre was obtained on non-lysogenic PAO1.
Southern blot analysis
Southern analysis was performed as previously described [56]. Specific probes were prepared using the digoxigenin (DIG) PCR labelling kit (Roche). DIG-labelled probes were designed to hybridize targets within either the LESφ2 int gene, the LESφ3 int gene, or the LESφ4 cI gene using primers: φ2intDIG F/R; φ3intDIG F/R and φ4cIDIG F/R (Table 4). Bacterial genomic DNA was extracted using a Wizard Genomic DNA extraction kit (Promega) and digested using PstI, AcuI or DraIII (NEB) according to the manufacturer’s instructions. Probes were hybridised to digested genomic DNA as described previously [53]. Hybridized probe was detected using alkaline phosphatase-conjugated anti-DIG antibody (1:10,000) and CPDstar substrate (1:100) (Roche) according to the manufacturer’s instructions.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CJ designed the study; carried out the purification and characterisation of the LES phages and rates of induction and drafted the manuscript. JL carried out initial induction of the phages from the native host. HK and CJ carried out the host range study. AH clone-typed each clinical P. aeruginosa isolate. JC prepared samples for electron microscopy of LESφ2 and LESφ3. MB and CW jointly conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chloe E James, Email: cejames@liv.ac.uk.
Joanne L Fothergill, Email: jofoth@liv.ac.uk.
Amanda J Hall, Email: mandyh@liv.ac.uk.
Jennifer Cottell, Email: jlc882@bham.ac.uk.
Michael A Brockhurst, Email: michael.brockhurst@york.ac.uk.
Craig Winstanley, Email: C.Winstanley@liv.ac.uk.
Acknowledgements
This work was supported by the Wellcome Trust (089215/Z/09/Z).
Thanks to Brian Getty (Institute of Infection and Global Health, University of Liverpool) for performing the electron microscopy; Dr Heather Allison for helpful discussions and to Professor Angus Buckling and Dr Rob Jackson for kindly supplying pil mutants and environmental Pseudomonas strains respectively.
References
- Hardalo C, Edberg SC. Pseudomonas aeruginosa: assessment of risk from drinking water. Crit Rev Microbiol. 1997;23:47–75. doi: 10.3109/10408419709115130. [DOI] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M. et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser S, Mabrouk A, Maher A. Colonization of burn wounds in Ain Shams University Burn Unit. Burns. 2003;29:229–233. doi: 10.1016/S0305-4179(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Chitkara YK, Feierabend TC. Endogenous and exogenous infection with Pseudomonas aeruginosa in a burns unit. Int Surg. 1981;66:237–240. [PubMed] [Google Scholar]
- Hutchison ML, Govan JR. Pathogenicity of microbes associated with cystic fibrosis. Microbes Infect. 1999;1:1005–1014. doi: 10.1016/S1286-4579(99)80518-8. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- Hassett DJ, Korfhagen TR, Irvin RT, Schurr MJ, Sauer K, Lau GW, Sutton MD, Yu H, Hoiby N. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin Ther Targets. 2010;14:117–130. doi: 10.1517/14728220903454988. [DOI] [PubMed] [Google Scholar]
- Fothergill JL, Walshaw MJ, Winstanley C. Transmissible strains of Pseudomonas aeruginosa in Cystic Fibrosis lung infections. Eur Respir J. 2012;40:227–238. doi: 10.1183/09031936.00204411. [DOI] [PubMed] [Google Scholar]
- Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, Heaf DP, van Saene H, Hart CA. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet. 1996;348:639–642. doi: 10.1016/S0140-6736(96)05169-0. [DOI] [PubMed] [Google Scholar]
- McCallum SJ, Corkill J, Gallagher M, Ledson MJ, Hart CA, Walshaw MJ. Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P aeruginosa. Lancet. 2001;358:558–560. doi: 10.1016/S0140-6736(01)05715-4. [DOI] [PubMed] [Google Scholar]
- Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax. 2004;59:334–336. doi: 10.1136/thx.2003.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagea S, Winstanley C, Parsons YN, Walshaw MJ, Ledson MJ, Hart CA. PCR-based detection of a cystic fibrosis epidemic strain of Pseudomonas aeruginosa. Mol Diagn. 2003;7:195–200. doi: 10.2165/00066982-200307030-00009. [DOI] [PubMed] [Google Scholar]
- Scott FW, Pitt TL. Identification and characterization of transmissible Pseudomonas aeruginosa strains in cystic fibrosis patients in England and Wales. J Med Microbiol. 2004;53:609–615. doi: 10.1099/jmm.0.45620-0. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Ramotar K, Giesbrecht-Lewis T, Tullis E, Freitag A, Paterson N, Jackson M, Lougheed MD, Dowson C. et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA. 2010;304:2145–2153. doi: 10.1001/jama.2010.1665. [DOI] [PubMed] [Google Scholar]
- Winstanley C, Langille MG, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F, Thomson NR, Winsor GL, Quail MA, Lennard N. et al. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome Res. 2009;19:12–23. doi: 10.1101/gr.086082.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan T, Liu J, Dubow M, Gros P, Pelletier J. Comparative genomic analysis of 18 Pseudomonas aeruginosa bacteriophages. J Bacteriol. 2006;188:1184–1187. doi: 10.1128/JB.188.3.1184-1187.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzio J, Kropinski AM. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J Bacteriol. 1983;155:203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmat S, Shapiro JA. Insertion and replication of the Pseudomonas aeruginosa mutator phage D3112. Mol Gen Genet. 1983;192:416–423. doi: 10.1007/BF00392184. [DOI] [PubMed] [Google Scholar]
- Ceyssens PJ, Lavigne R. Bacteriophages of Pseudomonas. Future Microbiol. 2010;5:1041–1055. doi: 10.2217/fmb.10.66. [DOI] [PubMed] [Google Scholar]
- Holloway BW, Cooper GN. Lysogenic conversion in Pseudomonas aeruginosa. J Bacteriol. 1962;84:1321–1324. doi: 10.1128/jb.84.6.1321-1324.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Baba T, Matsumoto H, Terawaki Y. Phage-conversion of cytotoxin production in Pseudomonas aeruginosa. Mol Microbiol. 1990;4:1703–1709. doi: 10.1111/j.1365-2958.1990.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Tay M, Hauser A, McDougald D, Webb JS, Kjelleberg S. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 2009;3:271–282. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill JL, Mowat E, Walshaw MJ, Ledson MJ, James CE, Winstanley C. Effect of antibiotic treatment on bacteriophage production by a cystic fibrosis epidemic strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2011;55:426–428. doi: 10.1128/AAC.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill JL, Mowat E, Ledson MJ, Walshaw MJ, Winstanley C. Fluctuations in phenotypes and genotypes within populations of Pseudomonas aeruginosa in the cystic fibrosis lung during pulmonary exacerbations. J Med Microbiol. 2010;59:472–481. doi: 10.1099/jmm.0.015875-0. [DOI] [PubMed] [Google Scholar]
- Ojeniyi B, Birch-Andersen A, Mansa B, Rosdahl VT, Hoiby N. Morphology of Pseudomonas aeruginosa phages from the sputum of cystic fibrosis patients and from the phage typing set. An electron microscopy study. APMIS. 1991;99:925–930. doi: 10.1111/j.1699-0463.1991.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, Morales G, Kohler T, van Delden C, Weinel C, Slickers P, Tummler B. Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RV, Pemberton JM, Clark AJ. Prophage F116: evidence for extrachromosomal location in Pseudomonas aeruginosa strain PAO. J Virol. 1977;22:844–847. doi: 10.1128/jvi.22.3.844-847.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refardt D. Within-host competition determines reproductive success of temperate bacteriophages. ISME J. 2011;5:1451–1460. doi: 10.1038/ismej.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess H, Kamp D, Kahmann R, Brauer B, Delius H. Nucleotide sequence of the immunity region of bacteriophage Mu. Mol Gen Genet. 1982;186:315–321. doi: 10.1007/BF00729448. [DOI] [PubMed] [Google Scholar]
- Berngruber TW, Weissing FJ, Gandon S. Inhibition of superinfection and the evolution of viral latency. J Virol. 2010;84:10200–10208. doi: 10.1128/JVI.00865-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanvliet F, Couturier M, Desmet L, Faelen M, Toussaint A. Virulent Mutants of Temperate Phage-Mu-1. Mol Gen Genet. 1978;160:195–202. doi: 10.1007/BF00267481. [DOI] [Google Scholar]
- Benzer S. Fine Structure of a Genetic Region in Bacteriophage. Proc Natl Acad Sci U S A. 1955;41:344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind MM, Botstein D, Wright A. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. III. Failure of superinfecting phage DNA to enter sieA+ lysogens. Virology. 1974;62:350–366. doi: 10.1016/0042-6822(74)90398-5. [DOI] [PubMed] [Google Scholar]
- Susskind MM, Botstein D. Superinfection exclusion by lambda prophage in lysogens of Salmonella typhimurium. Virology. 1980;100:212–216. doi: 10.1016/0042-6822(80)90571-1. [DOI] [PubMed] [Google Scholar]
- Susskind MM, Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978;42:385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YJ, Chung IY, Choi KB, Lau GW, Cho YH. Genome sequence comparison and superinfection between two related Pseudomonas aeruginosa phages, D3112 and MP22. Microbiology. 2007;153:2885–2895. doi: 10.1099/mic.0.2007/007260-0. [DOI] [PubMed] [Google Scholar]
- Brown SP, Le Chat L, De Paepe M, Taddei F. Ecology of microbial invasions: amplification allows virus carriers to invade more rapidly when rare. Curr Biol. 2006;16:2048–2052. doi: 10.1016/j.cub.2006.08.089. [DOI] [PubMed] [Google Scholar]
- Irvin RT, Doig P, Lee KK, Sastry PA, Paranchych W, Todd T, Hodges RS. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect Immun. 1989;57:3720–3726. doi: 10.1128/iai.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Mattick JS. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol Microbiol. 1994;13:1079–1091. doi: 10.1111/j.1365-2958.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Brockhurst MA, Buckling A, Rainey PB. The effect of a bacteriophage on diversification of the opportunistic bacterial pathogen, Pseudomonas aeruginosa. Proc Biol Sci. 2005;272:1385–1391. doi: 10.1098/rspb.2005.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibeu A, Ceyssens PJ, Hertveldt K, Volckaert G, Cornelis P, Matthijs S, Lavigne R. The adsorption of Pseudomonas aeruginosa bacteriophage phiKMV is dependent on expression regulation of type IV pili genes. FEMS Microbiol Lett. 2009;296:210–218. doi: 10.1111/j.1574-6968.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- Mowat E, Paterson S, Fothergill JL, Wright EA, Ledson MJ, Walshaw MJ, Brockhurst MA, Winstanley C. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Respir Crit Care Med. 2011;183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- Taylor TB, Buckling A. Competition and dispersal in Pseudomonas aeruginosa. Am Nat. 2010;176:83–89. doi: 10.1086/652995. [DOI] [PubMed] [Google Scholar]
- Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- Salunkhe P, Smart CH, Morgan JA, Panagea S, Walshaw MJ, Hart CA, Geffers R, Tummler B, Winstanley C. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J Bacteriol. 2005;187:4908–4920. doi: 10.1128/JB.187.14.4908-4920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill JL, White J, Foweraker JE, Walshaw MJ, Ledson MJ, Mahenthiralingam E, Winstanley C. Impact of Pseudomonas aeruginosa genomic instability on the application of typing methods for chronic cystic fibrosis infections. J Clin Microbiol. 2010;48:2053–2059. doi: 10.1128/JCM.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RM, Wiehlmann L, Ashelford KE, Preston SJ, Frimmersdorf E, Campbell BJ, Neal TJ, Hall N, Tuft S, Kaye SB, Winstanley C. Genetic characterization indicates that a specific subpopulation of Pseudomonas aeruginosa is associated with keratitis infections. J Clin Microbiol. 2011;49:993–1003. doi: 10.1128/JCM.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahenthiralingam E, Coenye T, Chung JW, Speert DP, Govan JR, Taylor P, Vandamme P. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J Clin Microbiol. 2000;38:910–913. doi: 10.1128/jcm.38.2.910-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison HE, Sergeant MJ, James CE, Saunders JR, Smith DL, Sharp RJ, Marks TS, McCarthy AJ. Immunity profiles of wild-type and recombinant shiga-like toxin-encoding bacteriophages and characterization of novel double lysogens. Infect Immun. 2003;71:3409–3418. doi: 10.1128/IAI.71.6.3409-3418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann H-W, Heldal M. In: Manual of Aquatic Viral Ecology. Wilhelm SW, Weinbauer MG, Suttle CA, Waco TX, editor. American Society of Limnology and Oceanography, Inc; 2010. Basic electron microscopy of aquatic viruses; pp. 182–192. [Google Scholar]
- Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, McCaughan GW, Eris JM, Bishop GA. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol. 2001;79:213–221. doi: 10.1046/j.1440-1711.2001.01002.x. [DOI] [PubMed] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/S0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]


