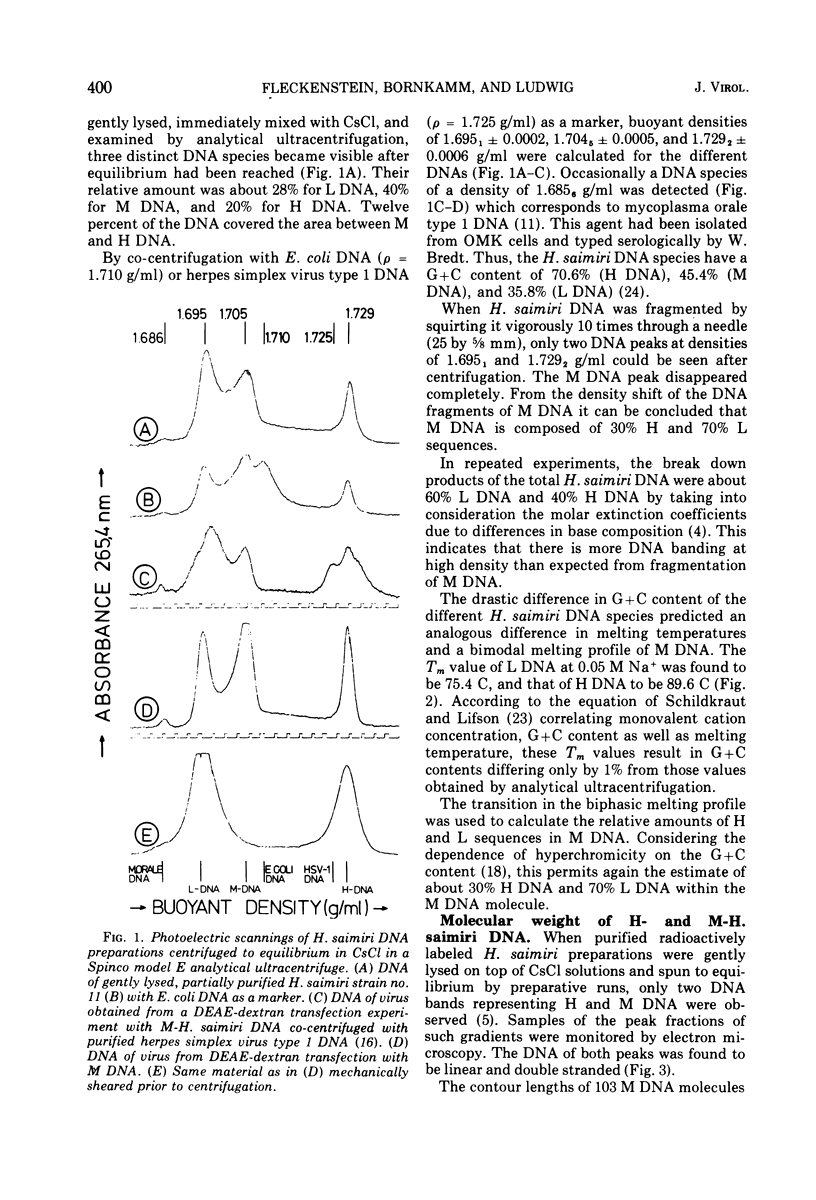
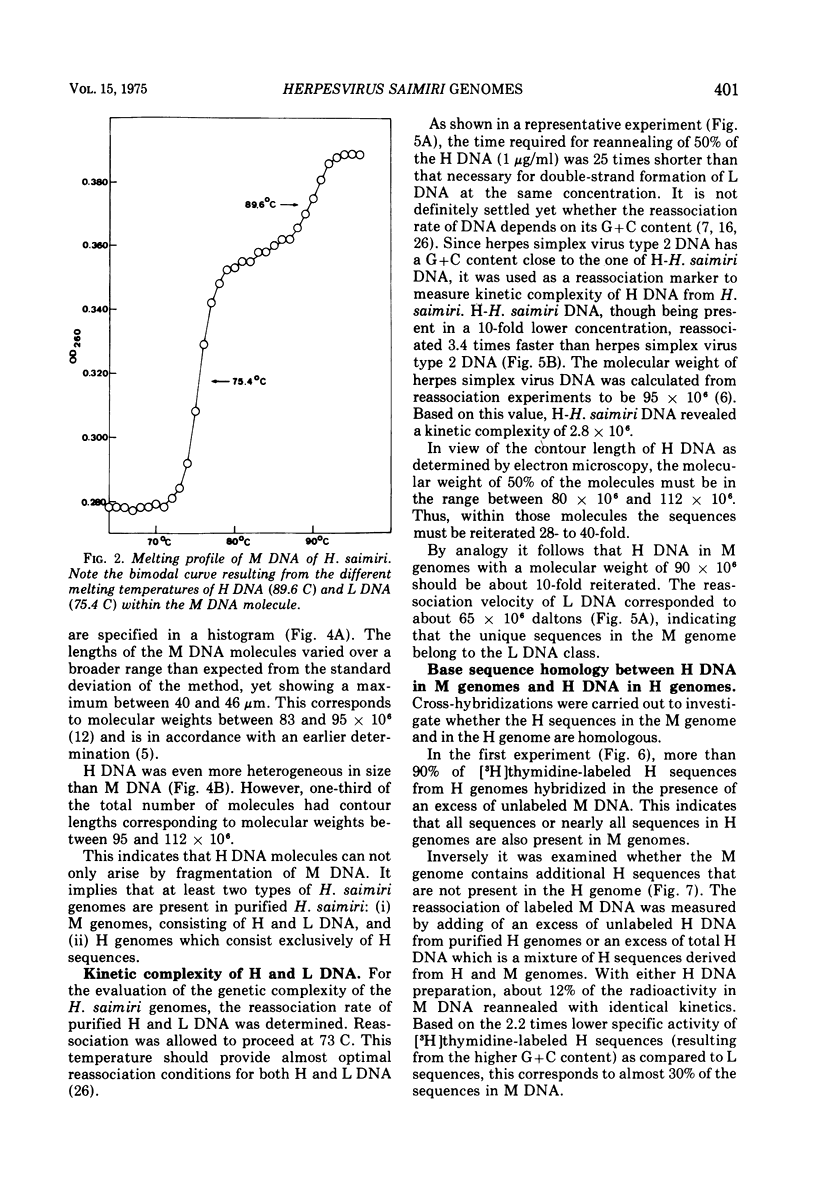
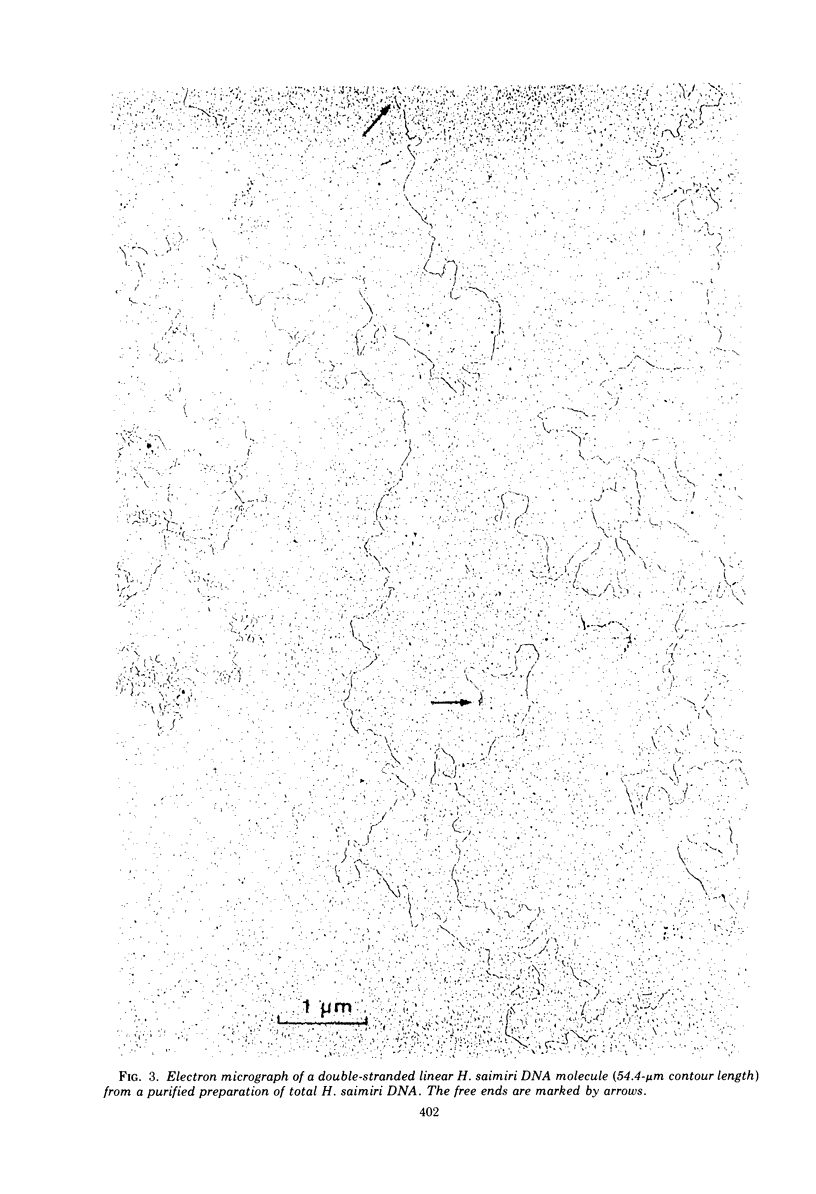
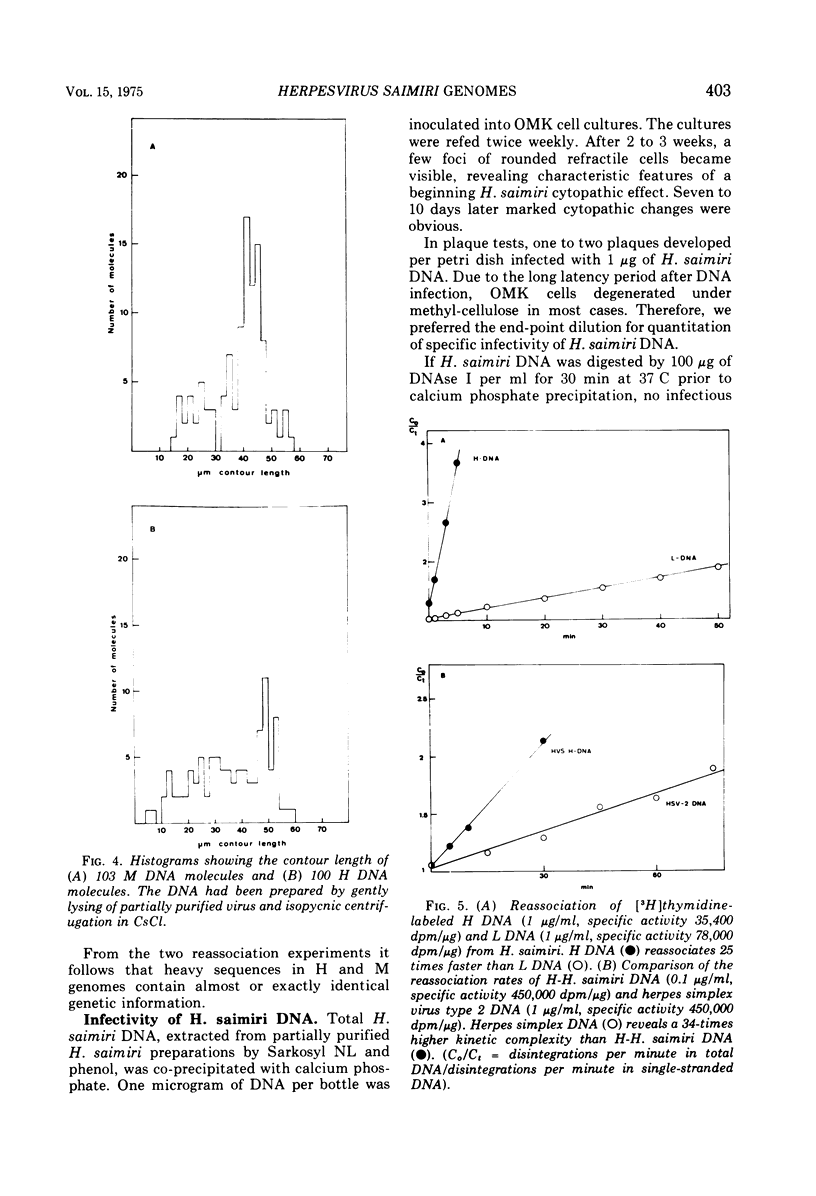
Abstract
Two types of Herpesvirus saimiri genomes can be isolated from purified virions: (i) the M genome is a double-stranded, liniear DNA molecule with a mean contour length corresponding to 89 times 10-6 daltons. The M genome contains about 70% of unique sequences (light DNA, 36% guanine plus cytosine) and 30% reiterated sequences (heavy DNA, 71% guanine plus cytosine). (ii) the H genome is composed of heavy DNA only and is more heterogeneous in size. The sequences in the H genome are up to 40-fold reiterated, indicating defectiveness of this type of genome. The repetitions in the H genome and the M genome cross-hybridize almost completely and have identical kinetic complexity (2.8 times 10-6 daltons). DNA infectivity studies by using the calcium phosphate and the DEAE-dextran method gave further evidence that H genomes are defective: no infectious virus was recovered from permissive cells treated with heavy DNA, whereas M genome-infected cells developed cytopathic changes after 11 to 56 days. Defective H genomes were present in the progeny virus two passages after transfection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Loeb W. F., Valerio M. G., Adamson R. H., Armstrong G. R., Bennett D. G., Heine U. Malignant lymphoma with lymphocytic leukemia induced in owl monkeys by Herpesvirus saimiri. J Natl Cancer Inst. 1971 Oct;47(4):837–855. [PubMed] [Google Scholar]
- Fareed G. C., Khoury G., Salzman N. P. Self-annealing of 4 S strands from replicating simian virus 40 DNA. J Mol Biol. 1973 Jul 5;77(3):457–462. doi: 10.1016/0022-2836(73)90451-8. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Herpes vimplex virus: genome size and redundancy studied by renaturation kinetics. J Virol. 1971 Oct;8(4):591–593. doi: 10.1128/jvi.8.4.591-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Veldhuisen G., Wilkie N. M. Infectious herpesvirus DNA. Nat New Biol. 1973 Oct 31;245(148):265–266. doi: 10.1038/newbio245265a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton W. H., Mandel M. Deoxyribonucleic acid base compositions of mycoplasma strains of avian orgin. J Gen Microbiol. 1969 May;56(2):131–135. doi: 10.1099/00221287-56-2-131. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Malignant lymphoma induced by partially purified Herpesvirus saimiri and recovery of infectious virus from tumorous lymph nodes. Med Microbiol Immunol. 1972;158(2):135–146. doi: 10.1007/BF02120479. [DOI] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Susceptibility to Herpesvirus saimiri and antibody development in old and new world monkeys. Med Microbiol Immunol. 1973 Mar 8;158(3):227–236. doi: 10.1007/BF02120558. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Wetmur J. G. On the kinetics of helix formation between complementary ribohomopolymers and deoxyribohomopolymers. Biopolymers. 1972;11(7):1485–1497. doi: 10.1002/bip.1972.360110712. [DOI] [PubMed] [Google Scholar]
- Ludwig H. Untersuchungen am genetischen Material von Herpesviren. I. Biophysikalisch-chemische Charakterisierung von Herpesvirus=Desoxyribonucleinsäuren. Med Microbiol Immunol. 1972;157(3):186–211. doi: 10.1007/BF02121161. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., KLINE B., MEHROTRA B. D. SOME OBSERVATIONS ON THE HYPOCHROMISM OF DNA. J Mol Biol. 1964 Sep;9:801–811. doi: 10.1016/s0022-2836(64)80186-8. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Henson D., Bowling C. P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology. 1969 Sep;39(1):134–137. doi: 10.1016/0042-6822(69)90355-9. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Laithier M., Lando D., Ryhiner M. L. Infectious DNA from herpes simplex virus: infectivity of double-stranded and single-stranded molecules. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3621–3625. doi: 10.1073/pnas.70.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Falk L. A., Deinhardt F. Oncogenicity of herpesvirus saimiri in marmoset monkeys. J Natl Cancer Inst. 1971 Nov;47(5):1145–1162. [PubMed] [Google Scholar]




