Abstract
The study aimed to understand the inter-annual variations of methane (CH4) emissions from an open fen on the Qinghai-Tibetan Plateau (QTP) from 2005 to 2007. The weighted mean CH4 emission rate was 8.37±11.32 mg CH4 m−2 h−1 during the summers from 2005 to 2007, falling in the range of CH4 fluxes reported by other studies, with significant inter-annual and spatial variations. The CH4 emissions of the year of 2006 (2.11±3.48 mg CH4 m−2 h−1) were 82% lower than the mean value of the years 2005 and 2007 (13.91±17.80 mg CH4 m−2 h−1 and 9.44±14.32 mg CH4 m−2 h−1, respectively), responding to the inter-annual changes of standing water depths during the growing season of the three years. Significant drawdown of standing water depth is believed to cause such significant reduction in CH4 emissions from wetlands in the year 2006, probably through changing the methanogen composition and decreasing its community size as well as activating methanotrophs to enhance CH4 oxidation. Our results are helpful to understand the inter-annual variations of CH4 emission and provide a more reasonable regional budget of CH4 emission from wetlands on the QTP and even for world-wide natural wetlands under climate change.
Introduction
Methane (CH4) is an important greenhouse gas, about 25 times more powerful in warming the atmosphere than carbon dioxide (CO2) for the time horizon of 100 years [1]. In particular, CH4 emissions have a larger impact on the climate than what was claimed in current carbon-trading schemes or in the Kyoto Protocol, which modified its radiative forcing as +0.48 W m−2 [2]. Given its atmospheric concentration, CH4 is regarded as an important greenhouse gas only second to CO2.
Due to the prevalence of waterlogged and anoxic conditions, wetlands are the largest natural source for atmospheric CH4 emission, about 148 Tg CH4 yr−1 (1 Tg = 1012 g) from natural wetlands [1], [3], contributing over 25% of the global CH4 emission to the atmosphere [4]. Moreover, wetlands represent not only one of the most important sources for methane emission, but also the most uncertain one. Such uncertainty arises primarily from the large spatiotemporal variation that occurs in different scales and the limited data of specific wetlands [1], [5]. Therefore, we need to fill into place the jigsaw pieces of data on specific wetlands from different regions, if we want to get the whole picture of CH4 emission from wetlands.
The Qinghai-Tibetan Plateau (QTP) is the largest and highest plateau in the world with an area of 2.5 million square kilometers. There are many lakes and wetlands on the plateau, with about 50% of wetlands and 51% of lakes of China unevenly distributed here [6]. On the eastern edge of QTP, there is the largest highland wetland in the world, Zoige alpine wetlands [7], which is, for its high altitude, a very important and sensitive area for climatic change [8], as well as hotspots for biodiversity in the world [9]. Though there are several studies about CH4 emission from wetlands on the plateau [10], [11], [12], [13], [14], [15], [16], these studies only discussed short-term variations of CH4 emission, not including inter-annual variation of CH4 emission and their determinants.
Wetlands on the QTP are sensitive to climate change and the plateau has experienced abrupt climate change [17]. In the past decades, trends of precipitation showed an overall slight increase with high inter-annual variations at the whole-plateau scale [18], [19]. This is also true for our study area. During the past 50 years, we observed a slight increase trend in the annual precipitation with high inter-annual variations. During our growing season measurements from 2005 to 2007, we encountered a dry year (2006) compared with the annual precipitation average during the period from 1957 to 2007. Moreover, in our study the chosen open fen was usually seasonally flooded, thus having obvious seasonal and inter-annual dynamics of standing water depths. This made an opportunity for us to test if CH4 emissions were significantly variable annually and if standing water depths were the dominant factor on inter-annual variations of CH4 emissions.
Materials and Methods
Ethics Statement
Our field studies were approved by Bureau of National Nature Reserve of Zoige Wetland. The study was observational, involving no cruelty to animals, no damage to habitats and no harm to endangered plants, and thus no review from the ethnic committee was required in China. All the work was carried out under the Wildlife Protection Law of the People's Republic of China.
Site Description
The investigations were carried out in an alpine wetland of National Nature Reserve of Zoige Wetland (33°56′N,102°52′E, 3430 m a.s.l.), located on the northeast edge of the QTP. Zoige wetlands is on the Ramsar List of Wetlands of International Importance (2008), with ubiquitous alpine wetlands on the plateau formed during the Early Holocene (9355±115 BP) [20]. The region is characterized by cold Qinghai-Tibetan climatic conditions with average annual precipitation 645±92 mm and temperature 274.21±273.75 K from 1957 to 2007 (Fig. 1a).
Figure 1. Weather conditions of Zoige.
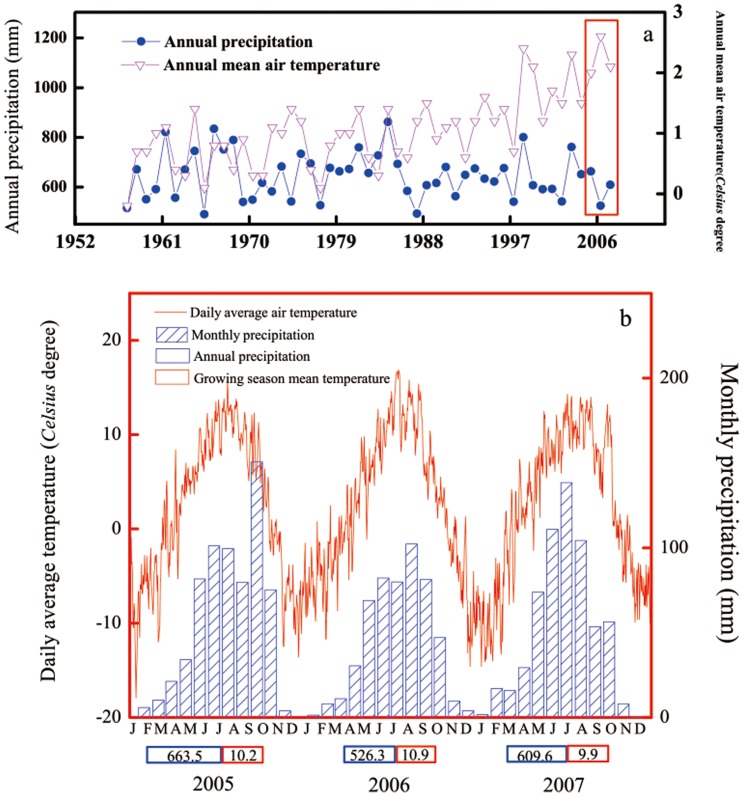
a. Annual air temperature and precipitation from 1957 to 2007 of Zoige County; b. Daily air temperature and precipitation from 2005 to 2007 in the study area.
A typical open fen was chosen in this study, which is about 28% of Zoige wetlands, covering an area of 7.08×105 hm2 [7]. The fen is consisted of three stands, including Kobresia tibetica on the hummock (covering about 40% of the whole site), which is almost never flooded, emergent Carex muliensis and Eleocharis valleculosa stands in the hollow (covering about 25% and 35%, respectively), which are usually flooded with some sporadically drainages. Due to warming and hydrological dynamics, this fen is usually confronted with water table drawdowns in the mid-summer, especially for dry years.
Weather and Soil Physical Characteristics
Local weather data were obtained from China Meteorological Data Sharing Service System (http://www.cma.gov.cn/2011qxfw/2011qsjgx/index.htm) from 1957 to 2007. During the monthly measurement of methane flux, air temperatures were also recorded.
Redox potentials and temperatures (soil and water) were taken with a portable digital meter (EcoScan pH6, Eutech Instruments Pte Ltd, Singapore). Water temperatures, ground surface temperatures and soil temperatures at the depth of 5 cm and 10 cm were manually recorded for each of the 18 plots. Standing water depths in the growing season were recorded with a ruler.
Sampling Plots Establishment and CH4 Flux Measurement
Eighteen plots in the study site were established for the consecutive three growing seasons (July to September) from 2005 to 2007. Among the 18 plots, six were for K. tibetica stand, six for C. muliensis stand and six for E. valleculosa stand.
In the three years, we took monthly measurements from July to September. The CH4 emission was measured with vented static chambers [21], [22]. The chambers (30 cm in diameter, 50 cm in height) were made of cylindrical polyvinyl chloride (PVC) pipe. Details about the chambers were described in reference [12].
Four air samples from each chamber were taken at 10-minute intervals over a 30 minute period after enclosure, stored in 5 ml air-tight vacuumed vials. The CH4 concentration was determined by a gas chromatography (PE Clarus 500, PerkinElmer, Inc., USA), equipped with a FID (flame ionization detector), operating at 350°C and a 2 m Porapak 80–100 Q Column. The column oven temperature was 35°C and the carrier gas was N2 with a flow rate of 30 cm3 min–1.
The flux J of CH4 was calculated as:
Where 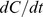 is the rate of concentration change; M is the molar mass of CH4; P is the atmosphere pressure of the sampling site; T is the absolute temperature of the sampling time; V0, P0, T0 is the molar volume (22.4 L mol−1), atmosphere pressure (101.325 kPa), and absolute temperature (273.15 K), respectively, under the standard condition; H is the chamber height over the water surface.
is the rate of concentration change; M is the molar mass of CH4; P is the atmosphere pressure of the sampling site; T is the absolute temperature of the sampling time; V0, P0, T0 is the molar volume (22.4 L mol−1), atmosphere pressure (101.325 kPa), and absolute temperature (273.15 K), respectively, under the standard condition; H is the chamber height over the water surface.
Calculation and Statistical Analysis
Mean CH4 emission, surface and soil temperature, Eh, and standing water depth for each stand type were calculated by averaging the replicates for each sampling date. A full general linear model in which stand and year were treated as fixed factors was used to compare the differences of environmental factors and CH4 emission in the three summers, and to assess the significance of the impacts of stand, year, and the combined effect of the two on CH4 emission and environmental factors. Multiple analysis of variance (MANOVA) was used to compare averages of CH4 emission for each stand of each sampling date and averages of CH4 emission for all stands in each year. The CH4 emissions were related to environmental variables by Pearson correlation analysis in each year. The effect of a certain variable was considered statistically significant for P<0.05. The above analyses were performed with the SPSS 11.5 for Windows.
Results
Variation in Air Temperature, Precipitation and Standing Water Depths
From the year 1957 to 2007, our study area showed a very obvious warming trend and a slight drying trend with significant inter-annual variations (P<0.01, Fig. 1a). During the past five decades, the average annual precipitation was 645±92 mm and the annual mean daily temperature was 1.06±0.6°C. For the experiment years (2005 through 2007) the annual mean precipitation and air temperature were 599 mm and 2.2°C (Fig. 1b). For each of the three years, the warmest month was July and the coldest month January. Also in all the three years more than 65% precipitation was distributed in the growing season (from June to September), about 431.1 mm in 2005, 354.7 mm in 2006 and 407.2 mm in 2007, with significantly less rainfall in 2006 than that in 2005 and 2007 (P<0.05). However, the growing season mean temperature was not significantly different among the three years (10.2°C in 2005, 10.9°C in 2006 and 9.9°C in 2007). During the three years, the lowest annual precipitation (526.3 mm) and the warmest mean daily air temperature (2.6°C) were recorded in 2006, a significantly drier and warmer year based on the annual averages of 1957 through 2007 (P<0.01).
During the summers of 2005 to 2007, standing water depths of the hollow stands (C. muliensis and E. valleculosa) varied markedly (Fig. 2). In the never-flooded hummock (K. tibetica stand), since water table was the height of hummocks from the surface of the standing water, it also varied greatly due to the dynamics of the standing water depths. Among the three stands, there were significant variations of standing water depths during the three summers (Table 1). However, standing water depths showed no significant difference between C. muliensis (6.8±3.7 cm) and E. valleculos stands (7.1±5.1 cm) except for that in July 2007. Moreover, the standing water depths of 2005 (10.6±4.5 cm) were significantly higher than that of 2006 (4.3±3.4 cm) and 2007 (6.0±2.7 cm), while there was no significant difference between the latter two.
Figure 2. Standing water depths of the hollow stands during the growing seasons from 2005 to 2007.
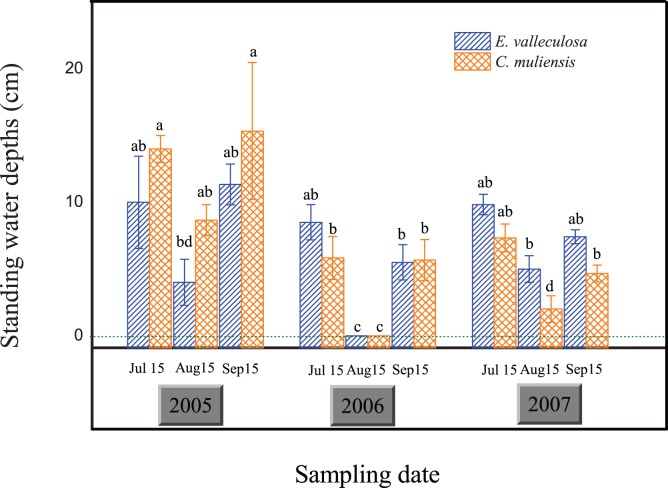
Different letters indicate significant difference (P<0.05).
Table 1. Significance of impacts of year, stand types and their combined effect on CH4 emission and environmental factors in growing season.
| Year | Stand types | The combined effect of year and stand | |
| CH4 emission | ** | ** | * |
| Surface temperature | ** | ns | ns |
| 5 cm temperature | ** | ns | ns |
| 10 cm soil temperature | ** | ns | ns |
| The standing water depth | * | ** | ** |
Significant impact P<0.05;
highly significant impact, P<0.01; ns, no significant impact.
CH4 fluxes from the Three Stands
We found different stands had different CH4 emission during the study period (Fig. 3). The CH4 emission (mean ± SD) from the open fen was about 8.68±14.33 mg CH4 m−2 h−1. The C. muliensis stand emitted CH4 at the highest rate, about 12.97±14.50 mg CH4 m−2 h−1. The K. tibetica stand emitted CH4 at the lowest rate, about 2.65±3.74 mg CH4 m−2 h−1, and the E. valleculosa stand emitted CH4 at an intermediate emission rate about 11.09±19.04 mg CH4 m−2 h−1. Comparing the three-year means of each stand, we also found that CH4 emission from C. muliensis and E. valleculosa stands was significantly higher than that from K. tibetica stand, with no significant difference between the former two (Fig. 3a). However, this trend was not the same for each year. For example, CH4 emission from C. muliensis and E. valleculosa stands was significantly higher than that from K. tibetica stand in the years 2005 and 2007, while there was no significant difference among the three stands in 2006 (Fig. 4).
Figure 3. Spatiotemporal variation of CH4 fluxes.
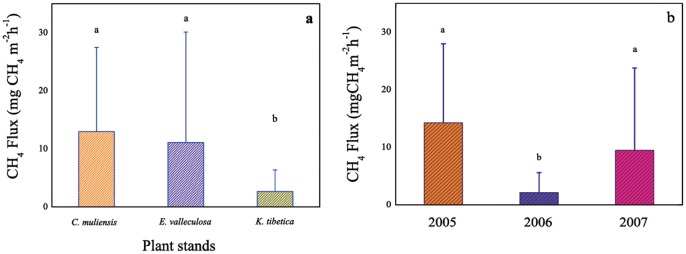
a. Mean CH4 fluxes in different stands during the growing seasons; b. Inter-annual variation of CH4 emission from the open fen of 2005 to 2007. Different letters indicate significant difference for each panel (P<0.05).
Figure 4. Seasonal variation of CH4 emission from the three plant stands from 2005 to 2007.
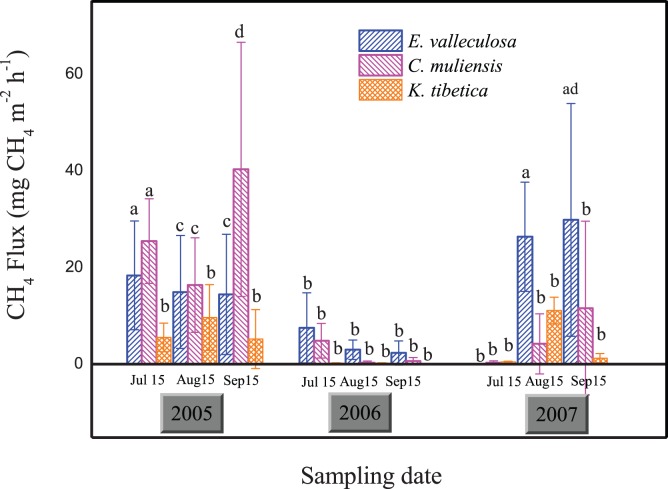
Different letters indicate significant difference (P<0.05).
Seasonal and Inter-annual Variations of CH4 Fluxes
In this study, we found CH4 emissions of the year of 2006 (2.11±3.48 mg CH4 m−2 h−1) were significantly lower than that of 2005 and 2007 (13.91±17.80 mg CH4 m−2 h−1 and 9.44±14.32 mg CH4 m−2 h−1, respectively), with no significant differences between 2005 and 2007 for all the three stands (Fig. 3b). The trend varied with stands. We observed significant inter-annual and seasonal variations of CH fluxes in C. muliensis and E. valleculosa stands of 2005 and 2007 (Table 1). There was no significant inter-annual or seasonal variation of CH4 emission in K. tibetica stand, with the emission rate markedly higher in 2005 and 2006 than in 2007 (Table 1).
Key Factors Controlling CH4 Fluxes
There were many factors influencing CH4 fluxes, including soil temperatures, soil redox potentials, standing water depth and the community height, etc (Table 2). However, in different years, they influenced CH4 fluxes differently. In the year of 2005, we found CH4 fluxes significantly related to surface temperature, soil redox potentials (at 5 cm, 10 cm and 15 cm soil depths), standing water depths and the plant community height. In the year of 2006, CH4 fluxes were related to soil redox potentials (at 10 cm and 15 cm soil depths), standing water depths and the plant community. In the year of 2007, CH4 fluxes were just related to soil temperatures (at 5 cm and 10 cm soil depths) and plant community heights. In different stands, the relations were also different during the three-year period. In the E. valleculosa stand, we found surface temperatures, soil temperatures (at 5 cm and 10 cm soil depths) and plant community heights significantly correlated to CH4 fluxes. In the C. muliensis stand, only standing water depths and plant community heights were significantly correlated with CH4 fluxes. In the K. tibetica stand, only soil temperatures (at 5 cm and 10 cm soil depths) and plant community heights were significantly correlated to CH4 fluxes.
Table 2. Significance of Pearson's rank correlations between CH4 emission and environmental factors.
| CH4 emission rates | ||||||
| 2005 | 2006 | 2007 | E.valleculosa | C.muliensis | K.tibetica | |
| Surfacetemperature | ** | ns | ns | * | ns | ns |
| 5 cm soiltemperature | ns | ns | * | * | ns | ** |
| 10 cm soiltemperature | ns | ns | ** | * | ns | * |
| 5 cm soil Eh | ** | ns | ns | ns | ns | nd |
| 10 cm soil Eh | ** | * | ns | ns | ns | nd |
| 15 cm soil Eh | ** | * | ns | ns | ns | nd |
| The Standingwater depth | ** | ** | ns | ns | ** | nd |
ns indicates the correlation is not significant.
indicates the correlation is significant (P<0.05);
indicates the correlation is highly significant (P<0.01); nd means no data.
Discussion
Comparisons with Other Studies on the QTP
The weighted mean CH4 emission rate was about 8.37±11.32 mg CH4 m−2 h−1 during the growing seasons from 2005 to 2007 with great inter-annual and spatial variations, falling in the range of CH4 fluxes during the growing seasons reported by other studies (summarized in Table 3). CH4 fluxes from wetlands show a significant spatial variations on the plateau [23], while their temporal variations are similar during the growing seasons [12], [13], [15]. With a large total area (ca. 1.33×105 km2) of wetlands on the QTP, the entire plateau is a source of CH4 in summer with high spatiotemporal variations [23]. Based on the distribution of wetlands, representative CH4 fluxes, and number of thaw days, a preliminary estimate is ca.0.7–0.9 Tg CH4 yr−1 emitting from wetlands on the plateau [15]. However, for a more reasonable estimate, we need a greater amount of observational field data at various temporal and spatial scales, hopefully through establishing a high-proficiency and long-term monitoring network in the future.
Table 3. Comparison with other studies about methane flux from wetlands on the Qinghai-Tibetan Plateau reported with the static chamber method.
| Location | Vegetation | Methane flux (mg CH4m-2 h-1 ) | Study period | Reference | ||
| In Zoige County | K. tibetica | 5.49±5.29 | Jun. to Sept.2005 to 2007 | This study | ||
| (33°56′N,102°52′E, a.s.l. 3430 m) | C. muliensis | 17.78±17.50 | ||||
| E. valleculosa | 13.35±12.27 | |||||
| In the Huashixia Region | Carex meadow | 0.41±0.79 | Apr. to Sept.1997 | [15] | ||
| (35°39′N, 98°48′E, a.s.l. 4300–4500 m) | Caltha scaposa | ±0.28 | ||||
| Hippuris vulgaris | 1.46±2.30 | |||||
| C. atrofusaskr | 3.00±4.25 | |||||
| In Hongyuan County | C. muliensis | 2.87 (0.51-8.20) | May to Sept.2001 | [16] | ||
| (32°47′N, 102°32′E, a.s.l. 3470 m) | C. meyeriana | 4.51 (0.36-10.04) | ||||
| In the Lanhaizhi wetland | Potamogeton | 1.38 | Jul. to Sept.2002 | [13] | ||
| (37°29′N, 101°12′E, a.s.l. 3250 m) | Hippuris | 8.92 | ||||
| Scirpus | 4.57 | |||||
| Carex | 8.19 | |||||
| In Haibei | Carex meadow | 0.80–1.41 | Jun. to Sept.2003 | |||
| (37°37′N, 101°19′E, a.s.l. 3280 m) | Carex and Hippuris | 2.91–16.25 | ||||
| In Zoige County (littoral wetlands) | K. tibetica | -0.1–26.3 | Jun. to Aug.2005 and 2006 | [10] | ||
| (33°56′N,102°52′E, a.s.l. 3430 m) | C. muliensis | -0.1–21.8 | ||||
| Non-vegetated | 0.1–3.8 | |||||
| H. vulgaris | 0.2–22.9 | |||||
| P. amphibium | 0.4–40.4 | |||||
| G. maxima | 12–90 | |||||
Role of Standing Water Depth in the Inter-annual Variations of CH4 Fluxes and its Implications for the Future
This study found that significant inter-annual and spatial variations of CH4 fluxes from the open fen (Table 1). In our previous studies and other related ones, standing water depths were regarded as the dominant factors controlling seasonal and spatial variations of CH4 fluxes [12], [24], [25]. Consistent with results from two Michigan peatlands [26], standing water tables were also the dominant factor influencing inter-annual variation of CH4 emissions, with their correlation relatively small but significant (r = 0.17, P<0.05). Furthermore, we noted that in the year 2006 with the thorough drainage (totally drying without standing water in the hollow) in July, CH4 fluxes from the open fen was 82% lower than the mean value of the years 2005 and 2007 (Fig. 3b). Similar to our study, a climate-induced drainage in summer was found to limit CH4 emission from newly created marshes [27]. In rice paddies, mid-season drainages also greatly reduced CH4 emissions [28], [29]. Consistent results were also found in stimulated drying experiments in wetlands [30], [31], [32], [33], [34]. Significant drawdown of water table position or standing water depth is believed to explain such significant reduction in CH4 emissions from the open fen in 2006 [34], [35], because more aerobic conditions enhanced CH4 oxidation and suppressed CH4 production and emission during the drawdown period [36], [37]. Also water table drawdown could have altered the structure of soil microbial communities related to methanogenesis and methanotrophs, which in turn limited CH4 production and enhanced CH4 oxidation [38]. In a very recent paper for the same studying site, we found methanogens community composition changed after the significant water table drawdown in 2006 and the community size was 10-time smaller in 2006 than that of 2007 [39]. The present study also showed that CH4 emission depended more on standing water depth in the relative dry year 2006 than 2005 and 2007, which were both relatively humid (Table 2). Furthermore, the water table drawdown in 2006 was also found to change the spatial patterns of CH4 emission among the three stands. In 2006, there were no significant variations of CH4 emission among the three stands; while in 2005 and 2007, CH4 emission was significantly higher in the flooded C. muliensis and E. valleculosa stands than in the dry K. tibetica stand [12]. This is partly because all three stands were dry in this year, and there was no significant variation of standing water depths among the hollow stands through significant drawdown of water tables (Fig. 2). For better understanding of CH4 dynamics of wetlands after significant water regime shifts, changes in soil microbial communities, vegetation cover and enzyme activities are the research priorities.
Although there is a slight wetting trend on the QTP with high inter-annual variations [19], [40], together with warming, wetlands on the plateau experienced great inter-annual changes and degraded during the last several decades [7], [41]. Such inter-annual dynamics in wetland area was believed to be the dominant cause of inter-annual variations in regional CH4 emissions from wetlands [42]. Moreover, inter-annual variations not only resulted in changes of wetland areas at large scales, but also in changes of water tables of specific wetlands. Therefore, inter-annual changes of water tables or hydrological processes should be another determinant of inter-annual variations of CH4 emissions from wetlands. In our study, the water table drawdown in 2006 may not only lead to reduction in wetland area but also to decreased CH4 emission rate from previous emission “hotspots” of CH4 [12], which could make the inter-annual variation of CH4 emission greater. Due to wetland degradation, the CH4 source strength of the entire QTP wetlands has declined during the past 50 years with highly inter-annual variations responding to highly inter-annual hydrological regime of wetlands under climate change [19]. As an example, sporadic drought events like that in 2006 may further decrease CH4 emission rate from typical wetlands on the plateau, making CH4 source of the entire plateau wetlands smaller and more variable. The results of the present research are meaningful to understanding the inter-annual variations of CH4 emission and getting a more reasonable regional budget of CH4 emission from wetlands on the QTP and even for world-wide natural wetlands under climate change.
Acknowledgments
The authors give special thanks to Ms. Wan Xiong for her editing and valuable comments on the manuscript. Two anonymous reviewers are thanked for detailed evaluation and constructive suggestion on our manuscript.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No.31100348), Natural Science Foundation Project of CQ CSTC (2009BB7182), Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant, and the China QianRen programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Denman KL, Brasseur G, Chidthaisong A, Ciais PMC, Dickinson RE, et al. (2007) Couplings Between Changes in the Climate System and Biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M et al.., editors. Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press.
- 2.Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, et al. (2007) Changes in Atmospheric Constituents and in Radiative Forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M et al.., editors. Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press.
- 3. Chen YH, Prinn RG (2006) Estimation of atmospheric methane emissions between 1996 and 2001 using a three-dimensional global chemical transport model. Journal of Geophysical Research 111: D10307 doi:10310.11029/12005JD006058. [Google Scholar]
- 4. Singh SN, Kulshreshtha K, Agnihotri S (2000) Seasonal dynamics of methane emission from wetlands Chemosphere - Global Change Science. 2: 39–46. [Google Scholar]
- 5. Middelburg JJ, Nieuwenhuize J, Iverson N, HØgh N, Dewilde H, et al. (2002) Methane distribution in European tidal estuaries. Biogeochemistry 59: 95–119. [Google Scholar]
- 6. Ding W, Cai Z, Wang D (2004) Preliminary budget of methane emissions from natural wetlands in China. Atmospheric Environment 38: 751–759. [Google Scholar]
- 7. Xiang S, Guo R, Wu N, Sun S (2009) Current status and future prospects of Zoige Marsh in Eastern Qinghai-Tibet Plateau. Ecological Engineering 35: 553–562. [Google Scholar]
- 8. Xu B, Yao T (2001) Dasuopu ice core record of atmospheric methane over the past 2000 years. Science in China Series D: Earth Sciences 44: 689–695. [Google Scholar]
- 9. Wu N (1997) Indigenous knowledge and sustainable approaches for the maintenance of biodiversity in nomadic society: experiences from the eastern Tibetan Plateau. Die Erde 128: 67–80. [Google Scholar]
- 10. Chen H, Wu N, Yao S, Gao Y, Zhu D, et al. (2009) High methane emissions from a littoral zone on the Qinghai-Tibetan Plateau. Atmospheric Environment 43: 4995–5000. [Google Scholar]
- 11. Chen H, Wu N, Yao SP, Gao YH, Wang YF, et al. (2010) Diurnal variation of methane emissions from an alpine wetland on the eastern edge of Qinghai-Tibetan Plateau. Environmental Monitoring and Assessment 164: 21–28. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Yao SP, Wu N, Wang YF, Luo P, et al. (2008) Determinants influencing seasonal variations of methane emissions from alpine wetlands in Zoige Plateau and their implications. Journal of Geophysical Research 113 D12303: 10.1029/2006JD008072. [Google Scholar]
- 13. Hirota M, Tang Y, Hu Q, Hirata S, Kato T, et al. (2004) Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland Soil Biology and Biochemistry. 36: 737–748. [Google Scholar]
- 14. Hirota M, Tang Y, Hu Q, Kato T, Hirata S, et al. (2005) The potential importance of grazing to the fluxes of carbon dioxide and methane in an alpine wetland on the Qinghai-Tibetan Plateau. Atmospheric Environment 39: 5255–5259. [Google Scholar]
- 15. Jin HJ, Wu J, Cheng GD, Tomoko N, Sun GY (1999) Methane emissions from wetlands on the Qinghai-Tibet Plateau. Chinese Science Bulletin 44: 2282–2286. [Google Scholar]
- 16.Wang DX, Lü XG, Ding WX, Cai ZC, Gao JF, et al.. (2002) Methane emission from marshes in Zoige Plateau. Advances in Earth Science 17: 877–880, in Chinese with an English abstract.
- 17. Li L, Yang S, Wang Z, Zhu X, Tang H (2010) Evidence of Warming and Wetting Climate over the Qinghai-Tibet Plateau. Arctic, Antarctic, and Alpine Research 42: 449–457. [Google Scholar]
- 18. Xu ZX, Gong TL, Li JY (2008) Decadal trend of climate in the Tibetan Plateau–regional temperature and precipitation. Hydrological Processes 22: 3056–3065. [Google Scholar]
- 19. Kang S, Xu Y, You Q, Flügel W-A, Pepin N, et al. (2010) Review of climate and cryospheric change in the Tibetan Plateau. Environmental Research Letters 5: 015101. [Google Scholar]
- 20. Wang MH (1987) Pollen composition, paleovegetation and paleoclimate of peatlands in Zoige Plateau. Scientia Geographica Sinica 18: 145–155 (in Chinese only).. [Google Scholar]
- 21. Mosier AR, Schimel DS, Valentine DW, Bronson KF, Parton WJ (1991) Methane and nitrous oxide fluxes in native, fertilized, and cultivated grasslands. Nature 335: 330–332. [Google Scholar]
- 22. Hutchinson GL, Mosier AR (1981) Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45: 311–316. [Google Scholar]
- 23. Kato T, Hirota M, Tang Y, Wada E (2011) Spatial variability of CH4 and N2O fluxes in alpine ecosystems on the Qinghai–Tibetan Plateau. Atmospheric Environment 45: 5632–5639. [Google Scholar]
- 24. Chen H, Wu N, Gao Y, Wang Y, Luo P, et al. (2009) Spatial variations on methane emissions from Zoige alpine wetlands of Southwest China. Science of The Total Environment 407: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 25. Ding W, Cai Z, Tsuruta H, Li X (2002) Effect of standing water depth on methane emissions from freshwater marshes in northeast China. Atmospheric Environment 36: 5149–5157. [Google Scholar]
- 26. Shannon RD, White JR (1994) A three-year study of controls on methane emissions from two Michigan peatlands. Biogeochemistry 27: 35–60. [Google Scholar]
- 27.Chen H, Wu YY, Yuan XZ, Gao YH, Wu N, et al.. (2009) Methane emissions from newly created marshes in the drawdown area of the Three Gorges Reservoir. Journal of Geophysical Research-Atmospheres 114: -.
- 28.Cai ZC, Tsuruta H, Minami K (2000) Methane emission from rice fields in China: Measurements and influencing factors. Journal of Geophysical Research 105(D13): 17,231–217,242.
- 29.Yan X, Cai Z, Ohara T, Akimoto H (2003) Methane emission from rice fields in mainland China: Amount and seasonal and spatial distribution. Journal of Geophysical Research 108(D16), 4505: doi:10.1029/2002JD003182.
- 30. Freeman C, Lock MA, Reynolds B (1993) Fluxes of CO2, CH4 and N2O from a Welsh peatland following simulation of water table draw-down: Potential feedback to climatic change. Biogeochemistry 19: 51–60. [Google Scholar]
- 31. Dowrick DJ, Freeman C, Lock MA, Reynolds B (2006) Sulphate reduction and the suppression of peatland methane emissions following summer drought. Geoderma 132: 384–390. [Google Scholar]
- 32. Davidson EA, Nepstad DC, Ishida FY, Brando PM (2008) Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Global Change Biology 14: 2582–2590. [Google Scholar]
- 33. Knorr K-H, Blodau C (2009) Impact of experimental drought and rewetting on redox transformations and methanogenesis in mesocosms of a northern fen soil. Soil Biology and Biochemistry 41: 1187–1198. [Google Scholar]
- 34. Estop-Aragonés C, Blodau C (2012) Effects of experimental drying intensity and duration on respiration and methane production recovery in fen peat incubations. Soil Biology and Biochemistry 47: 1–9. [Google Scholar]
- 35. Davidson EA, Ishida FY, Nepstad DC (2004) Effects of an experimental drought on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Global Change Biology 10: 718–730. [Google Scholar]
- 36. Knorr K-H, Oosterwoud MR, Blodau C (2008) Experimental drought alters rates of soil respiration and methanogenesis but not carbon exchange in soil of a temperate fen. Soil Biology and Biochemistry 40: 1781–1791. [Google Scholar]
- 37. Freeman C, Nevison GB, Kang H, Hughes S, Reynolds B, et al. (2002) Contrasted effects of simulated drought on the production and oxidation of methane in a mid-Wales wetland. Soil Biology and Biochemistry 34: 61–67. [Google Scholar]
- 38. Kim S-Y, Lee S-H, Freeman C, Fenner N, Kang H (2008) Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biology and Biochemistry 40: 2874–2880. [Google Scholar]
- 39.Tian JQ, Zhu YB, Kang XM, Dong XZ, Li W, et al.. (2012) Effects of drought on the archaeal community in soil of the Zoige Wetlands of the Qinghai-Tibetan Plateau. European Journal of Soil Biology: DOI: 10.1016/j.ejsobi.2012.1007.1003.
- 40. Wu Q, Zhang T, Liu Y (2010) Permafrost temperatures and thickness on the Qinghai-Tibet Plateau. Global and Planetary Change 72: 32–38. [Google Scholar]
- 41. Zhang Y, Wang GX, Wang YB (2011) Changes in alpine wetland ecosystems of the Qinghai-Tibetan plateau from 1967 to 2004. Environmental Monitoring and Assessment 180: 189–199. [DOI] [PubMed] [Google Scholar]
- 42.Ringeval B, Noblet-Ducoudré Nd, Ciais P, Bousquet P, Prigent C, et al.. (2010) An attempt to quantify the impact of changes in wetland extent on methane emissions on the seasonal and interannual time scales. Global Biogeochem Cy 24, GB2003: doi:10.1029/2008GB003354.


