Abstract
Background
Sonchus asper is traditionally used in Pakistan for the treatment of reproductive dysfunction and oxidative stress. The present investigation was aimed to evaluate chloroform extract of Sonchus asper (SACE) against potassium bromate-induced reproductive stress in male rats.
Methods
20 mg/kg body weight (b.w.) potassium bromate (KBrO3) was induced in 36 rats for four weeks and checked the protective efficacy of SACE at various hormonal imbalances, alteration of antioxidant enzymes, and DNA fragmentation levels. High performance chromatography (HPLC) was used for determination of bioactive constituents responsible.
Results
The level of hormonal secretion was significantly altered by potassium bromate. DNA fragmentation%, activity of antioxidant enzymes; catalase (CAT), peroxidase (POD), superoxide dismutase (SOD) and phase II metabolizing enzymes viz; glutathione reductase (GSR), glutathione peroxidase (GSHpx), glutathione-S-tansase (GST) and reduced glutathione (GSH) was decreased while hydrogen per oxide contents and thiobarbituric acid reactive substances (TBARS) were increased with KBrO3 treatment. Treatment with SACE effectively ameliorated the alterations in the biochemical markers; hormonal and molecular levels while HPLC characterization revealed the presence of catechin, kaempferol, rutin and quercetin.
Conclusion
Protective effects of Sonchus asper vs. KBrO3 induced lipid peroxidation might be due to bioactive compound present in SACE.
Keywords: Potassium bromate, Sonchus asper, Antioxidant, FSH, DNA fragmentation, TSH
Background
Potassium bromate (KBrO3) is found in drinking water, food additive [1] and hair solution [2], cause sensorineural hearing loss and adverse effects on the vestibuloocular reflex system [3,4]. KBrO3 induces chromosomal aberration and promoting tumorigenesis [5] due to elevation of ONOO- level and 8-hydroxydeoxyguanosine levels in DNA induced by oxidative stress [6]. Sever damage to sperm membranes, proteins and DNA is associated with alterations in signal transduction mechanisms that affect fertility [7,8]. Sertoli cells support the moment of sperm from testis into epididymis through efferent ducts [9]. Free radicals cause alterations in the spermatogenic cycle, hypogonadism, reduction of sperms, alterations of reproductive hormones and degeneration in seminiferous tubules [10]. 4–5 g Sonchus asper (L.) Hill powder is traditionally used in the treatment of human reproductive disorder [11], cardiac dysfunction [12] and cancer [13]. Phytochemical study revealed that Sonchus asper contains flavonoids glycosides, ascorbic acid and carotenoids, possess antioxidant, anticancer; anti-inflammatory properties. The present investigation was aim to conform the traditional use of Sonchus asper versus KBrO3 induced hormonal dysfunction, injuries and lipids peroxidation in rat testicular tissues.
Materials and methods
Plant collection
Sonchus asper at maturity was collected from District Bannu (Pakistan) during the September 2011, identified and a voucher specimen R-47 was submitted at herbarium of Pakistan, Quaid-i-Azam University Islamabad, Pakistan. All parts of the plant (leaves, stem, flowers, seeds and roots) were shade dried for two weeks, chopped, and ground mechanically.
Preparation of plant extract
2 kg powder of Sonchus asper was extracted in 5 L chloroform to get chloroform extract (SACE). The extract was cooled at room temperature, filtered and evaporated under reduced pressure through rotary evaporator. The extract was stored at 4°C for in vivo investigations.
High performance liquid chromatography of plant extract
250 mg plant powder was extracted with 10 ml of 25% hydrochloric acid and 25 ml chloroform for 1 h. The obtained extract was filtered and diluted to 100 ml. 10 μl samples were injected into the HPLC Agilent HPLC system. Separation was carried out through column C18, UV–VIS Spectra-Focus detector, injector-auto sampler. Trifluoroacetic acid and acetonitrile was used as solvent with flow rate was 1 ml/min. Different standards compounds (catechin, kaempferol, rutin and quercetin) were run for comparative detection. The calibration curves were defined for each compound in the range of sample quantity 0.02-0.5 μg. All samples were assayed in triplicate.
Animals
36 male albino rats (180–190 g) were procured from National Institute of Health Islamabad and were kept in ordinary cages at room temperature of 25 ± 3°C with a 12 h dark/light cycle. Standard laboratory feed and water was free accessed. The study protocol was approved by Ethical committee of Quaid-i-Azam University Islamabad. All these rats were used for in vivo screening of chloroform fraction of Sonchus asper against KBrO3-induced testicular toxicity and hormonal dysfunction in rats.
Experimental design
To study the antioxidant effects of Sonchus asper, male albino rats were equally divided into 6 groups (6 rats). SACE was dissolved in DMSO while 20 mg of KBrO3 in water. SACE was administered after 48 h of KBrO3 treatment for 30 days.
Group I Control; only allowed to food and water
Group II KBrO3 (20 mg/kg b.w.)
Group III KBrO3 (20 mg/kg b.w.) + SACE (100 mg/kg b.w. orally)
Group IV KBrO3 (20 mg/kg b.w.) + SACE (200 mg/kg b.w. orally)
Group V KBrO3 (20 mg/kg b.w.) + Silymarin (50 mg/kg b.w. orally)
Group VI SACE (200 mg/kg b.w. orally)
After 24 h of the last treatment, all the animals were weighted, sacrificed; collected their blood and urine while their testis were removed, weighted, perfuse in ice-cold saline solution and treated with liquid nitrogen for further enzymatic and DNA damage analysis.
Serum analysis of hormone
Serum level of testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol and prolactin was estimated using RIA gamma counter through kits.
Assessment of antioxidant enzymes
70 mg of tissue was homogenized in 10 volume of 100 mmol KH2PO4 buffer containing 1 mmol EDTA (pH 7.4) and centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was collected and used for enzymatic studies. Protein concentration of tissue supernatant was determined by the method of using crystalline BSA as standard. Various antioxidant enzymes including CAT [14], POD [15], SOD [16], TBARS [17], GST [18], GSR [19], GSHpx [20], GSH [21] and DNA fragmentation [22] were carried out.
Histopathological overview of testis
After weighting the portion specifies for histology; testis was fixed for 3–4 h in fixative sera followed by dehydration with ascending grades of alcohol (80%, 90%, and 100%) and transferred in cedar wood oil, when testis becomes clear then embedded in paraplast and prepared blocks for further microtomy. 3–4 μm thin slides were prepared with microtome; wax was removed, stained with hemotoxilin-eosin and photographed under light microscope at 40x.
Statistical analysis
To determine the treatment effects one way analysis of variance was carried by computer software SPSS 13.0. Level of significance among the various treatments was determined by LSD at 0.05% level of probability.
Results
HPLC characterization of polyphenolic constituents
The investigated compounds in the SACE were quantified by integration of the peak-areas at 220 nm using an external calibration method. Least-squares linear regression was used to determine the calibration parameters for each of standards. The main polyphenolic flavonoids compounds in the SACE include catechin, kaempferol, rutin and quercetin (Figure 1).
Figure 1.
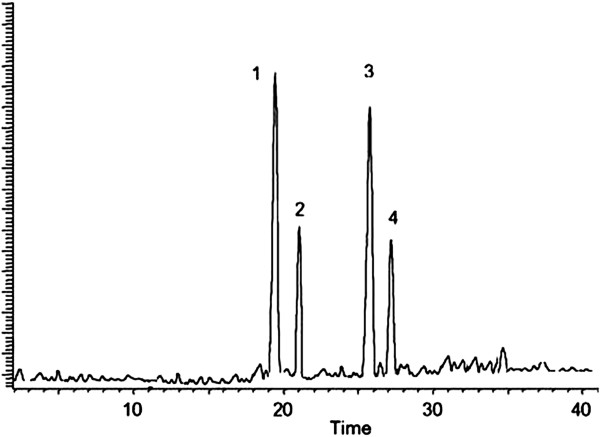
HPLC fingerprints obtained by chloroform extract of Sonchus asper eluted with mixtures of trifluoroacetic acid and acetonitrile indicated the presence of 4 compounds kaempferol, rutin, catechin and quercetin.
Effect of SACE on pituitary—gonadal axis
The mean values of the serum hormones; etradiol, prolactin, testosterone, luteinizing hormone and follicle stimulating hormone were shown in Table 1. Treatment of rats with KBrO3 for 4 weeks, significantly decreased (P < 0.01) the hormonal level of testosterone, luteinizing hormone and follicle stimulating hormone while increased markedly (P < 0.01) the secretion of estradiol, prolactin as against the control group. These alterations of hormones are significantly reversed (P < 0.01) by administrations of 100 mg/kg and 200 mg/kg b.w., SACE as well as 50 mg/kg b.w. Silymarin in KBrO3 treated rats however non significant changes were observed in non treated SACE alone rats.
Table 1.
Effect of SACE on FSH, LH, testosterone, prolactin and estradiol in rat
| Treatment |
FSH |
LH |
Testosterone |
Prolactin |
Estradiol |
|---|---|---|---|---|---|
| (mg/dl) | (mg/dl) | (mg/dl) | (mg/dl) | (mg/dl) | |
| Control |
21.5 ± 0.3++ |
25 ± 1.72++ |
47 ± 3.04++ |
24.5 ± 2.5++ |
41.0 ± 2.1++ |
| 20 mg/kg KBrO3 |
11.2 ± 0.2** |
10.5 ± 0.71** |
23 ± 2.7** |
48.5 ± 2.7** |
70.5 ± 3.0** |
| 50 mg/kg Silymarin + KBrO3 |
19.1 ± 0.74++ |
20.7 ± 2. 4++ |
30.3 ± 3. 9**++ |
23.5 ± 3.09**++ |
51.3 ± 2.4++ |
| 100 mg/kg SACE + KBrO3 |
12.8 ± 0.12** |
18.8 ± 2.04** |
34.7 ± 2.53++ |
30.7 ± 1.7**++ |
53 ± 2.6**++ |
| 200 mg/kg SACE + KBrO3 |
19.2 ± 0.80++ |
21 ± 1.85++ |
36 ± 2.53++ |
28.5 ± 1.51*++ |
48.3 ± 3.01++ |
| 200 mg/kg SACE alone | 24.0 ± 1.9++ | 24.0 ± 1.62++ | 45.7 ± 3.52++ | 23.7 ± 1.0++ | 40.0 ± 2.1++ |
Mean ± SE (n = 6 number), *, ** indicate significance from the control group at P < 0.01 probability level, ++ indicate significance from the KBrO3 group at P < 0.01 probability level.
Sonchus asper and antioxidant profile
The results regarding the protective effects of SACE against the toxic affect of KBrO3 in rat on activities of antioxidant enzymes such as CAT, POD, SOD and amount of tissue protein were shown in Table 2. Activities of antioxidant enzymes such as CAT, POD, SOD and amount of tissue protein were significantly (P < 0.01) reduced by treatment of KBrO3 as compared to control group. This reduction was improved significantly (P < 0.01) by post-administration of 100 mg/kg and 200 mg/kg b.w. SACE and 50 mg/kg b.w. silymarin to control rat. However, non significant changes (P > 0.05) were found by administration of SACE alone against the control group.
Table 2.
Effect of SACE on tissue protein, activity of CAT, POD and SOD
| Treatment |
Protein |
CAT |
POD |
SOD |
|---|---|---|---|---|
| (μg/mg tissue) | (U/min) | (U/min) | (U/mg protein) | |
| Control |
2.9 ± 0.09++ |
8. 9 ± 1. 8++ |
11.9 ± 2.17++ |
22.29 ± 2.07++ |
| 20 mg/kg KBrO3 |
2.07.087** |
4.7 ± 0.54** |
5.91 ± 0.983** |
13.7 ± 1.73** |
| 50 mg/kg Silymarin + KBrO3 |
2.82 ± 0.134++ |
6.804 ± 0.976++ |
9.45 ± 1.64*++ |
19.82 ± 2.31++ |
| 100 mg/kg SACE + KBrO3 |
2.97 ± 0.19**++ |
5.117 ± 0.575**+ |
7.63 ± 1.10**++ |
17.15 ± 1.62**++ |
| 200 mg/kg SACE + KBrO3 |
3.08 ± 0.057++ |
6.131 ± 0.779++ |
9.10 ± 1.39++ |
18.06 ± 2.00++ |
| 200 mg/kg SACE alone | 3.52 ± 0.173++ | 7.38 ± 1.25++ | 11.50 ± 2.14++ | 21.52 ± 3.02++ |
Mean ± SE (n = 6 number), ** indicate significance from the control group at P < 0.01 probability level, ++ indicate significance from the KBrO3 group at P < 0.01 probability level.
Effect of SACE on GSHpx, GST, GSR, GSH, TBARS
Effect of KBrO3 and the protective effects of SACE on tissue phase II metabolizing enzymes viz; GSHpx, GST, GSR, GSH and TBARS were shown in Table 3. KBrO3 treatment to rats significantly (P < 0.01) decreased the activities of GSHpx, GST, GSR and GSH while significantly (P < 0.01) increased the contents of TBARS in tissue homogenate as compared to control group. 100 mg/kg, 200 mg/kg b.w., SACE and 50 mg/kg b.w. silymarin showed significant protection (P < 0.01) and recovered the activity of enzymes near to control rat; increased the activities of GST, GSR and GSH while decreased the contents of TBARS in a dose dependent manner. SACE when administered alone did not show significant variations.
Table 3.
Effect of SACE on testis GST, GSH-Px, GSR, GSH and TBARS in rat
| Treatment | GSH-Px (nM/mg protein) | GSR (nM/min/ mg protein) | GST (nM/min/ mg protein) | GSH (μM/g tissue) | TBARS (nM/min/ mgprotein) |
|---|---|---|---|---|---|
| Control |
39.44 ± 3.86++ |
63.44 ± 3.86++ |
26.44 ± 3.86++ |
0.875 ± 0.0894++ |
19.78 ± 1.18++ |
| 20 mg/kg KBrO3 |
25.25 ± 1.87** |
44.25 ± 1.87** |
17.25 ± 1.87** |
0.570 ± 0.0443** |
27.17 ± 1.92** |
| 50 mg/kg Silymarin + KBrO3 |
34 ± 1.8++ |
58.2 ± 1.4++ |
26 ± 3.8++ |
0.726 ± 0.014++ |
20.0 ± 1.2++ |
| 100 mg/kg SACE + KBrO3 |
33.67 ± 2.14**++ |
53.67 ± 2.14**++ |
22.67 ± 2.14**++ |
0.757 ± 0.0523++ |
20.30 ± 1.27++ |
| 200 mg/kg SACE + KBrO3 |
37.83 ± 2.61++ |
56.03 ± 2.61++ |
23.03 ± 2.61++ |
0.797 ± 0.0611++ |
19.95 ± 1.20++ |
| 200 mg/kg SACE alone | 41.53 ± 3.91++ | 64.53 ± 3.91++ | 27.53 ± 3.91++ | 0.766 ± 0.018++ | 19.1 ± 1.3++ |
Mean ± SE (n = 6 number), ** indicate significance from the control group at P < 0.01 probability level, ++ indicate significance from the KBrO3 group at P < 0.01 probability level.
% DNA fragmentation, testis weight, relative testis weight
Protective effects of SACE and Silymarin against KBrO3 administration in rat on DNA fragmentation, testis weight and relative testis weight were shown in Table 4. Administration of KBrO3 significantly increased (P < 0.01) DNA fragmentation, testis weight and relative testis weight when compared to control group. Post-treatment with SACE and silymarin erased the KBrO3 toxication and significantly (P < 0.01) improved DNA damages, testis weight and relative testis weight kidney weight and relative tissue weight towards the control group in a dose dependent. However, non significant (P > 0.05) variations were observed by S. asper alone as compared to control group.
Table 4.
Effect of SACE on testis weight, relative testis weight in rat
| Treatment |
Tissue |
Relative testis |
%DNA |
|---|---|---|---|
| weight (g) | Weight (g) | Fragmentation | |
| Control |
6.03 ± 0.66++ |
0.060 ± 0.0166++ |
4.3 ± 0.0946++ |
| 20 mg/kg KBrO3 |
7.76 ± 0.504** |
0.077 ± 0.0024** |
14.0 ± 0.08** |
| 50 mg/kg Silymarin + KBrO3 |
7.03 ± 0.008++ |
0.035 ± 0.0018++ |
6.0 ± 0.063++ |
| 100 mg/kg SACE + KBrO3 |
6.66 ± 0.26++ |
0.066 ± 0.0073++ |
8.6 ± 0.060**++ |
| 200 mg/kg SACE + KBrO3 |
6.27 ± 0.451++ |
0.062 ± 0.0051++ |
6.8 ± 0.01**++ |
| 200 mg/kg SACE alone | 5.95 ± 0.189++ | 0.03590.00189++ | 4.33 ± 0.06++ |
Mean ± SE (n = 6 number), ** indicate significance from the control group at P < 0.01 probability level, ++ indicate significance from the KBrO3 group at P < 0.01 probability level.
Effect of SACE on histopathology of testis in rats
Microscopic examinations of male reproductive system from control group revealed the normal semeniferous tubules, sperms with normal morphology and concentration (Figure 2). Sertoli cells were inconspicuous. Histological appearance of prostate glands and surrounding fibro muscular stroma was found to be normal in appearance. Administration of KBrO3 caused degeneration of semeniferous tubules, loss of germ cells, abnormality of germinative epithelium, interruption in meiosis, and sperm with abnormal shape and concentration were visible. Orally-administration with SACE and silymarin revealed a marked repairing of testicular abnormalities induced by KBrO3; sperm with normal morphology and concentration near to control group were seen in repaired semeniferous tubules (Table 5).
Figure 2.
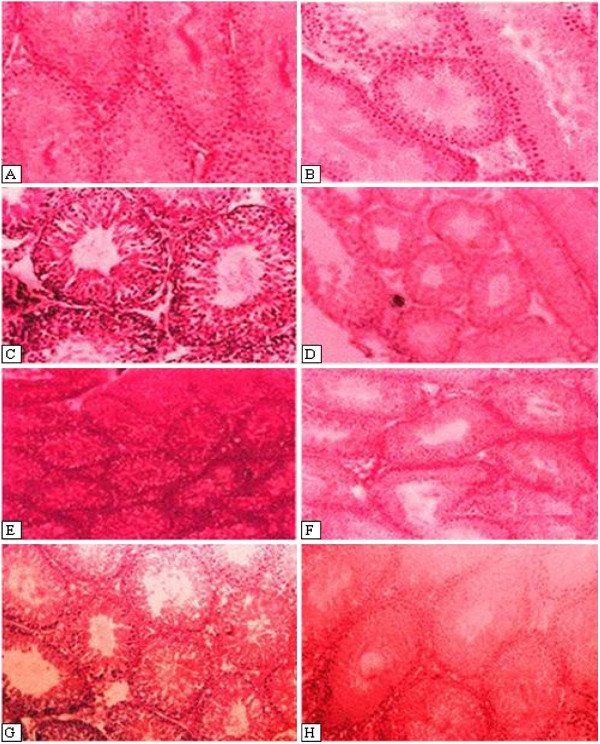
Histopathological changes caused by KBrO3 and preventive effect of SACE on testis in different groups; Slides (from left) Control (A, B), KBrO3 (C, D), 100 mg/kg SACE + KBrO3 (E), 200 mg/kg SACE + KBrO3 (F), 200 mg/kg SACE alone (G) and 50 mg/kg silymarine + KBrO3 (H).
Table 5.
Effect of SACE on testicular histopathology in rat
| Treatment | Seminiferous tubules degeneration | Meiosis interruption | Sperm concentration | Germ cell morphology | Germinative epithelium |
|---|---|---|---|---|---|
| Control |
- |
- |
- |
- |
- |
| 20 mg/kg KBrO3 |
+++ |
++ |
+++ |
++ |
++ |
| 50 mg/kg Silymarin + KBrO3 |
−/+ |
- |
- |
−/+ |
- |
| 100 mg/kg SACE + KBrO3 |
−/+ |
- |
−/+ |
−/+ |
−/+ |
| 200 mg/kg SACE + KBrO3 |
- |
- |
- |
- |
−/+ |
| 200 mg/kg SACE alone | - | - | - | - | - |
-, normal; −/+, mild; ++, medium; +++, severely damaged.
Discussion
Phenolic and polyphenolic compounds are potential functional foods or neutraceuticals possess a variety of biological activities and considered to detoxify oxidative stress [23,24]. Chromatogram of the present extract showed the presence of catechin, kaempferol, rutin and quercetin. The present investigation describes molecular and biochemical processes which may be involved in the toxicity and carcinogenicity of potassium bromate [25]. In this investigation, body weight, testis weight and relative testis weight changed significantly in KBrO3 treated rats, in agreement with previous studies using the same dose of 80 mg KBrO3/kg b.w. It has been shown that increased testis weight reflects the increment of lipidperoxidation that in turn related to fatty accumulation and alteration of weight [26]. The treatment with SACE effectively abolished this change since the values were not significantly different from those of the control group, suggesting that these fractions can prevent the toxic effects of KBrO3 in the testis. Antioxidant enzymes play important role in detoxification of oxidative damages constitute a mutually supportive team of defense against reactive oxygen species [27]. Present study revealed that the activities of antioxidant enzymes were significantly reduced with intoxication of KBrO3 in rats which might be due to the presence of catechin, kaempferol, rutin and quercetin which, propagating free radicals like peroxyl radicals and converting the reactive free radicals to inactive products. Similar results were recorded by other studies of during characterization of Digera muricata and Launaea procumbens in rats [28,29]. Glutathione and other sulfhydryls play a major role in the metabolism and excretion of bromate in the rat [30]. Thiol-mediated oxidation of DNA by bromine oxides and bromine radicals has been well characterized in vitro and are thought to play a role in DNA damage in vivo[31]. While extra cellular pools of glutathione may be important in protecting target organs from bromate uptake and oxidative DNA damage, intracellular glutathione may facilitate the formation of DNA reactive metabolites [32-35]. This decrease in antioxidant status permits further oxidation of cellular DNA resulting in the formation of the mutagenic lesion 8-oxodeoxyguanosine (8-oxodG) and an increase in cellular proliferation in the target tissue. More recent evaluations of oxidative DNA damage in response to acute bromate exposures have demonstrated 8-oxodG formation in rat kidney at doses as low as 80 mg/kg [36].
The successful and complete male germ cell development is dependent on the balanced endocrine interplay of hypothalamus, pituitary and testis. Gonadotropin releasing hormone (GnRH) secreted by the hypothalamus elicits the release of gonadotropins, i.e., FSH and LH from the pituitary gland. FSH stimulates spermatogenesis while LH stimulates the production of testosterone in Leydig cells, which in turn may act on the sertoli cells and peritubular cells of the seminiferous tubules and stimulates spermatogenesis [36]. In the present study, KBrO3 treatment decreased the serum level of testosterone, FSH and LH. Secretion of testosterone is probably impaired due to excessive oxidative stress and the degeneration of Leydig cells [37]. Toxic effects of KBrO3 may result in the failure of pituitary to secrete FSH and LH and that will result in testicular dysfunction leading to infertility. In the present study, estradiol and prolactin serum level are appeared to be positively associated in the control and all the treated groups but probably the direct stimulation of the pituitary by estradiol is only one of the factors determining prolactinemia, with hypothalamic dysfunction being associated, as observed in hypogonadism. This mechanism may be considered partly responsible for the central origin of hypogonadism in our study. Spermatogenesis is a complex differentiation process. Deterioration of spermatogenesis is an integral and important part of normal spermatogenesis [8]. However, spermatogonial degeneration can result from exposure to toxic chemicals [38-40]. Result of the present study revealed that KBrO3 intoxication caused marked tubular degeneration, meiotic interruption, depletion of sperm concentration and degradation of germinative epithelium were confirmed. Supplementation of SACE significantly improved the testicular injuries and reversed the cellular toxicity near to control rat [41].
Conclusion
Sonchus asper significantly improved the alteration of antioxidant status, DNA fragmentation and hormonal imbalance caused by KBrO3 in male rat which might be associated due the presence of catechin, kaempferol, quercetin and rutin. This study substantiated the scientific evidence in favors of its pharmacological use in male sexual dysfunction and hormonal imbalance in folk medicine.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RAK made significant contribution to acquisition and interpretation of data, conception and drafting of the manuscript. MRK and SS has made substantial contribution to conception and design, interpretation of data and drafting for intellectual content. All the authors read and approved the final manuscript.
Contributor Information
Rahmat Ali Khan, Email: Rahmatgul_81@yahoo.com.
Muhammad Rashid Khan, Email: mrkhanqau@yahoo.com.
Sumaira Sahreen, Email: Sumairasahreen@gmail.com.
References
- Himata K, Kuwahara T, Ando S, Maruoka H. Determination of bromate in bread by capillary gas chromatography with a mass detector (GC/MS) Food Addit Contam. 1994;11:559–569. doi: 10.1080/02652039409374257. [DOI] [PubMed] [Google Scholar]
- Chipman J, Davies J, Parsons J, Nair J, O’Neill G, Fawell J. DNA oxidation by potassium bromate; a direct mechanism or linked to lipid peroxidation? Toxicology. 1998;126:93–102. doi: 10.1016/S0300-483X(97)00174-1. [DOI] [PubMed] [Google Scholar]
- Hytonen M, Leino T, Sala E, Kanerva L, Tupasela O, Malmberg H. Nasal provocation test in the diagnostics of hairdressers occupational rhinitis. Acta OtoLaryngol Supplement. 1997;529:133–136. doi: 10.3109/00016489709124104. [DOI] [PubMed] [Google Scholar]
- Young YH, Chuu JJ, Liu SH, Lin-Shiau SY. Toxic Effects of Potassium Bromate and Thioglycolate an Vestibuloocular Reflex Systems of Guinea Pigs and Humans. Toxicol Appl Pharmacol. 2001;177:103–111. doi: 10.1006/taap.2001.9285. [DOI] [PubMed] [Google Scholar]
- Sai K, Umemura T, Takagi A, Hasegawa R, Kurokawa Y. The protective role of glutathione, cysteine and Vitamin C against oxidative DNA damage induced in rat kidney by potassium bromate. Japan J. Cancer Res. 1992;83:45–51. doi: 10.1111/j.1349-7006.1992.tb02350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenbe LW. Inhibition of carcinogenesis by minor a nutrient constituents of the diet. Proc Nutr Soc. 1990;49:173–183. doi: 10.1079/PNS19900022. [DOI] [PubMed] [Google Scholar]
- Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995;16(6):464–468. [PubMed] [Google Scholar]
- Russell LD, Alger LE, Nequin LG. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987;120:1615–1632. doi: 10.1210/endo-120-4-1615. [DOI] [PubMed] [Google Scholar]
- Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol. 2001;29(1):64–76. doi: 10.1080/019262301301418865. [DOI] [PubMed] [Google Scholar]
- Khan MR, Ahmed D. Protective effects of Digera muricata (L.) Mart. On testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009;47:1393–1399. doi: 10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G. Traditional medicine among the Embu and Mbeere peoples of Kenya. Afric J Trad Compl Alter Med. 2007;4:75–86. doi: 10.4314/ajtcam.v4i1.31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeen M, Ahmad AA. Exploring the folk medicinal flora of Abbotabad city Pakistan. Ethnobotnical Leaflets. 2009;13:810–833. [Google Scholar]
- Thomson B, Shaw IA. Comparison of risk and protective factors for colorectal cancer in the diet of New Zealand maori and non-maori. Asian Pac J Cancer Prev. 2002;3:319–324. [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;11:764–775. [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Iqbal M, Sharma SD, Zadeh HR, Hasan N, Abdulla M, Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Rep. 1996;2:385–391. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:4475–4480. [PubMed] [Google Scholar]
- Mohandas J, Marshal JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Wu B, Ootani A, Iwakiri R, Sakata Y, Fujise T, Amemori S, Yokoyama F, Tsunada S, Fujimoto K. T cell deficiency leads to liver carcinogenesis in Azoxymethane-treated rats. Exp Biol Med. 2005;231:91–98. doi: 10.1177/153537020623100111. [DOI] [PubMed] [Google Scholar]
- Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Van Marck E, Worobey M. The isolation of nucleic acids from fixed, paraffin- embedded tissues, which methods are useful when? PLoS One. 2007;2(6):e537. doi: 10.1371/journal.pone.0000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuricals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magne C, Abdelly C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083–2091. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Deangelo A, George M, Kilburn S, Moore T, Wolf D. Carcinogenicity of potassium bromate administered in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol Pathol. 1998;26:724–729. doi: 10.1177/019262339802600602. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Barja G. Resveratrol, Melatonin, Vitamine, and PBN protect against renal oxidative DNA damage induced by kidney carcinogen KBrO3. Free Rad Biol Med. 1999;26:1531–1537. doi: 10.1016/S0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma S, Sultana S. Nigella sativa (black cumin) ameliorates potassium bromate-induced early events of carcinogenesis: diminution of oxidative stress. Human Exp Toxicol. 2003;22:193–203. doi: 10.1191/0960327103ht349oa. [DOI] [PubMed] [Google Scholar]
- Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride induced nephrotoxicity in rat: Protective role of Digera muricata. J Ethnopharmcol. 2009;122:91–99. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Evaluation of launaea procumbens use in renal disorders: a rat model. J Ethnopharmacol. 2010;128:452–461. doi: 10.1016/j.jep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y, Takamura N, Matsuoka C, Imazawa T, Matsushima Y, Onodera H, Hayashi Y. Comparative studies on lipid peroxidation in the kidney of rats, mice, and hamsters and on the effect of cysteine, glutathione, and diethyl maleate treatment on mortality and nephrotoxicity after administration of potassium bromate. J Am Coll Toxicol. 1987;6:489–501. doi: 10.3109/10915818709075694. [DOI] [Google Scholar]
- Murata M, Bansho Y, Inoue S, Ito K, Ohnishi S, Midorikawa K, Kawanashi S. Requirement of glutathione and cysteine in guanine-specific oxidation of DNA by carcinogenic potassium bromate. Chem Res Toxicol. 2001;14:678–685. doi: 10.1021/tx000209q. [DOI] [PubMed] [Google Scholar]
- Parsons JL, Chipman JK. The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis. 2000;15:311–316. doi: 10.1093/mutage/15.4.311. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Hepatoprotection with chloroform extract of Launaea procumbens against CCl4 induced injuries in rat. BMC Compl Alter Med. 2012;12:114. doi: 10.1186/1472-6882-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J. 2012;6:12. doi: 10.1186/1752-153X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Brain antioxidant markers, cognitive performance and acetyl cholinesterase activity of rat: Efficiency of Sonchus asper. Behav Brain Funct. 2012;8:2. doi: 10.1186/1744-9081-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Kitamura Y, Kanki K, Maruyama S, Okazaki K, Imazawa T, Nishimura T, Hasegawa R, Nishikawa A, Hirose M. Dose-related changes of oxidative stress and cell proliferation in kidneys of male and female F344 rats exposed to potassium bromate. Cancer Sci. 2004;95:393–398. doi: 10.1111/j.1349-7006.2004.tb03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PM. The molecular basis of gonadotropin releasing hormone action. Endocrinol Rev. 1986;7(1):3–10. doi: 10.1210/edrv-7-1-3. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S, Naseer AS. Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: a randomized controlled trial. BMC Compl Alter Med. 2012;12:90. doi: 10.1186/1472-6882-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AM, Ferraz MR, Teixeira CV, Sampaio FJ, Da Fonte Ramos C. Effects of undernutrition on serum and testicular testosterone levels and sexual function in adult rats. Hormone Metab Res. 2004;36:27–33. doi: 10.1055/s-2004-814198. [DOI] [PubMed] [Google Scholar]
- Khan RA. Protective effects of Launaea procumbens on rat testis damage by CCl4. Lipids Health Dis. 2012;11:103. doi: 10.1186/1476-511X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testis. Toxicol Appl Pharmacol. 1996;137:42–50. doi: 10.1006/taap.1996.0055. [DOI] [PubMed] [Google Scholar]


