Abstract
The position of testudines in vertebrate phylogeny is being re-evaluated. At present, testudine morphological and molecular data conflict when reconstructing phylogenetic relationships. Complicating matters, the ecological niche of stem testudines is ambiguous. To understand how turtles have evolved to hear in different environments, we examined middle ear morphology and scaling in most extant families, as well as some extinct species, using 3-dimensional reconstructions from micro magnetic resonance (MR) and submillimeter computed tomography (CT) scans. All families of testudines exhibited a similar shape of the bony structure of the middle ear cavity, with the tympanic disk located on the rostrolateral edge of the cavity. Sea Turtles have additional soft tissue that fills the middle ear cavity to varying degrees. When the middle ear cavity is modeled as an air-filled sphere of the same volume resonating in an underwater sound field, the calculated resonances for the volumes of the middle ear cavities largely fell within testudine hearing ranges. Although there were some differences in morphology, there were no statistically significant differences in the scaling of the volume of the bony middle ear cavity with head size among groups when categorized by phylogeny and ecology. Because the cavity is predicted to resonate underwater within the testudine hearing range, the data support the hypothesis of an aquatic origin for testudines, and function of the middle ear cavity in underwater sound detection.
Introduction
Vocalizations indicate that hearing has behavioral importance for Testudines [1]. Sea turtles vocalize in air with “ [a] mercy cry and roars and grunts of anger” [2]. Many species of tortoise vocalize in air, most often in the context of mating or distress, including Gopherus agassizzi, Geochelone carbonaria, Geochelone travancorica, Geochelone gigantea, and Platysternon megacephalum [2]–[4]. Calls of G. agassizzi range from 500 to 1000 Hz [3]. Campbell and Evans characterized one of these calls as a possible distress signal because this particular animal was attempting to escape [3]. In one recorded instance, the male of a pair of G. carbonaria, vocalized in air while he attempted to mount the female. The vocalization is a “cluck” that is paired with head-bobbing behavior. The authors speculate that it is similar to the attraction calls observed in other species, which are used both in mating and in parent-offspring interactions [1], [3]. Campbell and Evans further characterize the vocalization of G. carbonaria [3]. The cluck previously described was in the range of 500–2500 Hz. Playbacks of “cluck” recordings elicit head movements. G. travancorica is, thus far, the only tortoise species that is known to call in chorus [2]. These vocalizations had the most energy from 1700–2000 Hz. P. megacephalum produces a two-part call with frequency components from 500–4000 Hz. Campbell and Evans observed this particular type of vocalization only in juveniles. Aside from these studies, little to nothing is known about the behavioral and social relevance of any testudine vocalizations.
The best candidate species for investigations of the behavioral relevance of vocalization among the Testudines is Chelodina oblonga (Oblong Turtle or Snake-necked Turtle), which exhibits an extensive vocal repertoire that can be divided into 17 categories, including both percussive and complex vocalizations [5]. Animals of different ages and both sexes were recorded vocalizing in air and underwater. These vocalizations range in frequency from 100 Hz to over 20,000 Hz, a much greater range than is found on other previously studied species. Despite this wide range, the calls are almost all under 4 kHz. The frequency spectra are also quite varied, from harmonic to noisy. This species inhabits turbid water, thus decreasing its ability to use visual cues [5]. This is would suggest a reliance on non-visual cues. The spectra covered by these calls do not necessarily imply that the animal can hear the calls throughout the entire range. Birds do not hear the entire spectra of their song [6]. Neither the anatomical structures involved in vocalization nor their hearing thresholds have yet been described for C. Oblonga.
Given this evidence for middle- and high-frequency vocalizations, it is possible that some pleurodires (side-necked turtles), including C. oblonga, may hear above 2 kHz, i.e. above reported hearing thresholds [5]. Taking new findings about vocalizations into account, the idea that turtles are relatively insensitive to sound should be reconsidered. If vocalizations are important for mating or other social interactions, there would be selective pressure for auditory acuity. Given that multiple species vocalize, some at frequencies higher than previously measured, hearing in these species should be more fully investigated.
Testudines are divided into two suborders: Cryptodira and Pleurodira. Extant cryptodires include three superfamilies: Chelonioidea, Testudinoidea, and Trionychoidea. Pleurodires, (Side-necked Turtles) include the superfamily Pelomedusoidea and the family Chelidae. Testudines, while monophyletic, have adapted to a wide variety of ecological niches and lifestyles [7]. Ecologies range from marine (Sea Turtles) to semi-arid desert biomes (Tortoises). Sound transmission, production, and reception are affected by the medium in which the animal lives and communicates. Environmental sounds, as well as those generated by predators, prey, and conspecifics provide essential information.
Multiple skull bones comprise the middle ear cavity [8]. As in other tetrapods, the inner ear is encased by the cavum labyrinthicum. The interior of the middle ear cavity is called the cavum tympani, which is formed from the quadrate and the squamosal. The middle ear is bordered anterolaterally and dorsally by the quadrate, dorsally by the opisthotic, medially by the prootic and opisthotic, and ventrally by pterygoid. The columella extends from the oval window, where it forms the stapedial footplate [9], through the cavum acustico-jugulare and incisura columellae auris, into the middle ear cavity. The columella is the primary transducer of sound as demonstrated by Wever and Vernon who showed that the hearing capability of an animal was greatly reduced after the columella was clipped [10]. The columella terminates on the extracolumella via a short, hinged joint [10], [11]. The extracolumella is cartilaginous and forms the tympanic disk.
In Trachemys scripta elegans (Red-eared Slider turtle), the tympanic disk is about 0.5 mm thick [10], [11]. The tympanic disk is visible on the animal through the relatively undifferentiated skin (Fig. 1), which adheres to the tympanic disk by a thin layer of connective tissue [9], [11]. The tympanic disk moves via a hinged connection to the bony capsule wall surrounding it [10], [11]. The disk is primary sound receiving structure of the turtle ear [9]–[12] (Fig. 1). Behind the tympanic disk is the middle ear cavity. Laser vibrometry measurements suggest that the air in the middle ear cavity resonates in the underwater sound field, driving the tympanic disk [11], Comparisons of hearing in air and under water in Trachemys scripta elegans show these turtles are more sensitive to sound under water [11].
Figure 1. Anatomical structures of the testudine audiotory system in Trachemys scripta elegans.
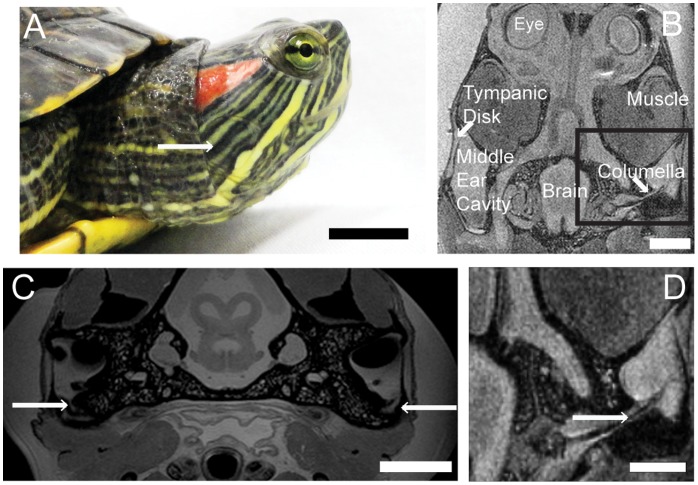
A. Lateral view of head (1 cm scale bar). B: Horizontal MR image. (500 mm scale bar) C: Transverse MRI at the level of the tectum. Arrows indicates Eustachian tubes (500 mm scale bar). “Muscle” is the splenius capitus. D: Horizontal MR image, enlarged from box in B. The columella runs through the middle ear cavity to the inner ear. Arrow indicates the columella (500 mm scale bar).
These findings raise many questions. Greater sensitivity to sound under water could be conferred by multiple adaptations. Christensen-Dalsgaard and colleagues suggest that the origin of greater sensitivity to underwater sound is the ability of the middle ear cavity to resonate in the underwater sound field, increasing sensitivity at resonant frequencies [11]. Is this type of middle ear cavity is a feature of all turtles and tortoises, or is it only found in those testudines that spend significant time underwater? How do variations in middle ear structures inform our understanding of the evolutionary history of testudines? We demonstrate here that middle ear scaling and morphology is similar across extant species, regardless of ecological niche or phylogenetic position.
Results
Anatomy
In all species examined, the Eustachian tubes were small and opened adjacent to the tympanic disk on the ventral wall of the middle ear cavity, connecting the cavity to the pharynx (Fig. 1) [10]. We used Trachemys scripta elegans as an example species for some more detailed anatomical studies. In T. scripta elegans, the Eustachian tubes are narrow but detectable on MR images (Fig. 1 C). The fluid-filled tube appeared as a grey duct, because the middle ears were filled with saline postmortem to optimize the image. At the opening of the Eustachian tube from the middle ear, on one sample of T. scripta elegans, the tube measured about 500 µm in diameter. All species examined had middle ear cavities in the general form of paraboloids with the long axis oriented rostrocaudally, parallel to the midline (Fig. 2).
Figure 2. Examples of middle ear morphology of extant turtles and tortoises.
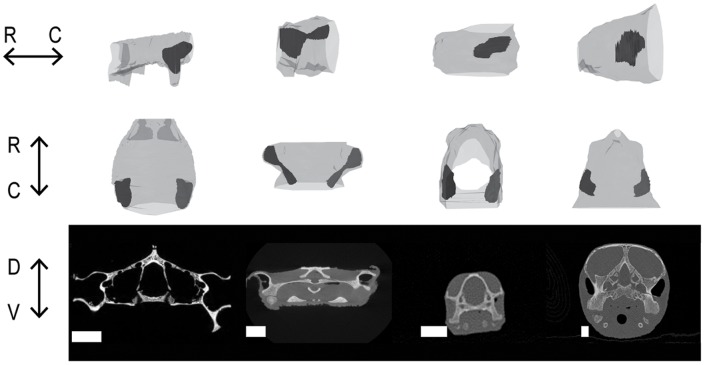
Middle ear cavities are in black with skulls in gray. Top row. Lateral view of the left side. Middle row: Dorsal view. Bottom row: Cross section CT images at the level of the middle ear cavity. Species in columns from left to right: Gopherus polyphemus, Chelus fimbriatus, Trachemys scripta elgans, Lepidochelys kempii. Scale bars = 1 cm. R = rostral. C = caudal. D = dorsal. V = ventral. Note that G. Polyphemus was scanned as only a skull.
Allometry of Middle Ear Cavity Volume in Trachemys Scripta Elegans
In order to assess changes over the lifespan of an animal, an allometric series of 5 Red-eared Sliders (T. scripta elegans) was analyzed separately from the other species [13] (Fig. 3 B) and included in the whole data set (Fig. 3 A). For T. scripta elegans,
with r2 = 0.89 showing that, during the growth of an animal, head size increases allometrically with body size. From visual inspection, the overall shape of the cavity did not change with the body size.
Figure 3. Allometry of middle ear cavities.
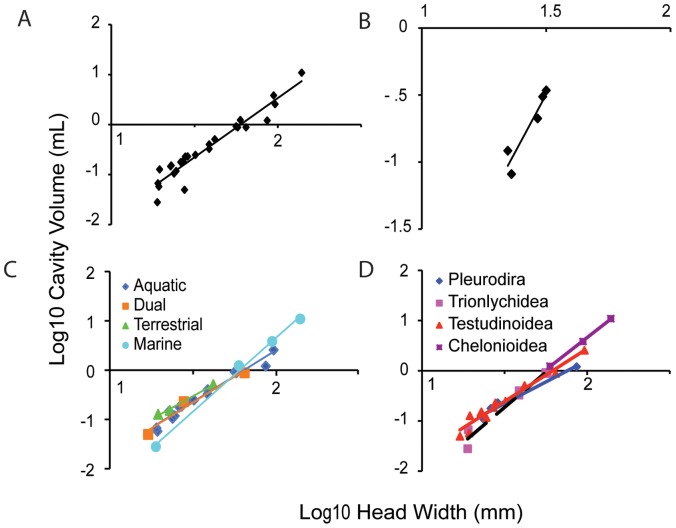
A: Scaling of middle ear cavity volume and head width across extant testudines B: Scaling of volume and head width in Trachemys scripta elegans. C: Scaling of middle ear cavity volume and head width across extant testudines divided by ecological niche. D: Scaling of middle ear cavity volume and head width across extant testudines divided by phylogenetic position.
Cross-species Comparisons
In the 25 species from 12 families examined (Table 1), the middle ear cavity was a paraboloid (Fig. 2) that scaled with head size (Fig. 3). The scaling followed the equation
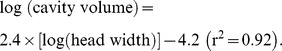 |
Table 1. Phylogenetic relationships of the species studied.
| Suborder | Superfamily | Family | Subfamily | Species | Ecology |
| Pleurodira | Chelidae | Elseya dentata | Aquatic | ||
| Chelus fimbriatus | Aquatic | ||||
| Pelomedusoidea | Podocnemididae | Pelusios sinuatus | Aquatic | ||
| Podocnemis unifilis | Aquatic | ||||
| Cryptodira | Trionychidea | Carettochelyidae | Carettochelys insculpta | Aquatic | |
| Dermochelyidae | Dermochelys coriacea | Marine | |||
| Kinosternidae | Staurotypinae | Staurotypus salvinii | Aquatic | ||
| Kinosterninae | Kinosternon bauri | Aquatic | |||
| Trionychidae | Trionychinae | Trionyx triunguis | Aquatic | ||
| Apalone mutica | Aquatic | ||||
| Testudinoidea | Platysternidae | Platysternon megacephalum | Dual | ||
| Bataguridae | Geoemydinae | Rhinoclemmys pulcherrima | Terrestrial | ||
| Cuora amboinensis | Dual | ||||
| Emydidae | Emydinae | Glyptemys (Clemmys) muhlenbergii | Dual | ||
| Emys orbicularia | Aquatic | ||||
| Malaclemys terrapin | Aquatic | ||||
| Deirochelyinae | Trachemys (Pseudemys) scripta elegans | Aquatic | |||
| Chrysemys picta picta | Aquatic | ||||
| Testudinidae (Tortoises) | Testudo horsfieldi | Terrestrial | |||
| Gopherus polyphemus | Terrestrial | ||||
| Chelydridae | Chelydra serpentina | Aquatic | |||
| Macroclemys temminckii | Aquatic | ||||
| Chelonioidea (Sea Turtles) | Cheloniidae | Carretta caretta | Marine | ||
| Chelonia mydas | Marine | ||||
| Lepidochelys kempii | Marine |
At least one representative from each family of testudines was included in this study, with the exception of the Dermatemydidae, a monotypic family containing Dermatemys mawii for which no museum specimen was available.
The exception to this morphology was the Matamata, Chelus fimbriatus, which has a hyperboloid (hourglass-shaped) middle ear cavity, which also scaled following the above equation. C. fimbriatus, is a pleurodire (Side-necked Turtle) and inhabits the Amazonian river basin. Its skull is dorsoventrally flattened, and its unusual skull morphology may constrain middle ear cavity dimensions.
The wavelengths of the sound range in question are much greater than the dimensions of the cavity and thus the effects of the shape of the cavity are negligible [14]. Because the volume of the cavity is the primary factor for acoustic characteristics of the middle ear cavity at frequencies relevant to testudines, we used a sphere equal to the measured paraboliod volume for resonance calculations for each middle ear cavity [14].
Scaling and Morphology do not Change with Ecology or Phylogeny
We compared the scaling of the middle ear cavity volumes, with head width as a covariate, among the ecological groups, using univariate ANOVA, and found no significant differences (p = 0.494, model 2 regression r2 = 0.942). When the scaling of the middle ear cavity volumes with head width was compared among the phylogenetic groups by using univariate ANOVA, no significant differences were found (p = 0.282, model 2 regression r2 = 0.773).
Middle Ear Cavity can Function as a Resonator
Calculations were performed for a model of an air-filled sphere vibrating in an underwater sound field [14]. Unlike the ears of lepidosaurs and archosaurs, testudine ears are not acoustically coupled [11] and because the wavelengths are large compared with the size of the cavity, calculations were based only on the volume of the middle ear cavity. Middle ear cavities ranged in volume from 0.03 mL to 10.9 mL; head widths ranged from 19–140 mm (Fig. 3). By modeling the middle ear cavity as a sphere vibrating underwater, we calculated the resonance frequencies of the cavities as ranging from 240–1740 Hz (Fig. 4).
Figure 4. Calculated best resonance underwater frequency of middle ear cavities of extant species, changing with head size.
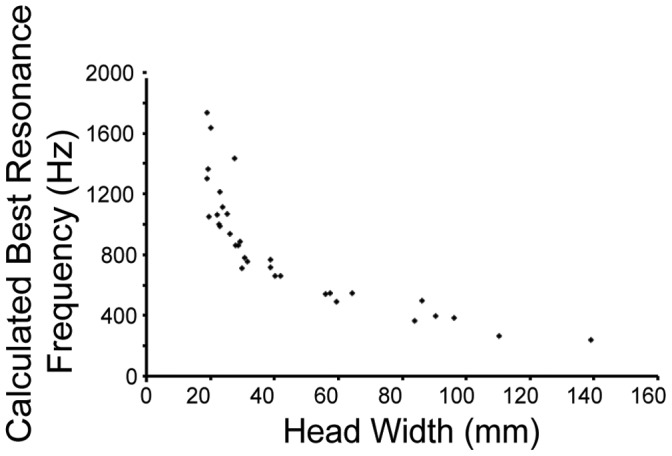
Sea Turtles (Family Cheloniidae)
Sea Turtle middle ear cavities contain varying amounts of fatty tissue adjacent to the tympanic disk, even differing bilaterally within the same animal [9], [15]. The amount of fatty connective tissue, and therefore the amount of residual air space in the middle ear, varied among the Sea Turtles examined, which complicated resonant frequency calculations. Because it was unclear what the exact volume of the middle ear fats might be and to what extent they compress with depth, our calculated resonance frequencies might be lower than the actual resonance frequencies experienced by the sea turtles (smaller effective resonating volume results in higher resonance frequencies). However, to date there are no published measurements of the maximal or minimal volumes for these fats nor of their elasticity or compressibility. Scans of both live and post-mortem sea turtle specimens demonstrate that the space occupied by soft tissue in the middle ear cavity can vary between individuals and even bilaterally within the same turtle, but it is not known whether these variations remain underwater. In the absence of such data, we calculated the maximal cavity volume based on skull morphology. Based on the skull structure, the allometry of the middle ear cavity of sea turtles did not scale differently from the other testudines (Fig. 3 C, D).
Extinct Species
CT scans of several extinct species, including Galianemys emringeri, Galianemys whitei, Nichollsemys baieri, and Hamadachelys escuilliei, revealed that Galianemys and Hamadachelys species have middle ears that are connected through the mouth, to the extent observable from the fossilized remains (Fig. 5), while Nichollsemys baieri has more isolated ears, like the extant testudines (Fig. 2). In the CT images of the Galianemys and Hamadachelys species, there is a clear opening from the middle ear cavity into the mouth (Fig. 5 C). This large opening is not seen in N. baieri. As the Eustachian tubes are comprised of soft tissue, the size of the Eustachian tubes could not be determined.
Figure 5. Examples of middle ear cavities of extinct testudines.
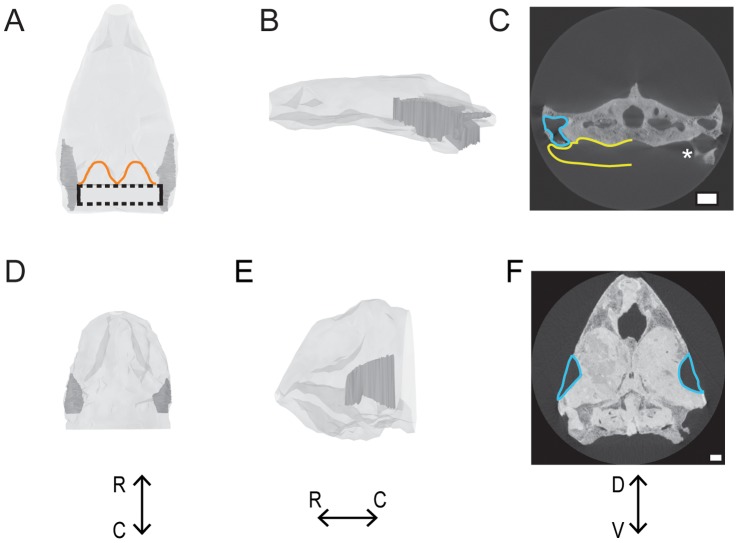
A-C: Connected ears of Galianemys emringeri. Connected middle ears are shown in dark gray; the skull is shown in light gray. The maximum space that the connected middle ears could possibly occupy is indicated by the dashed line. The dorsocaudal edge of the skull is outlined in orange. D-F: Separated ears of Nicholsemys baieri. Isolated middle ears are show in dark gray; skull is shown in light gray. A & D: Dorsal view. B & E: left lateral view. C & F: Transverse view from CT. Middle ear cavities are outlined in blue, and possible extent of middle ear cavity into pharynx is yellow. Asterisk indicates most caudal part of the middle ear cavity that can be seen intact before it opens into the pharynx. Scale bars = 1 cm. R = rostral. C = caudal. D = dorsal. V = ventral.
Connected ears were also shown in Proganochelys [16]. These specimens were not reconstructed in detail, nor used for volume calculations, because of the potential distortions derived from fossil compression. Galianemys emringeri, Galianemys whitei, Nichollsemys baieri were pleurodires, and Hamadachelys escuilliei a cryptodire. All of the specimens were found in Cretaceous formations.
Discussion
Middle Ear Cavities Enhance Hearing
Resonance via enlarged middle ear cavities has been shown to affect hearing in a number of vertebrate classes, both in air and under water. For example, the enlarged middle ear cavity of kangaroo rats underlies good hearing thresholds below 3 kHz, particularly in the 1–2 kHz range [17], [18]. Similarly, the bulla (middle ear cavity) in gerbillines acts like a Helmholtz resonator, lowering hearing thresholds [19]. One example of air-filled structures lowering hearing thresholds underwater is Ostariophysan fish, which couple swimbladders to Weberian ossicles, enabling sound pressure hearing, not just detection of particle motion [20]–[23]. Similarly, the ranid frog Lithobates (Rana) catesbeiana is more sensitive to sound below 200 Hz underwater than in air and is equally sensitive in air and in water for frequencies above 400 Hz, possibly due to specialization of the amphibian papilla [24]. The middle ear cavity of the African clawed frog (Xenopus laevis) provides hearing advantages underwater [25]. The ear of Xenopus works like the turtle ear, with cartilaginous tympanic disks and an air-filled resonating cavity. Xenopus also has further adaptations for underwater hearing, including a tighter coupling and lower lever ratio between the tympanic disk and ossicles than do the ranid frogs [26].
Wever and Vernon were aware of the potential for middle ear resonance in their studies of turtle hearing [10]. They calculated resonance frequencies for the middle ear cavities in Chrysemys picta picta and Trachemys (Pseudemys) scripta in air to be 6 kHz by using a closed tube model where the resonance frequency quarter wavelength matches the length of the tube. Volumes used in obtaining this value were not published. Because 6 kHz was well above measured highest audible frequency (about 2 kHz), they discounted any increased sensitivity modeling based on resonance. Recent studies, however, show that the ear of Trachemys scripta elegans is more sensitive to sound underwater than in air [11], where resonance frequencies are much lower. We hypothesize that the conserved structure of the testudine ear is an adaption for underwater hearing that was retained by neutral selection.
Middle ear cavities are also interesting from the perspective of understanding how a major vertebrate group processes sound. Hearing has been documented in multiple species of testudines, demonstrating that these animals have auditory sensitivity, albeit with higher thresholds in air than those of other reptiles [9]. Six testudine species have published in air audiograms (Table 2), with best hearing frequencies below 1000 Hz (around 400–600 Hz). There is much to be learned about how the testudine middle ear responds to sound underwater. Laser vibrometry studies, perhaps from post-mortem samples from a variety of species, could be used to test the hypothesis that both turtle and tortoise ears would respond well to underwater sound. The fossil specimens without isolated middle ear cavities could represent either the ancestral diapsid condition, or a secondary loss. As more extinct species are discovered, answers to this question should become clearer.
Table 2. Published testudine in-air audiograms.
| Species | Lowest TestedFrequency (Hz) | Highest TestedFrequency (Hz) | Best FrequencyRange (Hz) | Reference |
| Chelonia mydas | 30–40 | 2000 | 300–400 | [15] |
| Clemmys insculpta | 100 | 5000 | 500 | [48] |
| Chrysemys picta picta | 100 | 4000 | 400–500 | [48] |
| Caretta caretta | 250 | 1000 | 250–500 | [49] |
| Terrapene carolina carolina | 30 | 4000 | 400 | [50] |
| Trachemys scripta elegans | 100 | 3000 | 500 | [48] |
| Trachyemys scripta elegans | 64 | 1000 | 400–700 | [51] |
| Trachemys scripta elegans | 100 | 1000 | 400–500 | [11] |
Sea Turtle Ears
The function of the fatty tissue in Sea Turtle middle ears is unknown, while the high degree of variability in these structures adds to the mystery. There are a variety of hypotheses about their function, including their being an adaptation to the pressure resulting from deep diving [9], [15], or a secondary pathway for sound transmission, in a manner analogous to the fatty channels in the jaws of marine mammals [27]. While our data do not address the function of this tissue, they do suggest that fatty tissue in the middle ear may be secondary adaptation in Sea Turtles, because their skull elements and allometry are the same as the other testudines.
Phylogenetic Position of Testudines
As shown by Christensen-Dalsgaard and colleagues, at least one species of turtle hears well under water than in air, largely due to the middle ear cavity [11]. Given that the middle ear cavity resonates underwater within the published in-air testudine hearing range and that the middle ear cavity resonates beyond that range in air [10], our findings of unchanging middle ear cavity allometry among the testudines support the hypothesis of an aquatic origin for this group. Since the tortoises retained this allometric relationship, we further hypothesize that the middle ear cavity does not impede hearing in air.
Analyses of the hearing of testudines have been complicated by their ill-defined relationship to other major vertebrate groups. Since testudines are anapsids, they had been considered an extant representative of the parareptiles, which places them as a sister to the entire diapsid clade. This position was supported by some morphological analyses [28], [29]. Rieppel and deBraga, however, proposed that testudines were the sister group to lepidosaurs [30]. They state that the traditional view, in which the number of temporal fenestra is the deciding factor for determining vertebrate relationships, is too narrow. Their analyses included a much wider range of non-skull characters [30]. A recent study of mesosaurid skulls supports diapsid affinities of the testudines [31]. Interestingly, data that support testudines being either the sister group to the archosaurs or to the entire diapsid clade support a terrestrial origin of testudines [29]; conversely, the data that support testudines being the sister group to lepidosaurs support an aquatic origin [32].
The advent of molecular techniques and the application of these methods to phylogenetic problems called into question the traditional understanding of the position of testudines. Phylogenomic analyses have led to a reevaluation of the position of the testudines. These studies robustly support the position of testudines as sister to the archosaurs, with the archosaurs remaining monophyletic [33], [34]. Hedges and Poling found that in all but one gene, testudines were most closely related to archosaurs [35]. The position of testudines within the diapsid clade has been supported by other molecular analyses [36]–[39].
While our data do not directly address the phylogenetic position of testudines, they support an aquatic origin for this group. There is also support for this claim from the fossil record: Odontochelys, the most basal testudine discovered thus far, appears to have been aquatic [40]. It is parsimonious to assume that the common ancestor of archosaurs, lepidosaurs, and testudines had coupled ears that opened into the pharynx, since coupled ears are the ancestral condition for tympanic ears (Fig. 6) [41], [42]. Our data suggest that Testudines secondarily evolved acoustically isolated middle ear cavities because of the improved underwater sound sensitivity they provide.
Figure 6. Proposed middle ear structure across some extant vertebrate taxa.
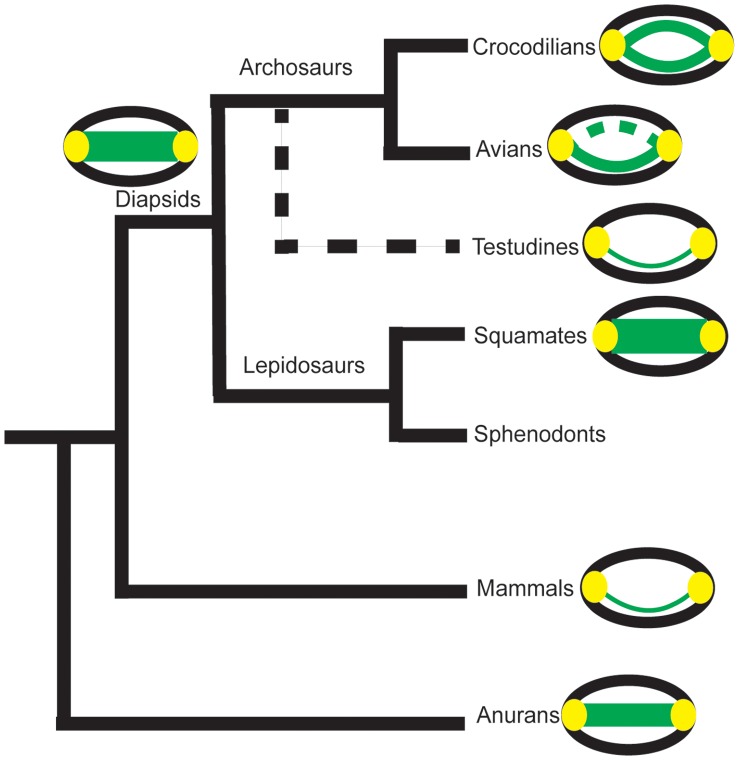
Skulls are shown in black, tympanic ears in yellow, connections between the ears (Eustachian tubes or through the buccal cavity) in green. The dashed line on the avian diagram indicates trabeculated bone. The proposed diapsid ancestral condition is also shown. The dashed branch to testudines indicates their suggested phylogenetic position [33]–[35].
Methods
Imaging
We examined the middle ear cavity and associated structures using X-ray computed tomography (CT) and magnetic resonance imaging (MRI) (Table 1). Specimens (Trachemys scripta elegans and Macroclemys temminckii) were prepared for magnetic resonance (MR) scanning by euthanasia via an overdose of Euthasol (Virbic Animal Health, Fort Worth, TX). The heads were then removed and immersion-fixed in 4% paraformaldehyde (PFA) in 0.01 M phosphate buffered saline (PBS) for a minimum of 1 week. The fixed heads were rehydrated in 0.01 M PBS a minimum of 24 hours before the scan. In order to optimize the image the middle ear cavities were filled with PBS: one syringe was inserted into the tympanic disk to remove air while another syringe was simultaneously used to inject 0.01 M PBS.
Trachemys scripta elegans was chosen as an example species for an allometric series because it is an amphibious invasive species and commercially available. Animals were obtained from a commercial dealer. Furthermore, the small head size allowed imaging in the most powerful MR scanner (9.4 T). MR images of Macroclemys temminckii and T. scripta elegans were acquired at the Armed Forces Institute of Pathology (Rockville, MD). Prior to imaging, larger heads were sealed in a plastic bag filled with 0.01 M PBS and imaged with a 72 mm volume coil on a Bruker Biospec spectrometer (Bruker Biospin, Inc. Billerica, MA) coupled to a horizontal-bore magnet (diameter: 20 cm) operating at 7 T (300 MHz for protons) using a Rapid Acquisition with Relaxation Enhancement (RARE) sequence with the following acquisition parameters: TR/TE = 1500/10 ms, NA = 4, RARE = 8. Small heads were immobilized in glass tubes (o.d. 25 mm) filled with PBS and imaged with a 25 mm RF insert on a Bruker DMX spectrometer (Bruker Biospin) coupled to a wide-bore magnet (dia. 89 mm) operating at 9.4 T (400.13 MHz for protons). Typical RARE images had a voxel resolution of 100×100×100 µm,) and the analyses were performed using 512 matrix TIFF images.
For all marine species, as well as Trachemys scripta elegans and Malaclemmys terrapin, submillimeter, ultrahigh resolution computerized tomography (UHRCT) images were obtained on a Siemens Volume Zoom CT scanner at the Woods Hole Oceanographic Institution Imaging Facility. Marine species were obtained post-mortem after death by natural causes. A spiral protocol was employed with 120 kV, 100 mA, 150 effective mAS, 0.5 mm collimation, 0.5 mm/sec table feeds and a 0.5 mm table pitch. Both live (physically restrained) and post-mortem turtles were scanned prone, head first, with scans acquired in the transaxial (shorter cross-section) plane. Images were reconstructed using soft, ultra-high bone, and lung kernels at 0.1 and 0.5 mm increments for the whole head and data based magnifications at smaller FOV of the ear regions alone. The 0.1 mm images provided image data sets with isotropic 100 µm voxel resolution, which were used for volume measurements and cavity reconstructions in 3D. Raw attenuation data and all 512 matrix DICOM images were archived onto CD and magneto-optical disks. In each of these programs, tissues were selected for auto-segmentation based on Hounsfield Unit values for tissue attenuations and air space attenuation. The auto-segmentations were reviewed visually and segmentation boundaries corrected when they incorporated inappropriate adjacent regions.
For all other species, CT images were obtained from DigiMorph (University of Texas, Austin). The images were 1024×1024 16-bit TIFF format. Scan parameters varied some depending on the specimen. A typically example follows: P250D, 420 kV, 1.8 mA, one brass filter, empty container wedge, 190% offset, integration time of 64 ms, slice thickness was 0.5 mm, S.O.D. was 698 mm, 1400 views, one ray averaged per view, one sample per view, interslice spacing of 0.4 mm, field of reconstruction of 268 mm (maximum field of view 280.1441), reconstruction offset of 6100, reconstruction scale of 3200. Ring-removal processing was based on correction of raw sinogram data using IDL routine “RK_SinoRingProcSimul” with parameter “BESTOF5.” This is a standardized process done for all CT scans by the imaging facilties. For an overview of the analysis of CT images, see [43]. The extinct species used were Galianemys emringeri (sample ID: AMNH 30035), Galianemys whitei (sample ID: AMNH 29987), Nichollsemys baieri (sample ID: TMP 97.99.1), and Hamadachelys escuilliei (sample ID: MDE-T-03).
Analysis
All scan files were converted to TIFF stacks and imported into Neurolucida (MicroBrightField Bioscience, Williston, VT). For species that were scanned using both MR and CT, all data sets were used. The outlines of the structures were all traced manually in serial sections. In CT scans, the lateral edge of the middle ear cavity was defined by connecting the most medial points of bone in images where the cavity was open with a straight line. In images where the soft tissue was visible, that line was drawn through the middle of the tympanic disk. Since some the CT images usually did not include the soft tissue tympanic disk, a straight line across the opening was the best approximation. These tracings were analyzed using the NeuroExplorer module to calculate the enclosed volume. Reconstructed area is accurate to one micrometer (MicroBrightField stated accuracy). Head widths were measured as a straight line across the widest part of the head, accurate to 0.1 micrometer. Approximate head widths were confirmed as the same with calipers when possible.
Resonance was calculated by modeling the middle ear cavity as an air-filled sphere vibrating underwater using the following equation:
(frequency in Hertz) [14]. Because the frequencies in question are low, and therefore the wavelengths much larger that the dimension of the cavity, the cavity can be treated as a lumped element with a resonance frequency that only depends on volume.
Univariate ANOVA tests were performed with the middle ear cavity volume co-varying with head width data categorized by ecological niche and phylogenetic position (Table 1). Ecological niche was defined by the medium in which the species spends the majority of its life. We divided the non-marine species according to how much time they spent in the water, in order to perform a univariate ANOVA test among the ecological niches. Animals that spent the majority (greater than 60%) of their time in non-marine environments (e.g. pond turtles) were categorized as aquatic. Sea turtles were categorized as marine. Animals spending the majority of their time on land (e.g. tortoises) were categorized as terrestrial. Those species spending approximately equal amounts of time on land and water were categorized as “dual”. We divided the Crypotodirae into superfamilies (Trionychidea, Testuinoidea, Chelonioidea), in order to perform a univariate ANOVA test among the phylogenetic groups. Phylogentic position was determined according to the species information from the University of Michigan Museum of Zoology [44]. Ecological niches were from the descriptions by [45]. We analyzed Pleurodirae as one group because of the small number of species available and because there are far fewer extant species relative to the cryptodires.
Experiments were performed according to the guidelines approved by the Marine Biological Laboratory (Woods Hole, MA, USA), the University of Maryland Institutional Animal Care and Use Committees (IACUC) and the Danish National Animal Experimentation Board (Dyreforsøgstilsynet).
Conclusions
After separating species by ecology and phylogeny (Fig. 4), there were no significant differences in the variation of middle ear cavity volume and head width, suggesting that there has been little modification among extant testudines. Since middle ear cavities enhance hearing under water [11], it follows that testudines should have lower hearing thresholds in water than in air. A lower hearing threshold under water than in air could only theoretically apply to the terrestrial species. Since not all extant testudines are aquatic or amphibious, the most probable explanation for this constancy is that neutral selection has maintained middle ear cavity scaling.
Given constancy in middle ear cavity scaling, we hypothesize that the most recent common ancestor of the extant testudines was primarily aquatic and had separated middle ears, an assertion supported by two observations from the fossil record. First, in some extinct species of testudines, including Galianemys emringeri, Galianemys whitei, and Hamadachelys escuilliei, the middle ear cavities opened into the mouth, as does the internally coupled, pressure-difference receiver ear of lizards [24], [46], [47]. It has been argued that coupled ears are both the simplest configuration of, and the ancestral condition for, tympanic ears (Fig. 6) [42]. Second, isolated middle ear cavities appeared in both the extinct marine cryptodire, Nichollsemys baieri [48], and independently in the mosasaurs (marine lizards) [49]. The evolution of isolated middle ear cavities in testudines would have provided some selective advantage, which we hypothesize was an increased sensitivity for conspecific vocalizations and auditory scene analysis in a primarily aquatic habit, which may then have been retained by neutral selection.
Acknowledgments
We acknowledge J. Arruda, C. Bell, D. Brinkman, K. Catania, S. Cramer, E. Gaffney, H. Jamniczky, J. Maisano, and K. Potter for assistance with scans and specimens, and H. Bierman, D. Hertz, R. Highton, T. Holtz, J. Merck, and D. Soares for advice and discussion. We thank the anonymous reviewers for their helpful comments.
Funding Statement
This work was supported by awards from the Danish National Science Foundation 09-065990 and Carlsberg Foundation 2009- 01-0684 (JCD), the Velux Foundation (Denmark), ONR N000140811231(DRK) and NIH (National Institutes of Health) DC00436 (CEC), and by NIH P30 DC0466 to the University of Maryland Center for Comparative and Evolutionary Biology of Hearing, by NSF IIS 9874781 and 0208675 to T. Rowe. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Carr A F (1969) Handbook of turtles; the turtles of the United States, Canada, and Baja California. Ithaca, N.Y.: Comstock Pub.
- 2.Campbell HW, Evans WE (1972) Observation on the vocal behavior of chelonians. Herpetologica 28, 277–280.
- 3.Campbell HW, Evans WE (1967) Sound production in two species of tortoises. Herpetologica 23, 204–209.
- 4.Frazier J, Peters G (1981) The call of the Aldabra tortoise (Geochelone gigantea) (Reptilia, Testudinidae). Amphibia-Reptilia 2, 1–17.
- 5.Giles JC, Davis JA, McCauley RD, Kuchling G (2009) Voice of the turtle: The underwater acoustic repertoire of the long-necked freshwater turtle, Chelodina oblonga J. Acoust. Soc. Am. 126, 434–443. [DOI] [PubMed]
- 6.Konishi M (1970) Comparative neurophysiological studies of hearing and vocalizations in songbirds. Z. vergl. Physiologie 66, 257–272.
- 7.Guillon JM, Guery L, Hulin V, Girondot M (2012) A large phylogeny of turtles (Testudines) using molecular data. Contributions to Zoology 81, 147–158.
- 8.Gaffney ES (1972) An Illustrated Glossary of Turtle Skull Nomenclature. American Museum Novitates, 1–33.
- 9.Wever EG (1978) The reptile ear: its structure and function. Princeton, N.J.: Princeton University Press.
- 10.Wever EG, Vernon JA (1956) Sound transmission in the turtle’s ear. PNAS 42, 292–299. [DOI] [PMC free article] [PubMed]
- 11.Christensen-Dalsgaard J, Brandt C, Willis KL, Christensen CB, Ketten D, et al. (2012) Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans. Proceedings of the Royal Society B: Biological Sciences 279, 2816–2824. [DOI] [PMC free article] [PubMed]
- 12.Adrian E, Craik K, Sturdy R (1938) The electrical response of the auditory mechanism in cold-blooded vertebrates. Proc Roy Soc B 125, 435–455.
- 13.Willis KL, Potter K, Carr CE (2011) Allometry of the Middle Ear Cavity in Trachemys scripta elegans. In. Society for Neuroscience Annual Meeting.
- 14.Urick RJ (1983) Principles of underwater sound (New York: McGraw-Hill).
- 15.Ridgway SH, Wever EG, McCormick JG, Palin J, Anderson JH (1969) Hearing in the giant sea turtle, Chelonia mydas PNAS 64, 884–890. [DOI] [PMC free article] [PubMed]
- 16.Gaffney ES (1983) Skull Morphology of the Oldest Turtles: A Preliminary Description of Proganochelys quenstedti J Vert Paleontol 3, 25–28.
- 17.Webster DB (1962) A function of the enlarged middle-ear cavities of the kangaroo rat, Dipodomys Physiol Zool 35, 248–255.
- 18.Ravicz ME, Rosowski JJ (1997) Sound-power collection by the auditory periphery of the Mongolian gerbil Meriones uguiculatus: III. Effect of variations in middle-ear volume. J Acoust Soc Amer 101, 2135–2147. [DOI] [PubMed]
- 19.Plassmann W, Kadel M (1991) Low-frequency selectivity in a gerbilline Rodent, Pachyurmys dupras Brain Behav Evol 38, 115–126. [DOI] [PubMed]
- 20.Evans HM (1925) A Contribution to the Anatomy and Physiology of the Air-Bladder and Weberian Ossicles in Cyprinidae. Proc Roy Soc B 97, 545–576.
- 21.Webb JF, Smith WL, Ketten DR (2006) The laterophysic connection and swim bladder of butterfly fishes in the genus Chaetodon (Perciformes: Chaetodontidae). J. Morphol. 267, 1338–1355. [DOI] [PubMed]
- 22.Zeddies DG (2005) Development of the acoustically evoked behavioral response in zebrafish to pure tones. Journal of Experimental Biology 208, 1363–1372. [DOI] [PubMed]
- 23.Polgar G, Malavasi S, Cipolato G, Georgalas V, Clack JA, et al. (2011) Acoustic Communication at the Water’s Edge: Evolutionary Insights from a Mudskipper. PLoS ONE 6, e21434. [DOI] [PMC free article] [PubMed]
- 24.Lombard RE, Fay RR, Werner YL (1981) Underwater hearing in the frog, Rana catesbeiana J. Exp. Biol 1981, 57–71.
- 25.Christensen-Dalsgaard J, Elepfandt A (1995) Biophysics of underwater hearing in the clawed frog. J comp Physiol A 176, 1–8. [DOI] [PubMed]
- 26.Mason M, Wang M, Narins PM (2009) Structure and function of the middle ear apparatus of the aquatic frog, Xenopus laevis Proc Inst Acoust 31, 13–21. [PMC free article] [PubMed]
- 27.Ketten DR, Merigo C, Chiddick E, Krum H (1999) Acoustic fatheads: Parallel evolution of underwater sound reception mechanisms in dolphins, turtles, and sea birds. In. Acoustical Society of America Annual Meeting.
- 28.Lee MSY (2001) Molecules, morphology, and the monophyly of diapsid reptiles. Contributions to Zoology 70, 1–22.
- 29.Lyson TR, Bever GS, Bhullar BAS, Joyce WG, Gauthier JA (2010) Transitional fossils and the origin of turtles. Biology Letters 6, 830–833. [DOI] [PMC free article] [PubMed]
- 30.Rieppel O, deBraga M (1996) Turtles as diapsid reptiles. Nature 384, 453–455.
- 31.Piñeiro G, Ferigolo J, Ramos A, Laurin M (2012) Cranial morphology of the Early Permian mesosaurid Mesosaurus tenuidens and the evolution of the lower temporal fenestration reassessed. Comptes rendus - Palevol 11, 379–391.
- 32.Rieppel O, Reisz RR (1999) The Origin and Early Evolution of Turtles. Annu Rev Ecol Syst 30, 1–22.
- 33.Shen XX, Liang D, Wen JZ, Zhang P (2011) Multiple Genome Alignments Facilitate Development of NPCL Markers: A Case Study of Tetrapod Phylogeny Focusing on the Position of Turtles. Molecular Biology and Evolution 28, 3237–3252. [DOI] [PubMed]
- 34.Crawford NG, Faircloth BC, McCormack JE, Brumfield RT, Winker K, et al. (2012) More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biology Letters 8, 1–4. [DOI] [PMC free article] [PubMed]
- 35.Hedges SB, Poling LL (1999) A molecular phylogeny of reptiles. Science 283, 998–1001. [DOI] [PubMed]
- 36.Mannen H, Li SSL (1999) Molecular evidence for a clade of turtles. Molecular Phylogenetics and Evolution 13, 144–148. [DOI] [PubMed]
- 37.Zardoya R, Meyer A (2001) The evolutionary position of turtles revised. Naturwissenschaften 88, 193–200. [DOI] [PubMed]
- 38.Iwabe N (2005) Sister Group Relationship of Turtles to the Bird-Crocodilian Clade Revealed by Nuclear DNA-Coded Proteins. Molecular Biology and Evolution 22, 810–813. [DOI] [PubMed]
- 39.Chiari Y, Cahais V, Galtier N, Delsuc F (2012) Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria). BMC Biol 10, 65. [DOI] [PMC free article] [PubMed]
- 40.Li C, Wu XC, Rieppel O, Wang LT, Zhao LJ (2008) An ancestral turtle from the Late Triassic of southwestern China. Nature 456, 497–501. [DOI] [PubMed]
- 41.Clack JA (2002) Gaining ground: the origin and evolution of tetrapods Bloomington: Indiana Univ. Press.
- 42.Christensen-Dalsgaard J, Carr CE (2008) Evolution of a sensory novelty: Tympanic ears and the associated neural processing. Brain Research Bulletin 75, 365–370. [DOI] [PMC free article] [PubMed]
- 43.Witmer LM, Ridgely RC, Dufeau DL, Sermones MC (2008) Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and non-avian dinosaurs. In Anatomical Imaging: Towards a new morphology, H. Endo and R. Frey, eds. Tokyo: Springer-Verlag, 67–88.
- 44.Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, et al. (2012) The Animal Diversity Web (online). Available: http://animaldiversity.org.
- 45.van Dijk PP, Iverson J, Shaffer B, Bour R, Rhodin A (2011) Conservation Biology of Freshwater Turtles and Tortoises First. A. Rhodin, P. Pritchard, P. P. van Dijk, R. Saumure, K. Buhlmann, J. Iverson, and R. Mittermeier, eds. Chelonian Research Foundation.
- 46.Tang Y, Christensen-Dalsgaard J, Carr CE (2012) Organization of the auditory brainstem in a lizard, Gekko gecko. I. Auditory nerve, cochlear nuclei, and superior olivary nuclei. J. Comp. Neurol. 520, 1784–1799. [DOI] [PMC free article] [PubMed]
- 47.Christensen-Dalsgaard J, Manley GA (2008) Acoustical Coupling of Lizard Eardrums. JARO 9, 407–416. [DOI] [PMC free article] [PubMed]
- 48.Brinkman DB (2005) A vertebrate assemblage from the marine shales of the Lethbridge Coal Zone. In P. J. Currie and E. B. Koppelhus (eds.) Dinosaur Provincial Park, A spectacular ancient ecosystem revealed. Indiana University Press, Bloomington and Indianapolis, Indiana.
- 49.Hetherington T (2008) Comparative Anatomy and Function of Hearing in Aquatic Amphibians, Reptiles, and Birds. In Sensory evolution on the threshold : adaptations in secondarily aquatic vertebrates, J. G. M. thewissen and S. Nummela, eds. Berkeley: University of California Press.
- 50.Wever EG, Vernon JA (1956) The sensitivity of the turtle’s ear as shown by its electrical potentials. PNAS 42, 213–220. [DOI] [PMC free article] [PubMed]
- 51.Bartol SM, Musick JA, Lendhardt ML (1999) Auditory evoked potentials of the loggerhead sea turtle (Caretta caretta) Copeia 1999, 836–840.
- 52.Wever EG, Vernon JA (1956) Auditory responses in the common box turtle. PNAS 42, 962–965. [DOI] [PMC free article] [PubMed]


