Abstract
Introduction
Human umbilical tissue-derived cells (hUTC) are a promising source of cells for regenerative treatment of stroke. In this study, we tested the efficacy of hUTC in experimental stroke and whether multiple injections of hUTC provide additional therapeutic benefits as compared to a single injection.
Methods
Adult male Wistar rats were subjected to 2 hours of middle cerebral artery occlusion (MCAo), and randomly selected animals were injected (i.v) with 3×106 hUTC or with vehicle control (at day: 1, 1&3 or 1&7 after MCAo, n = 8–9/group). A battery of functional outcome tests was performed at days 1, 7, 14, 21, 28, 35, 42, 49, 56 and 63 after MCAo. Rats were sacrificed at 63 days after MCAo and lesion volumes were measured. To investigate the underlying mechanism of hUTC treatment of stroke, Von Willebrand Factor (vWF), and Synaptophysin immunostaining were performed.
Results
All hUTC treated groups, single or multiple injections, had better functional recovery compared to control (p<0.01). There was no statistically significant difference between a single and multiple injections of hUTC (p = 0.23) or between different multiple injections groups (p>0.07) in functional outcome. All hUTC treatment groups showed significant increases in Synaptophysin, vascular density and perimeter compared to the control group (p<0.05). There was no statistically significant difference between a single and multiple injections of hUTC or between the two groups of multiple injections in all immunohistochemical measurements (p>0.1).
Conclusion
hUTC treatment significantly improves long term functional outcome after stroke and promotes vascular density and synaptic plasticity. At the proscribed doses, multiple injections of hUTC were not superior to single injection therapy in both functional outcome and histological assessments.
Introduction
Stroke remains the number one cause of disability and the third leading cause of death among Americans every year. Tissue plasminogen activator (tPA), the current treatment for ischemic stroke, while efficacious, is only effective if administered within 4.5 hours of the ischemic event [1], [2]. Therefore, there is a clear and unmet need to develop effective treatments with a wide therapeutic window capable of restoring neural function and reducing disabilities associated with stroke.
Stem cell transplantation to restore neurological function after stroke is a potential therapy [3]–[5]. However, there is a paucity of studies testing whether multiple injections of cells are superior to single injection [6] .
Cell-based treatments of stroke include neural stem cells, umbilical cord blood cells, bone marrow-derived mesenchymal stem cells (MSCs) [7]–[10], and human umbilical tissue-derived cells (hUTC) are promising sources of cells for regenerative treatment of stroke [11]–[13]. In contrast to other cell types (MSCs, embryonic stem cells etc.), hUTC are obtained by non-invasive methods and their use does not evoke ethical concerns.
In this study, we tested the efficacy of hUTC treatment when administered intravenously 24 hours after experimental stroke in rats and whether multiple injections of hUTC provides any additional beneficial effects as compared to a single injection.
Materials and Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Henry Ford Health System’s Institutional Animal Care and Use Committee (IACUC approval number: 1027). All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Preparation of hUTC
Human umbilical tissue derived cells (hUTC) were provided by Advanced Technologies and Regenerative Medicine, LLC. Cells were isolated and banked as previously described [12]. On the day of treatment, hUTC were thawed in a 37°C water bath and counted using a hemocytometer. Cells were diluted with cell cryopreservation buffer (Janssen R&D) containing 10% DMSO (Sigma, St. Louis) for 3×106/2 ml for injection. Cell viability was measured and exceeded 80%.
hUTC (3×106 cells in 2 ml) or vehicle control (cell cryopreservation buffer, 2 ml) were delivered intravenously at 1 day; 1 and 3 days (1&3d); or 1 and 7 days (1&7d) after stroke.
Middle Cerebral Artery Occlusion (MCAo) Model and Experimental Groups
Adult male Wistar rats weighing 270–300 g were used in all experiments. Transient right MCAo was induced for 2 hours by advancing a 4–0 surgical nylon suture (18.5–19.5 mm) determined by the animal weight, with its tip rounded by heating near a flame, to block the origin of the middle cerebral artery (MCA), using a method of intra-luminal vascular occlusion modified in our laboratory [14]. Rectal temperature was maintained at 37°C throughout the surgical procedure using a feedback regulated water heating system.
To test the efficacy of single and multiple doses of hUTC, rats were subjected to 2 hrs of transient MCAo and randomly selected animals were injected, via a tail vein, with:
3×106 hUTC in 2 ml administered at 1 day after MCAo (3 M/1d; n = 8).
3×106 hUTC in 2 ml administered at 1 and 3 days after MCAo (3 M/1&3d; n = 8).
3×106 hUTC in 2 ml administered at 1 and 7 days after MCAo (3 M/1&7d; n = 8).
Vehicle control (2 ml) administered at 1 day after MCAo (n = 9).
Vehicle control (2 ml) administered at 1 and 3 days after MCAo (n = 8).
Vehicle control (2 ml) administered at 1 and 7 days after MCAo (n = 9).
All rats were sacrificed at 63 days after MCAo.
Functional Tests
For each experimental animal, we performed functional tests at one day prior to MCAo and at one day after MCAo immediately prior to the first treatment (baseline), and weekly thereafter (7, 14, 21, 28, 35, 42, 49, 56 and 63 days after MCAo) by an investigator who was blinded to the experimental groups. The functional tests included a modified neurological severity score (mNSS), a foot- fault test and an adhesive removal test.
Modified neurological severity score (mNSS)
Modified neurological severity score (mNSS) is a composite of motor, sensory, balance and reflex tests. mNSS is graded on a scale of 0 to 18 (normal score 0; maximal deficit score 18). One score point is awarded for the inability to perform the test or for the lack of a tested reflex; thus, the higher the score, the more severe is the injury [9], [15].
Foot-fault test
Foot-fault is a test for placement dysfunction of forelimbs. Animals were placed on an elevated grid floor (45 cm×30 cm), 2.5 cm higher than a solid base floor, with 2.5 cm×2.5 cm diameter openings. Animals tend to move on the grid with their paws placed on the wire frame. When animals inaccurately place a paw, the front limb falls through one of the openings in the grid. When the paw falls or slips between the wires, this was recorded as a foot fault. A total 100 of steps (movement of each forelimb) were counted, and the total number of foot faults for the left forelimb was recorded. The percentages of foot faults are normalized to the number of total counted steps (100 steps) [16], [17].
Adhesive-removal somatosensory test
An adhesive removal test was used to measure somatosensory deficits [18], [19]. All rats were familiarized with the testing environment. In brief, two small pieces of adhesive-backed paper dots (of equal size, 113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The rats were then returned to their cages. The times to remove each stimulus from both forelimbs were recorded on 3 trials per day. Individual trials were separated by at least 5 min.
Histological and Immunohistochemical Assessment
At 63 days after MCAo, animals were sacrificed and subsequently brains were fixed by transcardial perfusion with saline containing heparin (2 units/ml), followed by perfusion and immersion in 4% Paraformaldehyde before being embedded in paraffin.
Lesion volume measurement: Seven coronal sections of tissue were processed and stained with hematoxylin and eosin (H&E) for calculation of volume of cerebral infarction [20]. The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated using the Global Lab Image analysis system (Data Translation, Malboro, MA) [20]. The percentages of infarct volume are normalized to the contralateral hemisphere and lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
Immunohistochemical Staining
A standard paraffin block was obtained from the center of the lesion (bregma –1 mm to +1 mm). A series of 6 µm thick sections were cut from the block. Brain sections obtained from hUTC treatment and vehicle control groups were used for immunostaining. Immunostaining was performed using antibodies against Von Willebrand Factor (vWF), an endothelial cell marker (1∶400; Dako, Carpenteria, CA) and Synaptophysin (1∶500, Chemicon).
Quantitation
For measurement of vascular density and perimeters, vWF immunostained coronal sections were used. Eight fields of view from the ischemic boundary zone (IBZ) were digitized using a 40X objective (Olympus BX40) via the MCID computer imaging analysis system (Imaging Research, St. Catharines, Canada). The number of vessels and their perimeters were counted throughout each field of view. The total number of vessels was divided by the total tissue-area to determine vascular density. Data are presented as total number of vessels per mm2.
For semi-quantification of Synaptophysin, immunostained coronal sections were used. Eight fields of view from the IBZ in each section were digitized under a 40x objective. The positive area was measured. Data are presented as percentage of positive area.
All cell counts were performed by observers blinded to the individual treatment status of the animals.
Statistical Analysis
Rats subjected to MCAo were randomized into one of the six groups. Animals were treated with hUTC (3×106 cells in 2 ml) or vehicle control (2 ml) intravenously at 1 day; 1 and 3 days (1&3d); or 1 and 7 days (1&7d) after stroke.
Behavior tests (adhesive test, foot-fault test and mNSS) were performed prior to MCAo, day 1 after MCAo prior to the study treatment (baseline), and at days 7, 14, 21, 28, 35, 42, 49, 56, and day 63 after MCAo. Histology evaluation was processed after sacrifice at day 63.
This study investigated the efficacy of hUTC treatment on the functional recovery (primary outcome), measured from three behavioral tests, and the histological evaluation (secondary outcomes). Data were evaluated for normality. Data transformation was considered if data were not normal. If the data were not distributed normally, the ranked data were used for the analysis. We first tested for differences between the three controls groups (group 4 to 6), if none were found; the controls would be combined as co-control to increase statistical power.
The global test using Generalize Estimating Equation (GEE) [21] was implemented to test the group difference on functional recovery measured from the three behavioral tests (adhesive test, foot-fault test and mNSS). The global test on multiple outcomes is more efficient than a single outcome, when the group effects are consistent on all the outcomes (e.g., the positive correlation). The analysis of the treatment effect started by testing for overall global effect and followed by a subgroup comparison for each individual behavioral test, if the overall global effect was detected at the 0.05 level. Otherwise, subgroup analysis would be considered as exploratory. Individual functional outcome within different groups and days was tested using contract statement.
One-way ANOVA was used to test the group difference on histology measurements at day 63. Correlation coefficients were calculated among the histology measurements as well as the correlation to the functional outcome at day 63 adjusting for treatment groups.
Results
Neurological Functional Outcome and Lesion Volume (Figure 1)
Figure 1. Neurological outcome and lesion volume measurements after stroke.
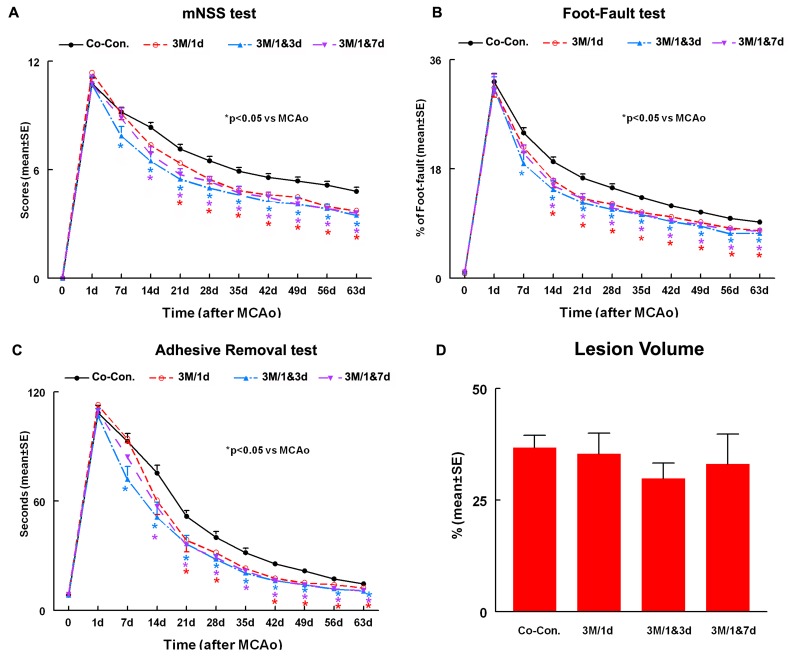
Panels A–C show mNSS (A) foot-fault (B) and adhesive-removal (C) tests after stroke in the 4 experimental groups: 1. Combined Control (Co-Con.). 2. 3×106 hUTC administered at 1 day after MCAo (3M/1d). 3. 3×106 hUTC administered at 1 and 3 days after MCAo (3M/1&3d). 4. 3×106 hUTC administered at 1 and 7 days after MCAo (3M/1&7d). Panel D shows the lesion volume in the 4 experimental groups. SE = standard error. *p<0.05 vs Co-control.
Functional outcome
To test whether hUTC treatment of stroke rats regulates functional outcome, a battery of functional tests was performed. Functional response was measured for each experimental animal, at day 1 (before treatment) and at 7, 14, 21, 28, 35, 42, 49, 56 and 63 days after MCAo.
All rats exhibited the same level of neurological functional deficits post stroke at 24 h immediately prior treatment with no significant differences among the groups (p>0.09).
Since the three control groups did not show significant differences in functional outcome from day 1 to day 63 after MCAo, the three control groups were combined into one as co-control (n = 26).
A significant overall group test based on the global test (p<0.0001) indicated that effects on functional recovery were different among the 4 groups from day 14 to 63. All hUTC treatments, single (1d) or multiple (1&3d and 1&7d), significantly improved functional recovery compared to co-control (p<0.01).
Rats receiving hUTC at 1d and at 1&7d showed significant improvement in neurological function from 14 days to 63 days compared with co-control group (p<0.05). hUTC treatment at 1&3d significantly improved functional outcome from day 7 to 63 compared to the co-control group (p<0.05).
The data for subgroup analysis for each individual functional test (foot fault, mNSS and adhesive removal tests) within different groups and days is presented in Figure 1.
There was no statistically significant difference between single and multiple injections of hUTC (p = 0.23) or between the two groups of multiple injections (1&3d vs. 1&7d, p>0.07) at all testing time points. Treatment with multiple injections of hUTC at 1&3d marginally improved functional outcome at days 56 (p = 0.08) and 63 (p = 0.07) compared to a single injection of hUTC given at day 1.
Lesion volume
No significant differences of ischemic lesion volumes in the hUTC treatment groups were detected compared with the co-control group (Figure 1D, p>0.05).
Synaptophysin Expression in the IBZ
Synaptophysin is a marker for presynaptic plasticity and synaptogenesis [22]. To test whether hUTC cell treatment regulates synaptic plasticity, Synaptophysin immunostaining was performed and the immunoreactive positive area was measured in the ischemic border.
All hUTC treatment groups showed significant increases in Synaptophysin expression in the IBZ compared to the co-control group (p<0.05, Figure 2A ). Synaptophysin expression in the IBZ was marginally correlated with the adhesive removal test (r = −0.26, p = 0.053). There was no statistically significant difference between single and multiple injections of hUTC or between the two groups of multiple injections in Synaptophysin expression in the IBZ (p>0.6).
Figure 2. hUTC treatment effect on vascular density, perimeter and synaptophysin expression.
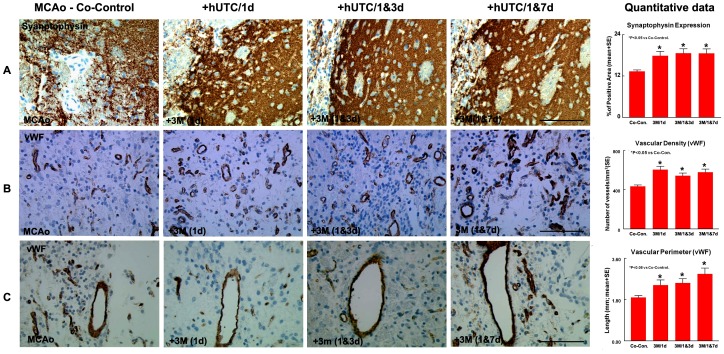
Panel A shows significant increase in Synaptophysin expression in the IBZ in all hUTC treatment groups compared to the co-control group. Panels B–C show significant increase in vascular density and perimeter in the IBZ in all hUTC treatment groups compared to the co-control group. *p<0.05 vs Co-control. SE = standard error. Scale bar in A,B and C = 100µm.
Vascular Density and Perimeter in the IBZ
To measure vascular perimeter and density, we performed vWF immunostaining. All hUTC treatment groups showed a significantly higher vascular density when compared to co-control group (p<0.05, Figure 2B ). There was no statistically significant difference between single and multiple injections of hUTC or between the two groups of multiple injections in vascular density in the IBZ (p>0.1).
All hUTC treatment groups showed significantly increased vascular perimeters compared to the co-control group (p<0.05, Figure 2C ). There was no statistically significant difference in vascular perimeter in the IBZ between the two groups of multiple injections (p = 0.13). However, there was marginal but not significant difference in perimeter between 3M/1d and 3M/1&7d (p = 0.07).
Discussion
Cell therapy has shown promising therapeutic potential in myocardial [23], limb [24], and brain ischemia [25]. Bone marrow-derived MSCs have been extensively studied in animal models of stroke and shown to promote neurovascular remodeling concomitantly with a significant improvement in neurological outcome [15], [26], [27]. However, little has been done to address the clinically relevant question whether treatment of stroke with multiple injections of cells are superior to a single injection. This is the first study to address the question whether multiple injections of hUTC when initiated 24 hours after experimental stroke in rats provides any additional beneficial effects as compared to a single injection.
Determining the optimal therapeutic protocol using stem cells for stroke therapy would enable us to improve the design of clinical trials to test the efficacy of hUTCs treatment in stroke patients. It is clearly beneficial to know whether multiple injections are superior to a single injection, as a single injection is more convenient, faster, requires lower number of cells and most likely, is accompanied with lower risks.
Omori et al. [6] compared the effects of a single intravenous injection of human MSCs (1×106 or 3×106) at a single time point (6 hours) with a low dose of 1×106 human MSCs injected at multiple time points (6 h, 24 h and 48 h or 6 h, 24 h and 1 week) after MCAo. Their results showed that the greatest therapeutic benefit was achieved following a single 3×106 cell dose injection at 6 hours post MCAo, rather than multiple lower cell infusions over multiple time points [6]. In a different study, injection of bone marrow cells at day 3 post experimental myocardial infarction in mice reduced infarct size and improved left ventricular function, while multiple injections (at day 3 and 7 or 3, 7 and 14) post myocardial infarction had no additive effect [28].
In a dose escalation study, Zhang et al. [13] demonstrated that endogenous neurorestorative response and neurological recovery were augmented after a single intravenous administration of hUTC at dose 3×106. Statistically significant improvements after intravenous administration of hUTC treatment were observed when treatment was initiated up to 30 days but not when hUTC were administered at 90 days after stroke [13]. Our data demonstrate that treatment of stroke with 3×106 hUTC improves functional outcome compared to control when treatment is initiated at 24 hours after MCAo. However, there was no statistically significant difference between single and multiple injections of hUTC or between the two groups of multiple injections both in functional outcome and immunohistochemical measurements. This may be due to ceiling effect with 3×106 hUTC providing the optimal number of cells to induce neurorestorative effects with no added benefit observed by multiple injections. Our data thus suggest that treatment with multiple injections is not superior to a single injection.
hUTC treatment was associated with a significantly higher vascular density and increased expression of the synaptic protein, Synaptophysin, when compared to the vehicle control group. Treatment of stroke with hUTC improved functional outcome with no change in the volume of the cerebral infarction. Thus, functional outcome after hUTC treatment likely results from a neurorestorative effect rather than neuroprotective effect when hUTC is administered 24 h after MCAo.
Vascular Density and Perimeter after hUTC Treatment
The adult brain vascular system is activated in response to pathological conditions including stroke [29]. Cerebral blood flow regulation is a critical step in maintaining neural function [30]. The formation of new blood vessels via angiogenesis is important in the pathophysiology of vascular disease [31]. In the rodent brain, capillary sprouting is initiated at the border of the infarct and new vessels develop in the ischemic boundary zone [32], [33].
Stroke patients with a greater cerebral blood flow appear to have a better outcome than patients with lower flow [34]–[36]. Neurorestorative treatments, either cell-based or pharmacological therapies, in animal models of stroke increase angiogenesis which is associated with and may underlie improvements in functional outcome [37].
Our data demonstrate that hUTC treatment improves functional recovery and has a positive influence on the vascular density and perimeter in the IBZ. Treatment with hUTC may enhance recovery after stroke through modulation of the brain vascular system.
Synaptophysin Expression after hUTC Treatment
Synaptic plasticity is an important mediator of functional recovery after stroke [38], [39]. Functional plasticity in the motor cortex is accompanied by changes in dendritic and synaptic structure, as well as by alterations in the regulation of cortical neurotransmitter systems [39], [40].
Synaptophysin is a presynaptic vesicle protein that is found in all nerve terminals [41].
Synaptophysin is used to quantify numbers of terminals during neuroanatomical remodeling and neural development [42]–[45].
Neurorestorative treatments of stroke increase synaptic plasticity in the IBZ [46], [47] as evidenced by the increased expression of synaptic proteins such as Synaptophysin and growth-associated protein 43 [41]. Reduction of neurological deficits after stroke has been attributed to extensive synaptic and functional reorganization.
Stroke rats treated with hUTC demonstrated significant increase in Synaptophysin expression in the ischemic brain compared to co-control. Functional benefits derived from hUTC treatment of stroke suggest an effect of hUTC on synaptic plasticity.
In summary, the data indicate that hUTC treatment started 24 h after MCAo significantly improves long term functional outcome after stroke and increases vascular density, perimeter and synaptic plasticity in the ischemic brain. Multiple injections of hUTC were not superior to single injection therapy in both functional outcome and histological assessments.
Acknowledgments
The authors wish to thank Qinge Lu and Supata Santra for technical assistance.
Funding Statement
This work was funded by Advanced Technologies and Regenerative Medicine, LLC, Somerville, NJ 08876, USA. The funders had no role in study design, data collection, analysis or preparation of the manuscript. Study design and decision to publish were approved by the funders.
References
- 1. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, et al. (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 2. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 3. Bliss T, Guzman R, Daadi M, Steinberg GK (2007) Cell transplantation therapy for stroke. Stroke 38: 817–826. [DOI] [PubMed] [Google Scholar]
- 4. Chopp M, Li Y, Zhang ZG (2009) Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40: S143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, et al. (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174: 11–20. [DOI] [PubMed] [Google Scholar]
- 6. Omori Y, Honmou O, Harada K, Suzuki J, Houkin K, et al. (2008) Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res 1236: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, et al. (2008) Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res 1234: 172–182. [DOI] [PubMed] [Google Scholar]
- 8. Mochizuki N, Takagi N, Kurokawa K, Onozato C, Moriyama Y, et al. (2008) Injection of neural progenitor cells improved learning and memory dysfunction after cerebral ischemia. Exp Neurol 211: 194–202. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Sanberg PR, Li Y, Wang L, Lu M, et al. (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 10. Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, et al. (2010) Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke 41: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alder J, Kramer BC, Hoskin C, Thakker-Varia S (2012) Brain-derived neurotrophic factor produced by human umbilical tissue-derived cells is required for its effect on hippocampal dendritic differentiation. Dev Neurobiol 72: 755–765. [DOI] [PubMed] [Google Scholar]
- 12. Sun J, Li X, Feng H, Gu H, Blair T, et al. (2011) Magnetic resonance imaging of bone marrow cell-mediated interleukin-10 gene therapy of atherosclerosis. PLoS One 6: e24529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, et al. (2011) Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 42: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Zacharek A, Li A, Zhang C, Ding J, et al. (2006) Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience 141: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Li Y, Wang L, Zhang Z, Lu D, et al. (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 16. Barth TM, Grant ML, Schallert T (1990) Effects of MK-801 on recovery from sensorimotor cortex lesions. Stroke 21: III153–157. [PubMed] [Google Scholar]
- 17. Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39: 777–787. [DOI] [PubMed] [Google Scholar]
- 18. Schallert T, Whishaw IQ (1984) Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci 98: 518–540. [DOI] [PubMed] [Google Scholar]
- 19. Hernandez TD, Schallert T (1988) Seizures and recovery from experimental brain damage. Exp Neurol 102: 318–324. [DOI] [PubMed] [Google Scholar]
- 20. Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, et al. (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10: 290–293. [DOI] [PubMed] [Google Scholar]
- 21. Lu M, Chen J, Lu D, Yi L, Mahmood A, et al. (2003) Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods 128: 183–190. [DOI] [PubMed] [Google Scholar]
- 22. Ujike H, Takaki M, Kodama M, Kuroda S (2002) Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci 965: 55–67. [DOI] [PubMed] [Google Scholar]
- 23. Yoon YS, Lee N, Scadova H (2005) Myocardial regeneration with bone-marrow-derived stem cells. Biol Cell 97: 253–263. [DOI] [PubMed] [Google Scholar]
- 24. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, et al. (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Chopp M (2009) Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett 456: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Chen J, Wang L, Lu M, Chopp M (2001) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56: 1666–1672. [DOI] [PubMed] [Google Scholar]
- 27. Rempe DA, Kent TA (2002) Using bone marrow stromal cells for treatment of stroke. Neurology 59: 486–487. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Sievers RE, Prasad M, Mirsky R, Shih H, et al. (2011) Timing of bone marrow cell therapy is more important than repeated injections after myocardial infarction. Cardiovasc Pathol 20: 204–212. [DOI] [PubMed] [Google Scholar]
- 29. Greenberg DA, Jin K (2005) From angiogenesis to neuropathology. Nature 438: 954–959. [DOI] [PubMed] [Google Scholar]
- 30. Pratt PF, Medhora M, Harder DR (2004) Mechanisms regulating cerebral blood flow as therapeutic targets. Curr Opin Investig Drugs 5: 952–956. [PubMed] [Google Scholar]
- 31. Zacharek A, Chen J, Cui X, Yang Y, Chopp M (2009) Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke 40: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia JH, Cox JV, Hudgins WR (1971) Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropathol 18: 273–285. [DOI] [PubMed] [Google Scholar]
- 33. Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, et al. (2002) Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 22: 379–392. [DOI] [PubMed] [Google Scholar]
- 34. Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, et al. (1997) A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 28: 2518–2527. [DOI] [PubMed] [Google Scholar]
- 35. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 36. Cramer SC, Chopp M (2000) Recovery recapitulates ontogeny. Trends Neurosci 23: 265–271. [DOI] [PubMed] [Google Scholar]
- 37. Zhang ZG, Chopp M (2009) Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolb B (1999) Synaptic plasticity and the organization of behaviour after early and late brain injury. Can J Exp Psychol 53: 62–76. [DOI] [PubMed] [Google Scholar]
- 39. Nudo RJ, Plautz EJ, Frost SB (2001) Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve 24: 1000–1019. [DOI] [PubMed] [Google Scholar]
- 40. Bohotin CR, Badescu M, Popescu DN, Bohotin V (2004) Motor cortex plasticity–from physiology to clinical neurology. Rom J Physiol 41: 99–108. [PubMed] [Google Scholar]
- 41.Stroemer RP, Kent TA, Hulsebosch CE (1998) Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29: 2381–2393; discussion 2393–2385. [DOI] [PubMed]
- 42. Masliah E, Fagan AM, Terry RD, DeTeresa R, Mallory M, et al. (1991) Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp Neurol 113: 131–142. [DOI] [PubMed] [Google Scholar]
- 43. Masliah E, Terry RD, DeTeresa RM, Hansen LA (1989) Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett 103: 234–239. [DOI] [PubMed] [Google Scholar]
- 44. Masliah E, Terry RD, Alford M, DeTeresa R (1990) Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem 38: 837–844. [DOI] [PubMed] [Google Scholar]
- 45. Cabalka LM, Ritchie TC, Coulter JD (1990) Immunolocalization and quantitation of a novel nerve terminal protein in spinal cord development. J Comp Neurol 295: 83–91. [DOI] [PubMed] [Google Scholar]
- 46. Cui X, Chopp M, Zacharek A, Roberts C, Buller B, et al. (2010) Niacin treatment of stroke increases synaptic plasticity and axon growth in rats. Stroke 41: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen J, Li Y, Wang L, Lu M, Chopp M (2002) Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAo in rats. J Neurol Sci 199: 17–24. [DOI] [PubMed] [Google Scholar]


