Abstract
Laminins, one of the major functional components of basement membranes, are found underlying endothelium, and encasing pericytes and smooth muscle cells in the vessel wall. Depending on the type of blood vessel (capillary, venule, postcapillary venule, vein or artery) and their maturation state, both the endothelial and mural cell phenotype vary, with associated changes in laminin isoform expression. Laminins containing the α4 and α5 chains are the major isoforms found in the vessel wall, with the added contribution of laminin α2 in larger vessels. We here summarize current data on the precise localization of these laminin isoforms and their receptors in the different layers of the vessel wall, and their potential contribution to vascular homeostasis.
Keywords: basement membrane, laminin, endothelium, vascular smooth muscle, blood vessels
The blood vasculature consists of different vessel types that vary in cellular and extracellular matrix (ECM) composition that can impact on vessel structural and functional integrity. Although considerable attention has been given to the cells comprising the vessel wall and their contribution to vessel function/physiology, comparatively little is known about the biochemical nature of the ECM of different vessel types and how it influences their function. This review summarizes current knowledge on the structure and function of the ECM of the vessel wall, with focus on capillaries, post-capillary venules and arterioles, and on the laminins, one of the major constituents of vascular basement membranes (BMs).
Cellular and Extracellular Matrix Layers Comprising the Vessel Wall
The blood vessel wall is composed of a luminal monolayer of endothelial cells that is surrounded by mural cells, the latter of which vary in phenotype and number, depending on blood vessel type and possibly also tissue,1 and are referred to as pericytes or vascular smooth muscle cells (vSMC). At the level of capillaries, post-capillary venules and venules, endothelial cells also express different marker molecules in different tissues.2 This suggests that the tissue milieu can impact on the phenotype of various cell types in the vessel wall.
In some tissues, pericytes and smooth muscle cells can share several marker molecules including platelet-derived growth factor (PDGF) receptor-β, desmin, NG2 and α-smooth muscle actin, depending on developmental stage and tissue type.3 However, the clearest distinction between pericytes and vascular smooth muscle cells is their localization, with pericytes being embedded within the endothelial BM, while smooth muscle cells secrete a BM that is independent and morphologically distinguishable from the endothelial BM. Pericytes and their processes ensheath the endothelial tube of capillaries and post-capillary venules, whereas in arterioles the vessel is surrounded by both pericytes and vSMC (Fig. 1). Only larger arteries/veins have several layers of vascular smooth muscle, which form the media. These large arteries additionally contain an outer fibrous coat, the adventitia, and have prominent elastin layers throughout the vessel wall to provide elasticity and resilience.
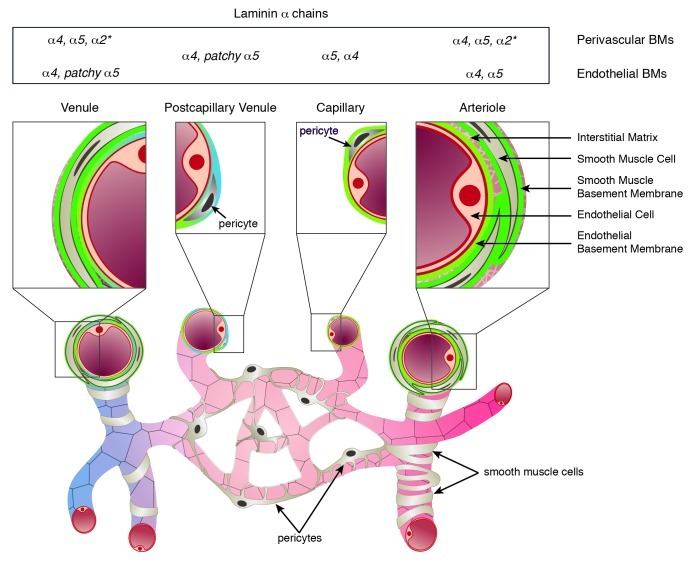
Figure 1. Schematic representation of the cellular and extracellular matrix layers that constitute the vessel wall of arterioles, capillaries, postcapillary venules and venules. Basement membranes underlie the endothelial cell monolayer and ensheath pericytes and smooth muscle cells, and vary in their laminin α chain expression and localization (summarized in the top panel). In arterioles and venules the interstitial matrix interconnects the different cellular and BM layers. *Laminin α2 has not been systematically studied in vascular smooth muscle BMs, but has been reported to occur in smooth muscle of the aorta and carotid arteries.
The ECM of vessel walls consists of BMs that underlie endothelium and encase pericytes, and ensheath individual smooth muscle cells, plus the fibrillar interstitial matrix that interconnects the endothelial and smooth muscle layers (Fig. 1) and forms the outer fibrous adventitial layer. As in all tissues, BMs of blood vessels contain collagen type IV isoforms, laminin isoforms, heparan sulfate proteoglycans (perlecan or agrin) and nidogen-1 and/or nidogen-2. Collagen type IV and laminins are the two major BM components that self-assemble to form independent networks that confer structural stability4 and biological activity,5 respectively. Based on intermolecular interactions identified mainly in in vitro studies, these two networks are considered to be cross-linked by perlecan6,7 or the nidogens.8,9 However, more recent data from BM networks isolated from the skin suggest that perlecan may have a dominant role in this cross-linking function in vivo.10 In addition to these four major ECM classes, many other glycoproteins, including netrin-4,11 fibulin-1 and -2,12 BM-40/osteonectin/SPARC,13 collagen types VII, VIII, XV and XVIII,14 are minor components of some vascular BMs that, nevertheless, contribute to their diversity.
In both endothelial and smooth muscle BMs of mature tissues, the major collagen type IV isoform is composed of α1 and α2 chains [(α1)2(α2)].15,16 However, there has been no detailed analysis of different vessel types and whether other isoforms composed of collagen IV α3, α4, α5 or α6 chains can also exist in vascular BMs is not clear. Laminins are composed of an α, β and γ chain that assemble to form an approximately crossed-shaped or Y-shaped molecule.17 Five α, four β and three γ chains have been identified that can combine to form up to 18 laminin isoforms18-20 that are named according to the three chain composition; i.e., laminin 211 is composed of α2, β1 and γ1 chains (Fig. 2).21 In general, the N-terminal domains of laminin chains mediate laminin self-assembly into the BM network, while their C-terminal domains (that are composed entirely of α chain sequences) carry the major receptor binding sites, making the α chains important for the transduction of specific cellular signals via defined receptors. Laminins containing laminin α4 and α5 chains are the predominant isoforms found in endothelial cell BMs,22-24 while those with α2, α4 and α5 chains occur in the vascular smooth muscle BMs24,25 (Fig. 3).
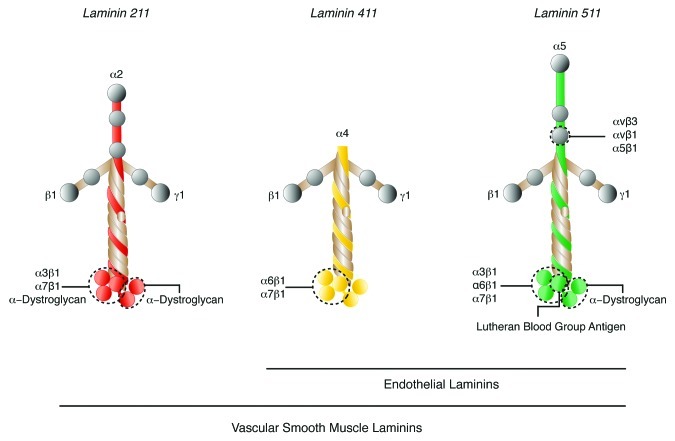
Figure 2. Model of laminin isoforms found in vascular BMs. Potential integrin and non-integrin receptors for each laminin isoform and their approximate interaction sites on the laminin α chains are shown.
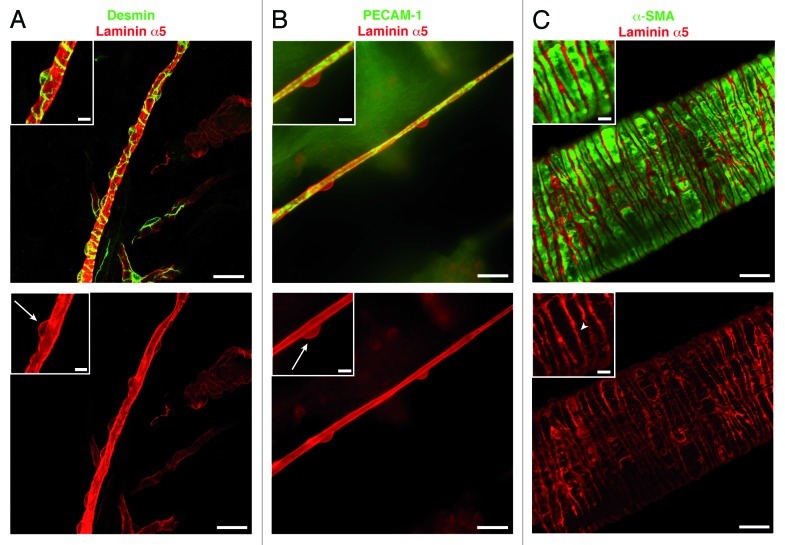
Figure 3. Immunofluorescence staining for laminin α5, as a BM marker, in capillaries and arterioles. Staining for laminin α5 together with (A) desmin, to mark pericytes, or (B) PECAM-1, to mark the endothelium, reveals pericyte cell bodies (arrows) and their extensive processes embedded in the endothelial cell BM. Double staining for laminin α5 and α-smooth muscle actin (α-SMA) (C) reveals the BM of the individual smooth muscle cells (arrowhead) enwrapping arterioles. Scale bars are 20 μm and 5 μm in the insets.
The fibrillar interstitial matrix underlies the endothelial BM and acts to interconnect the endothelial and smooth muscle BMs. It is composed largely of the fibrillar collagen types I and III (> 90%), together with chondroitin sulfate and dermatan sulfate proteoglycans such as decorin and biglycan,26 and multi-adhesive glycoproteins including fibronectin, osteopontin, thrombospondin,27 tenascin-C28,29 and vitronectin.30
Laminin α2, α4 and α5 in the Vessel Wall
Vascular endothelial cells and pericytes
Vascular endothelial cells express laminin α4 and α5 chains that combine with laminin β1 and γ1 chains to form laminins 411 and 511,22-24 respectively (Fig. 2). Laminin α chain distribution and expression depends on endothelial cell type, state of vessel growth and activation state.22,31-33 Laminin α4 is ubiquitously localized in endothelial BMs throughout the length of different vessel types independent of their stage of development, while laminin α5 appears postnatally, approximately at the time of pericyte recruitment, and its distribution varies with vessel type24 (Fig. 1). Laminin α5 chain is strongly expressed in most capillary BMs, except those of the fenestrated endothelium of some glands and the peri-tubular capillaries in the kidney. In postcapillary venules and venules, the distribution of laminin α5 is patchy, resulting in BM regions containing only laminin α4 or both laminin α4 and α5.34,35 Laminin α5 expression in endothelial cell BM of arteries has not been systematically studied but current data suggest that this too varies with tissue type (unpublished data from our laboratory). In addition, the expression of laminin α4 and α5 is differentially controlled by cytokines and growth factors; in vitro expression of laminin α4 by endothelial cells is strongly upregulated by proinflammatory cytokines such as TNF-α and IL-1, while laminin α5 is upregulated by progesterone and angiostatic factors.22,36 This differential expression of laminin α4 and α5 suggests functional distinction, which is being deciphered in our laboratory through the use of endothelial cell-specific knockout mice.
Pericytes are considered to contribute to the endothelial BM; however, the level of contribution is not yet clear. The reason for this is that it is difficult to isolate pericytes and to culture them in vitro without dedifferentiation, suggesting that the in vivo milieu impacts on their phenotype, which probably includes the ECM molecules they secrete. In vitro pericyte-endothelial cell co-cultures suggest that pericytes can secrete some BM components such as laminin and collagen IV37 and that this is a cooperative process.38 However, whether pericytes can express both of the endothelial cell laminin isoforms and thereby contribute to the differential expression of laminin α5 in endothelial BMs of different vessel types, described above, is not clear. Studies concerning the brain, which is particularly rich in pericytes, propose that pericytes can secrete laminin α2, an indication that pericyte ECM secretion may also vary with tissue type.39
Vascular smooth muscle cells
In comparison to endothelium, limited data exist for laminin isoform expression in vascular smooth muscle BMs. There is good evidence that laminin α440-42 and α5 chains24,32,43 occur at this site both during embryogenesis and in mature tissues, while vascular smooth muscle of larger vessels, such as aorta or the carotid arteries, has been reported to additionally express laminin α2,44 the major laminin α chain of myogenic tissues.45-47 In addition, the laminin β1 chain has been reported to be expressed in smooth muscle BMs of developing vessels and to be supplemented by laminin β2 in the mature vasculature,48 suggesting the existence of laminins 411, 511 and in some cases laminin 211 during development, and laminins 421, 521 and possibly 221 in mature vascular smooth muscle BMs.
Although limited, data suggest differential expression of laminin isoforms in vascular smooth muscle BMs during development and in different vessel types in mature tissues. Such variations are likely as fate mapping has revealed up to seven different cellular origins for vSMC, ranging from neural crest, splanchnic mesoderm, mesothelium and epicardium, or pericytes and adventitial myofibroblasts in mature vessels undergoing remodeling.49,50 Such diversity may also be reflected in their ECM secretion. To elucidate the contribution of laminins to vSMC function, smooth muscle specific laminin knockout mice are being generated in our laboratory.
Laminin Receptors on Endothelium and Perivascular Cells
Laminins are considered to be the major BM component responsible for the biological functions of BMs; i.e., for transducing signals that control cell migration, survival, proliferation and differentiation.5,20 These biological roles are largely due to the interaction of the laminin α chains with cell surface receptors. Three major classes of transmembrane receptors have been reported to interact with the vascular laminin α2, α4 and α5 chains, β1 and β3 integrins, α-dystroglycan of the dystrophin glycoprotein complex, best known from studies of dystrophic muscle, and lutheran blood group glycoprotein, a long-known blood group glycoprotein present on most cells but only recently identified as a laminin receptor.51-55Figure 2 illustrates the potential interactions between these receptors and the vascular laminin isoforms. All receptors, except integrin α7β1, which is expressed predominantly on muscle, and α-dystroglycan for which contradictory data exits,56-59 have been reported to be expressed on endothelium and smooth muscle (although not specifically vascular smooth muscle).60,61
In vitro, integrin α6β1 can interact with both laminin α4 and α5 chains.52,53,62-65 In addition, a recombinant fragment from the N-terminus of the laminin α5 which carries potentially two exposed RGD sequences (not present in other isoforms) has been shown to bind to αvβ3,54 a receptor that is better known for vitronectin and fibronectin binding (Fig. 2).54 Antibody inhibition studies suggest that β1 integrins, probably αvβ1 and α5β1, the latter of which is a well-known fibronectin receptor, can also interact with laminin α5 via the same RGD-site.54 Data suggests that integrin α5β1 acts cooperatively with the αv integrins during vascular remodeling.66 Integrin α3β1 has been reported to bind to laminin α552 and to laminin α2.67-69
Integrin α7β1 is a myogenic integrin that reacts primarily with laminin α2, but has also been shown in in vitro assays to interact with laminin α4 and α5 containing isoforms.51 It has been reported to occur on vSMC but not on endothelium and to play an important role in vascular integrity and development.70 However, the viability of mice lacking α7β1 suggests that other receptors may also act as laminin binding molecules in vSMC.71 Such an alternative receptor is likely to be α-dystroglycan, which has been shown to have high affinity binding for the laminin α2 chain (as well as the heparin sulfate proteoglycans, perlecan and agrin).72 There is no strong evidence for high affinity binding of α-dystroglycan to either laminin α4 or α5 chains,73 although weak binding to laminin α5 may occur.74
The lutheran blood group glycoprotein, also known as Lu/BCAM, and a spliced variant of the basal cell adhesion molecule (B-CAM), has been described to specifically bind laminin α5.55,75 Although reported to have a broad in vivo distribution,76 mice lacking this molecule have no overt phenotype,77 which contrasts with the early embryonic lethal phenotype of mice lacking laminin α5.78 Its role as a laminin α5 receptor in vivo therefore remains to be fully understood.
The precise in vivo contribution of these receptors to laminin binding in the vessel wall and the extent of cooperation between the different integrin and/or non-integrin receptors in vivo is not clear. In addition, there are increasing data that suggest that integrins are involved in functions independent of ECM binding, as recently shown for α3β1, which acts as a regulator of vascular endothelial growth factor (VEGF) expression by endothelium.79 Such studies raise the question of whether binding interactions identified in vitro are relevant to the in vivo situation, in particular in the vasculature where mechanical force and shear stress are likely to affect which adhesion structures are employed and which signals are transduced to the bound cells.
Laminin Functions in the Vasculature
Development
The in vivo expression pattern of laminin α4 and α522-24 and the absence of an overt phenotype in the laminin α4 knockout mouse41 suggest that laminin α4 and α5 are not crucial for angiogenesis during development. One exception is the retina where laminin α4 has recently been reported to regulate tip cell numbers and vascular density by inducing endothelial Delta-like 4 (Dll4)/Notch signaling. Laminin α4 was shown to be expressed exclusively at the growing vascular front in the postnatal retina, with most abundant expression in the leading tip cells; while laminin α5 was expressed by endothelial cells in more distal portions of the vascular tree, as well as by surrounding astrocytes. This is in contrast to the brain, where laminin α5 is expressed only by endothelium and not by astrocytes.36 Laminin α4 knockout mice have excessive filopodia and tip cell formation in the retina, similar to the phenotype observed when Notch is inhibited in vivo, which leads to aberrant sprouting angiogenesis and branching.80 It is hypothesized that laminin α4 directly induces Dll4 expression on the tip cells via an integrin β1-mediated mechanism.80,81
Barrier function
The best studied aspect of laminin function in the endothelial cell BM is that of permeability to extravasating immune cells. At the level of postcapillary venules, the patchy distribution of laminin α5 coincides with sites of preferred extravasation by T-cells35,36 and neutrophils.34 The ablation of laminin α4 in mice results in a ubiquitous expression of laminin α5 in all endothelial BMs and an associated severely reduced extravasation of T-cells in a neuroinflammation model,35 and also of monocytes and neutrophils in other inflammatory models.82 There are data demonstrating that laminin α5 acts to inhibit leukocyte transmigration.35 However, whether laminin α5 also impacts on the endothelium and affects the “tightness” of endothelial cell junctions and thereby reduces leukocyte transmigration is a possibility that has not been investigated (Fig. 4A).
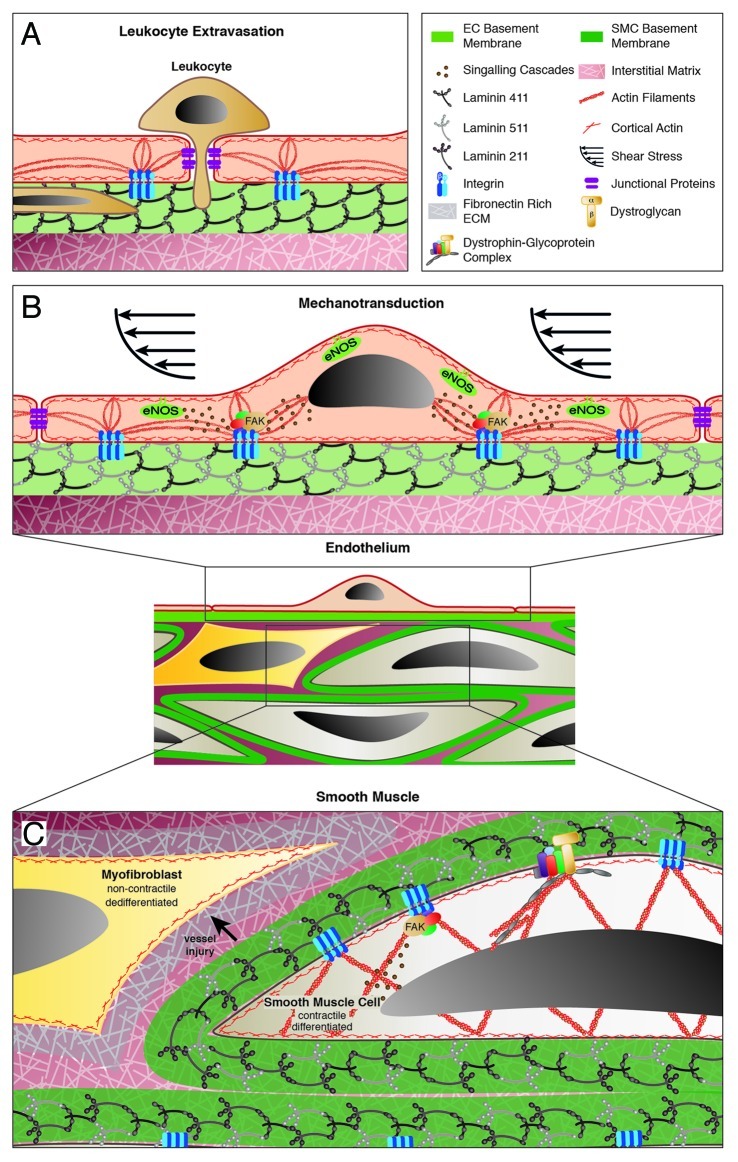
Figure 4 (See opposite page). Schematic representation of laminin functions in the endothelial and smooth muscle layers of the vessel wall. (A) In postcapillary venules, the absence of laminin α5 in the endothelial BM defines sites of leukocyte extravasation. Small (resistance) arterioles (B and C) are responsible for controlling vessel diameter in response to hemodynamics, where integrin-mediated anchorage to laminins in the endothelial BM via focal adhesions are implicated in shear sensing and transduction of signals to the underlying smooth muscle cells (B). (C) Vascular smooth muscle responds to shear and intraluminal pressure changes by contraction or relaxation, which requires firm anchorage between the individual vSMC and their BM. Vessel injury can induce changes in vSMC phenotype from a contractile to a dedifferentiated non-contractile phenotype, which is associated with changes in the surrounding BM and interstitial matrix.
It has been proposed that pericyte coverage of vessels also defines sites of neutrophil extravasation with areas of less coverage providing exit sites for leukocytes.83 The question that therefore arises is whether pericyte secretion of laminin α5 to the endothelial BM could account for the patchy laminin α5 in postcapillary venules.35 The fact that pericytes appear to secrete different laminins in different tissues argues against this possibility. However, pericytes are also considered to be oligopotential due to their ability to differentiate into several cells types (i.e., fibroblasts, osteoblasts, chondrocytes and adipocytes),84 suggesting high plasticity. Hence, factors such as proinflammatory cytokines released at sites of leukocyte extravasation may impact on pericyte mobility but also local ECM production. The latter is impossible to address; given that pericytes are small with extensive cytoplasmic processes (Fig. 3), focal changes in their ECM synthesis at sites of inflammation would therefore be beyond detection by northern blot or in situ hybridization.
Endothelial cells: shear sensing and mechanotransduction
Newer aspects of laminin function include whether endothelial cell anchorage to the laminins in the BM impacts on shear sensing and transduction of signals from the vessel lumen to other layers in the vessel wall. Shear sensing is crucial for hemodynamic control and occurs at the level of small arterioles, also referred to as resistance arterioles. The luminal location of the endothelium makes it perfectly positioned to sense changes in blood flow within vessels. In addition, endothelium can relay signals throughout the vessel wall by rapidly releasing vasodilating and vasoconstricting factors that regulate vascular tone in response to changes in hemodynamics. One such vasodilating molecule is nitric oxide (NO), produced by the endothelial nitric oxide synthase (eNOS), in response to increased shear stress,85 which diffuses to underlying vSMC or pericytes to induce relaxation. Endothelial cells can also induce vSMC contraction by producing vasoconstricting factors, including prostaglandins and thromboxane A2.86
Several mechanisms have been proposed to be involved in endothelial shear sensing and mechanotransduction (reviewed in ref. 87), all of which require firm anchorage of endothelial cells to their BM and to their neighboring cells.88,89 As described above, interaction of endothelial cells with the BM requires integrins that can aggregate to form “focal adhesions,” sites of multiple interconnection between the extracellular matrix and the actin cytoskeleton but also platforms containing molecules such as focal adhesion kinase (FAK) where intracellular signaling cascades are initiated90 (Fig. 4B and C). At present there is only indirect evidence that laminins may be involved in such mechanosensing and mechanotransduction functions; for example, shear induced eNOS synthesis in porcine endothelial cells has been shown to be RGD-dependent91 and several publications support a role for the RGD-binding integrins, α5β1 or αvβ3, in shear induced intracellular signaling in cultured endothelial cells.89,92,93 These studies focus on fibronectin and vitronection as ligands for α5β1 and αvβ3, which are interstitial matrix components and, therefore, not in direct contact with intact endothelium in vivo in the non-pathological situation (Fig. 1). Given that laminin α5 is the only BM molecule carrying an exposed RGD site that occurs in close proximity to endothelial cells and binds both α5β1 and αv series integrins, it may be that laminin α5 is the in vivo ligand of these integrins and thereby contributes to mechanosensing and transduction. Whether endothelial cell anchorage to laminins or any other component of the BM could influence the expression or function of junctional molecules has not been considered to date.
Functions of the vascular smooth muscle laminins
The maintenance of vascular tone and generation of contractile force against an increasing intraluminal pressure are independent of the endothelium and are functions inherent to the vascular smooth muscle. As discussed above for the shear sensing and mechanotransduction functions of endothelium, also the “mechano-response” of the vascular smooth muscle requires the interconnection of vSMC with each other and with their BM (Fig. 4C). While mechanisms of interconnections between vSMC have been investigated, revealing a role for N-cadherin,94 very little is known about vSMC interactions with their BM and how this influences contractility/phenotype.
Vascular smooth muscle differentiation: contractile (differentiated) vs. non-contractile (dedifferentiated) phenotype
Like endothelial cells, vSMC can interact with their surrounding BM via integrin and non-integrin receptors, which is considered to contribute to mechanical stability of the vessel and also permits the transduction of specific intracellular information to the smooth muscle cells that can influence vSMC proliferation, migration and differentiation state (Fig. 4C).
Vessel maturation is characterized by differentiation of the vSMC toward an enhanced contractile phenotype with associated increases in cytoskeletal proteins and an elongated morphology.95 Like the pericytes discussed above, vSMC also maintain their plasticity in the mature vasculature, thereby allowing them to undergo phenotypic changes in response to local stresses. In healthy vessels, vSMC in the contractile state contribute to the overall resistance of the vessel to hemodynamic changes. In response to vessel injury or neointima formation, vSMC dedifferentiate to a non-contractile phenotype, changing their expression of adhesion receptors and motility, which allows them to move into the affected tissue.96 Both the contractile and the non-contractile migratory phenotype are likely to be influenced by the interaction of the cells with their surrounding BM, but may also contribute to changes in the subjacent interstitial matrix.
There are no studies to date on the in vivo expression/distribution of smooth muscle BM components in vessels undergoing remodeling processes and how such changes impact on smooth muscle function (dilation or contraction of the vessel). However, there are some in vitro studies involving isolated arterial smooth muscle cells that suggest that laminins promote a contractile, differentiated phenotype while fibronectin, which is upregulated around vSMC after arterial injury, induces a switch to a synthetic, dedifferentiated state.97-99 While these studies implicate laminins in maintenance of the contractile vascular smooth muscle phenotype, all were performed with laminin isoforms that do not occur in the vasculature in vivo; namely laminin 111, which is commercially available and although highly adhesive for many cell types, has an extremely limited distribution in vivo.5,19
The potential involvement of laminins in the contractile phenotype of vascular smooth muscle is substantiated by more recent studies involving integrin α7β1 that is expressed on vSMC and can bind to laminins α2, α4 and α5.51 Ablation of integrin α7β1 in vivo results in reduced expression of contractile vSMC proteins and increased proliferation via a Ras-MAPK-mediated pathway,100 suggesting that α7β1-mediated interaction with one or more of the smooth muscle laminins inhibits smooth muscle cell growth in healthy arteries and that disturbances in this interaction removes this inhibition, thereby promoting a synthetic, proliferative vSMC phenotype.101 Similarly, integrins αvβ3 and α5β1, which can act as laminin α5 receptors,54 have been implicated in arterial myogenic constriction of healthy vessels.102 In isolated resistance arterioles with spontaneous tone, inhibition of integrin α5β1 using function-blocking antibodies (to integrin β1 and α5 subunits), and inhibition of αvβ3 (with RGD-sequences and anti-integrin β3 antibodies) impeded constriction in response to increased luminal pressure. While all these studies suggest a role for laminins in vSMC differentiation, they do not address the roles of specific laminin isoforms in phenotypic switching or vasoconstriction. It is therefore necessary to re-visit these questions and decipher which laminin isoforms and integrins are responsible.
Conclusion
Information on laminin isoform function in the different layers of the blood vessel wall remains limited and fragmented. However, the data to date support a role mainly for laminin α5 in inhibiting cell migration through or in the vessel wall and in promoting mural cell differentiation, and potentially also in mechanosensing and mechanotransduction. These processes are fundamental to vascular homeostasis and are altered in pathologies such as hypertension and arthrosclerosis where vascular remodeling occurs. To understand the molecular information imparted by the laminins to the endothelium and the mural cells of the vessel wall will aid in our understanding of vessel physiology and remodeling processes associated with vascular pathologies.
Acknowledgments
We thank Nina Gerigk for transforming our ideas and concepts into colorful illustrations, and Eva Korpos and Frank Arfuso for critical reading of the manuscript. Work reported here was funded by the German Research Foundation (DFG SFB 293A19, SO285/9-1) and by the FP-7 People Programme (Marie Curie Actions) under REA grant agreement 235711 (SmArt).
Glossary
Abbreviations:
- B-CAM
basal cell adhesion molecule
- BM(s)
basement membrane(s)
- Dll4
delta like 4
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- LuBCAM
lutheran blood group glycoprotein
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- vSMC
vascular smooth muscle cells
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22680
References
- 1.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 2.Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–32. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 5.Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–56. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- 6.Battaglia C, Mayer U, Aumailley M, Timpl R. Basement membrane heparan sulphate proteoglycan binds to laminin by its heparan sulfate side chains and to nidogen by sites in the protein core. Biochem J. 1992;289:313–30. doi: 10.1111/j.1432-1033.1992.tb17195.x. [DOI] [PubMed] [Google Scholar]
- 7.Hopf M, Göhring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–25. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–46. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerl M, Mann K, Aumailley M, Timpl R. Localization of a major nidogen-binding site to domain III of laminin B2 chain. Eur J Biochem. 1991;202:167–74. doi: 10.1111/j.1432-1033.1991.tb16358.x. [DOI] [PubMed] [Google Scholar]
- 10.Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, et al. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem. 2012;287:18700–9. doi: 10.1074/jbc.M111.336073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, et al. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol. 2000;151:221–34. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–89. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 13.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–80. doi: 10.1016/S0945-053X(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol. 2000;301:1179–90. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 15.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol. 1994;127:879–91. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–81. doi: 10.1016/0945-053X(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 18.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 19.Schéele S, Nyström A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med (Berl) 2007;85:825–36. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- 20.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–68. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 21.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Frieser M, Nöckel H, Pausch F, Röder C, Hahn A, Deutzmann R, et al. Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium. Eur J Biochem. 1997;246:727–35. doi: 10.1111/j.1432-1033.1997.t01-1-00727.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorokin LM, Girg W, Göpfert T, Hallmann R, Deutzmann R. Expression of novel 400-kDa laminin chains by mouse and bovine endothelial cells. Eur J Biochem. 1994;223:603–10. doi: 10.1111/j.1432-1033.1994.tb19031.x. [DOI] [PubMed] [Google Scholar]
- 24.Sorokin LM, Pausch F, Frieser M, Kröger S, Ohage E, Deutzmann R. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- 25.Rauch U, Saxena A, Lorkowski S, Rauterberg J, Björkbacka H, Durbeej M, et al. Laminin isoforms in atherosclerotic arteries from mice and man. Histol Histopathol. 2011;26:711–24. doi: 10.14670/HH-26.711. [DOI] [PubMed] [Google Scholar]
- 26.Thyberg J, Hedin U, Sjölund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–90. doi: 10.1161/01.ATV.10.6.966. [DOI] [PubMed] [Google Scholar]
- 27.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplàa C, et al. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002;53:952–62. doi: 10.1016/S0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 28.Weller A, Beck S, Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991;112:355–62. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aufderheide E, Ekblom P. Tenascin during gut development: appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol. 1988;107:2341–9. doi: 10.1083/jcb.107.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamazière JMD, et al. Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol. 1998;18:168–76. doi: 10.1161/01.ATV.18.2.168. [DOI] [PubMed] [Google Scholar]
- 31.Sorokin L, Girg W, Göpfert T, Hallmann R, Deutzmann R. Expression of novel 400-kDa laminin chains by mouse and bovine endothelial cells. Eur J Biochem. 1994;223:603–10. doi: 10.1111/j.1432-1033.1994.tb19031.x. [DOI] [PubMed] [Google Scholar]
- 32.Sorokin LM, Pausch F, Durbeej M, Ekblom P. Differential expression of five laminin alpha (1-5) chains in developing and adult mouse kidney. Dev Dyn. 1997;210:446–62. doi: 10.1002/(SICI)1097-0177(199712)210:4<446::AID-AJA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Iivanainen A, Sainio K, Sariola H, Tryggvason K. Primary structure and expression of a novel human laminin alpha 4 chain. FEBS Lett. 1995;365:183–8. doi: 10.1016/0014-5793(95)00462-I. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P, et al. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med. 2009;15:519–27. doi: 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- 36.Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–46. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brachvogel B, Pausch F, Farlie P, Gaipl U, Etich J, Zhou Z, et al. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp Cell Res. 2007;313:2730–43. doi: 10.1016/j.yexcr.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 40.Petäjäniemi N, Korhonen M, Kortesmaa J, Tryggvason K, Sekiguchi K, Fujiwara H, et al. Localization of laminin alpha4-chain in developing and adult human tissues. J Histochem Cytochem. 2002;50:1113–30. doi: 10.1177/002215540205000813. [DOI] [PubMed] [Google Scholar]
- 41.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, et al. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Bergmann U, Thesleff I, et al. Primary structure, developmental expression, and immunolocalization of the murine laminin alpha4 chain. J Biol Chem. 1997;272:27862–8. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- 43.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 44.McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP. Extracellular matrix gene expression in the developing mouse aorta. Adv Dev Biol. 2005;15:81–128. doi: 10.1016/S1574-3349(05)15003-0. [DOI] [Google Scholar]
- 45.Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988;85:1544–8. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990;87:3264–8. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci. 1995;108:3795–805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- 48.Glukhova M, Koteliansky V, Fondacci C, Marotte F, Rappaport L. Laminin variants and integrin laminin receptors in developing and adult human smooth muscle. Dev Biol. 1993;157:437–47. doi: 10.1006/dbio.1993.1147. [DOI] [PubMed] [Google Scholar]
- 49.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr., Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–9. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von der Mark H, Williams I, Wendler O, Sorokin L, von der Mark K, Pöschl E. Alternative splice variants of alpha 7 beta 1 integrin selectively recognize different laminin isoforms. J Biol Chem. 2002;277:6012–6. doi: 10.1074/jbc.M102188200. [DOI] [PubMed] [Google Scholar]
- 52.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113:869–76. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 53.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki T, Timpl R. Domain IVa of laminin alpha5 chain is cell-adhesive and binds beta1 and alphaVbeta3 integrins through Arg-Gly-Asp. FEBS Lett. 2001;509:181–5. doi: 10.1016/S0014-5793(01)03167-2. [DOI] [PubMed] [Google Scholar]
- 55.Parsons SF, Lee G, Spring FA, Willig TN, Peters LL, Gimm JA, et al. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind alpha5 chain-containing human laminin with high affinity. Blood. 2001;97:312–20. doi: 10.1182/blood.V97.1.312. [DOI] [PubMed] [Google Scholar]
- 56.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle α-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu H, Hosokawa H, Ninomiya H, Miner JH, Masaki T. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J Biol Chem. 1999;274:11995–2000. doi: 10.1074/jbc.274.17.11995. [DOI] [PubMed] [Google Scholar]
- 58.Hosokawa H, Ninomiya H, Kitamura Y, Fujiwara K, Masaki T. Vascular endothelial cells that express dystroglycan are involved in angiogenesis. J Cell Sci. 2002;115:1487–96. doi: 10.1242/jcs.115.7.1487. [DOI] [PubMed] [Google Scholar]
- 59.Durbeej M, Ekblom P. Dystroglycan and laminins: glycoconjugates involved in branching epithelial morphogenesis. Exp Lung Res. 1997;23:109–18. doi: 10.3109/01902149709074024. [DOI] [PubMed] [Google Scholar]
- 60.Terpe HJ, Stark H, Ruiz P, Imhof BA. Alpha 6 integrin distribution in human embryonic and adult tissues. Histochemistry. 1994;101:41–9. doi: 10.1007/BF00315830. [DOI] [PubMed] [Google Scholar]
- 61.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all α v integrins. Cell. 1998;95:507–19. doi: 10.1016/S0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 62.Katagiri F, Ishikawa M, Yamada Y, Hozumi K, Kikkawa Y, Nomizu M. Screening of integrin-binding peptides from the laminin α4 and α5 chain G domain peptide library. Arch Biochem Biophys. 2012;521:32–42. doi: 10.1016/j.abb.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276:17550–8. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 64.Kortesmaa J, Yurchenco P, Tryggvason K. Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification,and interactions with integrins. J Biol Chem. 2000;275:14853–9. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- 65.Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, et al. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–8. doi: 10.1074/jbc.M111228200. [DOI] [PubMed] [Google Scholar]
- 66.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, et al. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–49. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colognato H, MacCarrick M, O’Rear JJ, Yurchenco PD. The laminin alpha2-chain short arm mediates cell adhesion through both the alpha1beta1 and alpha2beta1 integrins. J Biol Chem. 1997;272:29330–6. doi: 10.1074/jbc.272.46.29330. [DOI] [PubMed] [Google Scholar]
- 68.Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, et al. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–15. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von der Mark H, Dürr J, Sonnenberg A, von der Mark K, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel β 1-series integrin and not alpha 6 β 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–601. [PubMed] [Google Scholar]
- 70.Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, et al. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- 71.Hultgårdh-Nilsson A, Durbeej M. Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr Opin Lipidol. 2007;18:540–5. doi: 10.1097/MOL.0b013e3282ef77e9. [DOI] [PubMed] [Google Scholar]
- 72.Talts JF, Andac Z, Göhring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–70. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu H, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J. 2003;371:289–99. doi: 10.1042/BJ20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, et al. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–54. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 75.Kikkawa Y, Moulson CL, Virtanen I, Miner JH. Identification of the binding site for the Lutheran blood group glycoprotein on laminin alpha 5 through expression of chimeric laminin chains in vivo. J Biol Chem. 2002;277:44864–9. doi: 10.1074/jbc.M208731200. [DOI] [PubMed] [Google Scholar]
- 76.Moulson CL, Li C, Miner JH. Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin alpha5 in vivo. Dev Dyn. 2001;222:101–14. doi: 10.1002/dvdy.1169. [DOI] [PubMed] [Google Scholar]
- 77.Rahuel C, Filipe A, Ritie L, El Nemer W, Patey-Mariaud N, Eladari D, et al. Genetic inactivation of the laminin alpha5 chain receptor Lu/BCAM leads to kidney and intestinal abnormalities in the mouse. Am J Physiol Renal Physiol. 2008;294:F393–406. doi: 10.1152/ajprenal.00315.2007. [DOI] [PubMed] [Google Scholar]
- 78.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.da Silva RG, Tavora B, Robinson SD, Reynolds LE, Szekeres C, Lamar J, et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am J Pathol. 2010;177:1534–48. doi: 10.2353/ajpath.2010.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stenzel D, Franco CA, Estrach S, Mettouchi A, Sauvaget D, Rosewell I, et al. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 2011;12:1135–43. doi: 10.1038/embor.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, et al. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res. 2011;109:172–82. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- 82.Kenne E, Soehnlein O, Genové G, Rotzius P, Eriksson EE, Lindbom L. Immune cell recruitment to inflammatory loci is impaired in mice deficient in basement membrane protein laminin alpha4. J Leukoc Biol. 2010;88:523–8. doi: 10.1189/jlb.0110043. [DOI] [PubMed] [Google Scholar]
- 83.Proebstl D, Voisin M-B, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–34. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collett GDM, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 85.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.HYP.8.1.37. [DOI] [PubMed] [Google Scholar]
- 86.Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–9. doi: 10.1016/S0165-6147(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 87.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi M, Berk BC. Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells. Essential role for a herbimycin-sensitive kinase. J Clin Invest. 1996;98:2623–31. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98:1042–6. doi: 10.1073/pnas.98.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–75. doi: 10.1161/01.RES.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 91.Gloe T, Riedmayr S, Sohn HY, Pohl U. The 67-kDa laminin-binding protein is involved in shear stress-dependent endothelial nitric-oxide synthase expression. J Biol Chem. 1999;274:15996–6002. doi: 10.1074/jbc.274.23.15996. [DOI] [PubMed] [Google Scholar]
- 92.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller JM, Chilian WM, Davis MJ. Integrin signaling transduces shear stress--dependent vasodilation of coronary arterioles. Circ Res. 1997;80:320–6. doi: 10.1161/01.RES.80.3.320. [DOI] [PubMed] [Google Scholar]
- 94.George SJ, Beeching CA. Cadherin:catenin complex: a novel regulator of vascular smooth muscle cell behaviour. Atherosclerosis. 2006;188:1–11. doi: 10.1016/j.atherosclerosis.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 95.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 96.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100(Suppl):S87–9. [PubMed] [Google Scholar]
- 97.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107:307–19. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45:837–46. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- 99.Hedin U, Sjölund M, Hultgårdh-Nilsson A, Thyberg J. Changes in expression and organization of smooth-muscle-specific alpha-actin during fibronectin-mediated modulation of arterial smooth muscle cell phenotype. Differentiation. 1990;44:222–31. doi: 10.1111/j.1432-0436.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 100.Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, et al. Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672–81. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- 101.Wilson E. Alpha 7 beta 1 integrin: putting the brakes on smooth muscle cell proliferation. Circ Res. 2007;101:651–3. doi: 10.1161/CIRCRESAHA.107.161877. [DOI] [PubMed] [Google Scholar]
- 102.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. αvβ3- and α5β1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol. 2005;289:H322–9. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]


