Abstract
Laminins are major constituents of basement membranes. At least 16 isoforms have now been described, each with distinct spatio-temporal expression patterns and functions. The laminin-511 heterotrimer (α5β1γ1) is one of the more recent isoforms to be identified and a potent adhesive and pro-migratory substrate for a variety of normal and tumor cell lines in vitro. As our understanding of its precise function in normal tissues and in pathologies is rapidly unraveling, current evidence suggests an important regulatory role in cancer. This review describes published data on laminin-511 expression in several malignancies and experimental evidence from both in vitro and in vivo studies supporting its functional role during tumor progression. A particular emphasis is put on more recent studies from our laboratory and that of others indicating that laminin-511 contributes to tumor dissemination and metastasis in advanced breast carcinomas and other tumor types. Collectively, the experimental evidence suggests that high expression of laminin-511 has prognostic significance and that targeting tumor-laminin-511 interactions may have therapeutic potential in advanced cancer patients.
Keywords: laminin, integrins, metastasis, extracellular matrix, breast cancer, adhesion, migration, invasion
Introduction
Laminins (LMs) are abundant extracellular matrix (ECM) proteins present predominantly in basement membranes (BM).1,2 At least 16 isoforms have been described and named according to their specific trimeric combination of α, β and γ chains using the new nomenclature.3 The existence of a LM-511 trimer (α5β1γ1, formerly LM-10) was first reported in 1997–98,4-6 some 15 y after the original discovery of laminin (now named LM-111, α1β1γ1 trimer).7 Given the more recent identification of LM-511, our understanding of its specific function in normal tissues and in diseases has lagged on other LM isoforms, in particular LM-111 and LM-332. Nevertheless, major breakthroughs in the field have rapidly closed this gap. Notably, the recognition of LMα5 as a distinct LM subunit8,9 and the characterization of various antibodies against human LM chains4-6,10 have clarified earlier discrepancies regarding the distribution of LM-511 in normal human and mouse tissues and in neoplasia. Genetic ablation of LMα5 in mouse11 marked the beginning of its functional characterization during development and later enabled investigations into the role of α5 laminins in specific organs.12-16 Functional studies in vitro and identification of LM-511 receptors have been facilitated by the triple cloning of recombinant human LM α5, β1 and γ1 chains17 as a rich source of intact LM-511 and the development of protocols for the purification of LM-511 from culture media.5,17-20 Together, these advances have contributed to the emerging view that LM-511 and its receptors regulate cancer progression. Studies documenting the high expression of LM-511 in several cancer types are briefly reviewed below and discussed in relation to more recent in vitro and in vivo experimental evidence indicating that LM-511 and its receptors regulate tumor cell migration, invasion and metastasis.
LM-511 Expression in Normal and Cancer Tissues
Establishing the precise tissue distribution of LM-511 has been complicated by earlier studies assuming the existence of a single LM isoform. Hence, many of the antibodies used for immunohistochemical (IHC) detection of LMs were raised against LM preparations containing more than one isoform (e.g., human placental laminin) or against LM subunits/fragments now known to be present in multiple isoforms including LM-511 (e.g., β1 and γ1 chains).10,21,22 These problems were compounded by the fact that the 4C7 antibody previously used for the detection of LMα1 chain23 actually recognizes the LMα5 chain.6 The latter study helped reconcile the discrepancies between the relatively restricted tissue expression reported for LMα1 mRNA and the broad LMα5 mRNA8 or protein distribution detected by the 4C7 antibody in human.23 Consequently, many of the earlier IHC studies need to be re-interpreted as evidence for the presence of LM-511 (and in some cases LM-521) rather than LM-111.23-25
Since the β1 and γ1 subunits are present in multiple isoforms and expressed in most tissues, the presence of LM-511 trimers in a given organ is often assumed solely based on the detection of the α5 chain. Nevertheless, co-localization of all three subunits of LM-511 has been demonstrated more conclusively in many tissues by IHC or co-immunoprecipitation. The results from these studies have shown that LM-511 is abundant in the basement membrane of most mature epithelia and endothelia and are consistent with LM-511 being the most widely distributed LM isoform in normal tissues and in carcinomas.24,26,27 Indeed, high expression of LM-511 subunits has been reported in many malignancies including colorectal, mammary, lung, thyroid, ovarian, prostate and basal cell carcinoma as well as in gliomas and melanomas.24,25,27-32 However, the precise pattern of LM-511 expression varies significantly between tumor types, from a continuous BM localization to a more diffuse stromal or tumor cell expression, and its level in tumor cells or in the associated vasculature is influenced by the stage of tumor progression.28-30,32,33
Many studies have shown that the expression of LM-511 is often maintained or even increased in advanced tumors. For example, Nagle et al.28,34 used a combination of antibodies to analyze the expression of LM chains in normal prostate, prostatic intraepithelial neoplasia and prostate carcinoma specimens. While the authors could detect the presence of multiple isoforms in normal prostate including α1, α3 and α5 LMs, they found a gradual loss of α1 and α3 isoforms whereas high expression of LMα5 (and therefore LM-511/521) was maintained in the majority of high grade tumors. Similarly, high tumor cell expression of all subunits of LM-511/521 has been reported in metastatic melanomas.31 These observations raise the possibility that high expression of α5 LMs may have prognostic significance in some tumor types. Consistent with this, detection of LMα5 in tumor cells of non-small cell lung carcinoma patients was found to provide the strongest independent prognostic value to identify patients with high risk of disease recurrence.35
A similar association is likely in breast carcinomas. Despite the gradual loss of BM deposition often seen during breast cancer progression,27,36 it is clear that some LM (unspecified isoform) remains expressed in advanced tumors and in metastases.21,29,37 High to moderate levels of LM-511 subunits have been reported in ductal carcinoma in situ, tubular carcinomas, fibroadenoma, atypical medullary carcinomas and carcinomas of no specific type.24,27 Our own investigation in a clinically relevant mouse model of spontaneous breast cancer metastasis revealed a direct correlation between tumor cell expression of LM-511 and metastatic potential.29,38 In particular, bone metastatic 4T1.2 tumors showed widespread expression of LM-511 and this phenotype was maintained in distant metastases.29 High tumor cell expression of LM-511 was observed also in advanced human breast cancers and bone metastases.29
This is in contrast to the downregulation of LM-111 and LM-332 expression that occurs in most (but not all)39,40 advanced breast cancers due to a reduced number of laminin-producing myoepithelial cells and/or decreased expression of these isoforms through promoter methylation.36,41-44 Thus, we have argued that while the loss of LM-111 and LM-332 may contribute to the initial disruption of BM integrity and tissue organization, high levels of LM-511 (or LM-521) in advanced breast tumors may be associated with increased risk of developing metastases and could predict poor clinical outcome. A more definitive demonstration of its prognostic value in breast cancer patients however, will require IHC analysis of LM-511 subunits in a large cohort of patients with known clinical outcome. Nevertheless, it is noteworthy that lung, prostate and breast cancers, for which high expression of LMα5 isoforms is most evident, have a high affinity for bone.45,46 Future studies should explore whether LM-511 expression has particular clinical relevance to tumors with a propensity to metastasize to bone.
Whether LM-511 expression is associated with a specific subtype of breast tumors has not been fully elucidated. Cell lines derived from the 4T1 mouse model of metastasis discussed above have a “basal-like” triple negative (TN) phenotype (lack expression of estrogen receptor, progesterone receptor and Her2/neu receptor) (ref. 47 and unpublished observations), a notoriously aggressive subtype of breast tumors.48 Interestingly, immunostaining of a small number of high grade human breast cancer specimens showed that expression of LMα5 is not limited to a specific breast tumor subtype but its expression is considerably higher in TN tumors (Fig. 1). These observations further support a potential association between high LM-511 expression and aggressive metastatic cancers. The predominance of LM-511 in advanced breast tumors however does not preclude a contribution by other LM isoforms during the early stage of tumor progression. For instance, the presence of LM-332-producing myofibroblasts at the tumor-stromal interface has been demonstrated in some invasive breast tumors.49,50 LM-332 is expressed also in TN tumors39 but it is not clear whether these represent a subset of breast cancer patients distinct from those with LM-511-expressing tumors.
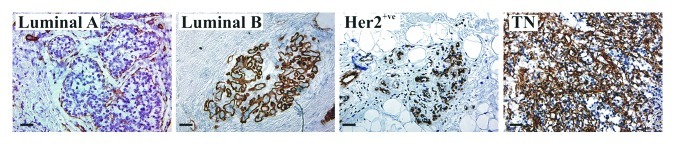
Figure 1. Representative IHC staining of LMα5 in grade 3 luminal A, luminal B, Her2+ve and TN human breast tumors showing highest expression (brown) in TN tumors. Blue, nuclear hematoxylin counterstain. Scale bar, 50 µm.
LM-511 Promotes Integrin-Dependent Tumor Cell Migration and Invasion
Consistent with tissue expression studies, many tumor lines synthesize, secrete and adhere to LM-511 in culture indicating that LM-511 mediates its effects in tumors partly via autocrine stimulation.5,18,29,31,51 LM-511 is a more potent adhesive and migratory substrate than many other matrices in vitro including several LM isoforms and generally shows potency similar to that of LM-332.5,17,18,52 However, commercial preparations of placental LM-511 commonly used in earlier functional studies can be contaminated by other isoforms and may be less active than LM-511 purified from culture media due to proteolytic degradation.53,54 Thus, the results from these earlier studies need to be interpreted with care. Nevertheless, it is clear that adhesion to LM-511 elicits a variety of tumor-specific cellular responses that are dictated in part by the repertoire and level of LM receptors expressed in each cell type as described below.
Multiple receptors including integrins, Lutheran/basal cell adhesion molecule (B-CAM) and α-dystroglycan, are known to bind LM-51155-59 but integrins have been by far the most extensively documented in tumor cells. For example, Tani et al.51 reported that JAR choriocarcinoma cells adhere to LM-511 via α6β1 integrin whereas PANC-1 pancreatic adenocarcinoma cells utilize the α3β1 receptor even though both lines express both receptors. Attachment of HuH-7 hepatocellular carcinoma cells to LM-511 involves at least three integrins, α1β1, α3β1 and α6β1.60 In contrast, the human lung adenocarcinoma A549 cell line adheres to LM-511 exclusively through the α3β1 integrin.5 While BE and M21 melanoma cells attach and migrate on recombinant LM-511 using both α3β1 and α6β1 integrins,31 we have shown that LIM1215 colon cancer cells adhere and spread on LM-511 via multiple integrins including α2β1, α3β1 and α6β4,18 and their migration on this substrate is mediated by α3β1 and α6β4.61 Simultaneous inhibition of all three receptors with function-blocking antibodies in LIM1215 cells resulted in significant death.18
Many of the functions of LMs in breast cancer progression and metastasis have been inferred from receptor studies. In particular, α3β1, α6β1 and α6β4 LM-binding integrins mediate the migration and invasion of breast tumor cells and/or promote their survival and proliferation at metastatic sites.62-69 Therefore, LM-binding receptors represent attractive targets for anti-metastatic therapies.66,67 However, the functions of these receptors in vivo have been interpreted largely as evidence for the role of LM-111 or LM-332 rather than LM-511 in metastasis owing to its more recent identification. Interestingly, a study comparing the ligand-binding specificity of these integrins to various LM isoforms clearly showed that α3β1 and α6β1 bind to LM-511/521 (purified from A549 lung carcinoma conditioned medium and containing predominantly LM-5115) with higher affinity than to LM-111 or LM-332.59 Integrin α6β4 was found to be selective for both LM-332 and LM-511/521 and to bind these isoforms with similar affinity. In light of these observations, it is probable that some of the functions assigned to LM-binding integrins in metastatic breast tumors are mediated through attachment to LM-511.
Recent results from our laboratory are consistent with this possibility. Specifically, we reported that metastatic breast tumor lines adhere and migrate more efficiently on LM-511 than non-metastatic lines.29 LM-511-induced haptotactic migration and invasion of human MDA-MB-231 metastatic breast tumor cells were inhibited strongly by lebein-1, a snake disintegrin targeting LM-binding β1 integrins,70 or an anti-α3 integrin-blocking antibody.29,71 Similar antibody perturbation experiments with metastatic MDA-MB-435 breast tumor cells showed that these cells utilize both α3β1 and α6β1 to migrate toward LM-511 (unpublished observations). Interestingly, migration of MDA-MB-231 and MDA-MB-435 on LM-332 is β1-integrin dependent but not inhibited by a α3 or α6 antibody.72 Together, these data indicate that LM-511 may be a more relevant migratory substrate than LM-332 in metastatic breast tumors expressing α3β1 and α6β1 integrins. LM-511-dependent haptotaxis and invasion of mouse mammary carcinoma lines were similarly inhibited by a β1 integrin-blocking antibody or by treatment with lebein-1.29,71 Consistent with the pro-migratory/invasive properties of LM-511, we found that soluble or coated LM-511 induces matrix metalloproteinase-9 expression in bone metastatic 4T1.2 cells and this activity could be blocked in vitro using the inhibitory LMα5-derived A5G27 peptide.73
To further demonstrate the relevance of these LM-511-tumor interactions to metastasis in vivo, we showed that selection of subsets of cells that migrate rapidly in response to LM-511 allowed the isolation of tumor variants more metastatic to multiple sites, particularly to bone.71 Importantly, enhanced metastasis was accompanied by a small increase in cell surface expression of β1 integrin and a more significant upregulation of β4 integrin. This finding is likely to be highly clinically relevant given that α6β4 integrin associates most significantly with the aggressive basal-like subtype of breast cancer and predicts shorter time to recurrence and decreased survival.74 The prognostic significance of LM and α6β4 integrin receptor expression in breast cancer patients was investigated in an earlier study by Tagliabue et al.22 They determined that the worst disease outcome was for patients with tumors co-expressing LM and α6β4, suggesting the involvement of a LM-integrin autocrine loop in invasive breast tumors. The precise isoform could not be identified in that study due to the use of polyclonal antibodies. However, the authors noted that the LM antiserum used stained throughout the tumor areas in a manner analogous to the widespread LM-511 staining observed in mouse29 and human TN tumors (Fig. 1).
If correct, blocking the production of LM-511 or the function/expression of its receptors would be expected to impact on metastatic potential. To begin to address this, we have generated variants of the 4T1.2 bone metastatic mammary carcinoma line with reduced LM-511 by stable retroviral expression of a short hairpin RNA targeting the LMα5 chain (unpublished, manuscript in preparation). IHC examination of LMα5 in primary tumors 35 d post-implantation confirmed the sustained downregulation of LMα5 compared with control tumors expressing a non-targeting construct (Fig. 2). As expected, LMα5 suppression was evident in the tumor cells but not in the vasculature. Preliminary characterization of metastatic spread in these mice revealed a reduction in bone metastasis (Pouliot et al., manuscript in preparation). Taken together, these observations strongly support a direct functional contribution of LM-511 to breast cancer metastasis. Whether targeting LMα5 also impact on the metastasis of other LM-511-expressing tumor types such as prostate or lung tumors is currently under investigation.
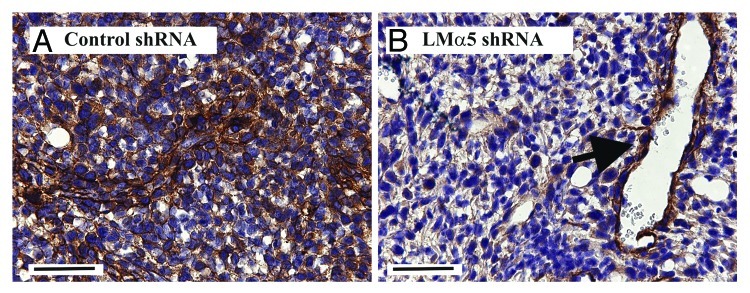
Figure 2. Representative 4T1.2 primary tumors expressing a non-targeting shRNA (A) or a LMα5-targeting shRNA (B). Note the loss of LMα5 in the tumor regions but not in the vasculature (arrow). Scale bar, 50 µm.
Conclusions and Future Perspectives
The increasing experimental evidence linking LM-511 to cancer progression supports the notion that LM-511 has a much broader role in tumor invasion and metastasis than previously appreciated. However, major questions remain regarding its prognostic significance, precise mechanism of action and potential as therapeutic target. Evidently, many advanced tumors express LM-511 and studies so far point to a possible association with bone metastasis and/or the basal-like/TN phenotype. Whether LM-332 and LM-511-expressing tumors represent overlapping or distinct TN subsets also needs to be addressed. A definitive answer to these questions will require a more in depth IHC analysis of larger cohorts of patients with known clinical outcome. However, the 4C7 anti-LMα5 antibody commonly used for this application does not work well on formalin-fixed paraffin embedded tissues, most likely because the 3D conformation of the epitope recognized by the 4C7 antibody in the LG1–3 modules of the LMα5 chain75 is destroyed by tissue fixation and processing. Thus, the generation of more robust antibodies for staining of archival material should be a priority.
Future studies will need also to clarify the precise contribution of LM-511 to the metastatic process and its relationship to LM-332. Both isoforms have been implicated in tumor invasion and metastasis and share many of their receptors suggesting redundant functions. Yet, observations in various normal physiological processes indicate that LM-511 and LM-332 also have distinct, non-overlapping and sometimes opposing functions. For instance, LM-511 stimulates hair growth whereas LM-332 antagonizes this LM-511 response.58 Moreover, LM-511 promotes long-term proliferation and maintenance of pluripotency in stem cell cultures, whereas LM-332 enables proliferation but not pluripotency.76,77
These properties could be relevant to metastasis and may be utilized by tumor cells at distinct stages of the metastatic cascade, including the epithelial to mesenchymal transition (EMT) required for tumor cell invasion. For example, downregulation of LM-511 and LM-332 occurring in cells undergoing EMT78,79 may be necessary to decrease the strength of adhesion and facilitate the early acquisition of a mesenchymal phenotype. Cells migrating away from the primary tumor, could utilize myofibroblast-derived LM-332 at the tumor-stroma interface.50 Conversely, re-expression of LM-511 at late stage of metastasis may provide the plasticity required for the reverse process of MET and facilitate the re-establishment of a proliferative epithelial phenotype at metastatic sites. This would be consistent with the recent demonstration that LM-511 promotes proliferation and partially blocks EMT in human β cells.80 An interesting possibility is that these distinct LM-511 and LM-332-dependent adhesive responses during EMT and MET may be differentially regulated by CD151, an important modulator of LM-binding integrins.66,81,82 CD151 overexpression was shown recently to be an independent prognostic marker of poor overall survival in invasive breast cancer, particularly in quintuple negative breast cancer, a subtype of TN breast cancer.83 A proposed model integrating these multiple LM-511 functions during metastasis is presented in Figure 3.
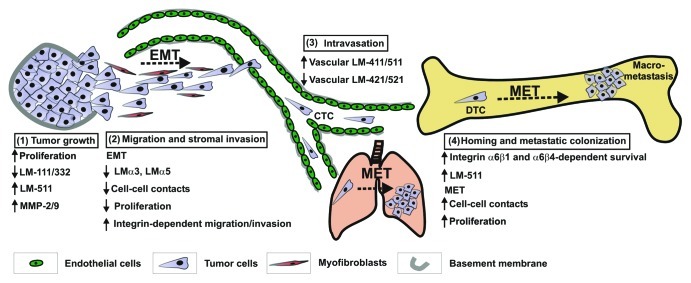
Figure 3. Proposed model of LM-511 expression and function during cancer progression and metastasis. The model depicted above is based primarily on observations made in breast cancer metastasis models but incorporates also findings from studies in other tumor types as referenced below and in the text. (1) Uncontrolled growth of the primary tumor and the loss of myoepithelial-derived LM-111 and LM-332 contribute to the initial disruption of tissue organization.36,41,44 Sustained expression of LM-511 further enhances the breakdown of the surrounding basement membrane through induction of MMP-2/9 gelatinases73,90 resulting in exposure of tumor cells to the surrounding stroma. (2) Under the influence of stromal factors, tumor cells undergo EMT and loss of cell-cell contacts. Snail-dependent downregulation of LMα3 and LMα578,79 may be required to reduce the strength of adhesive interactions and enhance α3β1, α6β1 or α6β4 integrin-dependent cell motility. Subsequent stromal invasion may be achieved by interaction of tumor cells with low level of tumor-derived LM-511 and/or LM-332 derived from myofibrobalsts present at the tumor-stromal interface.49 (3) Changes in the composition of LM isoforms in the tumor vasculature and the abundance of LM-511 are likely to contribute to tumor cell attachment and intravasation.24,29,30 (4) Homing of circulating tumor cells (CTC) and invasion into metastatic sites is mediated through α3β1 integrin interaction with vascular LMs (including LM-511) and MMP-9 proteolytic activity.64,73,91 Binding of integrin α6β1 or α6β4 to endogenous LM-332 or LM-511 (in organs rich in these isoforms) promotes survival and growth of disseminated tumor cells (DTC).62,68,69 Alternatively, enhanced expression of LM-511 and autocrine stimulation may be required in bone where LM-332 or LM-511 expression is more restricted29 to promote strong adhesion and α6β1/α6β4-dependent tumor cell survival. In addition, adhesion to LM-511 may provide the plasticity required for the process of MET and the acquisition of a proliferative epithelial phenotype at metastatic sites.
While current evidence supports the role of tumor-derived LM-511 in metastasis, the contribution (if any) of stromal/vascular LM-511 to metastasis is incompletely understood. The availability of various animal models of metastasis (e.g., MMTV-PyMT or 4T1 model) in combination with conditional Lama5−/− knockout mice could provide a useful in vivo platform to address this and guide future research.
The widespread expression of LM-511 in vivo makes direct targeting of this LM isoform impractical for cancer therapy. Hence, preclinical studies in animals have so far focused on targeting its receptors.66,67 For instance, many studies have employed inhibitory peptides derived from LMs, including all LM-511 subunits, to demonstrate the clinical relevance of targeting LM-511 in vivo for anti-metastatic therapies. Some of these peptides were shown to exert either potent anti-metastatic activity in experimental models of melanoma metastasis to lung or bone84-86 or anti-angiogenic effects on MDA-MB-231 mammary tumors in nude mice.87 Peptides may block metastasis by interfering with the function of tumor or host-derived LM-511 since LM-511 is also abundant in blood vessels, lung and bone sinusoids.29 While peptides are unlikely to be useful for long-term therapy due to their short half-life in vivo, the above studies nevertheless provide proof of principle that targeting LM receptors could have therapeutic benefits.
Disintegrin are a class of inhibitors increasingly investigated for their anti-tumor/metastatic activity. Lebein-1, unlike most other disintegrins that target β3-type integrins via RGD sequences, has the unusual characteristic of being selective for laminin-type integrins, inhibiting the α3β1, α6β1 and α7β1 integrins (but not α1β1 and α2β1 collagen receptors) in an RGD-independent manner.70,71,88 Our recent demonstration of its potent inhibitory properties against LM-511-mediated adhesion, migration and invasion in vitro provides the rationale for further testing in vivo.71 Consistent with this, pre-treatment of tumor cells with lebein-1 in vitro inhibits subsequent attachment to sinusoids and homing to the liver in vivo.89 Understanding the molecular mechanisms by which lebein-1 interacts with LM-511 receptors and inhibits LM-511-mediated responses in tumor cells could facilitate the design of specific and potent inhibitors of LM-511 receptors. Metastasis is responsible for the majority of cancer-related death and remains a major clinical challenge despite extensive efforts over the past decades aimed at understanding and targeting cancer. While much remains to be done, continuing research in the field of laminins could provide additional weapons to tackle this devastating disease and lead to the development of alternative strategies to prevent or delay metastatic progression.
Acknowledgments
We thank Dr. Delphine Denoyer for careful review of the manuscript and useful suggestions. N.P. is supported by Research Project Grants from the National Health and Medical Research Council of Australia and the PeterMac Cancer Foundation.
Glossary
Abbreviations:
- BM
basement membrane
- CTC
circulating tumor cell
- DCT
disseminated tumor cell
- ECM
extracellular matrix
- LM
laminin
- TN
triple negative
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22125
References
- 1.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–68. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 2.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–24. doi: 10.1016/S0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 3.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Church HJ, Aplin JD. BeWo choriocarcinoma cells produce laminin 10. Biochem J. 1998;332:491–8. doi: 10.1042/bj3320491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–9. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 6.Tiger CF, Champliaud MF, Pedrosa-Domellof F, Thornell LE, Ekblom P, Gullberg D. Presence of laminin alpha5 chain and lack of laminin alpha1 chain during human muscle development and in muscular dystrophies. J Biol Chem. 1997;272:28590–5. doi: 10.1074/jbc.272.45.28590. [DOI] [PubMed] [Google Scholar]
- 7.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–7. [PubMed] [Google Scholar]
- 8.Durkin ME, Loechel F, Mattei MG, Gilpin BJ, Albrechtsen R, Wewer UM. Tissue-specific expression of the human laminin alpha5-chain, and mapping of the gene to human chromosome 20q13.2-13.3 and to distal mouse chromosome 2 near the locus for the ragged (Ra) mutation. FEBS Lett. 1997;411:296–300. doi: 10.1016/S0014-5793(97)00686-8. [DOI] [PubMed] [Google Scholar]
- 9.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 10.Geberhiwot T, Wondimu Z, Salo S, Pikkarainen T, Kortesmaa J, Tryggvason K, et al. Chain specificity assignment of monoclonal antibodies to human laminins by using recombinant laminin beta1 and gamma1 chains. Matrix Biol. 2000;19:163–7. doi: 10.1016/S0945-053X(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 11.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, et al. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;281:5008–16. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–10. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahoney ZX, Stappenbeck TS, Miner JH. Laminin alpha 5 influences the architecture of the mouse small intestine mucosa. J Cell Sci. 2008;121:2493–502. doi: 10.1242/jcs.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, et al. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, et al. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–8. doi: 10.1074/jbc.M111228200. [DOI] [PubMed] [Google Scholar]
- 18.Pouliot N, Connolly LM, Moritz RL, Simpson RJ, Burgess AW. Colon cancer cells adhesion and spreading on autocrine laminin-10 is mediated by multiple integrin receptors and modulated by EGF receptor stimulation. Exp Cell Res. 2000;261:360–71. doi: 10.1006/excr.2000.5065. [DOI] [PubMed] [Google Scholar]
- 19.Sroka IC, Chen ML, Cress AE. Simplified purification procedure of laminin-332 and laminin-511 from human cell lines. Biochem Biophys Res Commun. 2008;375:410–3. doi: 10.1016/j.bbrc.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamurs L, Pouliot N, Gibson P, Hocking G, Nice E. Strategies for the purification of laminin-10 for studies on colon cancer metastasis. Biomed Chromatogr. 2003;17:201–11. doi: 10.1002/bmc.248. [DOI] [PubMed] [Google Scholar]
- 21.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. doi: 10.1016/S0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 22.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Ménard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–10. [PubMed] [Google Scholar]
- 23.Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sanes JR. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990;1:731–40. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewitt RE, Powe DG, Morrell K, Balley E, Leach IH, Ellis IO, et al. Laminin and collagen IV subunit distribution in normal and neoplastic tissues of colorectum and breast. Br J Cancer. 1997;75:221–9. doi: 10.1038/bjc.1997.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sollberg S, Peltonen J, Uitto J. Differential expression of laminin isoforms and beta 4 integrin epitopes in the basement membrane zone of normal human skin and basal cell carcinomas. J Invest Dermatol. 1992;98:864–70. doi: 10.1111/1523-1747.ep12457080. [DOI] [PubMed] [Google Scholar]
- 26.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 27.Määttä M, Virtanen I, Burgeson R, Autio-Harmainen H. Comparative analysis of the distribution of laminin chains in the basement membranes in some malignant epithelial tumors: the alpha1 chain of laminin shows a selected expression pattern in human carcinomas. J Histochem Cytochem. 2001;49:711–26. doi: 10.1177/002215540104900605. [DOI] [PubMed] [Google Scholar]
- 28.Brar PK, Dalkin BL, Weyer C, Sallam K, Virtanen I, Nagle RB. Laminin alpha-1, alpha-3, and alpha-5 chain expression in human prepubertal [correction of prepubetal] benign prostate glands and adult benign and malignant prostate glands. Prostate. 2003;55:65–70. doi: 10.1002/pros.10206. [DOI] [PubMed] [Google Scholar]
- 29.Chia J, Kusuma N, Anderson R, Parker B, Bidwell B, Zamurs L, et al. Evidence for a role of tumor-derived laminin-511 in the metastatic progression of breast cancer. Am J Pathol. 2007;170:2135–48. doi: 10.2353/ajpath.2007.060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, et al. Overexpression of beta1-chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:R411–21. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oikawa Y, Hansson J, Sasaki T, Rousselle P, Domogatskaya A, Rodin S, et al. Melanoma cells produce multiple laminin isoforms and strongly migrate on α5 laminin(s) via several integrin receptors. Exp Cell Res. 2011;317:1119–33. doi: 10.1016/j.yexcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Kawataki T, Yamane T, Naganuma H, Rousselle P, Andurén I, Tryggvason K, et al. Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin. Exp Cell Res. 2007;313:3819–31. doi: 10.1016/j.yexcr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Franz M, Wolheim A, Richter P, Umbreit C, Dahse R, Driemel O, et al. Stromal laminin chain distribution in normal, hyperplastic and malignant oral mucosa: relation to myofibroblast occurrence and vessel formation. J Oral Pathol Med. 2010;39:290–8. doi: 10.1111/j.1600-0714.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagle RB, Knox JD, Wolf C, Bowden GT, Cress AE. Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J Cell Biochem Suppl. 1994;19:232–7. [PubMed] [Google Scholar]
- 35.Szelachowska J, Jeleń M. Laminin, Her2/neu and Ki-67 as prognostic factors in non-small cell lung cancer. Rocz Akad Med Bialymst. 2004;49:256–61. [PubMed] [Google Scholar]
- 36.Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982;42:4763–70. [PubMed] [Google Scholar]
- 37.Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81. [PubMed] [Google Scholar]
- 38.Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed] [Google Scholar]
- 39.Carpenter PM, Wang-Rodriguez J, Chan OT, Wilczynski SP. Laminin 5 expression in metaplastic breast carcinomas. Am J Surg Pathol. 2008;32:345–53. doi: 10.1097/PAS.0b013e3181592201. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Angulo AM, Sahin A, Krishnamurthy S, Yang Y, Kau SW, Hortobagyi GN, et al. Biologic markers in axillary node-negative breast cancer: differential expression in invasive ductal carcinoma versus invasive lobular carcinoma. Clin Breast Cancer. 2006;7:396–400. doi: 10.3816/CBC.2006.n.056. [DOI] [PubMed] [Google Scholar]
- 41.Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: an immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology. 1999;34:305–9. doi: 10.1046/j.1365-2559.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 43.Korah R, Das K, Lindy ME, Hameed M, Wieder R. Coordinate loss of fibroblast growth factor 2 and laminin 5 expression during neoplastic progression of mammary duct epithelium. Hum Pathol. 2007;38:154–60. doi: 10.1016/j.humpath.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Martin KJ, Kwan CP, Nagasaki K, Zhang X, O’Hare MJ, Kaelin CM, et al. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4:602–13. [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–94. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 46.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 47.Bao L, Haque A, Jackson K, Hazari S, Moroz K, Jetly R, et al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011;178:838–52. doi: 10.1016/j.ajpath.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–36. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim BG, An HJ, Kang S, Choi YP, Gao MQ, Park H, et al. Laminin-332-rich tumor microenvironment for tumor invasion in the interface zone of breast cancer. Am J Pathol. 2011;178:373–81. doi: 10.1016/j.ajpath.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim BG, Gao MQ, Choi YP, Kang S, Park HR, Kang KS, et al. Invasive breast cancer induces laminin-332 upregulation and integrin β4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype during tissue remodeling. Breast Cancer Res. 2012;14:R88. doi: 10.1186/bcr3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tani T, Lehto VP, Virtanen I. Expression of laminins 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp Cell Res. 1999;248:115–21. doi: 10.1006/excr.1999.4399. [DOI] [PubMed] [Google Scholar]
- 52.Spessotto P, Gronkowska A, Deutzmann R, Perris R, Colombatti A. Preferential locomotion of leukemic cells towards laminin isoforms 8 and 10. Matrix Biol. 2003;22:351–61. doi: 10.1016/S0945-053X(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 53.Ferletta M, Ekblom P. Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J Cell Sci. 1999;112:1–10. doi: 10.1242/jcs.112.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Wondimu Z, Gorfu G, Kawataki T, Smirnov S, Yurchenco P, Tryggvason K, et al. Characterization of commercial laminin preparations from human placenta in comparison to recombinant laminins 2 (alpha2beta1gamma1), 8 (alpha4beta1gamma1), 10 (alpha5beta1gamma1) Matrix Biol. 2006;25:89–93. doi: 10.1016/j.matbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, et al. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–54. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 56.Kikkawa Y, Miner JH. Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues. Connect Tissue Res. 2005;46:193–9. doi: 10.1080/03008200500344074. [DOI] [PubMed] [Google Scholar]
- 57.Kikkawa Y, Sasaki T, Nguyen MT, Nomizu M, Mitaka T, Miner JH. The LG1-3 tandem of laminin alpha5 harbors the binding sites of Lutheran/basal cell adhesion molecule and alpha3beta1/alpha6beta1 integrins. J Biol Chem. 2007;282:14853–60. doi: 10.1074/jbc.M611706200. [DOI] [PubMed] [Google Scholar]
- 58.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008;17:473–80. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 59.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Kikkawa Y, Sudo R, Kon J, Mizuguchi T, Nomizu M, Hirata K, et al. Laminin alpha 5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp Cell Res. 2008;314:2579–90. doi: 10.1016/j.yexcr.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Pouliot N, Nice EC, Burgess AW. Laminin-10 mediates basal and EGF-stimulated motility of human colon carcinoma cells via alpha(3)beta(1) and alpha(6)beta(4) integrins. Exp Cell Res. 2001;266:1–10. doi: 10.1006/excr.2001.5197. [DOI] [PubMed] [Google Scholar]
- 62.Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005;65:10970–6. doi: 10.1158/0008-5472.CAN-05-2327. [DOI] [PubMed] [Google Scholar]
- 63.Mercurio AM, Bachelder RE, Chung J, O’Connor KL, Rabinovitz I, Shaw LM, et al. Integrin laminin receptors and breast carcinoma progression. J Mammary Gland Biol Neoplasia. 2001;6:299–309. doi: 10.1023/A:1011323608064. [DOI] [PubMed] [Google Scholar]
- 64.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–42. doi: 10.1002/1097-0215(20000801)87:3<336::AID-IJC5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Soung YH, Gil HJ, Clifford JL, Chung J. Role of α6β4 integrin in cell motility, invasion and metastasis of mammary tumors. Curr Protein Pept Sci. 2011;12:23–9. doi: 10.2174/138920311795659399. [DOI] [PubMed] [Google Scholar]
- 66.Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12:e3. doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subbaram S, Dipersio CM. Integrin α3β1 as a breast cancer target. Expert Opin Ther Targets. 2011;15:1197–210. doi: 10.1517/14728222.2011.609557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/S1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol. 1997;151:1191–8. [PMC free article] [PubMed] [Google Scholar]
- 70.Eble JA, Bruckner P, Mayer U. Vipera lebetina venom contains two disintegrins inhibiting laminin-binding beta1 integrins. J Biol Chem. 2003;278:26488–96. doi: 10.1074/jbc.M301860200. [DOI] [PubMed] [Google Scholar]
- 71.Kusuma N, Denoyer D, Eble JA, Redvers RP, Parker BS, Pelzer R, et al. Integrin-dependent response to laminin-511 regulates breast tumor cell invasion and metastasis. Int J Cancer. 2012;130:555–66. doi: 10.1002/ijc.26018. [DOI] [PubMed] [Google Scholar]
- 72.Plopper GE, Domanico SZ, Cirulli V, Kiosses WB, Quaranta V. Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat. 1998;51:57–69. doi: 10.1023/A:1006086218174. [DOI] [PubMed] [Google Scholar]
- 73.Kusuma N, Anderson RL, Pouliot N. Laminin α5-derived peptides modulate the properties of metastatic breast tumour cells. Clin Exp Metastasis. 2011;28:909–21. doi: 10.1007/s10585-011-9422-8. [DOI] [PubMed] [Google Scholar]
- 74.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin beta4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14:1050–8. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- 75.Ido H, Harada K, Yagi Y, Sekiguchi K. Probing the integrin-binding site within the globular domain of laminin-511 with the function-blocking monoclonal antibody 4C7. Matrix Biol. 2006;25:112–7. doi: 10.1016/j.matbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26:2800–9. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 77.Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 78.Takkunen M, Ainola M, Vainionpää N, Grenman R, Patarroyo M, García de Herreros A, et al. Epithelial-mesenchymal transition downregulates laminin alpha5 chain and upregulates laminin alpha4 chain in oral squamous carcinoma cells. Histochem Cell Biol. 2008;130:509–25. doi: 10.1007/s00418-008-0443-6. [DOI] [PubMed] [Google Scholar]
- 79.Takkunen M, Grenman R, Hukkanen M, Korhonen M, García de Herreros A, Virtanen I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem. 2006;54:1263–75. doi: 10.1369/jhc.6A6958.2006. [DOI] [PubMed] [Google Scholar]
- 80.Banerjee M, Virtanen I, Palgi J, Korsgren O, Otonkoski T. Proliferation and plasticity of human beta cells on physiologically occurring laminin isoforms. Mol Cell Endocrinol. 2012;355:78–86. doi: 10.1016/j.mce.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Ke AW, Shi GM, Zhou J, Huang XY, Shi YH, Ding ZB, et al. CD151 amplifies signaling by integrin α6β1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140:1629–41, e15. doi: 10.1053/j.gastro.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Yang W, Li P, Lin J, Zuo H, Zuo P, Zou Y, et al. CD151 promotes proliferation and migration of PC3 cells via the formation of CD151-integrin α3/α6 complex. J Huazhong Univ Sci Technolog Med Sci. 2012;32:383–8. doi: 10.1007/s11596-012-0066-y. [DOI] [PubMed] [Google Scholar]
- 83.Kwon MJ, Park S, Choi JY, Oh E, Kim YJ, Park YH, et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer. 2012;106:923–30. doi: 10.1038/bjc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–6. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- 85.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, et al. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–4. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 86.Nakai M, Mundy GR, Williams PJ, Boyce B, Yoneda T. A synthetic antagonist to laminin inhibits the formation of osteolytic metastases by human melanoma cells in nude mice. Cancer Res. 1992;52:5395–9. [PubMed] [Google Scholar]
- 87.Ponce ML, Kleinman HK. Identification of redundant angiogenic sites in laminin alpha1 and gamma1 chains. Exp Cell Res. 2003;285:189–95. doi: 10.1016/S0014-4827(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 88.McLane MA, Sanchez EE, Wong A, Paquette-Straub C, Perez JC. Disintegrins. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:327–55. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- 89.Rosenow F, Ossig R, Thormeyer D, Gasmann P, Schlüter K, Brunner G, et al. Integrins as antimetastatic targets of RGD-independent snake venom components in liver metastasis [corrected] Neoplasia. 2008;10:168–76. doi: 10.1593/neo.07898. [corrected] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu J, Nishiuchi R, Sekiguchi K. Matrix metalloproteinase-2 is involved in A549 cell migration on laminin-10/11. Biochem Biophys Res Commun. 2002;296:73–7. doi: 10.1016/S0006-291X(02)00831-8. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Fu W, Im JH, Zhou Z, Santoro SA, Iyer V, et al. Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164:935–41. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]


