Abstract
Laminin-111 is a large trimeric basement membrane glycoprotein with many active sites. In particular, four peptides active in tumor malignancy studies have been identified in laminin-111 using a systematic peptide screening method followed by various assays. Two of the peptides (IKVAV and AG73) are found on the α1 chain, one (YIGSR) of the β1 chain and one (C16) on the γ1 chain. The four peptides have distinct activities and receptors. Since three of the peptides (IKVAV, AG73 and C16) strongly promote tumor growth, this may explain the potent effects laminin-111 has on malignant cells. The peptide, YIGSR, decreases tumor growth and experimental metastasis via a 32/67 kD receptor while IKVAV increases tumor growth, angiogenesis and protease activity via integrin receptors. AG73 increases tumor growth and metastases via syndecan receptors. C16 increases tumor growth and angiogenesis via integrins. Identification of such sites on laminin-111 will have use in defining strategies to develop therapeutics for cancer.
Keywords: laminin-111, synthetic peptide, metastasis, tumor growth, angiogenesis, migration, adhesion, basement membrane, proteases
Introduction
The basement membrane glycoprotein laminin-111 is a large molecule found primarily in embryonic tissue-derived basement membranes. Laminin-111 is the most well-studied of the some 15 laminin isoforms because it can be isolated in quantity from the mouse Engelbreth-Holm-Swarm (EHS) tumor and is commercially available. It consists of three chains, α1 (400 kD), β1 (210 kD) and γ1 (200 kD), that associate to form a cruciform structure (Fig. 1). These chains are homologous in structure and have N-terminal globules separated by epidermal growth factor (EGF)-like repeat sequences. The α1 chain has three such globules and three EGF-like repeats while the other two chains are shorter with two globules and two EGF-like repeat sequences. All three chains have a coiled-coil structure of similar length that extends to the C-terminus. The laminin α1 chain C-terminal globular domain (LG domain) consists of LG1-LG5 tandems (100 kD) that play a critical role in the biological function of laminin-111.
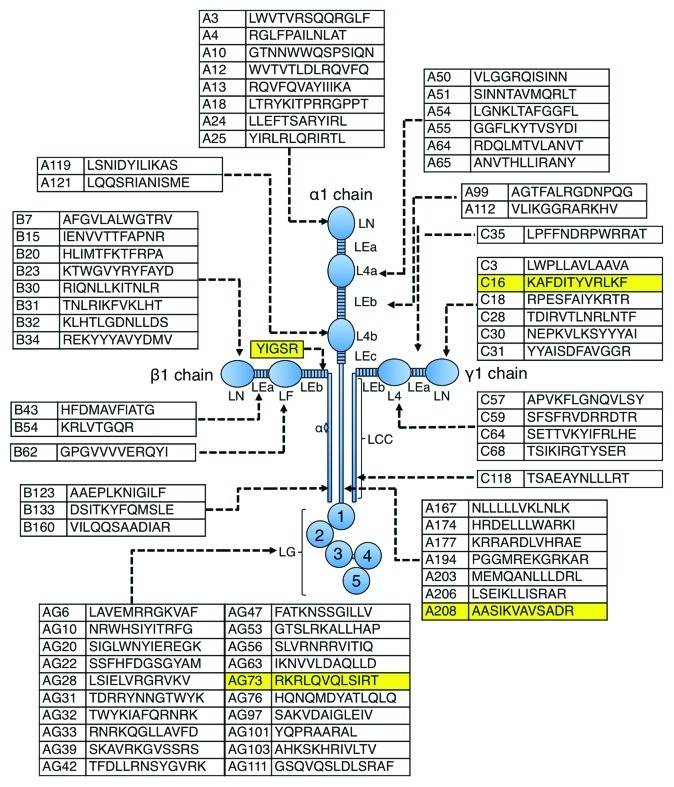
Figure 1. Schematic model of laminin-111 showing the location of peptides that exhibit cell attachment activity for human fibrosarcoma cells. Laminin-111 is composed of three subunits, α, β and γ chains. Forty-five active peptides are localized in α1 chain, 14 active peptides in β1 chain and 12 active peptides in γ1 chain. The four highlighted peptides described here are active in tumor malignancy and are also listed in Table 1. YIGSR and IKVAV were previously identified as active sequences.27,54 YIGSR peptide does not exhibit cell adhesion activity for fibrocarcoma cells. IKVAV sequence is contained in A208 peptides.
Laminin-111 binds to the other abundant basement membrane components, which include collagen IV, perlecan, entactin/nidogen and itself. Such interactions are specific and important in the assembly of the basement membrane matrix. Laminin-111 also interacts with cells and has multiple biological activities, including promoting cell adhesion, migration, neurite outgrowth and tumor growth and metastasis (Box 1). Proteolytic fragments as well as synthetic peptides have been used to localize and study these activities and demonstrate that it is a multifunctional protein with the potential for many active sites (Fig. 1). Furthermore, many different types of cell surface receptors have been identified that bind to these active sequences. Here, we describe four laminin-111-derived synthetic peptides that are active in malignancy (Fig. 1 and Table 1). One peptide (YIGSR) inhibits tumor growth and angiogenesis while the other three (IKVAV, RKRLQVQLSIR and KAFDITYVRLKF) promote tumor growth. These peptides appear to use different cellular receptors and mechanism to affect their activity.
Box 1. Biological activities of laminin-111
Adhesion
Migration
Differentiation
Protease secretion
Cell polarity
Angiogenesis
Tumor growth
Tumor metastasis
Table 1. Laminin-111-derived peptides active with tumor cells, sequence, location and activity.
| Peptide/location residues | Activity | Receptor |
|---|---|---|
| YIGSR/β1 929–933 |
↑ adhesion, ↑ migration, ↓ tumor growth, ↓ metastasis, ↓ invasion |
67 kD protein |
| IKVAV/α1 2097–2108 |
↑ metastasis, ↑ tumor growth, ↑ angiogenesis, ↑ proteases |
integrins α3β1, α6β1 |
| RKRLQVQLSIRT (AG73)/α2620–2631 |
↑ tumor growth, ↑ metastasis to lung, bone and liver, ↑ invasion, ↑ angiogenesis, ↑ proteases |
syndecans 1, 2 and 4 |
| KAFDITYVRLKF (C16)/γ1 139–150 | ↑ metastasis, ↑ angiogenesis | integrins αvβ3, α5β1 |
Laminin-111 and Malignancy
Laminin-111 has been shown to promote the malignant phenotype in many research laboratories using both in vitro and in vivo approaches (Table 2). It increases tumor cell adhesion, migration, growth and metastasis.1,2 Tumor cells selected for high laminin-111 adhesion are more malignant in vivo than either the non-adherent selected tumor cells, the parental cells, or those cells selected for high fibronectin adhesion.3,4 Additionally, levels of the Mr = 32/67 kD laminin receptor correlate positively with malignancy. Furthermore, protease production (urokinase-type plasminogen activator and matrix metalloproteases-2 and -9) is induced by laminin-111 in tumor cells,5,6 which likely facilitates metastatic spread by allowing tumor cells to penetrate tissues. The activity of these proteases and also of other proteins that the proteases release from the tissues and matrices, such as endogenous growth and angiogenic factors and protein fragments, further contributes to the metastatic spread and survival of tumor cells.
Table 2. Timeline of laminin-111 and laminin-111-derived peptide findings related to malignancy.
| 1979 |
Laminin isolated from EHS tumor |
| 1986 |
Laminin increased release of proteases from tumor cells |
| 1987 |
Laminin β1 chain sequenced |
| 1987 |
Laminin β1 peptide YIGSR promoted adhesion via a 67 kD receptor |
| 1987 |
YIGSR inhibited tumor growth and metastasis |
| 1992 |
Laminin α1 chain peptide IKVAV is angiogenic |
| 1993 |
YIGSR adhesion-selected tumor cells have increased malignancy |
| 1993 |
IKVAV promoted increased melanoma proteases |
| 1996 |
YIGSR inhibited angiogenesis |
| 1997 |
Laminin α1 chain peptide AG73 promoted liver metastasis |
| 1999 |
Laminin γ chain peptide C16 had angiogenic activity |
| 2001 |
C16 bound to integrins αvβ3 and α5β1 |
| 2002 |
AG73 promoted metastasis via heparan-containing proteoglycan |
| 2007 |
C16 increased melanoma extravascular migratory metastasis ex vivo |
| 2011 |
AG73 used for liposome targeting to cancer cells |
| 2011 | AG73 and C16 regulated cancer cell invadopodia |
The basement membrane is a barrier to tumor cell metastasis, separating the epithelium from connective tissue and the vascular endothelium. The anti-laminin-111 polyclonal antibody has been often used to identify the presence of this molecule in the tumor environment. Although the antibody cannot define the individual laminin-111 subunits, immunohistological studies have shown that laminin-111 is present in tumor tissues. Remodeling or loss of the basement membrane is believed to be required for tumor cells to move through the extracellular matrix (ECM) and to metastasize to distant sites.7 Collectively, active proteases can degrade all components of the ECM in vitro,8-10 and their expression is frequently found in vivo at sites where the ECM is cleaved.9,11-15 In tissues such as breast tumors, the laminin-111 staining is often discontinuous.12-17 Proteolytic cleavage of structural proteins may expose cryptic sites that have biological activity. The existence of such cryptic sites with biological activity within larger molecules is not unusual.10,18
When melanoma tumor cells were grown in culture in the presence of laminin-111 and then intravenously injected into mice more lung tumors formed over that observed with cells cultured in the absence of laminin-111 or in the presence of fibronectin.3,4 The reason for this increase in tumor metastasis is unclear but suggests a preferential growth of the more malignant subpopulations. Interestingly, laminin-111 also increased A375 human melanoma metastasis to bone in an intracardiac model.19 Finally, laminin-111 co-injected subcutaneously with tumor cells in mice increases the growth rate of some tumors over that observed with cells injected alone or with collagen I.3,4 Since collagen I forms a gel and had no effect, the role of laminin-111 in “holding the tumor cells in place” is not likely the mechanism for laminin-111-enhanced tumor cell growth. A possible explanation is that proteases degrade the lamnin-111 to active fragments that promote growth, protease production and angiogenesis.
Screening for Active Sites to Identify Laminin-111-Derived Peptides That Affect Malignancy
Several active sites on laminin-111 have been identified using proteolytic fragments, recombinant proteins and synthetic peptides.20,21 Some proteolytic fragments prepared from laminin-111 exhibit biological activity but there are not many enzymes that provide specific fragments. Thus, it is difficult to obtain a complete set of proteolytic fragments for defining active sites. Likewise, recombinant proteins provide another approach for identifying active sites on laminin-111 and have the advantage of providing specific desired sequences. However, it can be difficult to express these proteins in either bacteria or mammalian cells. Synthetic peptides are designed according to the amino acid sequence. A disadvantage of synthetic peptide is that it can be difficult to synthesize long peptides, mimic structure and include glycosylation. However, synthetic peptides do have major advantages over proteolytic fragments and recombinant proteins for probing active sites. The peptides are generally easier and more accurate in terms of sequence to obtain as well as having higher purity. We have developed systematic approaches for molecular dissection of laminin-111 functional sites using synthetic peptides (Fig. 2). All peptides were manually synthesized with a C-terminal amide and purified by HPLC. Peptides were generally designed with a length of 12 amino acid residues and overlapped with neighboring peptides by four amino acids. Cysteine residues were omitted to prevent the influence of disulfide bonds. Based on the amino acids sequence of laminin-111, we produced 673 overlapping synthetic peptides covering the entire protein.22-26
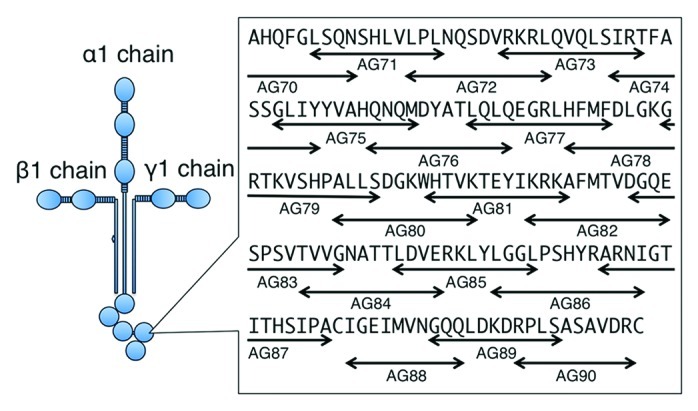
Figure 2. Design of synthetic peptides covering amino acid sequence of laminin-111. A set of synthetic peptides in laminin-α1 LG domain is shown. Arrows indicate the location of the peptides. Peptides were basically designed with a length of 12 amino acid residues and overlapped with neighboring peptides by four amino acids. Cysteine residues were omitted to prevent the influence of disulfide bonds.
Cell adhesion is a major function of laminin-111. Therefore, we first screened cell adhesion activity of synthetic peptides using plastic plates or Sepharose beads (Fig. 3). In the cell adhesion assay using plastic plates, synthetic peptides were added to each well followed by drying overnight. After drying, peptide-coated wells are blocked with BSA. As shown in Figure 3, a cell suspension is added to the wells and incubated for 1 h at 37°C. The cells adhering to the peptide-coated wells are stained with crystal violet and then quantified. In the cell adhesion assay, using Sepharose beads, synthetic peptides are coupled to CNBr-Sepharose beads. Cell suspension and peptide-beads are mixed and incubated for 1 h at 37°C. As described above, the cells adhering to the peptide-coupled Sepharose beads are stained with crystal violet and quantified by viewing with a phase-contrast microscope. However, both assays have limitations. The coating efficiency of a peptide depends on the property of the peptide. Additionally, the peptides coated on the wells may not be in the native conformation due to random binding to the dish which may result in loss of the structure needed for cell binding. Synthetic peptides coupled to Sepharose beads maintain their conformation due to binding of the peptide at one end via a peptide spacer with the remainder of the peptide in solution and available for interaction with cells. The quantification of the cells bound to the peptide-beads can be less accurate depending on the cell density. We evaluated cell adhesion activities using the both assays. Using this approach and additional assays with the identified active peptides, several peptides were discovered as having activity in malignancy (Table 1).23-26 The four peptides that have been most widely studied in malignancy will be reviewed here.
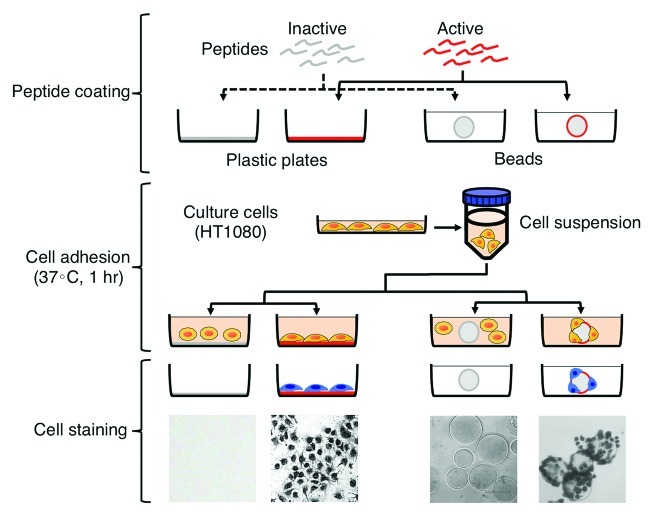
Figure 3. Cell adhesion assay using plastic plates and beads. Peptide coating: synthetic peptides are coated on plastic plates or beads. Cell adhesion: a cell suspension is added into the wells or mixed with peptide-beads. The cells are incubated for 1 h at 37°C. Cell staining: the cells adhering to the peptides are stained with crystal violet and then evaluated.
YIGSR
The first described and most studied laminin-111-derived active peptide, YIGSR, from the β1 chain binds to the 32/67 kD cell surface receptor and has many activities related to its inhibition of malignancy.27-29 To date, more than 240 papers have been published on this peptide documenting its biological activity and importance in cell behavior. In vivo, YIGSR blocks xenograft growth, experimental metastasis formation in the lungs (intravenous injection) and bones (intracardiac injection)19 and angiogenesis.30,31 The activity of this rather short five amino acid-containing peptide is enhanced with multimeric forms, such as a tandem repeat form and a multimeric form using a lysine branch, and when coupled to polyethylene glycol (PEG).33-36 The multimeric form offers more binding sites and enhances cell attachment activity while the PEG coupling may stabilize the structure and reduce degradation.36 In the circulation, the PEG-YIGSR would be expected to have a longer half-life.33 Cyclic forms of YIGSR also have increased activity, suggesting possible stabilization of the active conformation and/or reduced clearance from the circulation. Finally, conjugating YIGSR to chitosan also increased its antimetatatic activity as did conjugation to polyvinyl pyrrolidone.37,38 With the conjugation of YIGSR to polyvinyl pyrrolidone, there was a 15-fold increase in the plasma half-life over free peptide and a 100-fold increase in the antimetastatic effect.38 YIGSR also blocks angiogenesis in several assays, including the in vitro tube formation, chick chorioallantoic membrane (CAM) and rabbit eye pocket assays.31 Tumors grown in vivo in the presence of YIGSR have reduced numbers of blood vessels which is the likely mechanism for the smaller size of these tumors. The mechanism for the reduced angiogenesis is not known.
B16F10 melanoma cells which are adhesion-selected (adhesion-selected up to 30 times in a sequential manner) are more malignant in vivo with a relatively large increase in the number of lung colonies over either the parental cells or the YIGSR non-adherent cells.32 Additionally, the subcutaneous tumor growth is also accelerated with these adhesion-selected cells, suggesting the receptor for this peptide is important in tumor growth and metastasis. This is likely due to a selection for the 32/67 kD receptor-positive cells by adhesion. Levels of this receptor on malignant cells correlate with malignancy in cell lines and in patient-derived tumor tissue.39,40 The 32/67 kD receptor appears to be somewhat specific for tumor cells and is a potential target for cancer therapy. However, it should be noted that the nature of this receptor is uncertain.
Various groups have used the YIGSR peptide to localize tumors cells and to target tumor cells with drug delivery based on the peptide binding to the 32/67 kD receptor. Approaches have employed iodinated YIGSR, (99 min)Tc-YIGSR, YIGSR polymeric nanoparticles, nanospheres and micelles, liposomes and PEG liposomes.41-47 For tumor imaging, 99mTc-YIGSR was found to be an excellent radiotracer with rapid visualization (15 min) and high sensitivity and specificity with mice bearing Ehrlich ascites tumors.47 In related studies, YIGSR nanoparticles had a 2-fold increase in uptake over scrambled peptide nanoparticles in tumor cells, in vitro, and neither peptide was taken up by normal lung cells.45 Furthermore, the YIGSR-nanoparticles had a 5-fold increase over control scrambled peptide nanoparticles in tumor cell uptake in the lung, in vivo. In addition, no other tissues bound the nanoparticles. Similarly, YIGSR-conjugated etoposide loaded micelles have increased cellular uptake, significant reduction in colony formation in vitro and a marked inhibition of lung colony formation in vivo.46 These data demonstrate that enhanced cellular internalization of YIGSR-conjugated micelles via laminin receptor-mediated endocytosis resulted in higher cytotoxicity, specificity and enhanced anti-metastatic activity of the peptide against B16F10 melanoma cells. Finally, YIGSR peptide conjugated to liposomes has been used to deliver cancer chemotherapeutics. For example, YIGSR-PEG liposomes containing adriamycin have in vitro cytotoxicity with HT080 cells over control peptide control-PEG-liposomes.44 Using YIGSR peptide anchored liposomes bearing 5-fluorouracil, mice bearing B16F10 melanoma cells had a significantly greater tumor regression than the free drug or empty liposomes.42 These studies demonstrate that the YIGSR peptide when combined with the appropriate ligand can be used to visualize tumors, have a more effective and specific delivery of YIGSR for tumor destruction/prevention and be used to deliver chemotherapeutic agents. In addition, since YIGSR also affects angiogenesis, it can be expected that some of this activity may also be targeted to the vessels in the tumors.
Another novel approach with YIGSR has been to generate anti-idiotype antibodies.48 Here high titer anti-YIGSR serum from immunized rabbits was used to inoculate Lewis Lung Carcinoma-bearing mice. Mice injected with either the anti-id YIGSR or control rabbit serum developed anti-rabbit antibodies. However, only mice vaccinated with the anti-YIGSR serum had reduced tumor growth and metastasis compared with control serum-immunized mice. These data suggest again that YIGSR has a potent effect via its receptor on tumor growth and metastasis.
Little is known about the mechanisms by which YIGSR acts. Some labs have reported increased phosphorylation when tumor cells are treated with this peptide49 while others report increased apoptosis.50 Still other groups have reported an effect of YIGSR on epithelial mesenchymal transition (EMT): adenoid cystic carcinoma cells (CAC2) had a fibroblast-like morphology with decreased β catenin in the presence of YIGSR while untreated cells were epitheloid.51 Finally, with prostate cancer cells (PC3), YIGSR was found to inhibit growth and migration and decreased mitochondrial membrane potential, inhibited ATP synthesis and increased caspase-9 activity.52 These findings on the pathways involved are preliminary and require further investigation with multiple tumor cell types. It is possible that the tumor cellular response is dependent not only on the tumor cell type but also on the relative malignant potential of the tumor cells. Clearly, defining the mechanism by which YIGSR inhibits tumor spread and growth may lead to additional more potent therapeutics.
IKVAV
The IKVAV laminin-α1 chain peptide was initially described as promoting cell adhesion, migration and neurite outgrowth but it was soon found to be a potent stimulator of tumor growth, metastasis, protease activation/secretion and angiogenesis.53,54 When co-injected via the tail vein with B16F10 melanoma cells, a significant increase in the number of lung colonies is observed. Similar increases in metastasis are observed with other cell lines and this peptide. For example, colon cancer cells (HM7 and LiM6) show an increase in liver colonization when co-injected with IKVAV into the splenic portal vein in nude mice.55 This peptide also increases the growth of these tumor cells in xenografts when co-injected with the basement membrane extract BME/Matrigel (which is used to enhance tumor take and growth) over those tumors injected without peptide. These xenografts also showed a significant increase in vessel density.56 Further analysis of the angiogenic activity of IKVAV demonstrated that it increased vessel number and sprouting in an in vitro assay of tube formation on BME/Matrigel, in vivo in the chick CAM assay and in a subcutaneous BME/Matrigel plug assay.57 Thus, the ability of IKVAV to promote tumor growth and metastasis may be due in part to its role in promoting angiogenesis.
IKVAV also promotes protease activity based on several studies. It was found to initiate the invasive phenotype of melanoma K-1735 clones when added to cultures on BME/Matrigel.58 Analysis of the conditioned medium of these cells treated with IKVAV by zymography showed a dose-dependent increase in matrix metalloproteinase-2 (MMP) activity. Likewise, it increased protease activity in endothelial cells and adenoid cystic carcinoma cells cultured in a similar manner.57,59 While MMP-2 activity was increased by IKVAV in A-2058 melanoma cells, no effect on tissue inhibitor of metalloproteinase (TIMP) expression was found.60 In B16F10 melanoma cells, IKVAV increased production and activity of metastasis-associated proteases, such as tissue plasminogen activator (t-PA); however, this peptide had no effect on t-PA expression in the B16F1 cells (non-metastatic).61 This group also found that when the conditioned medium of IKVAV-treated B16F10 cells was incubated with plasminogen a significant increase in the direct activation of the zymogen to plasmin was observed in the absence of cells, suggesting that IKVAV stimulates B16F10 to increase protease activation. Finally, a 20-fold increase in urokinase-PA (u-PA) expression was observed with macrophages exposed to SIKVAV.5 These data suggest a possible mechanism involving protease increase and activation for the induced metastatic behavior of this peptide compounding and possibly contributing to the angiogenic affect. Therefore, IKVAV is a potent peptide when considering its effects on promoting both protease activity and angiogenic activity.
Preliminary studies suggest that the receptors for IKVAV appear to be two integrins, α3β1 and α6β1.62 IKVAV acts also through these integrins via extracellular signal-regulated kinase (ERK) 1/2 signaling to increase protease activity. Some limited studies have used the IKVAV for targeting and imaging tumors. 99mTc-IKVAV when injected intravenously in mice with lung tumors, localized in greater amounts to the lung than to other tissues.63 In addition, incorporating IKVAV on polymer-modified adenovirus allowed entry of the virus into PC-3 cells via integrin α6β164. Further study of various cancer cell lines showed a correlation between IKVAV-viral entry and expression of both integrin subunits. This suggests that IKVAV acts by a receptor-based mechanism to localize in tumors. Additionally, an enantiomer of IKVAV peptide also promoted cell adhesion and tumor growth.65 Furthermore, there are a considerable amount of studies focused on the use of this peptide with normal cells in tissue engineering biomaterials for tissue regeneration in the nervous system. Clearly more work needs to be done to determine how this peptide interacts with cells and its signaling mechanism.
RKRLQVQLSIRT (AG73)
The active sequence, RKRLQVQLSIRT, from the LG4 domain of the laminin-α1 chain is designated AG73 (Fig. 1). This peptide has been extensively studied in a variety of different cancer cell lines, including melanoma, oral squamous and salivary gland, breast and ovarian carcinoma cells.66-71 AG73 was first identified by its ability to promote cell adhesion of HT1080 human fibrosarcoma, B16F10 mouse melanoma and SW480 human colon adenocarcinoma cells.24 A scrambled sequence of AG73, called AG73T, (LQQRRSVLRTKI) does not promote cell adhesion. AG73 also inhibited the ability of these cells to spread on laminin-111, indicating it likely has physiological relevance. Similar to the IKVAV peptide, AG73 increased subcutaneous tumor growth and lung colonization of B16F10 melanoma cells. In addition, this peptide induces B16F10 liver metastases.67,68 AG73 is the only peptide tested in the tail vein injection experimental metastasis model that induces in addition to lung colonies B16F10 liver metastases in mice, suggesting that this peptide utilizes a different mechanism of action in promoting metastases. In vitro, B16F10 melanoma cell adhesion, migration, invasion and MMP-2 production are enhanced in the presence of AG73 compared with a scrambled control peptide. Both melanoma and breast cancer cell metastasis to the bone are increased by AG73.72 The cellular organization of actin filaments was examined in B16F10 and MDA-231 breast cancer cells attached to AG73 to determine if AG73 affected the cell shape. AG73 increased the formation of filament spikes, which resemble filopodia, compared with cells treated with scrambled peptide.72 Additionally, these increased filopodia are seen in fibroblasts bound to AG73.73 Filopodia are actin-rich structures associated with increased cell migration.74 Indeed, AG73 increases migration of several cells types, including breast and melanoma cells. Additionally, ovarian cancer growth and spread are also promoted and increased by AG73.69 AG73 may increase proliferation in these cells through increased expression of Bcl-2 and Mdm2, both survival genes. The increased tumor growth induced by AG73 in a variety of different tumor types may also be due to increased angiogenesis. AG73 promotes angiogenesis in the CAM and in subcutaneously injected BME/Matrigel supplemented with AG73, as well as in tube formation and sprouting of aortic rings assays.75 Thus, AG73 may enhance tumor growth and metastasis through increased tumor cell migration and invasion and increased angiogenesis.
The receptors for AG73 may also play an important role in tumor growth and metastasis. A subpopulation of B16F10 cells that were adhesion-selected to AG73 over 30 times have increased in vitro invasion, grow larger subcutaneously and form more lung and liver metastatic colonies than the parental population.68 These results were in the absence of added peptide, suggesting that receptors for AG73 are induced/selected for and are important in the growth and metastasis of cancer cells. This peptide sequence binds to cell surface proteoglycans, including syndecan-1, -2 and -4.70,76-78 Syndecans (Sdc) are a family consisting of four transmembrane proteoglycans that interact with integrins, growth factors and chemokine receptors. Although they are not the primary receptors for the ECM, growth factors, or chemokines, they synergize with these molecules’ prototypic receptors through simultaneous ligand engagement.79-81 These receptors play critical regulatory roles in a variety of physiological and pathophysiological functions, including wound healing, inflammation, neural patterning, tumor growth and angiogenesis.82,83 Interestingly, AG73 increases invadopodia of CAC2 adenoid cystic carcinoma cells. This increase is inhibited by silencing of β1 integrin and inhibition of RAC1 and ERK signaling,66 suggesting interactions between Sdcs and integrins may play a role in the ability of AG73 to increase invasion of tumor cells. Indeed, AG73 induces the co-localization of Sdc-1 and β1 integrin in oral squamous carcinoma cells and malignant and benign salivary gland tumors.71,84 The expression of these receptors is necessary for AG73-induced migration, invasion and increased MMP-9 activity in the oral squamous carcinoma cells.71 Similarly, these receptors are necessary for AG73-induced matrix remodeling and MMP-9 activity in the malignant and benign salivary gland tumors.84 These results suggest that downstream signaling of AG73 through interaction of Sdcs and integrin regulates adhesion and MMP production of several tumor types.
Elastase digestion of laminin-111 releases the E8 fragment containing LG1–3 and the E3 fragment containing LG4–5. Intact laminin-111 is cleaved in vitro,85 and the LG4–5 domain fragment has been found in the placenta cone in vivo.86 These studies suggest that the AG73 sequence may represent a cryptic epitope released by limited proteolytic modification of laminin-111 in tumor tissues. Laminin-111 mediated adhesion and migration in B16F10 cells is inhibited by this peptide,67 suggesting it is an active sequence in the laminin-111 molecule. Hozumi et al.76 have shown that the AG73 sequence is essential for binding of the proteoglycan receptors Sdcs-1, -2 and -4 to recombinant-LG4. The LG4 domain is detected in basement membrane extract (BME/Matrigel) and in laminin-111 isolated from EHS tumors (Koblinski, unpublished data). Taken together these results suggest that the AG73 sequence is likely bioavailable in the tumor microenvironment, and interaction with Sdcs can cause a variety of tumor promotion and metastatic events.
AG73 may also have the potential to selectively deliver gene therapy to target cancer cells overexpressing Sdcs.87 AG73-peptide labeled liposome can successfully deliver genes in syndecan-2 overexpressing cells.88 Furthermore, AG73 has potential for cell and tissue engineering. AG73 can be conjugated to polysaccharides, such as chitosan and alginate, and mixed with agarose gel.89-91 Depending on the stiffness of the agarose-AG73 matrix 3D functionality of cells was observed. For example, neuronal cells extend neuritic processes, endothelial cells formed capillary-like networks, and salivary gland cells formed acinar-like structures.91 In addition, AG73-collagen, AG73-laminin-111 and AG73-fibronectin matrices enhances cell attachment and spreading, suggesting that integrin-mediated activities are enhanced by this Sdc binding peptide, AG73.92 These types of cell culture scaffolds have the potential to be used for studying 3D tumor-stromal interactions and 3D migration.
KAFDITYVRLKF (C16)
In a specific screen for laminin- 111-derived γ1 chain peptide regulators of angiogenesis, 7 active peptides were identified as disrupting the formation of capillary-like endothelial structures and C16 from the N-terminal globular domain had the strongest activity at all concentrations tested. In additional assays, including sprouting from aortic rings and the chick CAM, C16 showed the most activity.93 C16 also promoted endothelial cell adhesion and blocked adhesion to laminin-111 but not to plastic or to fibronectin, suggesting that it is an important site for endothelial cells on the γ1 chain of laminin-111. Interestingly, an homologous active site (A13:RQVFQVATIIIKA) on the α1 chain was identified with similar activity.94,95 In addition to affecting angiogenesis, C16 peptide also promoted both B16F10 melanoma cell migration in vitro and lung metastases in vivo.96 Since C16 induced the production of MMP-9 by these cells, it is clear that this site on the γ1 chain is important in tumor cell metastasis as well as angiogenesis. Interestingly, human melanoma cells migrate to the vessel structures when added to the chick CAM and then these cells migrate along the outside of the vessels, which mimics one of the mechanisms by which melanoma cells spread throughout the body.97 When C16 is added to this extravascular migratory assay, the tumor cells were found to migrate further along the vessels than with peptide control-treated samples. Thus, C16 can promote tumor spread in extravascular migratory metastasis.
Integrins αvβ3 and α5β1 have been identified as the receptors for C16.98 Since this peptide also blocked attachment to both fibronectin and collagen I as well as to laminin-111, it was expected that a receptor common to these proteins would be found. The identification of the receptors was made based on affinity chromatography and blocking antibodies in adhesion assays. This peptide does not contain an RGD sequence which is the usual ligand for these integrins nor does it signal through MAP kinase, suggesting a different signaling pathway is involved. Many tumor cells use invadopodia as described above to migrate and degrade extracellular matrix barriers. Invadopodia are membrane protrusions enrich in degradative enzymes. Similar to AG73, C16 increased invadopodia in CAC2 cells (human adenoid cystic carcinoma cell line) and silencing of integrin β1 blocked these C16-induced invadopodia.99 Inhibition of Rac1 and ERK signaling pathways also blocked the ability of C16 to induce invadopodia suggesting that C16 increases invadopodia via integrin signaling through the Rac1 and ERK1/2 pathways. These data demonstrate that specific integrin receptors are involved in the malignant activity of C16.
Typically control peptides for the assays described above are scrambled versions of the active peptide. In the case of C16, it was found that a scrambled version, C16S (DFKLFAVTIKYR), acted as an antagonist. A more potent version, C16Y (DFKLFAVYIKYR) with a T to Y substitution was defined and found to be 5-fold more active in blocking C16 induced angiogenesis in the chick CAM than the original scrambled peptide.100 This peptide also blocked tumor growth and angiogenesis in vivo in animal models suggesting its potential as a therapeutic to treat cancer. Liposomes with C16Y for targeting endothelial and cancer cells which are enriched in integrins αvβ3 and α5β1 showed greater uptake in tumor cells over either empty liposomes or liposomes with a different and inactive scrambled peptide.101 This process was temperature-dependent and was blocked by recombinant integrin αvβ3 supporting that the activity is physiological. These data support the concept that C16Y peptide could be used in a drug or gene delivery carrier to target tumors and endothelial cells for cancer therapy.
Summary
Laminin-111 is a large trimeric basement membrane glycoprotein with many active sites. In particular, four peptides active in tumor malignancy studies have been identified in laminin-111 using a systematic peptide screening method followed by various assays. Two of the peptides (IKVAV and AG73) are found on the α1 chain, one (YIGSR) on the β1 chain and one (C16) on the γ1 chain. The four peptides have distinct activities and receptors. Since three of the peptides (IKVAV, AG73 and C16) strongly promote tumor growth, this may explain the potent effects laminin-111 has on malignant cells. The peptide, YIGSR, decreases tumor growth and experimental metastasis via a 32/67 kD receptor while IKVAV increases tumor growth, angiogenesis and increases protease activity via integrin receptors. AG73 increases tumor growth and metastases via syndecan receptors. C16 increases tumor growth and angiogenesis via integrins. Identification of such sites on laminin-111 will have use in defining strategies to develop therapeutics for cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22827
References
- 1.Engbring JA, Kleinman HK. The basement membrane matrix in malignancy. J Pathol. 2003;200:465–70. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman HK, Koblinski J, Lee S, Engbring J. Role of basement membrane in tumor growth and metastasis. Surg Oncol Clin N Am. 2001;10:329–38, ix. [ix.] [PubMed] [Google Scholar]
- 3.Terranova VP, Williams JE, Liotta LA, Martin GR. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984;226:982–5. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- 4.Terranova VP, Liotta LA, Russo RG, Martin GR. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982;42:2265–9. [PubMed] [Google Scholar]
- 5.Khan KM, Falcone DJ. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem. 1997;272:8270–5. doi: 10.1074/jbc.272.13.8270. [DOI] [PubMed] [Google Scholar]
- 6.Turpeenniemi-Hujanen T, Thorgeirsson UP, Rao CN, Liotta LA. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986;261:1883–9. [PubMed] [Google Scholar]
- 7.Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983;49:636–49. [PubMed] [Google Scholar]
- 8.Buck MR, Karustis DG, Day NA, Honn KV, Sloane BF. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J. 1992;282:273–8. doi: 10.1042/bj2820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113–35. doi: 10.1016/S0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 10.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–24. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visscher DW, Sloane BF, Sameni M, Babiarz JW, Jacobson J, Crissman JD. Clinicopathologic significance of cathepsin B immunostaining in transitional neoplasia. Mod Pathol. 1994;7:76–81. [PubMed] [Google Scholar]
- 12.Zheng WQ, Looi LM, Cheah PL. Correlation between laminin and cathepsin D expressions in breast carcinoma. Tumori. 2002;88:296–9. doi: 10.1177/030089160208800411. [DOI] [PubMed] [Google Scholar]
- 13.Ioachim E, Kamina S, Kontostolis M, Agnantis NJ. Immunohistochemical expression of cathepsin D in correlation with extracellular matrix component, steroid receptor status and proliferative indices in breast cancer. Virchows Arch. 1997;431:311–6. doi: 10.1007/s004280050104. [DOI] [PubMed] [Google Scholar]
- 14.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. doi: 10.1016/S0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 15.Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81. [PubMed] [Google Scholar]
- 16.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–62. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 19.Nakai M, Mundy GR, Williams PJ, Boyce B, Yoneda T. A synthetic antagonist to laminin inhibits the formation of osteolytic metastases by human melanoma cells in nude mice. Cancer Res. 1992;52:5395–9. [PubMed] [Google Scholar]
- 20.Yamada Y, Kleinman HK. Functional domains of cell adhesion molecules. Curr Opin Cell Biol. 1992;4:819–23. doi: 10.1016/0955-0674(92)90105-L. [DOI] [PubMed] [Google Scholar]
- 21.Yamada KM. Adhesive recognition sequences. J Biol Chem. 1991;266:12809–12. [PubMed] [Google Scholar]
- 22.Hozumi K, Akizuki T, Yamada Y, Hara T, Urushibata S, Katagiri F, et al. Cell adhesive peptide screening of the mouse laminin α1 chain G domain. Arch Biochem Biophys. 2010;503:213–22. doi: 10.1016/j.abb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Nomizu M, Kuratomi Y, Ponce ML, Song SY, Miyoshi K, Otaka A, et al. Cell adhesive sequences in mouse laminin beta1 chain. Arch Biochem Biophys. 2000;378:311–20. doi: 10.1006/abbi.2000.1828. [DOI] [PubMed] [Google Scholar]
- 24.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, et al. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270:20583–90. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 25.Nomizu M, Kuratomi Y, Malinda KM, Song SY, Miyoshi K, Otaka A, et al. Cell binding sequences in mouse laminin alpha1 chain. J Biol Chem. 1998;273:32491–9. doi: 10.1074/jbc.273.49.32491. [DOI] [PubMed] [Google Scholar]
- 26.Nomizu M, Kuratomi Y, Song SY, Ponce ML, Hoffman MP, Powell SK, et al. Identification of cell binding sequences in mouse laminin gamma1 chain by systematic peptide screening. J Biol Chem. 1997;272:32198–205. doi: 10.1074/jbc.272.51.32198. [DOI] [PubMed] [Google Scholar]
- 27.Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, et al. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, et al. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–4. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 29.Yamamura K, Kibbey MC, Jun SH, Kleinman HK. Effect of Matrigel and laminin peptide YIGSR on tumor growth and metastasis. Semin Cancer Biol. 1993;4:259–65. [PubMed] [Google Scholar]
- 30.Fridman R, Giaccone G, Kanemoto T, Martin GR, Gazdar AF, Mulshine JL. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:6698–702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–43. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 32.Yamamura K, Kibbey MC, Kleinman HK. Melanoma cells selected for adhesion to laminin peptides have different malignant properties. Cancer Res. 1993;53:423–8. [PubMed] [Google Scholar]
- 33.Kaneda Y, Yamamoto S, Kihira T, Tsutsumi Y, Nakagawa S, Miyake M, et al. Synthetic cell-adhesive laminin peptide YIGSR conjugated with polyethylene glycol has improved antimetastatic activity due to a longer half-life in blood. Invasion Metastasis. 1995;15:156–62. [PubMed] [Google Scholar]
- 34.Kawasaki K, Namikawa M, Murakami T, Mizuta T, Iwai Y, Hama T, et al. Amino acids and peptides. XIV. Laminin related peptides and their inhibitory effect on experimental metastasis formation. Biochem Biophys Res Commun. 1991;174:1159–62. doi: 10.1016/0006-291X(91)91542-K. [DOI] [PubMed] [Google Scholar]
- 35.Murata J, Saiki I, Azuma I, Nishi N. Inhibitory effect of a synthetic polypeptide, poly(Tyr-Ile-Gly-Ser-Arg), on the metastatic formation of malignant tumour cells. Int J Biol Macromol. 1989;11:97–9. doi: 10.1016/0141-8130(89)90049-4. [DOI] [PubMed] [Google Scholar]
- 36.Nomizu M, Yamamura K, Kleinman HK, Yamada Y. Multimeric forms of Tyr-Ile-Gly-Ser-Arg (YIGSR) peptide enhance the inhibition of tumor growth and metastasis. Cancer Res. 1993;53:3459–61. [PubMed] [Google Scholar]
- 37.Hojo K, Maeda M, Mu Y, Kamada H, Tsutsumi Y, Nishiyama Y, et al. Facile synthesis of a chitosan hybrid of a laminin-related peptide and its antimetastatic effect in mice. J Pharm Pharmacol. 2000;52:67–73. doi: 10.1211/0022357001773526. [DOI] [PubMed] [Google Scholar]
- 38.Mu Y, Kamada H, Kodaira H, Sato K, Tsutsumi Y, Maeda M, et al. Bioconjugation of laminin-related peptide YIGSR with polyvinyl pyrrolidone increases its antimetastatic effect due to a longer plasma half-life. Biochem Biophys Res Commun. 1999;264:763–7. doi: 10.1006/bbrc.1999.1567. [DOI] [PubMed] [Google Scholar]
- 39.Liotta LA, Stracke ML. Tumor invasion and metastases: biochemical mechanisms. Cancer Treat Res. 1988;40:223–38. doi: 10.1007/978-1-4613-1733-3_10. [DOI] [PubMed] [Google Scholar]
- 40.Stallmach A, Orzechowski HD, Feldmann P, Riecken EO, Zeitz M, Herbst H. 32/67-kD laminin receptor expression in human colonic neoplasia: elevated transcript levels correlate with the degree of epithelial dysplasia. Am J Gastroenterol. 1999;94:3341–7. doi: 10.1111/j.1572-0241.1999.01550.x. [DOI] [PubMed] [Google Scholar]
- 41.Dubey PK, Singodia D, Vyas SP. Polymeric nanospheres modified with YIGSR peptide for tumor targeting. Drug Deliv. 2010;17:541–51. doi: 10.3109/10717544.2010.490249. [DOI] [PubMed] [Google Scholar]
- 42.Dubey PK, Singodia D, Vyas SP. Liposomes modified with YIGSR peptide for tumor targeting. J Drug Target. 2010;18:373–80. doi: 10.3109/10611860903483388. [DOI] [PubMed] [Google Scholar]
- 43.Kouzi-Koliakos K, Koliakos G, Trontzos C, Papageorgiou A, Iliadis S, Triantos A, et al. In vivo binding of the radioiodinated peptide YIGSR on B16 melanoma cells. Invasion Metastasis. 1996;16:322–9. [PubMed] [Google Scholar]
- 44.Lopez-Barcons LA, Polo D, Reig F, Fabra A. Pentapeptide YIGSR-mediated HT-1080 fibrosarcoma cells targeting of adriamycin encapsulated in sterically stabilized liposomes. J Biomed Mater Res A. 2004;69:155–63. doi: 10.1002/jbm.a.20235. [DOI] [PubMed] [Google Scholar]
- 45.Sarfati G, Dvir T, Elkabets M, Apte RN, Cohen S. Targeting of polymeric nanoparticles to lung metastases by surface-attachment of YIGSR peptide from laminin. Biomaterials. 2011;32:152–61. doi: 10.1016/j.biomaterials.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Ukawala M, Chaudhari K, Rajyaguru T, Manjappa AS, Murthy RS, Gude R. Laminin receptor-targeted etoposide loaded polymeric micelles: a novel approach for the effective treatment of tumor metastasis. J Drug Target. 2012;20:55–66. doi: 10.3109/1061186X.2011.610799. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Zhang YX, Lan XL, Qin GM, Zhang J, Hu ZH. An imaging study using laminin peptide 99mTc-YIGSR in mice bearing Ehrlich ascites tumour. Chin Med J (Engl) 2005;118:753–8. [PubMed] [Google Scholar]
- 48.Koliakos KK, Sapountzi Z, Papageorgiou A, Trachana V, Kotsinou S, Koliakos G. Antiidiotypic antibodies carrying the “internal image” of peptide YIGSR inhibit spontaneous metastasis of Lewis lung carcinoma in mice. In Vivo. 2002;16:511–8. [PubMed] [Google Scholar]
- 49.Bushkin-Harav I, Littauer UZ. Involvement of the YIGSR sequence of laminin in protein tyrosine phosphorylation. FEBS Lett. 1998;424:243–7. doi: 10.1016/S0014-5793(98)00180-X. [DOI] [PubMed] [Google Scholar]
- 50.Kim WH, Schnaper HW, Nomizu M, Yamada Y, Kleinman HK. Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res. 1994;54:5005–10. [PubMed] [Google Scholar]
- 51.Morais Freitas V, Nogueira da Gama de Souza L, Cyreno Oliveira E, Furuse C, Cavalcanti de Araújo V, Gastaldoni Jaeger R. Malignancy-related 67kDa laminin receptor in adenoid cystic carcinoma. Effect on migration and beta-catenin expression. Oral Oncol. 2007;43:987–98. doi: 10.1016/j.oraloncology.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Yu HN, Zhang LC, Yang JG, Das UN, Shen SR. Effect of laminin tyrosine-isoleucine-glycine-serine-arginine peptide on the growth of human prostate cancer (PC-3) cells in vitro. Eur J Pharmacol. 2009;616:251–5. doi: 10.1016/j.ejphar.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 53.Sweeney TM, Kibbey MC, Zain M, Fridman R, Kleinman HK. Basement membrane and the SIKVAV laminin-derived peptide promote tumor growth and metastases. Cancer Metastasis Rev. 1991;10:245–54. doi: 10.1007/BF00050795. [DOI] [PubMed] [Google Scholar]
- 54.Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–82. [PubMed] [Google Scholar]
- 55.Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin alpha 1 chain Ile-Lys-Val-Ala-Val (IKVAV)-containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–80. [PubMed] [Google Scholar]
- 56.Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–8. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- 57.Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, et al. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992;153:614–25. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- 58.Royce LS, Martin GR, Kleinman HK. Induction of an invasive phenotype in benign tumor cells with a laminin A-chain synthetic peptide. Invasion Metastasis. 1992;12:149–55. [PubMed] [Google Scholar]
- 59.Freitas VM, Scheremeta B, Hoffman MP, Jaeger RG. Laminin-1 and SIKVAV a laminin-1-derived peptide, regulate the morphology and protease activity of a human salivary gland adenoid cystic carcinoma cell line. Oral Oncol. 2004;40:483–9. doi: 10.1016/j.oraloncology.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Mackay AR, Gomez DE, Nason AM, Thorgeirsson UP. Studies on the effects of laminin, E-8 fragment of laminin and synthetic laminin peptides PA22-2 and YIGSR on matrix metalloproteinases and tissue inhibitor of metalloproteinase expression. Lab Invest. 1994;70:800–6. [PubMed] [Google Scholar]
- 61.Stack MS, Gray RD, Pizzo SV. Modulation of murine B16F10 melanoma plasminogen activator production by a synthetic peptide derived from the laminin A chain. Cancer Res. 1993;53:1998–2004. [PubMed] [Google Scholar]
- 62.Freitas VM, Vilas-Boas VF, Pimenta DC, Loureiro V, Juliano MA, Carvalho MR, et al. SIKVAV, a laminin α1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol. 2007;171:124–38. doi: 10.2353/ajpath.2007.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamora PO, Eshima D, Graham D, Shattuck L, Rhodes BA. Biological distribution of 99mTc-labeled YIGSR and IKVAV laminin peptides in rodents: 99mTc-IKVAV peptide localizes to the lung. Biochim Biophys Acta. 1993;1182:197–204. doi: 10.1016/0925-4439(93)90141-M. [DOI] [PubMed] [Google Scholar]
- 64.Stevenson M, Hale AB, Hale SJ, Green NK, Black G, Fisher KD, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007;14:335–45. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 65.Nomizu M, Utani A, Shiraishi N, Kibbey MC, Yamada Y, Roller PP. The all-D-configuration segment containing the IKVAV sequence of laminin A chain has similar activities to the all-L-peptide in vitro and in vivo. J Biol Chem. 1992;267:14118–21. [PubMed] [Google Scholar]
- 66.Nascimento CF, de Siqueira AS, Pinheiro JJ, Freitas VM, Jaeger RG. Laminin-111 derived peptides AG73 and C16 regulate invadopodia activity of a human adenoid cystic carcinoma cell line. Exp Cell Res. 2011;317:2562–72. doi: 10.1016/j.yexcr.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 67.Kim WH, Nomizu M, Song SY, Tanaka K, Kuratomi Y, Kleinman HK, et al. Laminin-alpha1-chain sequence Leu-Gln-Val-Gln-Leu-Ser-Ile-Arg (LQVQLSIR) enhances murine melanoma cell metastases. Int J Cancer. 1998;77:632–9. doi: 10.1002/(SICI)1097-0215(19980812)77:4<632::AID-IJC25>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 68.Song SY, Nomizu M, Yamada Y, Kleinman HK. Liver metastasis formation by laminin-1 peptide (LQVQLSIR)-adhesion selected B16-F10 melanoma cells. Int J Cancer. 1997;71:436–41. doi: 10.1002/(SICI)1097-0215(19970502)71:3<436::AID-IJC22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida Y, Hosokawa K, Dantes A, Kotsuji F, Kleinman HK, Amsterdam A. Role of laminin in ovarian cancer tumor growth and metastasis via regulation of Mdm2 and Bcl-2 expression. Int J Oncol. 2001;18:913–21. doi: 10.3892/ijo.18.5.913. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki N, Ichikawa N, Kasai S, Yamada M, Nishi N, Morioka H, et al. Syndecan binding sites in the laminin alpha1 chain G domain. Biochemistry. 2003;42:12625–33. doi: 10.1021/bi030014s. [DOI] [PubMed] [Google Scholar]
- 71.Siqueira AS, Gama-de-Souza LN, Arnaud MV, Pinheiro JJ, Jaeger RG. Laminin-derived peptide AG73 regulates migration, invasion, and protease activity of human oral squamous cell carcinoma cells through syndecan-1 and beta1 integrin. Tumour Biol. 2010;31:46–58. doi: 10.1007/s13277-009-0008-x. [DOI] [PubMed] [Google Scholar]
- 72.Engbring JA, Hossain R, VanOsdol SJ, Kaplan-Singer B, Wu M, Hibino S, et al. The laminin alpha-1 chain derived peptide, AG73, increases fibronectin levels in breast and melanoma cancer cells. Clin Exp Metastasis. 2008;25:241–52. doi: 10.1007/s10585-007-9138-y. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki N, Nakatsuka H, Mochizuki M, Nishi N, Kadoya Y, Utani A, et al. Biological activities of homologous loop regions in the laminin alpha chain G domains. J Biol Chem. 2003;278:45697–705. doi: 10.1074/jbc.M304667200. [DOI] [PubMed] [Google Scholar]
- 74.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 75.Mochizuki M, Philp D, Hozumi K, Suzuki N, Yamada Y, Kleinman HK, et al. Angiogenic activity of syndecan-binding laminin peptide AG73 (RKRLQVQLSIRT) Arch Biochem Biophys. 2007;459:249–55. doi: 10.1016/j.abb.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 76.Hozumi K, Suzuki N, Nielsen PK, Nomizu M, Yamada Y. Laminin alpha1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin alpha2beta1. J Biol Chem. 2006;281:32929–40. doi: 10.1074/jbc.M605708200. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman MP, Engbring JA, Nielsen PK, Vargas J, Steinberg Z, Karmand AJ, et al. Cell type-specific differences in glycosaminoglycans modulate the biological activity of a heparin-binding peptide (RKRLQVQLSIRT) from the G domain of the laminin alpha1 chain. J Biol Chem. 2001;276:22077–85. doi: 10.1074/jbc.M100774200. [DOI] [PubMed] [Google Scholar]
- 78.Engbring JA, Hoffman MP, Karmand AJ, Kleinman HK. The B16F10 cell receptor for a metastasis-promoting site on laminin-1 is a heparan sulfate/chondroitin sulfate-containing proteoglycan. Cancer Res. 2002;62:3549–54. [PubMed] [Google Scholar]
- 79.Bass MD, Morgan MR, Humphries MJ. Syndecans shed their reputation as inert molecules. Sci Signal. 2009;2:pe18. doi: 10.1126/scisignal.264pe18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopes CC, Toma L, Pinhal MA, Porcionatto MA, Sogayar MC, Dietrich CP, et al. EJ-ras oncogene transfection of endothelial cells upregulates the expression of syndecan-4 and downregulates heparan sulfate sulfotransferases and epimerase. Biochimie. 2006;88:1493–504. doi: 10.1016/j.biochi.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 82.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–28. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gama-de-Souza LN, Cyreno-Oliveira E, Freitas VM, Melo ES, Vilas-Boas VF, Moriscot AS, et al. Adhesion and protease activity in cell lines from human salivary gland tumors are regulated by the laminin-derived peptide AG73, syndecan-1 and beta1 integrin. Matrix Biol. 2008;27:402–19. doi: 10.1016/j.matbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Paulsson M, Deutzmann R, Timpl R, Dalzoppo D, Odermatt E, Engel J. Evidence for coiled-coil α-helical regions in the long arm of laminin. EMBO J. 1985;4:309–16. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schéele S, Falk M, Franzén A, Ellin F, Ferletta M, Lonai P, et al. Laminin alpha1 globular domains 4-5 induce fetal development but are not vital for embryonic basement membrane assembly. Proc Natl Acad Sci U S A. 2005;102:1502–6. doi: 10.1073/pnas.0405095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iijima H, Negishi Y, Omata D, Nomizu M, Aramaki Y. Cancer cell specific gene delivery by laminin-derived peptide AG73-labeled liposomes. Bioorg Med Chem Lett. 2010;20:4712–4. doi: 10.1016/j.bmcl.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 88.Negishi Y, Omata D, Iijima H, Hamano N, Endo-Takahashi Y, Nomizu M, et al. Preparation and characterization of laminin-derived peptide AG73-coated liposomes as a selective gene delivery tool. Biol Pharm Bull. 2010;33:1766–9. doi: 10.1248/bpb.33.1766. [DOI] [PubMed] [Google Scholar]
- 89.Hozumi K, Otagiri D, Yamada Y, Sasaki A, Fujimori C, Wakai Y, et al. Cell surface receptor-specific scaffold requirements for adhesion to laminin-derived peptide-chitosan membranes. Biomaterials. 2010;31:3237–43. doi: 10.1016/j.biomaterials.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 90.Mochizuki M, Kadoya Y, Wakabayashi Y, Kato K, Okazaki I, Yamada M, et al. Laminin-1 peptide-conjugated chitosan membranes as a novel approach for cell engineering. FASEB J. 2003;17:875–7. doi: 10.1096/fj.02-0564fje. [DOI] [PubMed] [Google Scholar]
- 91.Yamada Y, Hozumi K, Aso A, Hotta A, Toma K, Katagiri F, et al. Laminin active peptide/agarose matrices as multifunctional biomaterials for tissue engineering. Biomaterials. 2012;33:4118–25. doi: 10.1016/j.biomaterials.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 92.Yamada Y, Katagiri F, Hozumi K, Kikkawa Y, Nomizu M. Cell behavior on protein matrices containing laminin α1 peptide AG73. Biomaterials. 2011;32:4327–35. doi: 10.1016/j.biomaterials.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 93.Ponce ML, Nomizu M, Delgado MC, Kuratomi Y, Hoffman MP, Powell S, et al. Identification of endothelial cell binding sites on the laminin gamma 1 chain. Circ Res. 1999;84:688–94. doi: 10.1161/01.RES.84.6.688. [DOI] [PubMed] [Google Scholar]
- 94.Nomizu M, Yokoyama F, Suzuki N, Okazaki I, Nishi N, Ponce ML, et al. Identification of homologous biologically active sites on the N-terminal domain of laminin alpha chains. Biochemistry. 2001;40:15310–7. doi: 10.1021/bi011552c. [DOI] [PubMed] [Google Scholar]
- 95.Ponce ML, Kleinman HK. Identification of redundant angiogenic sites in laminin alpha1 and gamma1 chains. Exp Cell Res. 2003;285:189–95. doi: 10.1016/S0014-4827(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 96.Kuratomi Y, Nomizu M, Tanaka K, Ponce ML, Komiyama S, Kleinman HK, et al. Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer. 2002;86:1169–73. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lugassy C, Kleinman HK, Vernon SE, Welch DR, Barnhill RL. C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br J Dermatol. 2007;157:780–2. doi: 10.1111/j.1365-2133.2007.08120.x. [DOI] [PubMed] [Google Scholar]
- 98.Ponce ML, Nomizu M, Kleinman HK. An angiogenic laminin site and its antagonist bind through the alpha(v)beta3 and alpha5beta1 integrins. FASEB J. 2001;15:1389–97. doi: 10.1096/fj.00-0736com. [DOI] [PubMed] [Google Scholar]
- 99.Nascimento CF, de Siqueira AS, Pinheiro JJ, Freitas VM, Jaeger RG. Laminin-111 derived peptides AG73 and C16 regulate invadopodia activity of a human adenoid cystic carcinoma cell line. Exp Cell Res. 2011;317:2562–72. doi: 10.1016/j.yexcr.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 100.Ponce ML, Hibino S, Lebioda AM, Mochizuki M, Nomizu M, Kleinman HK. Identification of a potent peptide antagonist to an active laminin-1 sequence that blocks angiogenesis and tumor growth. Cancer Res. 2003;63:5060–4. [PubMed] [Google Scholar]
- 101.Hamano N, Negishi Y, Fujisawa A, Manandhar M, Sato H, Katagiri F, et al. Modification of the C16Y peptide on nanoparticles is an effective approach to target endothelial and cancer cells via the integrin receptor. Int J Pharm. 2012;428:114–7. doi: 10.1016/j.ijpharm.2012.02.006. [DOI] [PubMed] [Google Scholar]


