Abstract
Basement membranes (BMs) evolved together with the first metazoan species approximately 500 million years ago. Main functions of BMs are stabilizing epithelial cell layers and connecting different types of tissues to functional, multicellular organisms. Mutations of BM proteins from worms to humans are either embryonic lethal or result in severe diseases, including muscular dystrophy, blindness, deafness, kidney defects, cardio-vascular abnormalities or retinal and cortical malformations. In vivo-derived BMs are difficult to come by; they are very thin and sticky and, therefore, difficult to handle and probe. In addition, BMs are difficult to solubilize complicating their biochemical analysis. For these reasons, most of our knowledge of BM biology is based on studies of the BM-like extracellular matrix (ECM) of mouse yolk sac tumors or from studies of the lens capsule, an unusually thick BM. Recently, isolation procedures for a variety of BMs have been described, and new techniques have been developed to directly analyze the protein compositions, the biomechanical properties and the biological functions of BMs. New findings show that native BMs consist of approximately 20 proteins. BMs are four times thicker than previously recorded, and proteoglycans are mainly responsible to determine the thickness of BMs by binding large quantities of water to the matrix. The mechanical stiffness of BMs is similar to that of articular cartilage. In mice with mutation of BM proteins, the stiffness of BMs is often reduced. As a consequence, these BMs rupture due to mechanical instability explaining many of the pathological phenotypes. Finally, the morphology and protein composition of human BMs changes with age, thus BMs are dynamic in their structure, composition and biomechanical properties.
Keywords: basement membrane, mass spectrometry, atomic force microscopy, extracellular matrix, laminin, collagen IV, proteoglycans
Introduction
Basement membranes (BMs) are thin sheets of extracellular matrix (ECM) at the basal side of every epithelium. They outline muscle fibers, and they are present at the basal surface of the vascular endothelial cells and Schwann cells.1 BMs in situ are detected by immunocytochemistry (Fig. 1A) or by transmission electron microscopy (TEM; Fig. 1B). According to high-power TEM micrographs, the thickness of BMs ranges from 100 nm for average BMs to over 10 µm for the lens capsule, the thickest BMs in the body. BMs are not static structures; their morphologies and compositions change with age, particularly obvious in long-lived humans.2 The direct protein analysis of in vivo-derived BMs is complicated by the fact that BMs proteins are problematic to solubilize. The identification of main BM constituents was possible after realizing that mouse yolk sac tumors produce large quantities of a BM-like extracellular matrix (ECM; see also refs. 3 and 4). BM proteins are multi-domain proteins of high molecular weight that either polymerize or bind to other BM proteins. The binding of BM proteins to cellular receptors, such as integrins5,6 and dystroglycan,7 is required for BM assembly. BM proteins emerged about 500 million years ago during the evolution of metazoan species, and they are the evolutionary oldest ECM proteins.8 The importance of BMs is evident by the dramatic phenotypes from worms to humans with mutations that affect the assembly or stability of BMs. The phenotypes include early embryonic death, muscular dystrophy, kidney and cardiovascular defects and eye, ear and brain malformations.9-21 The current model states that BMs are composed of a two-dimensional scaffold of collagen IV and a network of polymerized laminin.1 Nidogen-1 has been proposed to provide the connection between the two polymers.22-25
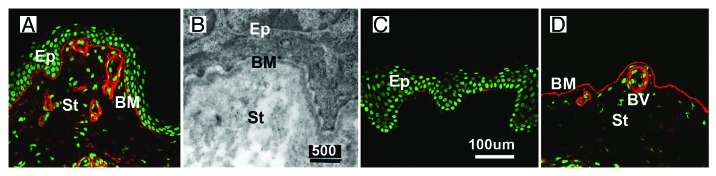
Figure 1. BMs are detected in tissue sections of human skin by immunostaining with antibodies to BM-specific proteins, such as collagen IV (A) (red), or by TEM (B). The epidermal BM (BM) of the skin is located between the epidermis (Ep) and the underlying dermal stroma (St). The vascular BMs are detected along the endothelial lining of blood vessels (BV). When the skin is experimentally split into the epidermal (C) and the dermal layers (D), the epidermal BM consistently stays with the dermal connective tissue, as shown by anti-collagen IV staining (C and D). It is inseparable from the dermal connective tissue, making a clean preparation of this BM impossible (D). The sections were counter-stained with SytoxGreen. Bars: (A, C and D), 100 µm; (B) 500 nm.
Isolation of BMs
BMs are not trivial to isolate. The main reason is that BMs are often inseparably connected to an underlying connective tissue stroma. This is best illustrated with the epidermal BM of human skin (Fig. 1A and B). Epidermis and dermis can be manually separated after incubation of skin with EDTA or high salt (Fig. 1C and D). In all cases, the epidermal BM stays with the dermis, and attempts to isolate the BM results in a thick sheet of dermal ECM with a thin BM on top (Fig. 1D). The tight connection of a thin BM with a thick stromal layer also applies to human amnion BM preparations that are frequently used to test the transmigration of tumor cells and neutrophils in vitro.26,27 In cases where the connection of the BMs to the adjacent stroma are less tight and the BMs are thicker and, therefore, easier to grasp, such the lens capsules (LC) or the corneal-derived Descemet’s membrane (DM), BMs can be isolated by microdissection followed by removal of adherent epithelial cells with detergent (Fig. 2A). SEM images show that such BMs are clean and not contaminated with cellular debris or stromal ECM (Fig. 2B). BM isolation procedures have also been described for tissues with BMs that have epithelial cell layers on both sides, such as the kidney glomeruli or the retinal or cortical capillaries.28-30 In both cases, the glomeruli and the blood vessels are isolated first, and the glomeruli or capillaries are then treated with detergent to remove the adherent epithelial cells. Our laboratory developed several procedures to isolate BMs as large, coherent sheets. In a mechanical procedure, the entire chick and mouse inner limiting membrane (ILM), a BM from the retina, is isolated by splitting the retina along the vitro-retinal border followed by several rinses with detergent.31 The procedure results in single-layered, up to finger nail-sized BM sheets that are tightly connected to a glass slide or tissue culture plastic. The BM preparation is useful for biomechanical studies and for tissue culture, but less usable for biochemical analysis. In another procedure, segments of dissected human retinas are incubated in Triton-X-100 followed by deoxycholate, and the detergent-insoluble, free-floating ILM and vascular BM sheets are isolated and cleaned by repeated detergent washes.2 The ILMs and the vascular BMs from the retina are separated under a dissection microscope (Fig. 2C and D). Dark field illumination is essential to see the otherwise transparent BMs. The BM sheets can be used for biochemical analysis and, after mounting on a solid support, also for biomechanical studies and tissue culture. Detergent treatment is the most widely used method to remove cellular contaminants during BM preparations. Experiments showed that when LCs, isolated by microdissection only, are extracted with detergent and the extracts analyzed by protein assays, SDS PAGE and western blots, little if any proteins, laminin or collagen IV are detectable in the extracts.32 The data indicate that BMs are resistant to detergent, and detergent treatment does not lead to a loss of BM proteins.
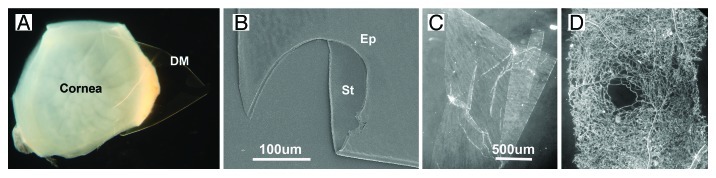
Figure 2. Isolation of BMs and the gross morphology of isolated BMs. The DM is peeled off the inner surface of a detergent-treated human cornea (A). The BM is completely transparent. When imaged by SEM, the DM appears as clean ECM sheet without cellular contamination (B) (St, stromal surface; Ep, epithelial surface). Sheets of isolated ILM and retinal vascular BMs are shown in (C and D). The micrographs show the appearance of the BMs under a dissecting microscope using dark field. The vascular BM sheet (D) was from the foveal area of the retina. Note the foveal avascular zone in the middle of the sample. Bars: (B), 100 µm; (C and D), 500 µm.
A different approach to obtain BMs is to culture epithelial or endothelial cells either on plastic or on a collagen gel and allow the cell to assemble a BM. After weeks in culture, the cells are lysed, leaving behind a BM-like extracellular matrix.33,34 Compositionally, the in vitro-synthesized BMs are similar to in vivo-derived BMs, but it is unclear whether they also share their high biomechanical strength.
Protein Composition of BMs
The first BM proteins were identified by the analysis of the BM-like ECM from yolk sac tumors.3,4 In contrast to intact, in vivo-derived BMs, the tumor ECM can be dissolved in buffers containing chaotropic or chelating agents and analyzed by conventional biochemistry.35 Laminin 111, collagen IV, perlecan and nidogen 1 were identified in this first round of studies. Additional laminins, collagen IVs, nidogens and two more proteoglcyans, agrin and collagen XVIII, were later identified by means of antibodies and molecular cloning.36
While most of the BM proteins have been identified, the composition and the protein stoichiometry of in situ-derived BMs are still unknown. The most commonly used approach to determine the proteome of an intact, in vivo BM has been immunocytochemistry.37,38 A drawback of this method is that it (1) restricts itself to candidate proteins, (2) depends on the availability and quality of antibodies and (3) provides no data on protein abundance. A more comprehensive method is SDS-PAGE followed by mass spectrometry: the procedure is unbiased, does not require specific probes and provides measures of protein abundance. The first mass spectrometry-based proteome analysis of an in vivo-derived BM was pioneered using the embryonic chick ILM as a model.32
As shown in Figure 3A, the typical banding pattern of an in vivo-derived BM shows protein bands with molecular weights between 150 kD and 1,000 kD; most BM proteins have molecular weights around 200 kD, and very few, if any, below 100 kD (Fig. 3A). The embryonic chick ILM consists of approximately 20 proteins, including several members of the laminin family, nidogen 1 and 2, perlecan, agrin, collagen XVIII and collagen IV α5/6.32 FREM and FRAS, two members of the bleb protein family, were also detected, confirming previous immunocytochemistry that detected both proteins in the mouse ILM.39 Semi-quantitative evaluation of the data, based on the emPAI values (number of sequenced peptides per molecular weight of the protein40), showed that the laminins and the nidogens are the most prominent proteins in this embryonic BM (Fig. 3B). Laminin 121 and 521 are equally abundant; collagen IV accounts for less than 10% of the total ILM protein.32 The data were confirmed by western blotting. When adult human BMs were analyzed by western blots, results showed that collagen IV accounts for 50% in ILM and 80% of the total protein in LC. Thus, collagen IV is the most prominent protein in adult human BMs, and its abundance increases with age.2 The chick and human data combined indicate that BMs undergo an age-related compositional change from a laminin to a collagen IV-dominated ECM.
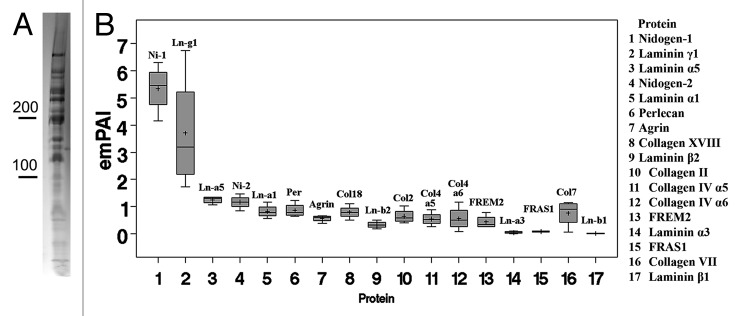
Figure 3. Proteome analysis of a BM. A Coomassie-stained SDS PAGE of chick ILM proteins is shown in (A). Note that BM proteins typically have molecular weights between 150 kD and 1,000 kD. Semi-quantitative mass spectrometry analysis of the E10 chick ILM proteome based on emPAI values (B) shows that laminin and nidogens are the most prevalent proteins. The collagen IV members represent only a minor portion of the ILM proteome.
A caveat for mass spectrometry analysis of BMs is that not all of the BM protein mass can be solubilized; we estimate that 80% of the human ILM, 60% of the LC and 70% of the DM total protein can be solubilized and successfully analyzed. The insoluble part of the BMs consists most likely of crosslinked collagen IV polymers, but the exact composition of this part of BM proteome remains to be established.
Biomechanical Properties of BMs
The phenotype analysis of fish, mice and humans with mutations of BM proteins suggests that a central function of BMs is to provide mechanical strength to epithelial tissues. By using atomic force microscopy (AFM), our laboratory has confirmed that BMs from mice with BM protein mutations are, indeed, mechanically weaker than BMs from non-mutant control mice. This indicates that decreased mechanical strength of BMs is a causal contributor to the phenotypes of blood vessel ruptures, muscular dystrophy and neural ectopias that are typical for mice and humans with BM protein mutations.41 With the exception of the lens capsule, the thickest BM in the body,42,43 biophysical measurements of BMs have been out of reach for experimentation.44 Several years ago, we introduced AFM as a new method for probing BMs. The AFM uses a sharp tip mounted at the end of a flexible cantilever to probe the sample; the deflection of the cantilever is recorded by means of a laser reflecting from the cantilever tip. By choosing a very soft cantilever, the imaging force can be in the range of interatomic forces (about 10−9 newtons), thus the name “atomic force” microscopy.45 The state of art AFM uses a Z-scanner to control the vertical position of the probe and a XY scanning stage to control the horizontal position of the sample. In the imaging mode, the AFM tip raster-scans over the sample to show its surface topography and to measure its thickness (Fig. 4C). The main advantage of using the AFM is that the microscope can operate in PBS, thus, the native surface structure can be imaged and the thickness of fully hydrated BMs can be probed under physiological conditions.
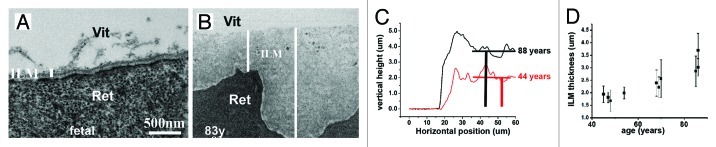
Figure 4. The thickness of human BMs. TEM images of a fetal (A) and an 83-year-old retina (B). White bars indicate the height of the ILMs. The ILM increases in thickness by a factor of 20 from 100 nm at fetal stages (A) to 1.5 µm at old age (B). In contrast to the even, regular and text-book-like fetal ILM (A), the retinal surface (Ret) of the aged ILM is highly irregular, whereas the vitreal side (Vit) is even and smooth (B). Representative AFM height measurements from a 44- and 88-y-old ILM confirm that the thickness of the ILMs increases with age (C). The scans extend from the flat glass surface on the left to the convoluted edge and further deeper into the BM sheet on the right. The thickness is measured by comparing the height difference of the glass surface and deep averaged ILM surface. The graph in (D) shows the age-related increase in ILM thickness as measured by AFM. The thickness of the ILMs, as measured by AFM, ranges from 0.4–4 µm, on average four-times greater than measured by TEM. Bar: (A and B), 500 nm.
Conventional TEM of human ILM, human corneal BMs and human epidermal BM showed that these BMs increase in thickness with age2,46,47 (Fig. 4A and B). AFM studies confirmed that human ILMs undergo an age-dependent increase in thickness (Fig. 4C and D). However, the thickness of BMs measured by AFM are on average four-times greater than measured by TEM.2,48 The difference is due to the tight binding of water to the BM sheets that is removed during the dehydration and embedding of the samples for TEM, but that remains when the samples are probed by AFM under PBS. Experiments also showed that the glycosaminoglycans of the proteoglycan are responsible for binding large quantities of water to the ECM and thereby determine to a major degree the thickness of BMs.2,32 While AFM can probe very thin as well as thick BMs, measurements are restricted to BMs that can be mounted as a single layered, flat sheet on a solid support. This kind of preparation is problematic for the narrow and tubular vascular BMs and the BM tubes from muscle fibers.
In addition to thickness, the AFM also allows to measure biomechanical characteristics of BMs: in the force indentation mode, the tip of the AFM is advanced toward the sample with a predetermined force that produces a small indentation; the repulsion force deflects the cantilever, whereby the degree of the deflection is dependent on the stiffness of the sample;49-51 the deflection of the cantilever relative to the applied force is represented by a force curve (Fig. 5E). The elastic or Young’s modulus of the sample can be calculated from the force curves using the Hertzian and Sneddon model.2,49-51 A steep slope of the curves means a high, a shallow slope a low Young’s modulus. Stiffness measurements showed that the Young’s modulus for adult human ILMs, LC and DM is in the single digit MPa-range (1–4 MPa) and increases with age.2,42 The stiffness of BMs is approximately 1,000 times greater than that of epithelial cell layers (1–4 kPa), and similar to that of articular cartilage, both measured by AFM,52,53 explaining why defects of BMs leads to the disruption of the corresponding epithelia. AFM is currently the only method to measure the biomechanical properties of nanometer-thin to micrometer-thick BMs.2,32,41,48
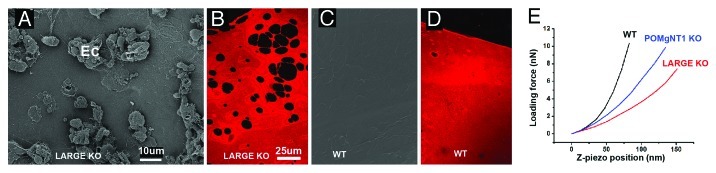
Figure 5. Stiffness measurements of mutant and wild type mouse ILMs. SEM images of the vitreal surfaces of an intact retina from a LARGE mutant (LARGE KO) and of a wild type mouse (WT). The images show numerous ectopias (Ec) in the retina of the mutant mouse (A) and a continuous, smooth surface of the retina from the control mouse (C). ILMs isolated from the mutant mice have numerous perforations (B), while wild type mice have a continuous ILM (D); the ILM flat mounts had been stained for collagen IV. Representative force curves of ILMs from two different mutant mouse lines (LARGE and POMgNT1) show a much shallower slope than the force curves of ILMs from wild type mice (E) indicating a lower Young’s modulus. LARGE and POMgNT1 mutations affect the glycosylation of dystroglycan, one of the laminin receptors that are essential for BM assembly. Bars: (A and C), 10 µm; (B and D), 25 µm.
Data showed that a variety of BMs from mice with mutation of BM proteins are instable. The ILMs from these mice, for example, rupture at random locations and retinal cells migrate through the gaps into the vitreal cavity, forming retinal ectopias (Fig. 5A; see also refs. 17, 41 and 54). ILMs isolated from those mutant mice show numerous perforations at random locations (Fig. 5B). AFM force measurements reveal that ILMs of these mutant mice have an up to 50% lower Young’s modulus than ILMs of wild type mice (Fig. 5E and ref. 41). Mechanical weakness provides a straightforward explanation for the disruption of the BMs during the expansion of the retina during development. Contrary to previous beliefs, it appears that the laminins are at least as important for BM stability as collagen IVs that traditionally have been considered to be the main stabilizing components of BMs.
Cell Adhesion Properties of BMs
BMs in vivo provide a solid support for epithelial cells to attach. In cases where BMs are defective, the adjacent epithelial layers are in danger of falling apart and to undergo apoptosis. Laminins are the top candidates to provide cell adhesive property to BMs by binding to integrins and dystroglycan as their corresponding cell surface receptors. Indeed, mutations of integrins and dystroglycan result in phenotypes that are similar if not identical to mutation of key BM proteins.5-7 Cell adhesion, cell migration, cell invasion and neurite outgrowth assays have been tested in vitro using intact, in vivo-derived BM as substrates, but more widely used for these assays is matrigel, an ECM extract from the EHS mouse tumor.55,56 Matrigel, however, forms a soft gel and is not comparable with the much tougher and thinner in situ BMs. In vivo-derived BMs that have been used as substrates for in vitro assays include human amnion and rat peritoneal BMs.57-59 These BMs were mostly used in assays investigating tumor cell transmigration and leucocytes diapedesis through BMs.58,59 We have used the embryonic chick ILM as a substrate for cell adhesion, cell migration (Fig. 6A and B) and neurite outgrowth assays.31,60,61 We also developed methods to immobilize several in vivo-derived human BMs and used them in cell adhesion and neurite outgrowth assays as well.2 Experiments showed that cells of all origin adhere to embryonic chick and adult human BMs in less than 30 min and survive in short-term and long-time cultures (Fig. 6C).
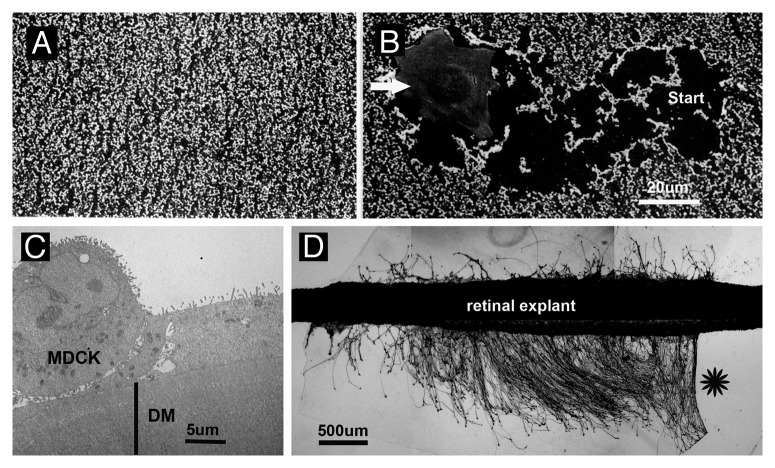
Figure 6. Cell adhesion/migration and axonal growth on embryonic chick ILM (A and B) and adult human DM (C and D). SEM micrograph showing an ILM whole mount from the embryonic chick retina. The BM is covered by a dense monolayer of neuroepithelial endfeet (A). When cells (Bowes human melanoma cells, arrow) are plated at low density onto this BM, the cells adhere and migrate on the BM by displacing the endfeet, and the cells leave behind a track as a record of their migratory behavior (B; start: site, where the cell adhered and started migration for 10 h). TEM micrograph showing a crossection of MDCK cells on a human DM after 4 d of culture. The cells attached to the BM and formed microvilli at the apical surface (C). When chick embryonic retina strips are cultured on an adult human DM flat mount, numerous axons grow out from the explants during 24 h in vitro. The neurites were visualized by anti-tubulin staining. Note, neurite outgrowth was restricted to the BM substrate; there was no neurite outgrowth on the poly-lysine coated plastic (star). Bars: (A and B), 20 µm; (C), 5 µm; (D) 500 µm.
Histology of embryonic brain and retina showed that many newly forming nerve fiber trajectories form along the pial BM of the brain or along the ILM of the retina. Further, peripheral nerve regeneration occurs along the BM sheaths of Schwann cells. The data suggest that BMs provide a favorable substratum for de novo neurite outgrowth and axon regeneration. The supportive activity of BMs has been confirmed in neurite outgrowth assays using human amnion BM and embryonic chick ILM as substrates in vitro: chick and mouse retinal explants and dorsal root ganglia grow out neurites on BM substrates with a density and growth rate that is superior to that of purified laminin or fibronectin.31,57 Surprisingly, the growth rate of axon on adult human BMs was as profuse and the rate of growth was as high as on embryonic BMs (Fig. 6D) demonstrating that BMs from adult or even aged human tissues retain their neurite outgrowth promoting properties for their entire life. Thus, BMs provide a preferred substrate for axon regeneration in the adult organisms.
Concluding Remarks and Open Questions
There is a wealth of information on individual BM proteins, including members of the laminin, nidogen and collagen IV families.62-64 The functions and interplay of these proteins in the context of the complete BM structure, however, are much less known. It is still unclear which proteins and protein family members are present in all BMs and which proteins vary from one BM to another. Further, it is unclear what the differences in the proteomes mean in terms of the specific function of the BMs. Extending the proteome analysis to more and different BMs will certainly help to address these questions. With the availability of AFM as a method to analyze the biomechanical properties of BMs, future experiments analyzing the mechanical stability of BMs from mice with different BM protein mutations will help pinpointing the importance of specific proteins for the biomechanical properties of BMs. Further, BMs are excellent substrates for cell adhesion and cell migration; a potential application is to use BMs for medical proposes, such as in wound healing and tissue repair. This is particularly interesting where transparency is an issue, such as in the eye. Finally, the medical relevance of BM thickening at old age is an unsolved mystery. It is well possible that BM thickening contributes to age-related chronic diseases, particularly in long-term diabetes, where BM thickening is further exacerbated.65
Acknowledgments
We would like to thank Jonathan Franks and Mara Sullivan in the laboratory of Simon Watkins of the University of Pittsburgh for technical support in the preparation of the TEM and SEM samples. Funding was provided by an in-house grant from the University of Pittsburgh School of Medicine.
Glossary
Abbreviations:
- AFM
atomic force microscopy
- BM
basement membrane
- DM
Descement’s membrane
- ECM
extracellular matrix
- ILM
inner limiting membrane
- LC
lens capsule
- PBS
phosphate-buffered saline
- TEM
transmission electron microscopy
- SDS PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22479
References
- 1.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29:402–10. doi: 10.1016/j.matbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–32. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 4.Chung AE. Embryonal carcinoma and the basement membrane glycoproteins laminin and entactin. Int J Dev Biol. 1993;37:141–50. [PubMed] [Google Scholar]
- 5.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–95. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 6.Fässler R, Meyer M. Consequences of lack of β 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 7.Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–70. doi: 10.1016/S0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196:671–9. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–35. doi: 10.1016/S0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 10.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–8. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 12.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–22. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–60. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sertie AL, Sossi V, Camargo AA, Zatz M, Brabe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retina structure and in neural tube closure. Hum Mol Genet. 2000;9:2051–8. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]
- 15.Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–44. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, et al. Specific ablation of the nidogen-binding site in the laminin γ1 chain interferes with kidney and lung development. Development. 2002;129:2711–22. doi: 10.1242/dev.129.11.2711. [DOI] [PubMed] [Google Scholar]
- 17.Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–40. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 19.Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–56. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Gross JM. Laminin beta1 and gamma1 containing laminins are essential for basement membrane integrity in the zebrafish eye. Invest Ophthalmol Vis Sci. 2007;48:2483–90. doi: 10.1167/iovs.06-1211. [DOI] [PubMed] [Google Scholar]
- 22.Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–46. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981;120:203–11. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 24.Schittny JC, Timpl R, Engel J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparan sulfate proteoglycan in epithelial basement membrane of mouse cornea reveals different topological orientations. J Cell Biol. 1988;107:1599–610. doi: 10.1083/jcb.107.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987;105:2559–68. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986;47:487–98. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- 27.Azzarelli B, Lafuze J. Amniotic basement membrane: a barrier to neutrophil invasion. Am J Obstet Gynecol. 1987;156:1130–6. doi: 10.1016/0002-9378(87)90125-6. [DOI] [PubMed] [Google Scholar]
- 28.Meezan E, Hjelle JT, Brendel K, Carlson EC. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975;17:1721–32. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 29.Carlson EC, Brendel K, Hjelle JT, Meezan E. Ultrastructural and biochemical analyses of isolated basement membranes from kidney glomeruli and tubules and brain and retinal microvessels. J Ultrastruct Res. 1978;62:26–53. doi: 10.1016/S0022-5320(78)80028-8. [DOI] [PubMed] [Google Scholar]
- 30.Duhamel RC, Meezan E, Brendel K. Selective solubilization of two populations of polypeptides from bovine retinal basement membranes. Exp Eye Res. 1983;36:257–67. doi: 10.1016/0014-4835(83)90010-6. [DOI] [PubMed] [Google Scholar]
- 31.Halfter W, Reckhaus W, Kröger S. Nondirected axonal growth on basal lamina from avian embryonic neural retina. J Neurosci. 1987;7:3712–22. doi: 10.1523/JNEUROSCI.07-11-03712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasubramani M, Schreiber EM, Candiello J, Balasubramani GK, Kurtz J, Halfter W. Molecular interactions in the retinal basement membrane system: a proteomic approach. Matrix Biol. 2010;29:471–83. doi: 10.1016/j.matbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Ferrell N, Groszek J, Li L, Smith R, Butler RS, Zorman CA, et al. Basal lamina secreted by MDCK cells has size- and charge-selective properties. Am J Physiol Renal Physiol. 2011;300:F86–90. doi: 10.1152/ajprenal.00484.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–36. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulsson M, Aumailley M, Deutzmann R, Timpl R, Beck K, Engel J. Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur J Biochem. 1987;166:11–9. doi: 10.1111/j.1432-1033.1987.tb13476.x. [DOI] [PubMed] [Google Scholar]
- 36.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291–306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 37.Libby RT, Champliaud M-F, Claudepierre T, Xu Y, Gibbons EP, Koch M, et al. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–28. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, et al. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44:1469–79. doi: 10.1177/44.12.8985139. [DOI] [PubMed] [Google Scholar]
- 39.Kiyozumi D, Sugimoto N, Nakano I, Sekiguchi K. Frem3, a member of the 12 CSPG repeats-containing extracellular matrix protein family, is a basement membrane protein with tissue distribution patterns distinct from those of Fras1, Frem2, and QBRICK/Frem1. Matrix Biol. 2007;26:456–62. doi: 10.1016/j.matbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–72. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Hu H, Candiello J, Zhang P, Ball SL, Cameron DA, Halfter W. Retinal ectopias and mechanically weakened basement membrane in a mouse model of muscle-eye-brain (MEB) disease congenital muscular dystrophy. Mol Vis. 2010;16:1415–28. [PMC free article] [PubMed] [Google Scholar]
- 42.Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Prog Retin Eye Res. 2003;22:749–67. doi: 10.1016/S1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 43.Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009;88:151–64. doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–4. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 45.Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930–3. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 46.Murphy C, Alvarado J, Juster R. Prenatal and postnatal growth of the human Descemet’s membrane. Invest Ophthalmol Vis Sci. 1984;25:1402–15. [PubMed] [Google Scholar]
- 47.Vázquez F, Palacios S, Alemañ N, Guerrero F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas. 1996;25:209–15. doi: 10.1016/S0378-5122(96)01066-3. [DOI] [PubMed] [Google Scholar]
- 48.Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, et al. Biomechanical properties of native basement membranes. FEBS J. 2007;274:2897–908. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 49.A-Hassan E, Heinz WF, Antonik MD, D’Costa NP, Nageswaran S, Schoenenberger CA, et al. Relative microelastic mapping of living cells by atomic force microscopy. Biophys J. 1998;74:1564–78. doi: 10.1016/S0006-3495(98)77868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–74. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laney DE, Garcia RA, Parsons SM, Hansma HG. Changes in the elastic properties of cholinergic synaptic vesicles as measured by atomic force microscopy. Biophys J. 1997;72:806–13. doi: 10.1016/S0006-3495(97)78714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 53.Loparic M, Wirz D, Daniels AU, Raiteri R, Vanlandingham MR, Guex G, et al. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite. Biophys J. 2010;98:2731–40. doi: 10.1016/j.bpj.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:1000–9. doi: 10.1167/iovs.04-1185. [DOI] [PubMed] [Google Scholar]
- 55.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Albini A, Noonan DM. The ‘chemoinvasion’ assay, 25 years and still going strong: the use of reconstituted basement membranes to study cell invasion and angiogenesis. Curr Opin Cell Biol. 2010;22:677–89. doi: 10.1016/j.ceb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Davis GE, Blaker SN, Engvall E, Varon S, Manthorpe M, Gage FH. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science. 1987;236:1106–9. doi: 10.1126/science.3576223. [DOI] [PubMed] [Google Scholar]
- 58.Hotary K, Li X-Y, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–86. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Halfter W, Diamantis I, Monard D. Migratory behavior of cells on embryonic retina basal lamina. Dev Biol. 1988;130:259–75. doi: 10.1016/0012-1606(88)90432-0. [DOI] [PubMed] [Google Scholar]
- 61.Halfter W, Von Boxberg Y. Axonal growth on solubilized and reconstituted matrix from the embryonic chick inner limiting membrane. Eur J Neurosci. 1992;4:840–52. doi: 10.1111/j.1460-9568.1992.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 62.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 63.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho MS, Böse K, Mokkapati S, Nischt R, Smyth N. Nidogens-Extracellular matrix linker molecules. Microsc Res Tech. 2008;71:387–95. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- 65.Roy S, Ha J, Trudeau K, Beglova E. Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res. 2010;35:1045–56. doi: 10.3109/02713683.2010.514659. [DOI] [PubMed] [Google Scholar]


