Abstract
The importance of laminin-containing basement membranes (BM) for adult muscle function is well established, in particular due to the severe phenotype of congenital muscular dystrophies in patients with mutations disrupting the BM-muscle cell interaction. Developing muscles in the embryo are also dependent on an intact BM. However, the processes controlled by BM-muscle cell interactions in the embryo are only beginning to be elucidated. In this review, we focus on the myotomal BM to illustrate the critical role of laminin-111 in BM assembly and function at the surface of embryonic muscle cells. The myotomal BM provides also an interesting paradigm to study the complex interplay between laminins-containing BM and growth factor-mediated signaling and activity.
Keywords: laminin-111, myotome, basement membrane, development, Sonic hedgehog signaling
Introduction
Basement membranes (BMs) are pericellular extra-cellular matrix (ECM) structures that assemble at the surface of mainly epithelial, endothelial, nerve and muscle cells.1 BMs contain laminins, isoforms of collagen IV, nidogen (1 and 2) and proteoglycans such as perlecan. The main component of BMs is laminin, a heterotrimeric glycoprotein composed of three subunits, α, β and γ.2 In mammals, there are five Lama, four Lamb and three Lamc genes, encoding for laminin α, β and γ chains, respectively.3 A limited number of combinations of these subunits are used to make up 18 different laminins with distinct binding specificity to integrins and dystroglycan.3,4 Laminins share several structural motifs that are important for their interactions with other BM components, their deposition into BMs and their interaction with cell surface receptors, including the N-terminal LN domain that mediates laminin self-assembly and also interaction with integrin α1β1, α2β1,5,6 the EGF repeat-containing LE domains that mediate the interaction of laminin polymers with collagen IV polymers and perlecan through their binding to nidogen7-9 and the C-terminal globular LG (laminin G domain-like) domains that interact with fibulin and perlecan, as well as the laminin receptors integrin and dystroglycan (Fig. 1).10 Consequently, through their association with both ECM components and cell surface receptors, laminin-containing basement membranes (BMs) provide an essential link between the extracellular environment, the intracellular cytoskeleton and the nucleus. For instance, laminins participate to the control of muscle cell activity through their specific interaction with the cell surface receptors, dystroglycan and integrins, and to the cytoarchitecture of muscle cells through their indirect interaction with dystrophin.11-13 Therefore, BMs have not only a structural role at the surface of muscle fibers, providing support and eliciting mechanical properties of fibers, they also have signaling functions that are crucial to muscle cell proliferation, survival and differentiation.14,15 Consistent with this, several congenital muscular dystrophies are associated with mutations that disrupt the interaction BM-muscle cell. These include mutations affecting laminin,16 dystroglycan17,18 and integrin.19,20
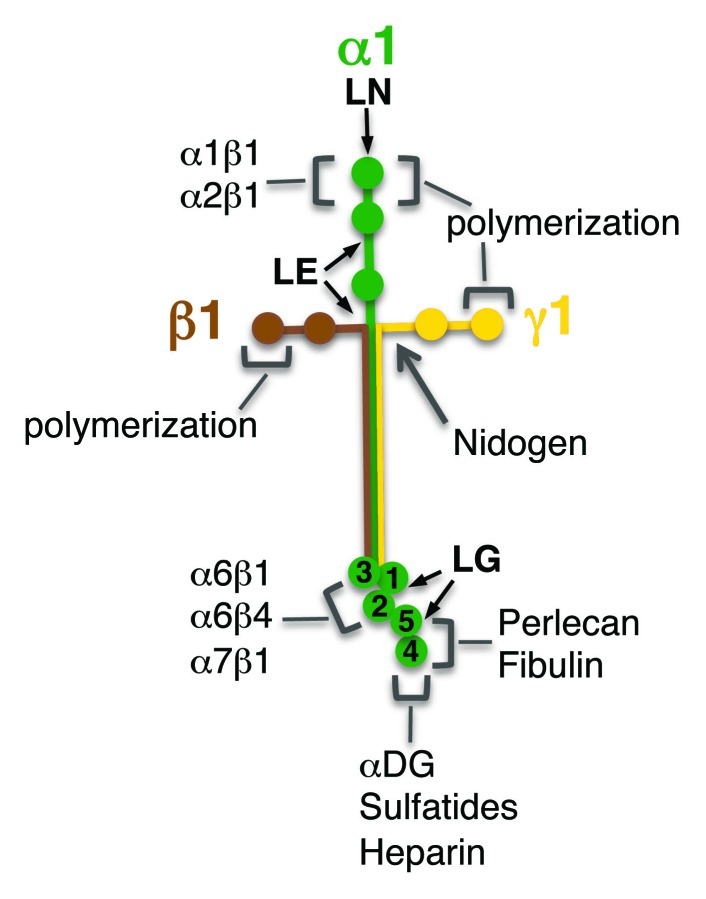
Figure 1. Schematic representation of laminin-111 structure. Each laminin chain is represented with a different color (green, α1; brown, β1; yellow, γ1) and the main interaction domains are indicated (LN, LE, LG). Binding sites for laminin receptors and for other BM components are shown, together with the domains involved in laminin self-assembly. DG, dystroglycan.
While the association of BM function with muscular dystrophies has resulted in a surge of studies on laminin function in adult muscles, there are relatively fewer reports on laminin function during embryonic myogenesis. This review will focus on the myotomal basement membrane and use it as a paradigm to gain insights into its role during muscle formation in the embryo. Finally, the complex regulatory network that ensures correct spatio-temporal development of muscles will be addressed in the context of laminin regulation and interaction with growth factor activity.
Embryonic Development of Skeletal Muscles and Formation of the Myotomal Basement Membrane
The embryonic origin of skeletal muscles
Skeletal muscles are mesoderm-derived tissues with distinct embryonic origins depending on their anatomical position and function. Specifically, while the head musculature (also known as cranio-facial musculature) largely derives from the cranial mesoderm, the trunk musculature originates from the segmented paraxial mesoderm.21,22 The development of cranio-facial and trunk muscles is also controlled by independent gene networks and regulatory mechanisms.21,22 Progenitor cells for trunk muscles reside in a transient structure of the segmented paraxial mesoderm, called somite (Fig. 2A). Somites form in a sequential manner along the rostro-caudal axis of the embryo, during a stereotypical process called somitogenesis (for review, see refs. 23 and 24), which results in somites budding off the anterior unsegmented paraxial mesoderm in a coordinated manner on the left and right sides of the vertebrate embryo. Newly-formed somites appear as a sphere of pluripotent epithelial cells that express the paired transcription factor Pax3, with a mesenchymal core, termed the somitocoel (Fig. 2A). Somite-derived cells give rise to smooth and striated muscles, brown fat, cartilage and bones, tendon and ligament cells, the dermis of the back and endothelial cells. As somites mature, distinct compartments are generated along the dorso-ventral and medio-lateral axes of the somite. First, cells in the ventro-medial compartment undergo an epithelial-to-mesenchymal transition to form the sclerotome that gives rise to cartilage and bones (Fig. 2A).25,26 In the dorsal somite, cells remain epithelial and form the dermomyotome, which continues to express Pax3 and contains proliferative progenitor cells for the skeletal muscles, brown fat and the dermis (Fig. 2A).27,28 At the medial lip of the dermomyotome (the dorsal medial lip), cells become committed to the myogenic lineage through the activation of the myogenic regulatory factors Myf5 and MyoD (for review, see ref. 29), initiate an epithelial-to-mesenchymal transition and translocate from the dermomyotome to the underlying nascent myotome. Subsequently, cells from the lateral lip of the dermomyotome and from the rostral and caudal lips of the dermomyotome contribute to the lateral and central dermomyotome, respectively.30-33 Once in the myotome, myotomal cells exit the cell cycle, initiate myogenin expression and begin differentiating into primary myotubes. These primary myotubes are mono-nucleated and elongate bi-directionally in a rostro-caudal manner (Fig. 2A). The medial myotome contains precursors for the epaxial muscles of the back, while the lateral myotome contains precursors for the hypaxial muscles of the abdominal wall. At cervical and occipital levels, dermomyotomal cells will migrate as a cohort in the hypoglossal chord and participate to the tongue and diaphragm muscles.34,35 At limb levels, dermomyotomal cells will migrate along a dorsal and ventral route to the limb mesenchyme, where they will establish the dorsal and ventral limb muscle masses, containing precursor cells of the limb musculature.36 At later stages of embryonic development, a population of Pax3 and Pax7-expressing cells located in the central dermomyotome populates the myotome and contributes to the embryonic and fetal growth of the myotome. The central dermomyotome contains also progenitor cells for satellite cells that are muscle-specific stem cells essential for the growth and repair of adult muscles.37-39 The final somitic compartment to form is the syndetome that contains precursors for the tendons and is sandwiched between the myotome and the sclerotome.40 Blood vascular endothelial cells do not appear to form a specific compartment and are scattered within the sclerotome and dermomyotome, whereas lymphatic endothelial cells derive from the dermatome.41
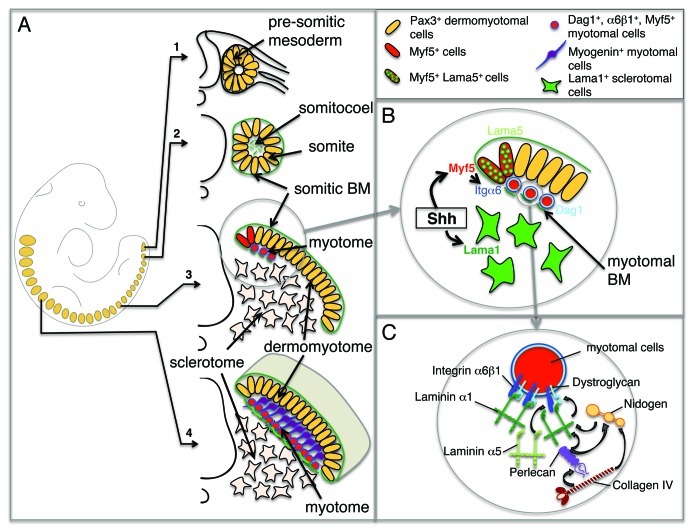
Figure 2. Assembly of the myotomal basement membrane. (A) Myogenesis along the antero-posterior axis of the mouse embryo. A schematic representation of an E9.5 mouse embryo is shown on the left with somites indicated in orange. Panels 1–4 depict the maturation of somites along the rostro-caudal axis with pluripotent, undetermined Pax3+ cells in the anterior presomitic mesoderm (1), Pax3+ epithelial somitic cells and fragment of laminins secreted in the somitocoele (2), the initiation of myotome formation following Myf5 activation in the dorsal medial lip of the dermomyotome. Myf5+ cells begin assembling the myotomal BM as they translocate to the myotome (3), in rostral somites, the myotome is separated from the sclerotome by a fully assembled myotomal BM (shown in green). Cells deeper within the myotome begin differentiating and expressing myogenin (4). (B) Magnification of the steps taking place in the dorsal medial lip of the dermomyotome illustrated in (3). Shh induces the activation of Myf5 in the dermomyotome. Myf5+ cells translocate to the myotome and upregulate α6β1 integrin and dystroglycan, allowing the initiation of the myotomal BM assembly using primarily laminin α1 produced by sclerotomal cells, and laminin α5 produced by the dorsal medial lip of the dermomyotome. (C) Magnification of a myotomal cell illustrating the steps leading to the assembly of the myotomal BM. Laminin-111 polymers are shown binding to and assembling at the surface of Myf5+ myotomal cells. The nascent ultrastructure is stabilized by the incorporation of nidogen and perlecan that link collagen IV to laminin chains. Laminin-511 is likely to be intercalated in the myotomal BM.
Extracellular matrix and basement membrane deposition during somitogenesis
A fibronectin-rich matrix envelops the anterior unsegmented paraxial mesoderm and newly-formed immature somites, and participates in the formation of somite boundaries during somitogenesis and the maintenance of the epithelial organization of somitic cells, a process likely to be mediated through fibronectin interaction with integrin α5β1 (Fig. 2A).42-44 As segmentation initiates in the anterior unsegmented paraxial mesoderm, a new laminin-containing BM is deposited at the surface of epithelial Pax3-positive cells.45-47 This laminin-based BM will surround the whole newly-formed somite. As somites mature and the sclerotome forms, the somitic BM disintegrates in the ventral somite. In the chick embryo, this takes place in somite III-V (third-fifth newly-formed somites)26,47 and correlates with the timing of sclerotome induction.48 In the dermomyotome, the laminin-containing somitic BM remains tightly associated with the basal (dorsal) side of the dermomyotome,46,49,50 and the myotomal BM begins assembling at the interface between the myotome and the sclerotome, as myotomal cells enter the myotome (Fig. 2A and B).50-52 As somites mature and more myogenic cells populate the myotome, the myotomal BM extends laterally such that in rostral somites, it forms a near continuous BM.50-52 However, in the central myotome where myotomal growth occurs, the myotomal BM is discontinuous and this is proposed to facilitate myogenic differentiation in the first instance, and myotomal growth subsequently.50,51
The Roles of the Myotomal Basement Membrane
Myotomal basement membrane function in somite patterning
The main role of the myotomal BM is to physically separate migrating myotomal cells from the underlying sclerotome, and thus help patterning the myotome by maintaining myotomal cells tightly packed underneath the dermomyotome. Consequently, mutations affecting directly or indirectly the formation of the myotomal BM result in defects in myotome patterning, with myotomal cells aberrantly migrating to the ventro-medial somite, failing to elongate and losing their antero-posterior orientation. This is the case in mouse embryos deficient for Myf5, Paraxis, Dmrt2 (doublesex and mab-3 related transcription factor 2) and Shh (Sonic hedgehog) signaling.49,51,53-55
Myotomal basement membrane function in myogenic cell fate specification and differentiation
However, the myotomal BM does not solely function as a barrier separating myotome from sclerotome, it is also essential for the control of myogenic specification and differentiation. A compelling example illustrating the role of basement membranes in the control of cell fate specification is that of sleepy (sly) zebrafish mutant embryos. Sleepy (sly) zebrafish embryos, which carry a mutation in Lamc1 (encoding for laminin γ1) (and prevent the formation of laminin-111), fail to induce the expression of engrailed 2a, which specifies muscle pioneer cells.56 Engrailed 2a activation is regulated positively by Hh and negatively by BMP signaling in the zebrafish.57 Interestingly, sly mutant embryos present ectopic BMP signaling in the progenitor domain of muscle pioneer cells, suggesting that Lamc1 (hence, laminin-111) participate in muscle pioneer cell fate specification by maintaining a BMP-free environment in progenitor cells, necessary for the activity of Shh.56 A similar BMP-free environment exists in the dorsal medial lip of the dermomyotome in the amniote embryo, and is required for the specification of epaxial muscle progenitor cells by Shh signaling.58,59 Although there is no evidence that disruption of the dermomyotomal basement membrane causes ectopic BMP signaling in amniotes, the similar environments suggests that the triangular relationship between laminins, Shh and BMP signaling may be a reiterated module in embryonic development. The mechanism by which laminins control BMP activity is currently unknown, and may involve either a direct effect on the presentation of BMP ligands to their receptor through the binding of laminins to heparan sulfate proteoglycans (HSPG), which are known to regulate BMP signaling,60 or an indirect effect on the control of Noggin expression, an inhibitor of BMP signaling.58,61 Knowing that in other systems (embryonic stem cells), Smad4, a BMP mediator, controls BM deposition through a dual control of metalloproteinase and laminin α1 synthesis, these observations suggest the existence of complex feedback mechanisms between Shh, BMP and laminin α1 in the control of BM deposition and myogenesis.
Interestingly, blocking the interaction of laminin with integrin α6β1 in dermomyotomal myogenic progenitor cells prevents their translocation to the myotome and causes their premature differentiation within the dermomyotome.51 Thus, a laminin-containing basement membrane may have dual roles in myogenesis, first contributing in shielding cells from BMP signaling to create a micro-environment favorable to combinatorial Shh and Wnt signaling; second, controlling myogenic differentiation through integrin-mediated signaling. Of interest is the observation that in Shh-deficient mouse embryos, which fail to form a myotomal basement membrane, myotomal cells fail to exit the cell cycle, maintain Pax3 expression and delay the differentiation program.49,59 This phenotype suggests that the loss of the myotomal BM in Shh-deficient somites contributes in part to the aberrant behavior of myotomal cells, and that the disruption of myogenic cell-ECM interaction yields distinct outcomes depending on the micro-environment myogenic cells are in, and most likely also depending on their intrinsic status (progenitor vs. committed muscle cells). In particular, as alluded to above, the signaling environment is very different in these two locations: the dorsal medial lip of the dermomyotome is BMP signaling free, and Wnt, Notch and Shh signaling responsive, whereas the myotome is Notch signaling free, and responsive to FGF and Shh signaling.59,62-69 Also, one of the differences between myogenic progenitor cells residing in the dermomyotome and those that have already translocated to the myotome is the presence or not of dystroglycan at their cell surface.50 Finally, the fact that integrin α6-mediated interaction with laminin promotes differentiation of myoblasts in vitro, whereas integrin α5-mediated interaction with fibronectin represses differentiation of myoblasts,70,71 raises also the possibility that the differential defect observed when cell-ECM interactions are blocked in the dermomyotome or in the myotome depends on the substrate. Consistent with this possibility is the enrichment in fibronectin matrix on the dorsal and medial sides of the dermomyotome in the mouse somite.46 Furthermore, downregulation of fibronectin in the zebrafish embryo is observed at the time slow-twitch muscle cells initiate their outward migration and fast-muscle cells undergo fusion, providing additional support to the idea that fibronectin may have a negative influence on myoblast differentiation.46,72,73 In amniotes, dermomyotomal cell interaction with fibronectin is probably not mediated by integrin α5 though, as it is mainly expressed in the myotome.74 Instead, integrin α4, which is initially expressed in the dermomyotome, is more likely to be the fibronectin receptor in vivo.74
Myotomal basement membrane function in cell migration, orientation and elongation
The myotomal BM is finally acting as a scaffolding to support cell migration, orientation and elongation. There is some evidence that it plays a role in the migration of muscle progenitor cells populating the myotome.49,51 For instance, the absence of basement membrane in either Myf5 or Shh mutant embryos correlates with the aberrant migration of muscle progenitor cells to the ventral and dorsal somitic domains.49,54 The myotomal basement membrane provides also a substrate for neural crest cell migration.52 Indeed, although neural crest cells would successfully migrate and enter the somite in the absence of the myotomal BM, they appear to preferentially migrate along this matrix on entering the somite.52,75 Disruption of the myotomal BM in amniotes and intersomitic BM in the zebrafish causes also defects in myofiber elongation, and interestingly in the zebrafish fast-twitch muscles laminin-111 cooperates with Shh signaling in the control of fiber elongation.76
Thus, an intact myotomal BM is critical for the correct patterning of the myotome, the control of myotomal cell fate and differentiation, the migration of myotomal and neural crest cells and the orientation and elongation of myofibers. It is also likely that as in other tissues, the myotomal BM may elicit local increase or decrease in cell signaling by concentrating or sequestering growth factors at the cell surface through binding between growth factors and BM components or through the binding of BM-associated heparan sulfate proteoglycans to growth factors.77-80 Finally, laminin-containing BMs are involved in complex feedback mechanisms with Shh and BMP signals during myogenesis.
Composition and assembly of the myotomal basement membrane
Thus, BMs have distinct roles, which are mainly dictated by their composition in collagen types and laminin isoforms. The laminin composition is of particular interest, as different laminins signal through different receptors with different affinities, providing a potential molecular mechanism for the diversity of responses triggered by BMs. Laminin-111 and laminin-511 are the first laminin proteins to be produced during embryogenesis,81,82 and remain the primary laminins present during early embryogenesis. Consistent with this, the myotomal BM is composed of both laminin-111 and laminin-511.49,51,52,83 The study of the expression pattern of somitic laminins is compelling, and illustrates the interplay and relationship between developing bone and muscle tissues. In this regards, it is important to remember that laminin β1 and γ1 subunits, which are incorporated in the embryonic laminins 111 and 511, are ubiquitously expressed in the embryo and depend on the presence of α subunits for their secretion.84 Thus, the expression of laminin α subunits within a cell dictates its ability to produce and secrete laminin heterotrimers. Both Lama1 and Lama5, which encode laminin α1 and α5, respectively, are expressed in newly-formed somites and laminin-111 and 511, as well as other BM components, are first immunodetected as punctate staining at the surface of somitocoele cells in newly-formed somites (Fig. 3). As somites mature, Lama5 is transcribed in epithelial cells of the dermomyotome, whereas Lama1 is transcribed in sclerotomal cells (Fig. 4),49 suggesting that although both incorporated into the myotomal BM, laminin-111 and 511 are produced by different sources in mature somites. Yet, both laminins are deposited in the BM at the surface of myotomal cells, at the interface with the sclerotome only. While the presence of laminin receptors on myotomal, but not on sclerotomal cells explains the selective assembly of the myotomal BM on myotomal cells, its preferred positioning at the interface with the sclerotome remains unexplained and is likely to involve other partners. In this respect, we have reported that dystroglycan proteins are initially uniformly distributed at the surface of myotomal cells but cluster rapidly on the side facing the myotomal BM.50 However, the clustering of dystroglycan appears to follow, and not to precede, myotomal BM assembly, suggesting that clustering is triggered by laminin binding to dystroglycan.85
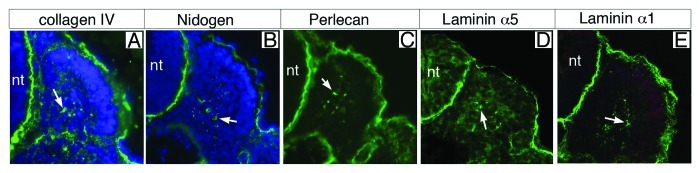
Figure 3. Basement membrane component distribution in newly-formed somites. Immunodetection of collagen IV (A), nidogen (B), perlecan (C), laminin α5 (D) and laminin α1 (E) shows the presence of polymers secreted in the somitocoele (white arrows). Note the presence of a BM surrounding the neural tube (nt) and the somite.
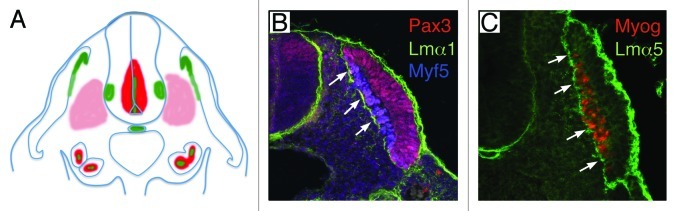
Figure 4. Laminin distribution in the myotomal basement membrane. (A) Cartoon showing the expression pattern of Lama1 (red) and Lama5 (green) in interlimb somites. Different shades of red indicate low and high levels of expression. Lama1 is expressed in the sclerotome, ventral neural tube and promesonephros. Lama5 is expressed in the dorsal medial lip of the dermomyotome, notochord, ventral neural tube and promesonephros. (B) Laminin α1 distribution in mouse interlimb somites. The myotomal basement membrane (white arrows) deposited at the interface between myotomal (Myf5-expressing cells in blue) and sclerotomal cells contains laminin α1 (green). The dermomyotome is labeled with Pax3 (red). Note the absence of laminin α1 in the BM surrounding the notochord. (C) Laminin α5 distribution in mouse interlimb somites. The myotomal basement membrane (white arrows) deposited at the interface between myotomal (myogenin-expressing cells in red) and sclerotomal cells contains laminin α5 (green). Note the presence of laminin α5 in the BM surrounding the notochord.
In vertebrates, there is little knowledge of the mechanisms driving the polarity of BM component secretion and assembly. Extracellular matrix proteins are secreted at the basal side and the BM assembles at the basal surface of epithelial cells. Different intracellular trafficking pathways have been suggested to drive the preferential secretion of BM components, but no specific player has been identified. In contrast, proteins with specific function in the polarized secretion or the polarized extracellular accumulation of BM components, including laminin, perlecan and collagenIV, have been found in invertebrates.86,87 Although myotomal cells are not epithelial cells, it is possible that similar proteins and mechanisms promote the preferential accumulation and assembly of BM components at the interface between sclerotomal and myotomal cells.
A comprehensive investigation of the expression pattern of each BM component, in addition to the analyses already reported,88-90 and the study of putative factors controlling the polarity of BM assembly would highlight the complex relationship and interdependence between tissues during ECM deposition at the surface of myotomal cells.
In the mouse, the deposition of the myotomal BM coincides with the translocation of Myf5-expressing myoblasts into the epaxial myotome at E9.5.49-51 Therefore, although short laminin polymers, likely to be already complexed to collagen IV through interactions with nidogen and perlecan, are present in the somitocoele of newly-formed somites (Fig. 3), the myotomal BM does not assemble until several hours after the initial secretion of laminins (approximately 6–8 h—i.e., 3–4 somites later). This is intriguing and raises the question of the rate limiting factor preventing earlier assembly of the myotomal BM. Previous studies have shown that, although dispensable for BM assembly, the laminin receptors integrin and dystroglycan play a crucial role by sequestering and clustering laminin polymers at the cell surface, and facilitating their assembly into a BM.91,92 In particular, the LG4 domain of laminin α1, which binds heparin/sulfatides and α-dystroglycan, seems to be critical for BM assembly.93 It has been suggested that the interaction between the laminin LG4 domain and cell surface receptors make cells “competent” for the assembly of a BM.94 Consistent with this idea, we noted that the timing of myotomal BM assembly correlates with the onset of dystroglycan immunodetection at the surface of myotomal cells,50 and not with that of integrin α6β1, which is already expressed by muscle progenitor cells in the dermomyotome (Fig. 2B).51,74,95 However, studies in other systems indicate that BM assembly does not require laminin α1 LG4–5 domains (see below). Consistent with this, Bajanca et al. (2006) demonstrated that blocking laminin binding to integrin α6 prevents Myf5-positive cells from populating the myotome and causes disruption in the myotomal BM,51 suggesting that integrin α6β1 is indispensable for the assembly of the myotomal BM. In fact, the requirement for dystroglycan and integrin for BM assembly at the surface of muscles appears to vary across species and developmental stages, as there are severe defects in laminin deposition at the myoseptum in Dag1 (dystrophin associated glycoprotein 1) morphant Xenopus,96 whereas loss of Dag1 has a moderate effect on laminin deposition at the myoseptum in the zebrafish97,98 or the muscle basal lamina in adult mouse muscles.99 Further genetic analyses using conditional targeted deletion of Dag1 and ItgB1 are required to determine their respective requirement in myotomal BM assembly.
Laminin-111, a Critical Laminin in Myotomal Basement Membrane Assembly
Studies of embryoid bodies showed that laminins are a prerequisite for BM assembly.93 Thus, the synthesis and distribution of laminins, in addition to the expression of integrins and dystroglycan, is a critical factor in BM assembly. Both Dmrt2 and Shh-deficient embryos fail to synthesize laminin α1. Thus, laminin-111 plays a central role in muscle specification and patterning. Its critical role may be conferred by its unique ability to initiate basement membrane assembly in the myotome, a property that is not shared by laminin-511 (Fig. 2C),49 and probably not shared by laminin-411 and -421, which are unable to compensate for loss of Lama2 and Lama1 in the zebrafish.100,101 Furthermore, the addition of laminin-111 in embryos defective in their myotomal basement membrane as a result of impaired Shh signaling or in adult muscles with defective basal lamina as a result of loss of Lama2 or Integrin α7 is sufficient to restore some levels of basement membrane assembly.49,102-104 This characteristic may have been conserved during vertebrate evolution, as loss of Lama1 alone causes some muscle fiber detachment and loss of Lama1 and Lama2 results in the loss of myoseptum and severe muscle fiber detachment in the zebrafish larvae.100 Collectively, these observations suggest that laminin α1 presents distinctive features allowing it to trigger assembly of a basement membrane. It is worth noting that although exclusive requirement for laminin α1 has been reported in pre-implantation embryos for the generation of the Reichert’s membrane, embryonal BM formation can be compensated for by laminin α5.82,105 What are the features of laminin α1 that confer its roles in BM assembly? The crystal structure of laminin α1 and α2 LG4–5 domains, which bind to sulfatides, heparin sulfate proteoglycans and dystroglycan, is informative as it highlights the unusual absence of specific conserved residus between the two proteins and instead the requirement for basic residus that confer a positively charged surface.106,107 Likewise, the crystal structure of laminin α2 LG1–3 domains, thought to be predominantly involved in integrin binding, reveals that integrin binding involves the cooperation of several sites, including within the γ chain.108 This may provide an explanation for the known differential binding affinities among laminin α subunits for their receptors.109-111 Of particular interest for muscle-associated basement membranes is the fact that laminins α1, α2 and α5 display distinct affinities for integrin α6β1, which is the main integrin receptor in early myogenesis, integrin α7β1, which is the main integrin receptor in adult myogenesis, and for dystroglycan.
Interestingly, although LG4–5 domains have been proposed to aid laminin deposition into BMs, the deletion of domains LG4–5 within laminin α1 does not affect Reichert’s membrane formation to the same extent than loss of laminin α1.112 Likewise, laminin-111 therapy in Lama2-deficient mice using transgenic mice lacking domains LG4–5 of laminin α1 leads to improvement of muscle structure and function, although limb muscles remain affected.113 In both cases although BM assembly occurred to some extent, BM were discontinuous.112,113 In addition, there were clear functional deficits (apoptosis or polarization), indicating that laminin α1 interaction with dystroglycan and sulfatides, or indeed another unknown receptor, has essential signaling roles.
Synthesis of Laminins and Role of Sonic Hedgehog Signaling
Thus, the assembly of the myotomal BM requires Lama1 expression in somites to produce laminin-111, which upon binding to dystroglycan, newly upregulated, and integrin α6β1 at the surface of Myf5-expressing cells translocating to the myotome, facilitates anchoring of laminin polymers and initiation of the myotomal BM assembly (Fig. 2). This has established the primary requirement for laminin α1 as an initiating event.
Both Dmrt2 and Shh-deficient embryos fail to synthesize laminin α1. In Shh−/− embryos, absence of laminin α1 results from a failure to transcribe Lama1.49 In Dmrt2−/− embryos, it is still unknown whether absence of laminin α1 is caused by a transcriptional defect. Dmrt2 is required for Myf5 expression in epaxial muscle progenitor cells,114 and mutations in Myf5 cause a loss of myotomal BM, suggesting that the phenotype observed in Dmrt2−/− embryos results from loss of Myf5.54 However, loss of laminin α1 has not been observed in Myf5−/− somites and Dmrt2−/− embryos are more severely affected than Myf5−/− embryos, suggesting that additional defects contribute to the severe phenotype of Dmrt2−/− embryos. In particular, loss of laminin α1 in Dmrt2-deficient somites, although not shown to result from a failure in Lama1 transcription, suggests a disruption in Shh signaling. In the zebrafish, Dmrt2 has been subject to gene duplication to generate dmrt2a (also known as terra), the true homolog to mouse Dmrt2, and dmrt2b. Terra/dmrt2a, like mouse Dmrt2, is expressed in the presomitic mesoderm and plays a role in somite segmentation and left-right asymmetry.115 It has not been reported to affect Hh signaling, but its expression is negatively regulated by Shh and positively regulated by BMP signaling.116 In contrast, Dmrt2b, which plays a role in left-right asymmetry, but not in somitogenesis, acts on Hh signaling by controlling Gli processing downstream of Su(Fu).117,118 Collectively, these observations suggest that a partitioning of Dmrt2 functions may have occurred upon gene duplication in the zebrafish. Further characterization of Dmrt2−/− mouse embryos would establish whether Shh signaling is disrupted, causing a loss of Lama1 expression.
Thus, Shh signaling and Dmrt2, by regulating both Myf5, which in turns controls integrin α6, and Lama1, operates a dual control on the assembly of the myotomal BM. Interestingly, in the cerebellum granule cell precursors, the laminin-containing BM binds and locally increases Shh signals,77 suggesting a possible feedforward mechanism within the somite whereby Shh controls Lama1 expression and the assembly of the myotomal BM, allowing subsequently the concentration of signals in the vicinity of the myotome. Supporting this possibility is the intense expression of Ptc1 (Patched 1) and Ptc2 (Patched 2) in the myotome, a sign that high levels of Shh signaling is taking place.69 Another possible feedforward mechanism could involve laminin α5. Indeed, loss of Lama5 function in the dermal papilla results in the loss of primary cilia, which are known to be essential for Shh signaling.119,120 Interestingly, primary cilia formation can be restored upon ectopic addition of laminin-511, but not laminin-111. This suggests a 2-fold mechanism to enhance Shh signaling in myotomal cells: (1) through laminin-mediated binding to Shh proteins as described in cerebellum granule cell precursors and (2) through the deposition of laminin-511 in the myotomal BM, following its initial assembly mediated by laminin-111, which would promote the formation of primary cilia at the surface of myotomal cells and allow Shh signaling. It remains to demonstrate that myotomal cells have primary cilia and that laminin-511 contributes to their formation.
It is presently unknown whether Shh controls Lama1 transcription directly or indirectly. Further studies are necessary to unravel the determinants of Lama1 transcription. Nevertheless, the relationship between laminin α1 and Shh is reminiscent of the combinatorial activities between laminin-111 and Shh in the control of myogenesis in the zebrafish embryo (see the section “Basement membranes function in myogenic cell fate specification and differentiation” above). Thus, there is a complex interplay between Shh signaling and laminin expression or activity in the control of muscle cell fate specification, fiber elongation and attachment and muscle patterning during embryogenesis.
Concluding Remarks and Future Perspectives
There are few in vivo models that allow research into the molecular mechanisms underlying the initiation of basement membrane assembly. Previous investigations in this area of research have utilized either pre-implantation embryos to examine the formation of the Reichert’s and embryonic BMs or embryoid bodies.121 The myotomal basement membrane provides a novel paradigm for studying BM assembly and unraveling the essential players in this process. Data collected so far suggest a critical role for laminin-111 in myotomal BM assembly, an area that requires further investigation to decipher the biochemistry underlying this property. Crystal structure studies to unravel differences between laminin α subunits would be decisive, as well as analyses to identify putative novel laminin α binding partners and further investigations into the differential binding affinities between laminin isoforms and their receptors, integrins and dystroglycan.
The study of the myotomal basement membrane has also provided novel insight into the complex relationship between extracellular matrix components, in particular laminins, and growth factors. This connection should be further explored, as it will have important impact not only for our understanding of developmental processes, but also for our comprehension of the role of basement membranes within stem cell niches. Specifically, the transcriptional control of laminin α subunits needs to be solved. Future studies will also establish the connections between Shh signaling and laminins, including in the control of cilia formation and signaling activity, signal binding and release and growth factor cooperation.
Together, these studies will shed light on the role of basement membranes in the control of cell behavior (proliferation, cell survival, differentiation and migration). Notably, it is paramount to decode how the laminin composition of basement membranes may trigger distinct intracellular responses as a result of differential cell surface receptor and/or membrane-associated protein binding affinities.
Acknowledgments
We are grateful to Association Francaise contre les Myopathies (AFM) for their continuous financial support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23411
References
- 1.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–38. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Nishiuchi R, Murayama O, Fujiwara H, Gu J, Kawakami T, Aimoto S, et al. Characterization of the ligand-binding specificities of integrin alpha3beta1 and alpha6beta1 using a panel of purified laminin isoforms containing distinct alpha chains. J Biochem. 2003;134:497–504. doi: 10.1093/jb/mvg185. [DOI] [PubMed] [Google Scholar]
- 5.Ettner N, Göhring W, Sasaki T, Mann K, Timpl R. The N-terminal globular domain of the laminin alpha1 chain binds to alpha1beta1 and alpha2beta1 integrins and to the heparan sulfate-containing domains of perlecan. FEBS Lett. 1998;430:217–21. doi: 10.1016/S0014-5793(98)00601-2. [DOI] [PubMed] [Google Scholar]
- 6.Yurchenco PD, Cheng YS. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem. 1993;268:17286–99. [PubMed] [Google Scholar]
- 7.Aumailley M, Wiedemann H, Mann K, Timpl R. Binding of nidogen and the laminin-nidogen complex to basement membrane collagen type IV. Eur J Biochem. 1989;184:241–8. doi: 10.1111/j.1432-1033.1989.tb15013.x. [DOI] [PubMed] [Google Scholar]
- 8.Hopf M, Göhring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–25. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 9.Mayer U, Kohfeldt E, Timpl R. Structural and genetic analysis of laminin-nidogen interaction. Ann N Y Acad Sci. 1998;857:130–42. doi: 10.1111/j.1749-6632.1998.tb10113.x. [DOI] [PubMed] [Google Scholar]
- 10.Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–17. doi: 10.1016/S0945-053X(00)00072-X. [DOI] [PubMed] [Google Scholar]
- 11.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–23. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore CJ, Winder SJ. Dystroglycan versatility in cell adhesion: a tale of multiple motifs. Cell Commun Signal. 2010;8:3. doi: 10.1186/1478-811X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonen KJ, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev. 2008;14:419–31. doi: 10.1089/ten.teb.2008.0045. [DOI] [PubMed] [Google Scholar]
- 15.Thorsteinsdóttir S, Deries M, Cachaço AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol. 2011;354:191–207. doi: 10.1016/j.ydbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, et al. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–8. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- 17.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–22. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 18.Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltrán-Valero de Bernabé D, Gündeşli H, et al. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364:939–46. doi: 10.1056/NEJMoa1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–7. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 20.Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–23. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 21.Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- 22.Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–15. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- 23.Dequéant ML, Pourquié O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–82. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- 24.Maroto M, Bone RA, Dale JK. Somitogenesis. Development. 2012;139:2453–6. doi: 10.1242/dev.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christ B, Huang R, Scaal M. Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 2004;208:333–50. doi: 10.1007/s00429-004-0408-z. [DOI] [PubMed] [Google Scholar]
- 26.Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–96. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Yair R, Kalcheim C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development. 2005;132:689–701. doi: 10.1242/dev.01617. [DOI] [PubMed] [Google Scholar]
- 28.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pownall ME, Gustafsson MK, Emerson CP., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–83. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 30.Venters SJ, Thorsteinsdóttir S, Duxson MJ. Early development of the myotome in the mouse. Dev Dyn. 1999;216:219–32. doi: 10.1002/(SICI)1097-0177(199911)216:3<219::AID-DVDY1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Gros J, Scaal M, Marcelle C. A two-step mechanism for myotome formation in chick. Dev Cell. 2004;6:875–82. doi: 10.1016/j.devcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Denetclaw WF, Jr., Berdougo E, Venters SJ, Ordahl CP. Morphogenetic cell movements in the middle region of the dermomyotome dorsomedial lip associated with patterning and growth of the primary epaxial myotome. Development. 2001;128:1745–55. doi: 10.1242/dev.128.10.1745. [DOI] [PubMed] [Google Scholar]
- 33.Kahane N, Cinnamon Y, Kalcheim C. The cellular mechanism by which the dermomyotome contributes to the second wave of myotome development. Development. 1998;125:4259–71. doi: 10.1242/dev.125.21.4259. [DOI] [PubMed] [Google Scholar]
- 34.Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K. Origin and development of the avian tongue muscles. Anat Embryol (Berl) 1999;200:137–52. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- 35.Clugston RD, Greer JJ. Diaphragm development and congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:94–100. doi: 10.1053/j.sempedsurg.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Birchmeier C, Brohmann H. Genes that control the development of migrating muscle precursor cells. Curr Opin Cell Biol. 2000;12:725–30. doi: 10.1016/S0955-0674(00)00159-9. [DOI] [PubMed] [Google Scholar]
- 37.Gros J, Manceau M, Thomé V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–8. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- 38.Relaix F, Rocancourt D, Mansouri A, Buckingham MA. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–53. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 39.Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomès D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–31. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. doi: 10.1016/S0092-8674(03)00268-X. [DOI] [PubMed] [Google Scholar]
- 41.Wilting J, Becker J. Two endothelial cell lines derived from the somite. Anat Embryol (Berl) 2006;211(Suppl 1):57–63. doi: 10.1007/s00429-006-0120-2. [DOI] [PubMed] [Google Scholar]
- 42.Rifes P, Carvalho L, Lopes C, Andrade RP, Rodrigues G, Palmeirim I, et al. Redefining the role of ectoderm in somitogenesis: a player in the formation of the fibronectin matrix of presomitic mesoderm. Development. 2007;134:3155–65. doi: 10.1242/dev.003665. [DOI] [PubMed] [Google Scholar]
- 43.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, et al. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–98. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 44.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 45.Krotoski DM, Domingo C, Bronner-Fraser M. Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol. 1986;103:1061–71. doi: 10.1083/jcb.103.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deries M, Gonçalves AB, Vaz R, Martins GG, Rodrigues G, Thorsteinsdóttir S. Extracellular matrix remodeling accompanies axial muscle development and morphogenesis in the mouse. Dev Dyn. 2012;241:350–64. doi: 10.1002/dvdy.23703. [DOI] [PubMed] [Google Scholar]
- 47.Rifes P, Thorsteinsdóttir S. Extracellular matrix assembly and 3D organization during paraxial mesoderm development in the chick embryo. Dev Biol. 2012;368:370–81. doi: 10.1016/j.ydbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Borycki AG, Strunk KE, Savary R, Emerson CP., Jr. Distinct signal/response mechanisms regulate pax1 and QmyoD activation in sclerotomal and myotomal lineages of quail somites. Dev Biol. 1997;185:185–200. doi: 10.1006/dbio.1997.8555. [DOI] [PubMed] [Google Scholar]
- 49.Anderson C, Thorsteinsdóttir S, Borycki AG. Sonic hedgehog-dependent synthesis of laminin alpha1 controls basement membrane assembly in the myotome. Development. 2009;136:3495–504. doi: 10.1242/dev.036087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson C, Winder SJ, Borycki AG. Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev Dyn. 2007;236:2627–35. doi: 10.1002/dvdy.21259. [DOI] [PubMed] [Google Scholar]
- 51.Bajanca F, Luz M, Raymond K, Martins GG, Sonnenberg A, Tajbakhsh S, et al. Integrin alpha6beta1-laminin interactions regulate early myotome formation in the mouse embryo. Development. 2006;133:1635–44. doi: 10.1242/dev.02336. [DOI] [PubMed] [Google Scholar]
- 52.Tosney KW, Dehnbostel DB, Erickson CA. Neural crest cells prefer the myotome’s basal lamina over the sclerotome as a substratum. Dev Biol. 1994;163:389–406. doi: 10.1006/dbio.1994.1157. [DOI] [PubMed] [Google Scholar]
- 53.Seo KW, Wang Y, Kokubo H, Kettlewell JR, Zarkower DA, Johnson RL. Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev Biol. 2006;290:200–10. doi: 10.1016/j.ydbio.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Tajbakhsh S, Rocancourt D, Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature. 1996;384:266–70. doi: 10.1038/384266a0. [DOI] [PubMed] [Google Scholar]
- 55.Wilson-Rawls J, Hurt CR, Parsons SM, Rawls A. Differential regulation of epaxial and hypaxial muscle development by paraxis. Development. 1999;126:5217–29. doi: 10.1242/dev.126.23.5217. [DOI] [PubMed] [Google Scholar]
- 56.Dolez M, Nicolas JF, Hirsinger E. Laminins, via heparan sulfate proteoglycans, participate in zebrafish myotome morphogenesis by modulating the pattern of Bmp responsiveness. Development. 2011;138:97–106. doi: 10.1242/dev.053975. [DOI] [PubMed] [Google Scholar]
- 57.Maurya AK, Tan H, Souren M, Wang X, Wittbrodt J, Ingham PW. Integration of Hedgehog and BMP signalling by the engrailed2a gene in the zebrafish myotome. Development. 2011;138:755–65. doi: 10.1242/dev.062521. [DOI] [PubMed] [Google Scholar]
- 58.Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–63. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 59.Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP., Jr. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126:4053–63. doi: 10.1242/dev.126.18.4053. [DOI] [PubMed] [Google Scholar]
- 60.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 61.Fürthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–96. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 62.Han JK, Martin GR. Embryonic expression of Fgf-6 is restricted to the skeletal muscle lineage. Dev Biol. 1993;158:549–54. doi: 10.1006/dbio.1993.1212. [DOI] [PubMed] [Google Scholar]
- 63.Holowacz T, Zeng L, Lassar AB. Asymmetric localization of numb in the chick somite and the influence of myogenic signals. Dev Dyn. 2006;235:633–45. doi: 10.1002/dvdy.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jory A, Le Roux I, Gayraud-Morel B, Rocheteau P, Cohen-Tannoudji M, Cumano A, et al. Numb promotes an increase in skeletal muscle progenitor cells in the embryonic somite. Stem Cells. 2009;27:2769–80. doi: 10.1002/stem.220. [DOI] [PubMed] [Google Scholar]
- 65.Brauner I, Spicer DB, Krull CE, Venuti JM. Identification of responsive cells in the developing somite supports a role for beta-catenin-dependent Wnt signaling in maintaining the DML myogenic progenitor pool. Dev Dyn. 2010;239:222–36. doi: 10.1002/dvdy.22098. [DOI] [PubMed] [Google Scholar]
- 66.Sela-Donenfeld D, Kalcheim C. Localized BMP4-noggin interactions generate the dynamic patterning of noggin expression in somites. Dev Biol. 2002;246:311–28. doi: 10.1006/dbio.2002.0672. [DOI] [PubMed] [Google Scholar]
- 67.McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, et al. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148:273–84. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borycki A, Brown AM, Emerson CP., Jr. Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–87. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- 70.Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–84. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von der Mark K, Ocalan M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation. 1989;40:150–7. doi: 10.1111/j.1432-0436.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 72.Snow CJ, Henry CA. Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish. Gene Expr Patterns. 2009;9:37–42. doi: 10.1016/j.gep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snow CJ, Peterson MT, Khalil A, Henry CA. Muscle development is disrupted in zebrafish embryos deficient for fibronectin. Dev Dyn. 2008;237:2542–53. doi: 10.1002/dvdy.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajanca F, Luz M, Duxson MJ, Thorsteinsdóttir S. Integrins in the mouse myotome: developmental changes and differences between the epaxial and hypaxial lineage. Dev Dyn. 2004;231:402–15. doi: 10.1002/dvdy.20136. [DOI] [PubMed] [Google Scholar]
- 75.Loring JF, Erickson CA. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol. 1987;121:220–36. doi: 10.1016/0012-1606(87)90154-0. [DOI] [PubMed] [Google Scholar]
- 76.Peterson MT, Henry CA. Hedgehog signaling and laminin play unique and synergistic roles in muscle development. Dev Dyn. 2010;239:905–13. doi: 10.1002/dvdy.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, et al. Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci. 2004;24:3402–12. doi: 10.1523/JNEUROSCI.5241-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 79.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–28. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dziadek M, Timpl R. Expression of nidogen and laminin in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol. 1985;111:372–82. doi: 10.1016/0012-1606(85)90491-9. [DOI] [PubMed] [Google Scholar]
- 82.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–56. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 83.Gullberg D, Tiger CF, Velling T. Laminins during muscle development and in muscular dystrophies. Cell Mol Life Sci. 1999;56:442–60. doi: 10.1007/PL00000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, et al. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A. 1997;94:10189–94. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen MW, Jacobson C, Yurchenco PD, Morris GE, Carbonetto S. Laminin-induced clustering of dystroglycan on embryonic muscle cells: comparison with agrin-induced clustering. J Cell Biol. 1997;136:1047–58. doi: 10.1083/jcb.136.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–64. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorrosal G, Pérez L, Herranz H, Milán M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010;11:373–9. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 89.Zagris N, Chung AE, Stavridis V. Entactin and laminin gamma 1-chain gene expression in the early chick embryo. Int J Dev Biol. 2005;49:65–70. doi: 10.1387/ijdb.041812nz. [DOI] [PubMed] [Google Scholar]
- 90.Thomas T, Dziadek M. Expression of laminin and nidogen genes during the postimplantation development of the mouse placenta. Biol Reprod. 1993;49:1251–9. doi: 10.1095/biolreprod49.6.1251. [DOI] [PubMed] [Google Scholar]
- 91.Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–70. doi: 10.1016/S0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 92.Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–31. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, et al. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–24. doi: 10.1016/S1534-5807(03)00128-X. [DOI] [PubMed] [Google Scholar]
- 95.Bronner-Fraser M, Artinger M, Muschler J, Horwitz AF. Developmentally regulated expression of alpha 6 integrin in avian embryos. Development. 1992;115:197–211. doi: 10.1242/dev.115.1.197. [DOI] [PubMed] [Google Scholar]
- 96.Hidalgo M, Sirour C, Bello V, Moreau N, Beaudry M, Darribère T. In vivo analyzes of dystroglycan function during somitogenesis in Xenopus laevis. Dev Dyn. 2009;238:1332–45. doi: 10.1002/dvdy.21814. [DOI] [PubMed] [Google Scholar]
- 97.Gupta V, Kawahara G, Gundry SR, Chen AT, Lencer WI, Zhou Y, et al. The zebrafish dag1 mutant: a novel genetic model for dystroglycanopathies. Hum Mol Genet. 2011;20:1712–25. doi: 10.1093/hmg/ddr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parsons MJ, Campos I, Hirst EM, Stemple DL. Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development. 2002;129:3505–12. doi: 10.1242/dev.129.14.3505. [DOI] [PubMed] [Google Scholar]
- 99.Côté PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23:338–42. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 100.Sztal TE, Sonntag C, Hall TE, Currie PD. Epistatic dissection of laminin-receptor interactions in dystrophic zebrafish muscle. Hum Mol Genet. 2012;21:4718–31. doi: 10.1093/hmg/dds312. [DOI] [PubMed] [Google Scholar]
- 101.Sztal T, Berger S, Currie PD, Hall TE. Characterization of the laminin gene family and evolution in zebrafish. Dev Dyn. 2011;240:422–31. doi: 10.1002/dvdy.22537. [DOI] [PubMed] [Google Scholar]
- 102.Gawlik KI, Li JY, Petersén A, Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum Mol Genet. 2006;15:2690–700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 103.Gawlik KI, Mayer U, Blomberg K, Sonnenberg A, Ekblom P, Durbeej M. Laminin alpha1 chain mediated reduction of laminin alpha2 chain deficient muscular dystrophy involves integrin alpha7beta1 and dystroglycan. FEBS Lett. 2006;580:1759–65. doi: 10.1016/j.febslet.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 104.Rooney JE, Gurpur PB, Yablonka-Reuveni Z, Burkin DJ. Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am J Pathol. 2009;174:256–64. doi: 10.2353/ajpath.2009.080522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alpy F, Jivkov I, Sorokin L, Klein A, Arnold C, Huss Y, et al. Generation of a conditionally null allele of the laminin alpha1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- 106.Harrison D, Hussain SA, Combs AC, Ervasti JM, Yurchenco PD, Hohenester E. Crystal structure and cell surface anchorage sites of laminin alpha1LG4-5. J Biol Chem. 2007;282:11573–81. doi: 10.1074/jbc.M610657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wizemann H, Garbe JH, Friedrich MV, Timpl R, Sasaki T, Hohenester E. Distinct requirements for heparin and alpha-dystroglycan binding revealed by structure-based mutagenesis of the laminin alpha2 LG4-LG5 domain pair. J Mol Biol. 2003;332:635–42. doi: 10.1016/S0022-2836(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 108.Carafoli F, Clout NJ, Hohenester E. Crystal structure of the LG1-3 region of the laminin alpha2 chain. J Biol Chem. 2009;284:22786–92. doi: 10.1074/jbc.M109.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talts JF, Andac Z, Göhring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–70. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andac Z, Sasaki T, Mann K, Brancaccio A, Deutzmann R, Timpl R. Analysis of heparin, alpha-dystroglycan and sulfatide binding to the G domain of the laminin alpha1 chain by site-directed mutagenesis. J Mol Biol. 1999;287:253–64. doi: 10.1006/jmbi.1999.2606. [DOI] [PubMed] [Google Scholar]
- 111.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Schéele S, Falk M, Franzén A, Ellin F, Ferletta M, Lonai P, et al. Laminin alpha1 globular domains 4-5 induce fetal development but are not vital for embryonic basement membrane assembly. Proc Natl Acad Sci U S A. 2005;102:1502–6. doi: 10.1073/pnas.0405095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gawlik KI, Akerlund M, Carmignac V, Elamaa H, Durbeej M. Distinct roles for laminin globular domains in laminin alpha1 chain mediated rescue of murine laminin alpha2 chain deficiency. PLoS One. 2010;5:e11549. doi: 10.1371/journal.pone.0011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sato T, Rocancourt D, Marques L, Thorsteinsdóttir S, Buckingham MA. A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis. PLoS Genet. 2010;6:e1000897. doi: 10.1371/journal.pgen.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saúde L, Lourenço R, Gonçalves A, Palmeirim I. terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat Cell Biol. 2005;7:918–20. doi: 10.1038/ncb1294. [DOI] [PubMed] [Google Scholar]
- 116.Meng A, Moore B, Tang H, Yuan B, Lin S. A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development. 1999;126:1259–68. doi: 10.1242/dev.126.6.1259. [DOI] [PubMed] [Google Scholar]
- 117.Liu S, Li Z, Gui JF. Fish-specific duplicated dmrt2b contributes to a divergent function through Hedgehog pathway and maintains left-right asymmetry establishment function. PLoS One. 2009;4:e7261. doi: 10.1371/journal.pone.0007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lourenço R, Lopes SS, Saúde L. Left-right function of dmrt2 genes is not conserved between zebrafish and mouse. PLoS One. 2010;5:e14438. doi: 10.1371/journal.pone.0014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22:2111–24. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- 121.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]


