Abstract
Laminins (LM) are extracellular matrix molecules that contribute to and are required for the formation of basement membranes. They participate in the modulation of epithelial/mesenchymal interactions and are implicated in organogenesis and maintenance of organ homeostasis. Among the LM molecules, the LM α5 chain (LMα5) is one of the most widely distributed LM in the developing and mature organism. Its presence in some basement membranes during embryogenesis is absolutely required for maintenance of basement membrane integrity and thus for proper organogenesis. LMα5 also regulates the expression of genes important for major biological processes, in part by repressing or activating signaling pathways, depending upon the physiological context.
Keywords: basement membranes, cell receptors, extracellular matrix, stem cells, signaling pathways
Introduction
Development and homeostasis of organisms are complex processes requiring interactions between cells and the extracellular matrix (ECM). Basement membranes are specialized ECMs that play a mechanical supporting role for various cell types and also mediate interactions between epithelial sheets and the adjacent mesenchyme or stroma. Among the major basement membrane proteins, laminins (LM) have been shown to regulate cell adhesion, migration and differentiation. LM are composed of α, β and γ chains that assemble into αβγ heterotrimers to form 16 different isoforms.1 Each heterotrimer is assigned a three digit Arabic numeral based on the respective αβγ chain composition.2 The laminin α5 (LMα5) chain is one of the major α chains in the epithelial basement membranes of developing and adult organs.3,4
Diverse experimental approaches, such as cell culture on LM-511 (α5β1γ1) or LM-521 (α5β2γ1) substrates, knockout of LMα5, and generation of LMα5 transgenic mice have been employed to study this LM chain. The importance of LMα5 in morphogenesis was demonstrated by the embryonic lethality of Lama5 knockout mice at ~17 d of embryonic development. Embryos lacking LMα5 exhibit multiple developmental defects, including failure of anterior neural tube closure (exencephaly), failure of digit septation (syndactyly) and dysmorphogenesis of the placental labyrinth.4 Although mutations in LAMA5 have not yet been described in human pathologies, alterations of LMα5 have been reported to be associated with Crohn disease, Hirschsprung disease and tufting enteropathy.5
Here we review the current literature devoted to studies of the role of LMα5 in development and homeostasis of various organs and summarize the possible signaling pathways that are triggered by LM-511/-521. The potential contributions of LMα5 chain to vascular homeostasis are discussed elsewhere in this issue by Yousif et al., and the implications of LMα5 for regulating tumor cell migration, invasion and metastasis are discussed by Pouliot and Kusuma.
The Laminin α5 Chain
Although LMα5 was first visualized on protein gels and in tissues as a presumed component of a LM trimer, its actual identity was not known until it was cloned by RT-PCR using degenerate oligonucleotides designed to amplify any LM α chain cDNA from mouse kidney. The mature mouse LMα5 protein is composed of ~3,630 amino acids and has a molecular weight of approximately 400 kDa.3 Northern blot analysis reveals a single band of ~11 kb in most organs studied,6-8 although an additional 13 kb band was found in certain mouse organs, including lung and kidney.7 Comparison between the human (3,695 amino acids) and the mouse LMα5 sequence shows an overall amino acid identity of 79%.9 In Drosophila, the LMα3,5 chain is most similar to vertebrate α3 and α5 and is part of the laminin A trimer.10 Studies of the origin of LM suggest that the early evolution of this family of molecules involved duplication and extensive domain rearrangement.11
LMα5 (Fig. 1) is very widely expressed in mammalian tissues. Through a combination of immunoprecipitation, non-denaturing gel electrophoresis, mass spectroscopy, and colocalization experiments using chain-specific antibodies, it has been shown that the α5 chain can combine with β1, β2, γ1 and γ3 chains to form three distinct isoforms: LM-511 (in basement membranes underlying epithelium, endothelium and smooth muscle), LM-521 (in basement membranes of epithelium, endothelium, smooth muscle, neuromuscular junctions and kidney glomerulus) and LM-523 (in the retina and central nervous system).1,2,12 The existence of LM-522 (α5β2γ2), reported so far solely in bone marrow,13 was not confirmed in a complete analysis of LM chain assembly using recombinant coiled-coil fragments of the LMγ2 domain.12

Figure 1. Schematic representation of LMα5 binding sites for its major receptors. Binding sites to known cellular receptors interacting with the LMα5 chain are depicted on the main functional domains LN and LG (LG1-LG5). The LG3 module (*) is indispensable for α3β1 and α6β1 binding; the precise binding site for α6β4 integrin (★) has not been determined.
Each LMα5-containing trimer appears as a cross-like structure, similar to most other LM heterotrimers. The base of the cross is formed by the C-terminal part of the α chain called the laminin globular (LG) domain, which is subdivided into five globular subdomains (LG1-LG5).14 The LG domain represents the principal site for interactions with cell surface receptors, whereas the short arm of α5 contains the laminin N-terminal (LN) domain, which is involved in spontaneous heterotrimer polymerization via intertrimer interactions with LN domains present in the β and γ chains.15,16 A recent study using Biacore analysis investigated the nature of the bonds formed among LM subunits and identified the amino acids involved by analyzing the crystal structure of the α5 LN domain.17 In the skin it has been hypothesized that LM-511 and LM-521 can co-polymerize by interactions among the LN domains; lateral aggregation will then occur to allow further polymerization into a heterotypic LM superstructure that is also capable of integrating LM-332, which lacks typical LN domains. Then, the separate LM and collagen IV networks can be connected either by nidogen or perlecan.16,18
Receptors for the LMα5 Chain
The functionality of extracellular LM is mediated through interactions with cell membrane receptors. The best-established type of LM receptor is the integrin family, which plays a central role in cell-matrix interactions in most tissues. Besides integrins, LM can also bind to more specific receptors, depending on the tissue or the constituent α chain. Regarding LMα5 (Fig. 1), it is known to bind specifically to particular integrins, to α-dystroglycan, to syndecan-4, and to the Lutheran/basal cell adhesion molecule (Lu/B-CAM).19
Integrins
Integrins are transmembrane αβ heterodimers. Eighteen α and eight β subunits are known, generating 24 heterodimers.20,21 They form a superfamily of cell adhesion receptors that bind to extracellular matrix ligands, cell-surface ligands and soluble ligands. Integrins can function as traction receptors that can transmit and detect changes in mechanical forces acting on the ECM. Some integrins are limited to certain cell types or tissues, whereas others are widely distributed. In regard to ligand specificity, integrins can be grouped into laminin-binding integrins, collagen-binding integrins, leukocyte integrins and RGD-recognizing integrins. Among the laminin-binding integrins, the LMα5 chain can potentially bind the α2β1, α3β1, α6β1, α6β4, α7β1 and αvβ3 integrins.1,22,23 Interestingly, double homozygous integrin α3/α6-null mouse embryos show developmental phenotypes similar to those observed in LMα5-null embryos, suggesting that these two integrin subunits are part of the major receptors for LM-511/-521 during development and that the interaction of LMα5 with these receptors is essential for the development of certain tissues.24
α-dystroglycan and syndecan-4
Dystroglycan is a member of the dystrophin-glycoprotein complex, a large complex of plasma membrane-associated proteins that is critical for the integrity of skeletal muscle fibers. Dystroglycan is composed of an α and a β subunit encoded by a single gene, DAG1 (dystrophin-associated glycoprotein 1), and it provides a link between the ECM and the cytoskeleton (for review, see ref. 25). Dystroglycan is mainly expressed in skeletal muscle, in the central and peripheral nervous systems, as well as in the retina and in a subset of epithelial cells. Besides its high affinity binding to LMα1 and α2 chains, α-dystroglycan was also shown to bind to a recombinant LMα5 fragment, supporting the idea that this receptor could work as a LM receptor in vivo.26 Very little is known about the signaling mediated by the binding of LMα5 to α-dystroglycan, except that it could inhibit extracellular-signal-regulated kinase (ERK) activation, in contrary to the binding of LMα5 to integrin α6β1, which activates ERK.27
Syndecans are transmembrane heparan sulfate proteoglycans. Four syndecans (syndecan-1 to -4) have been identified in mammals, and the expression of each is developmental stage- and cell type-specific. Among the syndecans, syndecan-4 is widely distributed in development, expressed in many different cell types.28 When adhesion assays were performed with conjunctival epithelial cells on a peptide from the LG4 domain of LMα5, an antibody to the ectodomain of syndecan-4 inhibited adhesion to the peptide. In addition, cells adhering to this peptide showed increased phosphorylation of focal adhesion kinase (FAK). These results suggest that cell adhesion to a peptide from the LMα5 LG4 domain results in increased tyrosine phosphorylation of FAK through binding to syndecan-4.29
Lutheran/B-CAM
The Lutheran blood group antigen (Lu) is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily.30,31 This receptor is also known as basal cell adhesion molecule (B-CAM). In contrast to the integrins that can bind several ECM molecules, Lu only binds to laminin isoforms containing the α5 chain.32 Supporting this, it was shown that Lu/B-CAM expression is dramatically reduced in tissues from mouse embryos lacking LMα5, while it is significantly increased in the hearts of transgenic mice overexpressing LMα5.33 Similarly, in the intestines of LMα5 deficient mice, there was decreased Lu staining, especially in the muscle region where LMα5 is usually present at high levels.34 Using a soluble recombinant protein containing the Lu extracellular domain, Kikkawa and colleagues demonstrated specific binding of Lu to LM-511/521.32 The recombinant protein used as a probe was also able to bind LMα5-containing basement membranes in normal but not in LMα5 knockout tissue sections. But surprisingly, mice with targeted disruption of the Lu gene are viable, fertile, and appear healthy with no major defects in organ development or function. However, loss of Lu causes structural alterations in the kidney (thickening of the glomerular basement membrane) and intestine (enlargement of the smooth muscle layers and abnormal organization of the cells).35,36
Binding sites for LMα5 receptors
Binding sites for receptors have been reported to be localized to three regions of the LMα5 chain. The C-terminal LG domain appears to be the major one (Fig. 1), as there are multiple lines of evidence both in vitro and in vivo, but the N-terminal LN domain and the L4 domain present in the central part of the molecule have also been implicated in a few studies.
Concerning the LG domain, in vivo experiments using transgenic mice expressing full length and chimeric laminin α5/α1 chains on the LMα5 null-background revealed that the LG1–2 tandem harbors most of the functionality of the α5LG domain for developmental processes, while the α5LG3–5 tandem is somehow involved in maintaining integrity of the glomerular filtration barrier.37,38 To understand which subdomain is specific for which receptor, antibody-inhibition assays performed on two α5LG fragments showed that α3β1 and α6β1 integrins bind to the LG1–3 domain.39 Interestingly, the LG3 module is indispensable for binding to α3β1 and α6β1 integrins, but this module alone is not sufficient to recapitulate integrin binding activity.40 Moreover, by producing a large series of recombinant LM trimers containing modified chimeric α1/α5 chains, it was determined that only a complete α5LG1–3 tandem with the LCC (laminin coiled coil) domain could reproduce the activity of Lu/B-CAM binding to LMα5. This specific binding site may overlap with that of integrins α3β1 and α6β1, as soluble Lutheran can inhibit integrin-mediated cell adhesion to LMα5.41 The binding site for α6β4 integrin has not yet been characterized, but comparison with LMα1 suggests that this integrin may also bind the LMα5 LG1–3 domain.22 Furthermore it was demonstrated that α-dystroglycan and syndecan-4 binding was localized to the LG4 domain.29,39,40
Regarding receptor binding sites on the α5LN domain (previously known as domain VI), experiments using integrin blocking antibodies demonstrated α3β1 to potentially be a major receptor. Unexpectedly, weak interactions with integrins containing the α2, α4 and α6 subunits were also found. Furthermore, heparin could inhibit cellular binding to the LN domain by ~90%, suggesting a potential role for syndecans and/or α-dystroglycan in mediating adhesion.42 Finally, the LMα5 L4b domain (previously called domain IVa) contains two RGD sequences (Arg-Gly-Asp; conserved in human LMα5) that could theoretically contribute to cell adhesion. Antibodies against integrins β1, αvβ5 or α5 partially inhibited specific binding to this domain, whereas antibodies against αv, α3 or α6 integrins had either no or only a marginal effect.43 Binding to those integrins was rather surprising, since some of them are not classical LM receptors. One hypothesis could be that the L4 domain of LMα5 represents a cryptic site, but whether and how it is released from or exposed on the intact molecule is not known.
LMα5 Can Trigger Either Stem Cell Renewal or Differentiation
Recently, there has been significant interest in developing defined feeder cell-free in vitro culture systems for the establishment and maintenance of undifferentiated mammalian ES cells. The goal is to identify appropriate substrates suitable for promoting self-renewal of embryonic stem cells (ES) without loss of pluripotency. Matrigel that contains LM-111 is the most common non-feeder cell coating used so far for human ES (hES) cell cultures. As the LMα5 chain contacts the inner cell mass of blastocysts, which is the natural origin of ES cells, some groups investigated whether LM-511 may be useful for culturing ES cells. The first attempts tested specific LM isoforms for their ability to serve as substrates for maintaining mouse ES cells in the absence of any differentiation inhibitors or feeder cells.44 Only when mouse ES cells were cultured on LM-511 were they able to self-renew for up to 31 passages, without undergoing differentiation. Under these conditions, LM-511 allowed expansion of pluripotent cells, as assessed by the expression of markers such as Oct4, Sox2 or Nanog, and they could contribute to various mouse tissues in chimeras upon blastocyst injection. The same investigators later used recombinant human LM-511 for the culture of hES cells. The hES cells exhibited more spreading behavior on LM-511 as compared with other LM isoforms or to Matrigel. The hES cells could be maintained on LM-511 for more than 20 passages, expressed hES cell markers (Oct4) and, most importantly, retained pluripotency as demonstrated by teratoma formation. Using function-blocking antibodies, they showed that the binding of hES cells to LM-511 is solely through integrin α6β1.45 In parallel, Hongisto and colleagues studied the characteristics of various types of hES cells feeder cells, and showed that the cells that supported the maintenance of hES pluripotency indeed produced LM-511 and expressed an appropriate set of LM-binding integrins (α3β1, α6β1 and α7β1).46
Regarding the fact that ECM is also important for cell differentiation, a new approach is to use LM-511 to help ES cells or induced pluripotent stem (iPS) cells to differentiate into a particular cell type under defined culture conditions. Using a synthesized basement membrane substratum containing LM-511 (derived from HEK293 cells stably expressing LM-511), differentiation of mouse ES or iPS cells into definitive endoderm and further to insulin-expressing pancreatic β-cells in vitro was achieved.47 When β1 integrin was knocked-down in differentiating ES cells, a significantly reduced proportion of pancreatic cells was observed, suggesting that the guiding signal from the LM-511-enriched matrix was transduced through β1 integrin. Similarly, the ability of this LM-511 substratum to support hepatic differentiation of both mouse and human ES cells was shown to involve integrin β1 and Akt signaling activation.48
These studies indicate that LM-511, alone or in combination with other molecules, plays a dual role in both the maintenance/renewal of ES cells and in their differentiation into particular cell lineages in vitro, depending upon the culture context.
LMα5 and Tissue Patterning during Development
The LMα5 chain is required during embryogenesis (Fig. 2), as shown by the embryonic lethality of the Lama5 knockout. LMα5 is present in virtually all basement membranes of early somite stage embryos and then becomes restricted to specific basement membranes as development proceeds, including those of the surface ectoderm and placental vasculature.4 LMα5 plays multiple roles in various processes of early embryogenesis: structural support, adhesion, stop signal; yet it is also important in cell renewal and/or differentiation.
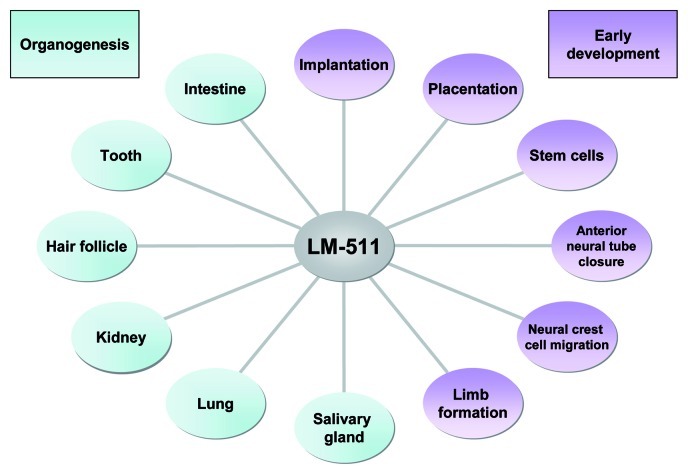
Figure 2. A diagrammatic summary of the diverse developmental processes in which LM-511 and the basement membranes that contain it are involved. Results from studies of Lama5 mutant mice, transgenic mice, and cells cultured on LMα5-containing substrates reveal roles in organogenesis (left) and early developmental events (right). See text for details.
LMα5 expression during implantation
Implantation of the mammalian embryo into the uterus requires changes in adhesive cell molecules. LM-511 and LM-111 are the major isoforms expressed in the embryo and the uterine decidua during implantation.49,50 In the uterus, LMα5 is strongly expressed in the decidualizing matrix of the stroma.49 In the implanting blastocyst, LMα5 is expressed only in the inner cell mass and the polar trophectoderm.50 During implantation, LMα5 promotes spreading and persistent migration, as assessed by culturing trophoblasts on LM-511 substrata.51 Although the roles of each LM chain appear to generally be distinct, it has been shown that LMα5 can partially compensate for the absence of LMα1 in embryonic basement membrane formation, and can promote embryonic ectoderm cavitation and parietal endoderm differentiation.50,52 Moreover, overexpression of LMα5 from a transgene in LMα1 null-embryos is able to rescue the phenotype until the initiation of gastrulation, but it fails to rescue Reichert’s membrane.50
LMα5 is involved in the establishment of the placenta
In LMα5 knockout mice, the placental labyrinth was clearly malformed, in contrast to Reichert’s membrane or yolk sac, two other extraembryonic tissues that were unaffected in Lama5 mutants. The placental labyrinth is composed of trophoblasts and endothelial cells that derive from the embryo. Normally, a basement membrane that is rich in LMα5 separates the endothelium (which forms the vessels through which the embryonic blood circulates) from the trophoblast layer. In Lama5 mutants, the labyrinth is significantly smaller and underdeveloped. The network of fetal blood vessels is present and associated with a basement membrane, but the complexity of vessel branching is greatly reduced. These defects reduce the surface area available for exchange of molecules between the fetal and maternal bloodstreams, which probably leads to placental insufficiency in mutants, and then to embryonic lethality. Additionally, an ultrastructural study of the mutant labyrinth showed that endothelial cells and trophoblasts become separated by a cell-free space due to detachment of the trophoblasts from the intervening basement membrane, which is discontinuous and of heterogeneous thickness. These data suggest that the LMα5 chain is necessary to maintain trophoblast adhesion to the basement membrane and to promote blood vessel branching in the placenta.4 Interestingly, Kikkawa and colleagues generated mice expressing chimeric LMα5/α1 chains with different LMα5 LG domains on the LMα5 null background. By this approach they could show that expression of chains containing the LMα5 LG1-LG2 tandem were able to rescue placental vascularization, probably through a binding site recognized by a trophoblast cell surface receptor.38
LMα5 defect leads to exencephaly
One of the most dramatic developmental defects of Lama5-null mice is exencephaly. This phenotype appears in approximately 60% of mutant fetuses.4 Exencephaly is caused by a failure in anterior neural tube closure. This results in an “inside out” neuroepithelium, meaning that the proliferative cells are found on the outer surface. In mutant mice, the ectodermal basement membrane was thin and patchy at the peak of the neural folds. The authors concluded that weakness of the basement membrane in this region decreased the amount of lateral forces the ectoderm could generate on the neural folds, thereby leading to sporadic failure of cranial neural tube closure.4
LMα5 influences neural crest cell migration
In addition to the exencephaly, LMα5 null-mice exhibit craniofacial abnormalities that can result from abnormal neural crest migration. Separation of neural crest cells from the dorsal neural epithelium and their migration involve alterations in cell-cell adhesion but also in cell-matrix interactions. While it was already shown that ECM molecules are involved in neural crest cell migration,53 investigating the function of LMα5 in this embryonic process was undertaken by Coles and colleagues.54 Although changes in LMα5 expression levels precede neural crest cell migration,55 LMα5 appears not to be implicated in the initiation of migration, since no differences were observed between wild type and Lama5-null embryos. Yet, in LMα5 null-mice, an expansion in neural crest migratory streams was observed, suggesting that LMα5 normally restricts neural crest migration pathways. This is corroborated by in vitro data showing that a LMα5-containing substratum was able to reduce cranial neural crest cell migration.54 Examination of Lama5 transcripts in chick and mouse to identify the migratory paths showed that these transcripts had minimal overlap with migratory neural crest cells. These data support the hypothesis that LMα5 may act as a non-permissive or inhibitory signal that restricts and/or guides neural crest cells to defined routes.
Limb defects occur in the absence of LMα5
Syndactyly (failed digit separation) is one of the most severe and visible defects observed in the LMα5 null-mice. This defect is visible at E12.5, as soon as septation normally begins. Until E9, the ectodermal and dermal layers appear similar in mutants and controls. Like the cranial defect, syndactyly is associated with localized structural deficiencies in regions of basement membrane that are likely to be subjected to greater mechanical stress than are neighboring regions. In mutants, the outgrowth that normally occurs in the distal portion of the limb bud (under the direction of the apical ectodermal ridge) may stress the LMα5 deficient basement membrane, leading in turn to rupture of the overlying ectoderm and extrusion of mesenchymal cells onto the outer surface of the distal limb. This is a mechanical explanation for the defect, but one cannot exclude the deregulation of signaling pathways linked to the absence of LMα5.4
Implications of Laminin α5 in Organogenesis
Although Lama5 expression is regulated both temporally and spatially during development, its expression can still be described as widespread, as LMα5 is found in many fetal and adult tissues including salivary gland, lung, kidney, skin, tooth and intestine (Fig. 2). Several defects that will be summarized here were observed in internal organs of Lama5 null-embryos,4 arguing for a major role for LMα5-containing molecules during organogenesis. Additional studies suggest that LMα5 is important in the maintenance of homeostasis in mature tissues.
LMα5 controls lobular ramification of the submandibular gland
Branching morphogenesis is a key developmental process that leads to organ growth as well as increased surface area for efficient gas or fluid exchange across an epithelium in organs such as the lung, kidney (ureteric bud), mammary gland and salivary gland. One of the classic and well-described systems for studying branching morphogenesis is the mouse embryonic submandibular salivary gland, which develops via repetitive bifurcations of a cluster of epithelial cells that leads to formation of a tree-like duct system. At the ends of the ducts, buds differentiate into acini that contain well-polarized secretory cells surrounded by myo-epithelial cells. At the beginning of gland formation (at E13), LMα5 is found in the basement membrane underlying the acini and the duct, when ducts and buds are emerging.56
The first strategy used to test if LMα5 could play a role in development was to screen LMα5 LG domain peptides for their ability to interfere with branching morphogenesis of cultured submandibular glands. These in vitro experiments suggested that one peptide derived from the LG4 domain (A5G77) was crucial, as it inhibited epithelial morphogenesis, although, surprisingly, a continuous intact basement membrane was still present. Peptides corresponding to the A5G77 sequence but derived from other LMα chains (α1, α3 or α4) did not inhibit epithelial branching, suggesting for the specificity of action of the LMα5 peptide.56 In a different strategy, examination of Lama5−/− mice revealed that the submandibular glands were developmentally delayed at E13; the first round of cleft formation and branching had not occurred, although the end bud was enlarged, and the sublingual salivary gland had not formed.57 Finally, addition of Lama5-siRNA to wild-type submandibular gland explants in culture decreases branching morphogenesis, clearly indicating that LMα5 controls the lobular ramification of the salivary gland.57
LMα5 is required for complete lung lobar septation but not for lung branching morphogenesis
A major role for LM in lung branching morphogenesis was first identified by Schuger and colleagues, who showed that polyclonal antibodies to LM (recognizing several isoforms, including LM-511) inhibit lung morphogenesis when added to organotypic cultures of mouse embryonic lung.58 In situ hybridization revealed the presence of LAMA5 transcripts throughout human lung development, with preferential expression in the alveolar type II cells, similar to mouse lung.7,8 Antibodies to LMα5 stain nearly all basement membranes of the lung, including alveolar and visceral pleural basement membranes, showing that the expression of the protein is continuous from early lung development through adult life.8 In LMα5 null-mice, there is incomplete lobar septation and absence of the visceral pleura basement membrane, whereas lung branching morphogenesis and vasculogenesis are normal.59 In contrast, in other branching organs such as salivary glands or kidney, alterations of morphogenesis were reported.57,60 One explanation offered is that the normal branching morphogenesis observed in the Lama5-null lung is probably due to compensation linked to ectopic deposition of LMα4 in the airway basement membrane.59 The defect in lobar septation is clearly linked to the absence of LMα5 and subsequently to the lack of the pleural basement membrane, as LMα5 was found to be the only LMα chain present in the normal visceral pleura. As LMα5 null-mice are embryonic lethal, an inducible lung epithelial cell-specific LMα5 deletion was generated.61 Using the human surfactant protein C promoter, the reverse tetracycline transactivator, and the Cre/LoxP system, mice lacking LMα5 in lung epithelial cells survived to birth. But more significant defects in lung morphogenesis were observed, including dilated airspaces, perturbed epithelial cell differentiation (reduction or even absence of alveolar cells) and decreased proliferation. Expression of α3 integrin was increased, raising the hypothesis that epithelial cells may attempt to compensate for the lack of interaction with its ligand (a situation also observed in LMα5 null-intestine34). Finally the Lama5-deficient lungs also showed a decrease in VEGF production (normally expressed by alveolar cells), which might explain the vascular defects observed in the mutants.61
LMα5 in kidney development and function
The kidney is an organ rich in basement membranes that is particularly dependent on LMα5 for proper development and function due to its deposition in practically all kidney basement membranes. In Lama5 null-embryos, there is a low incidence of renal agenesis, usually unilateral, for unknown reasons.60 Although the kidneys that do form in these mutants are of practically normal size, when grown in organ culture there is a defect in ureteric bud branching morphogenesis, suggesting that LM-511 in the early ureteric bud basement membrane could be important for providing morphogenetic signals, as discussed above for salivary gland. Although renal agenesis is a partially penetrant phenotype in Lama5 null-embryos, kidneys that do form exhibit failed vascularization of glomeruli, the part of the nephron that filters the blood.60 Thus, Lama5 null-kidneys cannot filter the blood or produce urine and so are nonfunctional. Ultrastructural analysis of the developing glomerulus shows that the glomerular basement membrane (GBM), a normal component of the filtration barrier, breaks down in the absence of LMα5. This leads to disorganization of the three cell types that are necessary to form the glomerular capillaries: podocytes, endothelial cells and mesangial cells. Results from several studies suggest that the LG domain of LMα5 in the GBM is normally bound by α3β1 integrin on podocytes and mesangial cells.37,38 Exactly how endothelial cells adhere to the GBM is not known.
LMα5 is not only important for development of the kidney and the glomerular vasculature; it is also a crucial component of the glomerular filtration barrier to plasma albumin, due to its presence in the mature GBM as part of the LM-521 trimer. Replacement of the α5LG3–5 domain with that of α1 results in leakage of albumin across the GBM,38 as does podocyte specific knockout of Lama5 using the Cre/loxP system.62 Mutations that affect expression of laminin β2 in humans and mice also cause leakage of plasma albumin, likely due to reduced overall levels of LM-521 trimers in the GBM that could lead to increased porosity.63-65
LMα5 promotes hair follicle development
The skin contains hair follicles formed during embryogenesis as appendages of the epidermis. As in other organs, epithelial-mesenchymal crosstalk is required to complete morphogenesis and differentiation, and basement membrane molecules are possible mediators involved in this communication.66 LM-511 (assessed by immunostaining with anti-α5, anti-β1 and γ1) was prominently expressed in the specialized basement membrane of elongating hair germs, which lacked LMα1, β2 and LM-332.67 The first evidence showing that LM-511 plays an important role in hair development was provided by injection of anti-human LMα5/LM-511 blocking antibody into nude mice bearing embryonic human scalp skin xenografts. The circulating antibody targeted the xenografts and produced alopecia (loss of hair).67 As a next step, the authors examined embryonic Lama5 null mouse skin and showed that it contained fewer hair germs. Upon further development of E16.5 skin after grafting, it was shown that hair germs of Lama5−/− skin progressively underwent complete regression. Interestingly, this phenotype was restored by applying exogenous LM-511 to mutant skin prior to grafting.67-69 Similarly, in mature hair follicle cultures, addition of human LM-511/521 enhanced hair growth, suggesting that LM-511 is crucial not only for initial hair morphogenesis, but also for hair growth in the adult. Given that, the authors hypothesized that the gradual loss of LM-511 from the epidermal basement membrane with aging70 could be responsible in part for age-related hair loss.71
LMα5 has been described to be a potent adhesive ligand for keratinocytes through binding to integrins α3β1 and α6β4. LMα5 also regulates keratinocyte proliferation and migration, suggesting a role in normal epithelial replacement, wound healing and tumor growth.70 A microarray analysis designed to discover genes expressed by human dermal pericytes that could potentially promote epidermal regeneration identified the LAMA5 gene. Moreover, LMα5 deposition into the dermal space was shown to derive from pericytes. Hence, it was concluded that pericytes compensate for the inherently decreased levels of LMα5 synthetized by differentiating keratinocytes, thereby promoting epidermal regeneration.72
LMα5 promotes cell proliferation in the developing tooth
Tooth morphogenesis in mice starts at E11.5, when the oral ectoderm invaginates into the underlying mesenchyme. This process results in the formation of epithelial tooth buds. A homogeneous strong expression of Lama5 transcripts, confined to the epithelia but not to the dental mesenchyme, was seen throughout several stages of tooth development, although its expression was downregulated as development proceeded. The corresponding LMα5 protein is the major LM in the tooth basement membrane and showed an even distribution.73 Lama5-null mice presented with basement membrane disorganization and corresponding defects in tooth germ development. Defects include smaller tooth germs with defective cusp formation and impaired polarity of the inner dental epithelium. Proliferation of the dental epithelium was reduced, and consistent with this in vivo finding, α5-containing LM trimers (-511/-521) were able to promote proliferation of primary cultures of dental epithelial cells.74
Absence of LMα5 in the intestine leads to morphological defects
The development and homeostasis of the intestine are mainly regulated by interactions between cells and the extracellular matrix. The deficiency of LMα5 in the intestine leads to morphological and biochemical alterations in the smooth muscle layer, whereas no obvious morphological defects were observed in villous outgrowth and crypt formation, probably due to a compensatory mechanism involving the overproduction of other LMα chains (i.e., overexpression of LMα4 and ectopic expression of LMα2 in the epithelial basement membrane). However, excessive folding of the intestinal loops was observed in LMα5 null-mice that could lead to fusion of the muscular layers in the most severe cases.34 Due to the embryonic lethality of LMα5 null-mice, two strategies have been used to allow generation of intestines at later stages. The first was the engrafting of LMα5−/− intestinal fragments into the subcutaneous space of adult nude mice.34 Unfortunately, here again a compensatory phenomenon occurred, as LM α2, α4, and α1 were deposited into the LMα5-deficient basement membrane. The second strategy consisted of a transgenic rescue of the LMα5 null mice by crossing them with transgenic mice expressing a full-length mouse LMα5 transgene. The LMα5 transgene was widely expressed in several organs but was silenced in the intestine after embryogenesis.75 Interestingly, the diminution of LMα5 in the intestine at later developmental stages led to drastic morphological changes in the mature intestinal architecture with many similarities to a colon-like phenotype. These changes included disruptions of normal crypt-villus architecture with fusion of villi and an increase in the number of goblet cells.75,76
Cellular Origins of the Laminin α5 Chain
Basement membrane assembly in many cases requires intimate contact of both epithelial cells and stromal cells, as exemplified in the intestine. In contrast to the original notion that the basement membrane was strictly of epithelial origin, it has become clear that some molecules, including LM, can be of mesenchymal origin.76 In the intestine, the origin of the LMα5 chain was studied during development by creating hybrid intestines consisting of mouse and chick endodermal or mesenchymal tissues. Deposition of α5 in well-developed hybrid intestines was then assayed by mouse-specific LMα5 antibodies. This experiment demonstrated that both epithelial and mesenchymal cells synthesize and deposit the LMα5 chain into the basement membrane. The LMα5 chain produced by each cell type depended on the developmental stage and location along the crypt-villus axis; in the crypt region (where cell proliferation occurs), LMα5 is predominantly produced by the mesenchyme, and in the villus (where cells migrate and differentiate) it is predominantly produced by the endoderm (epithelium).6 In LMα5 knockout mice, a compensation process takes place in which α2 and α4 laminins are deposited precociously or ectopically by mesenchymal cells.6,76
Similar to the intestine, there is a mesenchymal contribution to the skin’s epidermal basement membrane.77 Fetal bovine keratinocytes cultured on collagen gels without fibroblasts were unable to produce an ultrastructurally recognizable basement membrane, whereas the addition of dermal fibroblasts facilitated basement membrane formation. When dual species cultures of bovine keratinocytes and human fibroblasts were analyzed by immunofluorescence with human specific antibodies, dermal fibroblasts were shown to synthesize and deposit LMα5 in a linear manner into the basement membrane zone.77 Therefore it is likely that the hypothesized necessity of heterotypic cell/cell interactions for basement membrane formation will be applicable to other organs. This is exemplified by the fact that there is an upregulation of Lama5 mRNA levels in endothelial/pericyte cocultures as compared with endothelial cells or pericytes cultured alone.78
As discussed above, the kidney glomerular basement membrane is situated between endothelial cells and podocytes, also known as visceral epithelial cells. It is clear that both podocytes and endothelial cells synthesize the LMα5 in the GBM, and that as long as one of the two cell types is able to synthesize it, then GBM structure is maintained and vascularization can occur.79,80 Similarly, in the placental labyrinth, the vascular basement membranes containing LMα5 lie between the fetal endothelial cells and the trophoblasts. Although both trophoblasts and endothelial cells produce LMα5 and contribute to this basement membrane, expression from only one of the two cell types is sufficient for proper placental vascularization.80
LMα5 and Signaling Pathways
In addition to its mechanical role in maintaining the basement membrane that provides structural support to cells and tissues, LMα5 is also involved in signaling pathways that mediate instructions for differentiation, migration or proliferation. Here we will summarize what is currently known about this subject. Through the study of developing organs, three major pathways have been correlated with LMα5 activities: the Sonic hedgehog pathway, the Wnt pathway and the PI3Kinase/Akt pathway (Fig. 3).
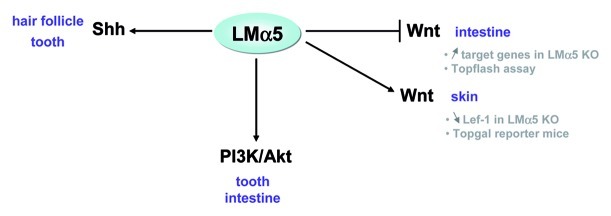
Figure 3. The LMα5 chain modulates signaling pathways. Three important signaling pathways have been described so far to be modulated by LMα5, as shown by studies of LMα5-null mice, by microarray experiments, or in reporter assays (in vitro or in mice). These include Shh, Wnt/β-catenin and PI3K/Akt pathways in the indicated tissues.
Sonic hedgehog (Shh) signaling
The Shh pathway plays a role in multiple developmental processes by mediating interactions between stromal and epithelial cells. The Shh pathway has three effectors in mammalian cells: the Glioblastoma (Gli) transcription factors Gli1, Gli2 and Gli3.81,82 The first direct demonstration of a link between LMα5 and the Shh pathway was provided by Li and colleagues in hair follicle development; they showed by in situ hybridization that Shh and Gli1 were not expressed in the embryonic Lama5-null skin, either before or after grafting, in contrast to controls.67 In addition, grafting of LMα5 null skin incubated with Shh-expressing adenovirus showed that the exogenous Shh can rescue follicle regression.68 Likewise, in teeth, the expression level of Shh mRNA was reduced in the enamel knot region of the dental epithelium of LMα5-deficient mice, which correlated with a reduction of cell proliferation.74 Interestingly, the abnormal lung lobar septation observed in the Lama5-null mice resembled the defects observed in Gli2-null mice.83 These experiments argue for a functional link between LMα5 and the Shh pathway in at least two developing organs.
The Wnt pathway
During hair development, it was suggested that Shh is not the only factor influenced by LM-511, as shown by the existence of a signaling loop between Shh and noggin coupled with Wnt/β-catenin activation.68 Gao and colleagues showed that secreted LM-511, after interacting with β1 integrin, leads to an increase of Shh; then the combined action of PDGF and Shh signaling promotes expression of noggin, thus inhibiting BMP signaling. This inhibition leads to Lef-1 expression, which, coupled with the Wnt/β-catenin-induced activation, drives further Shh expression.68 The relationship between the Wnt pathway and LM-511, although indirect, has been confirmed by the decreased expression of the downstream Wnt transcription factor Lef-1 in the embryonic LMα5 null-skin.68 Furthermore, when LMα5 null-mice were crossed with mice harboring Topgal, a β-galactosidase transgene under control of a Lef/TCF and β-catenin-inducible promoter, the skin showed a loss of Topgal activation.68 Recently, a microarray analysis comparing deregulated genes in LMα5 null vs. wild-type intestines revealed genes that belong to the Wnt pathway, among which the Fzd2 Wnt receptor and some Wnt targets such as Pitx2, Myod1 or Msx1 were upregulated in mutants. Activation of Wnt signaling in the absence of LMα5 suggests that Wnt signaling is repressed in a Lama5-expressing tissue. Indeed, in vitro TopFlash experiments showed inhibition of Wnt signaling when intestinal epithelial cells were seeded on LM-511 but not on LM-111.5 Mechanisms by which LMα5 functions to regulate the Wnt pathway in the intestine are not known, although one can argue for the involvement of cellular receptors as some, such as β4 integrin and Lutheran, were found to be altered.5
The PI3Kinase/Akt pathway
The PI3Kinase/Akt pathway is known to be interconnected with the Wnt pathway through the phosphorylation of its effecting factor Akt, which can stimulate β-catenin.84 The PI3Kinase/Akt pathway regulates, among other processes, cell growth and survival. The link between this pathway and LMα5 was first demonstrated by an experiment in which dental epithelium was allowed to grow on a LM-511/521 substratum with wortmannin, an inhibitor of PI3Kinase. Under these conditions, filopodia formation was inhibited.74 Furthermore, in the intestine, expression of genes in this pathway was altered in the absence of LMα5.5 It was then shown that Akt was phosphorylated in vitro when epithelial intestinal cells were seeded onto a LM-511 substratum. Through this pathway, LMα5 stimulated cell spreading and survival, as assessed by experiments in which cells were seeded onto LM-511 with or without wortmannin.5 Similarly, in vitro data provided a mechanistic link between LM-511/LM-521 and cell survival, as cell adhesion to the substratum, via the α3β1 integrin, was shown to trigger phosphorylation of p130cas, leading to activation of downstream cascades involving PI3K/Akt that prevents apoptosis.85
Conclusion and Perspectives
LMα5 is widely expressed in the organism and provides both structural and functional support for interactions between epithelium and mesenchyme.76 It has been shown that LMα5 transcripts, but not protein, decrease with aging, suggesting a great stability of this molecule within the basement membrane once organogenesis is complete.6 Moreover, LMα5 is deposited by both epithelial and mesenchymal cells, and the origin of this deposition varies throughout embryonic and perinatal life, at least in the intestine.6,76 The lack of this LM chain leads to multiple defects that can sometimes be rescued by compensatory mechanisms with secretion and deposition of other LMα chains, as observed in the intestine for example.34 As LMα5 is implicated in divergent physiological processes such as adhesion, migration, proliferation and differentiation, one can understand why distinct signaling pathways can be engaged, in particular those employed during morphogenesis and homeostasis of mature organs. At this time, little is known about the exact mechanisms by which LMα5 regulates such signaling pathways, but there are likely to be multiple cellular receptors involved. Future research will help to further clarify the role of LMα5 in pathological processes, where signals between epithelium and mesenchyme/fibroblasts are often disrupted. Uncontrolled expression of this chain could also lead to pathologies. Finally, increasing interest in LM−511 is illustrated by its use as an efficient substratum for adhesion and self-renewal of embryonic stem cells.
Acknowledgments
The authors sincerely thank O. Lefebvre and Y. Kikkawa for scientific interactions, and C. Arnold and A. Klein for their technical assistance throughout the years. The authors’ recent work has been supported by grants from the Institute National Contre le Cancer (INCa), ANR, ARC, Oncosuisse, Contrat d`interface with the Hautepierre Hospital (to G.O.), the Ligue Nationale Contre le Cancer (CCIR-GE to P.S.-A.), Strasbourg University, INSERM and the US National Institutes of Health (R01DK078314 and R01GM060432, to J.H.M.).
Glossary
Abbreviations:
- ECM
extracellular matrix
- LM
laminin
- GBM
glomerular basement membrane
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22236
References
- 1.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–68. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 2.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 4.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritié L, Spenlé C, Lacroute J, Bolcato-Bellemin AL, Lefebvre O, Bole-Feysot C, et al. Abnormal Wnt and PI3Kinase signaling in the malformed intestine of lama5 deficient mice. PLoS One. 2012;7:e37710. doi: 10.1371/journal.pone.0037710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefebvre O, Sorokin L, Kedinger M, Simon-Assmann P. Developmental expression and cellular origin of the laminin alpha2, alpha4, and alpha5 chains in the intestine. Dev Biol. 1999;210:135–50. doi: 10.1006/dbio.1999.9270. [DOI] [PubMed] [Google Scholar]
- 7.Pierce RA, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Sanes JR, et al. Expression of laminin alpha3, alpha4, and alpha5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol. 1998;19:237–44. doi: 10.1165/ajrcmb.19.2.3087. [DOI] [PubMed] [Google Scholar]
- 8.Pierce RA, Griffin GL, Miner JH, Senior RM. Expression patterns of laminin alpha1 and alpha5 in human lung during development. Am J Respir Cell Mol Biol. 2000;23:742–7. doi: 10.1165/ajrcmb.23.6.4202. [DOI] [PubMed] [Google Scholar]
- 9.Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, et al. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–8. doi: 10.1074/jbc.M111228200. [DOI] [PubMed] [Google Scholar]
- 10.Urbano JM, Torgler CN, Molnar C, Tepass U, López-Varea A, Brown NH, et al. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–76. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahey B, Degnan BM. Origin and evolution of laminin gene family diversity. Mol Biol Evol. 2012;29:1823–36. doi: 10.1093/molbev/mss060. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald PR, Lustig A, Steinmetz MO, Kammerer RA. Laminin chain assembly is regulated by specific coiled-coil interactions. J Struct Biol. 2010;170:398–405. doi: 10.1016/j.jsb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siler U, Rousselle P, Müller CA, Klein G. Laminin gamma2 chain as a stromal cell marker of the human bone marrow microenvironment. Br J Haematol. 2002;119:212–20. doi: 10.1046/j.1365-2141.2002.03800.x. [DOI] [PubMed] [Google Scholar]
- 14.Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–27. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Garbe JH, Göhring W, Mann K, Timpl R, Sasaki T. Complete sequence, recombinant analysis and binding to laminins and sulphated ligands of the N-terminal domains of laminin alpha3B and alpha5 chains. Biochem J. 2002;362:213–21. doi: 10.1042/0264-6021:3620213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, et al. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem. 2012;287:18700–9. doi: 10.1074/jbc.M111.336073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain SA, Carafoli F, Hohenester E. Determinants of laminin polymerization revealed by the structure of the α5 chain amino-terminal region. EMBO Rep. 2011;12:276–82. doi: 10.1038/embor.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki N, Yokoyama F, Nomizu M. Functional sites in the laminin alpha chains. Connect Tissue Res. 2005;46:142–52. doi: 10.1080/03008200591008527. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113:869–76. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 23.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–68. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- 25.Carmignac V, Durbeej M. Cell-matrix interactions in muscle disease. J Pathol. 2012;226:200–18. doi: 10.1002/path.3020. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu H, Hosokawa H, Ninomiya H, Miner JH, Masaki T. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J Biol Chem. 1999;274:11995–2000. doi: 10.1074/jbc.274.17.11995. [DOI] [PubMed] [Google Scholar]
- 27.Ferletta M, Kikkawa Y, Yu H, Talts JF, Durbeej M, Sonnenberg A, et al. Opposing roles of integrin alpha6Abeta1 and dystroglycan in laminin-mediated extracellular signal-regulated kinase activation. Mol Biol Cell. 2003;14:2088–103. doi: 10.1091/mbc.E03-01-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–37. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Kurpakus-Wheater M. Laminin alpha5 chain adhesion and signaling in conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:2615–21. [PubMed] [Google Scholar]
- 30.Eyler CE, Telen MJ. The Lutheran glycoprotein: a multifunctional adhesion receptor. Transfusion. 2006;46:668–77. doi: 10.1111/j.1537-2995.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 31.Kikkawa Y, Miner JH. Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues. Connect Tissue Res. 2005;46:193–9. doi: 10.1080/03008200500344074. [DOI] [PubMed] [Google Scholar]
- 32.Kikkawa Y, Moulson CL, Virtanen I, Miner JH. Identification of the binding site for the Lutheran blood group glycoprotein on laminin alpha 5 through expression of chimeric laminin chains in vivo. J Biol Chem. 2002;277:44864–9. doi: 10.1074/jbc.M208731200. [DOI] [PubMed] [Google Scholar]
- 33.Moulson CL, Li C, Miner JH. Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin alpha5 in vivo. Dev Dyn. 2001;222:101–14. doi: 10.1002/dvdy.1169. [DOI] [PubMed] [Google Scholar]
- 34.Bolcato-Bellemin AL, Lefebvre O, Arnold C, Sorokin L, Miner JH, Kedinger M, et al. Laminin alpha5 chain is required for intestinal smooth muscle development. Dev Biol. 2003;260:376–90. doi: 10.1016/S0012-1606(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 35.Rahuel C, Filipe A, Ritie L, El Nemer W, Patey-Mariaud N, Eladari D, et al. Genetic inactivation of the laminin alpha5 chain receptor Lu/BCAM leads to kidney and intestinal abnormalities in the mouse. Am J Physiol Renal Physiol. 2008;294:F393–406. doi: 10.1152/ajprenal.00315.2007. [DOI] [PubMed] [Google Scholar]
- 36.Colin Y, Rahuel C, Wautier MP, El Nemer W, Filipe A, Cartron JP, et al. Red cell and endothelial Lu/BCAM beyond sickle cell disease. Transfus Clin Biol. 2008;15:402–5. doi: 10.1016/j.tracli.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–96. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kikkawa Y, Miner JH. Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function. Dev Biol. 2006;296:265–77. doi: 10.1016/j.ydbio.2006.04.463. [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J. 2003;371:289–99. doi: 10.1042/BJ20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, et al. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–54. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 41.Kikkawa Y, Sasaki T, Nguyen MT, Nomizu M, Mitaka T, Miner JH. The LG1-3 tandem of laminin alpha5 harbors the binding sites of Lutheran/basal cell adhesion molecule and alpha3beta1/alpha6beta1 integrins. J Biol Chem. 2007;282:14853–60. doi: 10.1074/jbc.M611706200. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen PK, Yamada Y. Identification of cell-binding sites on the Laminin alpha 5 N-terminal domain by site-directed mutagenesis. J Biol Chem. 2001;276:10906–12. doi: 10.1074/jbc.M008743200. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki T, Timpl R. Domain IVa of laminin alpha5 chain is cell-adhesive and binds beta1 and alphaVbeta3 integrins through Arg-Gly-Asp. FEBS Lett. 2001;509:181–5. doi: 10.1016/S0014-5793(01)03167-2. [DOI] [PubMed] [Google Scholar]
- 44.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26:2800–9. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 45.Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 46.Hongisto H, Vuoristo S, Mikhailova A, Suuronen R, Virtanen I, Otonkoski T, et al. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. 2012;8:97–108. doi: 10.1016/j.scr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi Y, Shiraki N, Yamane K, Qin Z, Mochitate K, Araki K, et al. Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages. J Cell Sci. 2010;123:2733–42. doi: 10.1242/jcs.066886. [DOI] [PubMed] [Google Scholar]
- 48.Shiraki N, Yamazoe T, Qin Z, Ohgomori K, Mochitate K, Kume K, et al. Efficient differentiation of embryonic stem cells into hepatic cells in vitro using a feeder-free basement membrane substratum. PLoS One. 2011;6:e24228. doi: 10.1371/journal.pone.0024228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klaffky E, Williams R, Yao CC, Ziober B, Kramer R, Sutherland A. Trophoblast-specific expression and function of the integrin alpha 7 subunit in the peri-implantation mouse embryo. Dev Biol. 2001;239:161–75. doi: 10.1006/dbio.2001.0404. [DOI] [PubMed] [Google Scholar]
- 50.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–56. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 51.Klaffky EJ, Gonzáles IM, Sutherland AE. Trophoblast cells exhibit differential responses to laminin isoforms. Dev Biol. 2006;292:277–89. doi: 10.1016/j.ydbio.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 52.Alpy F, Jivkov I, Sorokin L, Klein A, Arnold C, Huss Y, et al. Generation of a conditionally null allele of the laminin alpha1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- 53.Duband JL, Rocher S, Yamada KM, Thiery JP. Interactions of migrating neural crest cells with fibronectin. Prog Clin Biol Res. 1986;226:127–39. [PubMed] [Google Scholar]
- 54.Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Dev Biol. 2006;289:218–28. doi: 10.1016/j.ydbio.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 55.Gammill LS, Bronner-Fraser M. Genomic analysis of neural crest induction. Development. 2002;129:5731–41. doi: 10.1242/dev.00175. [DOI] [PubMed] [Google Scholar]
- 56.Kadoya Y, Mochizuki M, Nomizu M, Sorokin L, Yamashina S. Role for laminin-alpha5 chain LG4 module in epithelial branching morphogenesis. Dev Biol. 2003;263:153–64. doi: 10.1016/S0012-1606(03)00446-9. [DOI] [PubMed] [Google Scholar]
- 57.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, et al. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuger L, O’Shea S, Rheinheimer J, Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev Biol. 1990;137:26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol. 2002;246:231–44. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- 60.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–89. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 62.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol. 2010;21:579–86. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–9. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YM, Kikkawa Y, Miner JH. A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J Am Soc Nephrol. 2011;22:849–58. doi: 10.1681/ASN.2010060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–58. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–10. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22:2111–24. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeRouen MC, Zhen H, Tan SH, Williams S, Marinkovich MP, Oro AE. Laminin-511 and integrin beta-1 in hair follicle development and basal cell carcinoma formation. BMC Dev Biol. 2010;10:112. doi: 10.1186/1471-213X-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pouliot N, Saunders NA, Kaur P. Laminin 10/11: an alternative adhesive ligand for epidermal keratinocytes with a functional role in promoting proliferation and migration. Exp Dermatol. 2002;11:387–97. doi: 10.1034/j.1600-0625.2002.110501.x. [DOI] [PubMed] [Google Scholar]
- 71.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008;17:473–80. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 72.Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salmivirta K, Sorokin LM, Ekblom P. Differential expression of laminin alpha chains during murine tooth development. Dev Dyn. 1997;210:206–15. doi: 10.1002/(SICI)1097-0177(199711)210:3<206::AID-AJA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 74.Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, et al. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;281:5008–16. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- 75.Mahoney ZX, Stappenbeck TS, Miner JH. Laminin alpha 5 influences the architecture of the mouse small intestine mucosa. J Cell Sci. 2008;121:2493–502. doi: 10.1242/jcs.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simon-Assmann P, Spenle C, Lefebvre O, Kedinger M. The role of the basement membrane as a modulator of intestinal epithelial-mesenchymal interactions. Prog Mol Biol Transl Sci. 2010;96:175–206. doi: 10.1016/B978-0-12-381280-3.00008-7. [DOI] [PubMed] [Google Scholar]
- 77.Marinkovich MP, Keene DR, Rimberg CS, Burgeson RE. Cellular origin of the dermal-epidermal basement membrane. Dev Dyn. 1993;197:255–67. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- 78.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abrahamson DR, St John PL, Isom K, Robert B, Miner JH. Partial rescue of glomerular laminin alpha5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 2007;18:2285–93. doi: 10.1681/ASN.2007020207. [DOI] [PubMed] [Google Scholar]
- 80.Kim ST, Adair-Kirk TL, Senior RM, Miner JH. Functional consequences of cell type-restricted expression of laminin alpha5 in mouse placental labyrinth and kidney glomerular capillaries. PLoS One. 2012;7:e41348. doi: 10.1371/journal.pone.0041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chari NS, McDonnell TJ. The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol. 2007;14:344–52. doi: 10.1097/PAP.0b013e3180ca8a1d. [DOI] [PubMed] [Google Scholar]
- 82.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 83.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 84.Anderson EC, Wong MH. Caught in the Akt: regulation of Wnt signaling in the intestine. Gastroenterology. 2010;139:718–22. doi: 10.1053/j.gastro.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu J, Fujibayashi A, Yamada KM, Sekiguchi K. Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J Biol Chem. 2002;277:19922–8. doi: 10.1074/jbc.M200383200. [DOI] [PubMed] [Google Scholar]


