Abstract
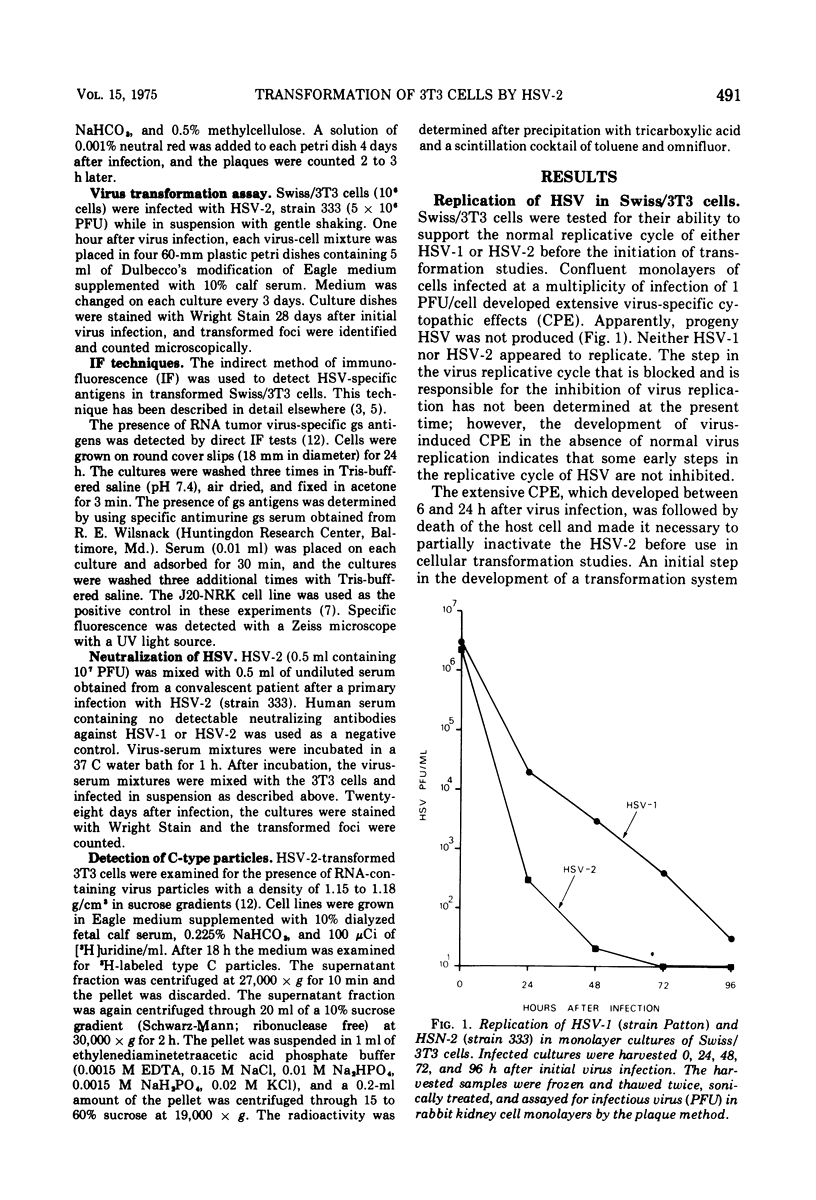
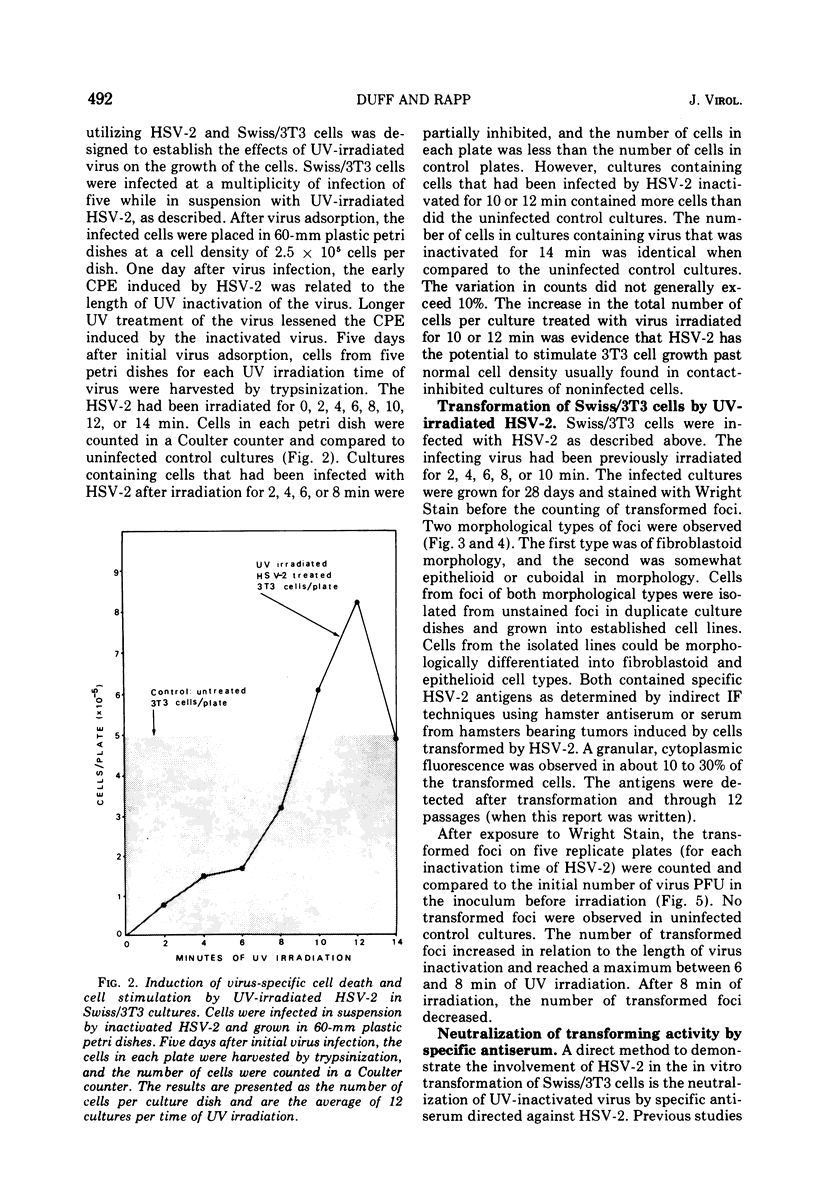
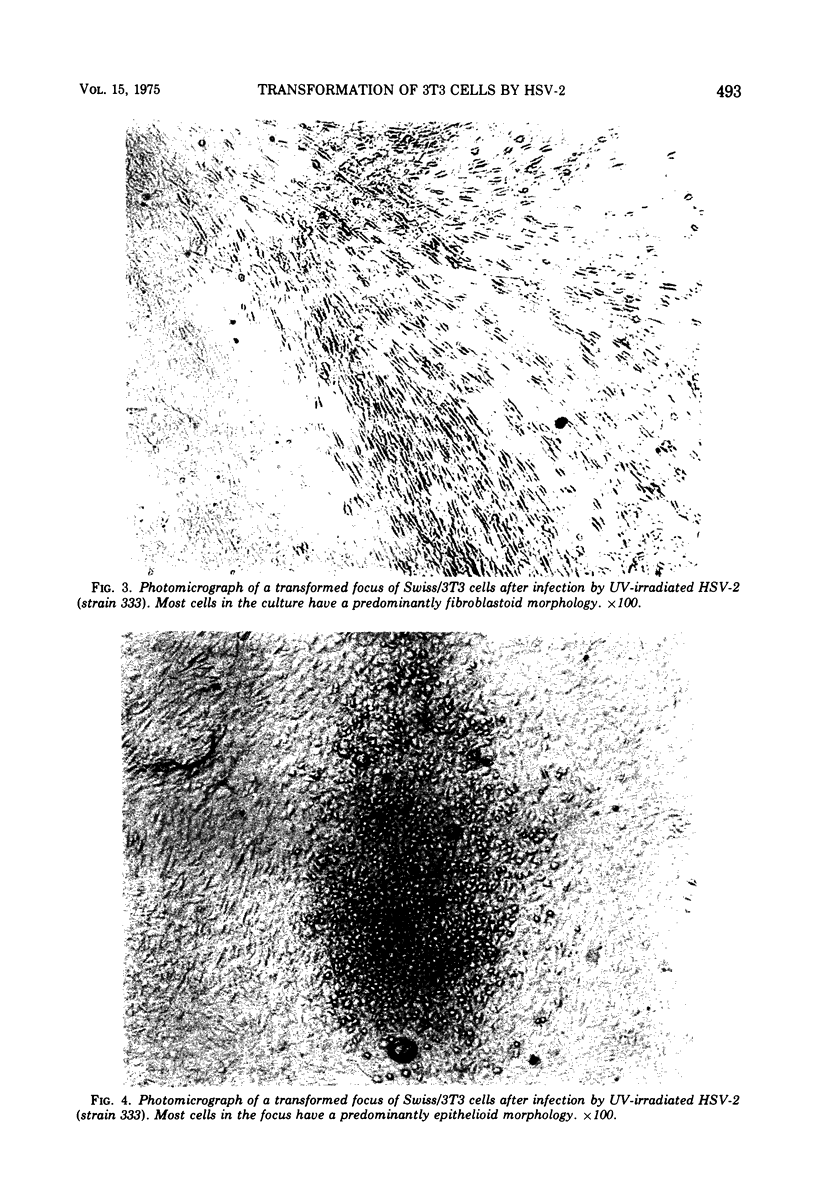
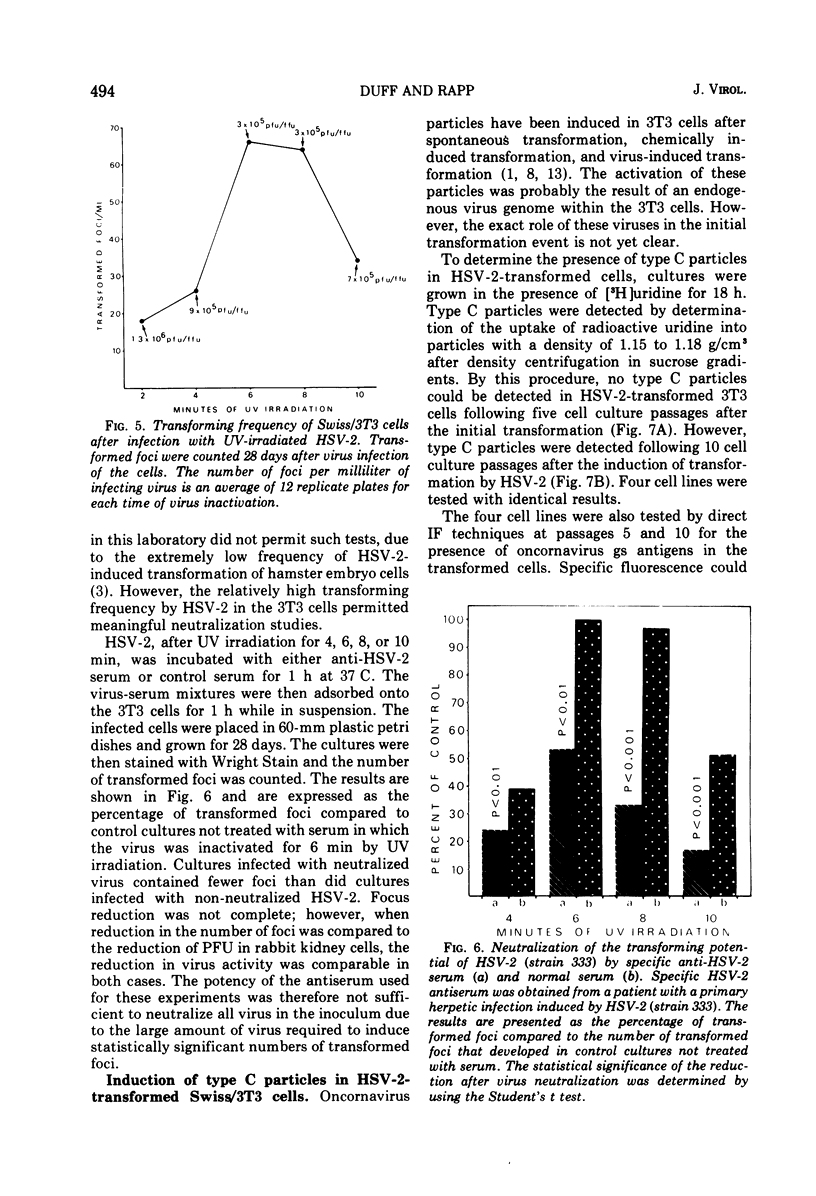
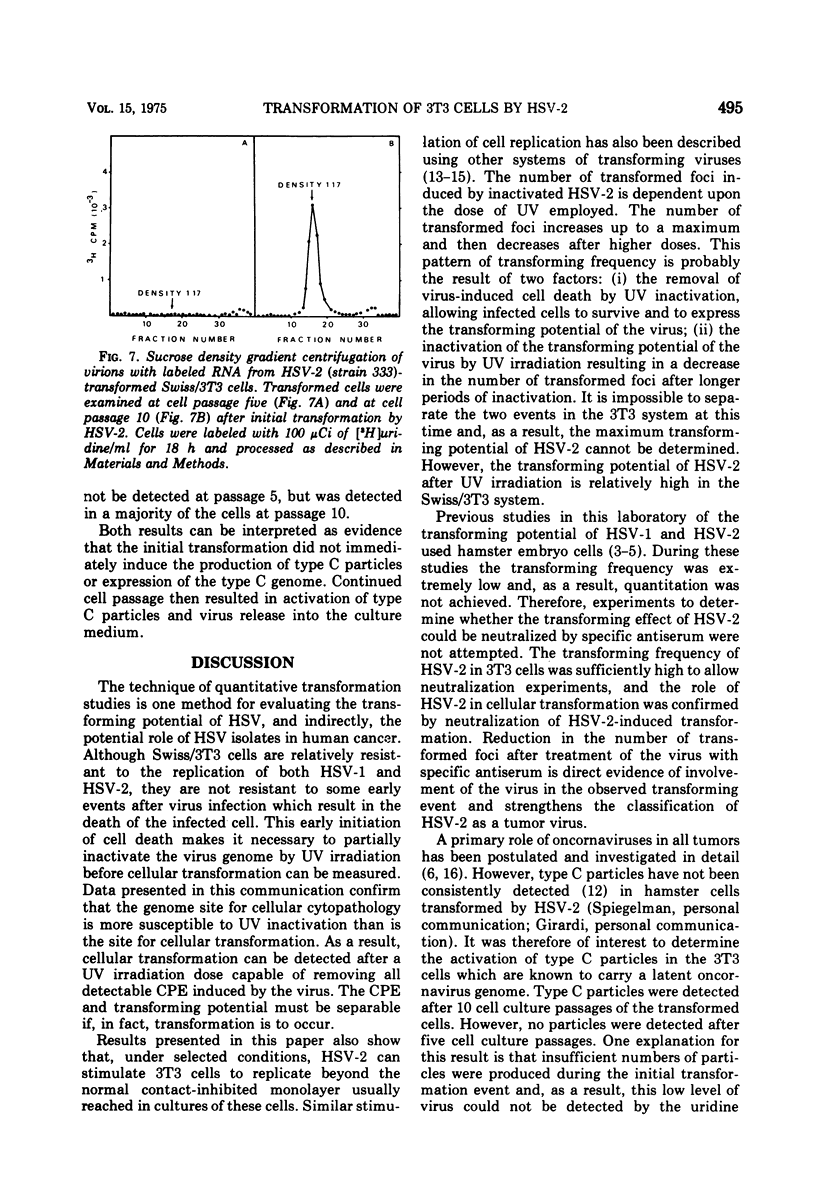
The interaction of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) with Swiss/3T3 cells was investigated. Virus-induced cytopathic effects developed in the absence of production of infectious virus. HSV-2 inactivated with UV light (2, 4, 6, and 8 min) also induced cell death in the absence of virus replication. Cell death was not detectable after infection by HSV-2 that had been inactivated by UV irradiation for 10, 12, and 14 min. 3T3 cells infected with UV-inactivated virus (10 and 12 min) continued to replicate past the contact-inhibited monolayer normally associated with these cells. Infection of 3T3 cells with UV-irradiated USV-2 also induced the development of transformed foci. Transformed cells with an epithelioid of fibroblastoid morphology were identified and isolated. All HSV-2-transformed cell lines contained HSV-2-specific antigens detectable by immunofluorescence techniques. The maximum frequency of HSV-2-induced transformation was 3 times 105 PFU per transformed focus, and the observed transformation could be inhibited by pretreatment of the virus with specific antiserum. No type C particles were detected within five cell culture passages after transformation by HSV-2. Type C virus particles were detected after 10 cell culture passages of the HSV-2-transformed cell lines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Todaro G. J. Antigenic properties of endogenous type-C viruses from spontaneously transformed clones of BALB-3T3. Proc Natl Acad Sci U S A. 1973 May;70(5):1598–1602. doi: 10.1073/pnas.70.5.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Livingston D. M., Todaro G. J. Superinduction of endogenous type C virus by 5-bromodeoxyuridine from transformed mouse clones. Science. 1973 Aug 3;181(4098):443–444. doi: 10.1126/science.181.4098.443. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Conner R., Glaser R., Duff R. Absence of leukosis virus markers in hamster cells transformed by herpes simplex virus type 2. J Virol. 1972 Jun;9(6):1059–1063. doi: 10.1128/jvi.9.6.1059-1063.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H. Cell growth and the initiation of transformation by SV40. Proc Natl Acad Sci U S A. 1966 Feb;55(2):302–308. doi: 10.1073/pnas.55.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]