Abstract
The activation mechanisms of recombinant N-methyl-d-aspartate receptors (NRs) have been established in sufficient detail to account for their single channel and macroscopic responses; however, the reaction mechanism of native NRs remains uncertain due to indetermination of the isoforms expressed and possible neuron-specific factors. To delineate the activation mechanism of native NRs, we examined the kinetic properties of currents generated by individual channels located at the soma of cultured rat neurons. Cells were dissociated from the embryonic cerebral cortex or hippocampus, and on-cell single channel recordings were done between 4 and 50 days in vitro (DIV). We observed two types of kinetics that correlated with the age of the culture. When we segregated recordings by culture age, we found that receptors recorded from early (4–33 DIV) and late (25–50 DIV) cultures had smaller unitary conductances but had kinetic profiles that matched closely those of recombinant 2B- or 2A-containing receptors, respectively. In addition, we examined the effects of cotransfection with postsynaptic density protein 95 or neuropilin tolloid-like protein 1 on recombinant receptors expressed in human embryonic kidney-293 cells. Our results add support to the view that neuronal cultures recapitulate the developmental patterns of receptor expression observed in the intact animal and demonstrate that the activation mechanism of somatic neuronal NRs is similar to that described for recombinant receptors of defined subunit composition.
Keywords: native receptors, N-methyl-d-aspartate receptors, patch clamp, single channel kinetics, gating mechanism
ionotropic glutamate receptors (iGluRs) mediate the majority of excitatory neurotransmission throughout the mammalian brain. Based on pharmacology, there are three main classes of glutamate-activated channels: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainate receptors (KARs), and N-methyl-d-aspartate receptors (NRs) (Traynelis et al. 2010). Among iGluRs, NRs are exceptional in their high unitary conductance, high Ca2+ permeability, and remarkably slow gating kinetics (Ascher and Nowak 1988; Jahr and Stevens 1993; MacDermott et al. 1986). These unique biophysical properties are essential to the critical functions performed by NRs, including neuronal development and synaptic plasticity (Cull-Candy et al. 2001). These properties are also central to excitotoxicity, the glutamate-initiated neuronal demise that has been associated with several neurodegenerative conditions including stroke and Alzheimer's disease (Bowie 2008; Lau and Zukin 2007; Waxman and Lynch 2005). Thus, delineating the biophysical properties of NRs and how they change during physiological and pathological events represent important goals that remain to be fully achieved.
NRs are composed of two glycine-binding GluN1 (N1) subunits and two glutamate-binding GluN2 (N2) subunits (Clements and Westbrook 1991; Hirai et al. 1996; Laube et al. 1997). Throughout the brain, eight N1 splice variants (1a,b–4a,b) and four N2 subunit genes (A–D) are differentially expressed during development and in distinct neuronal populations (Hollmann et al. 1993; Kutsuwada et al. 1992; Monyer et al. 1992; Zukin and Bennett 1995). The exact composition of native receptors is not known and represents an area of current investigation. The N1 subunit is obligatory to all functional NRs and is ubiquitously expressed throughout the mammalian brain; the N2B (2B) and N2A (2A) subunits are the principal N2 subtypes expressed in the cortex and hippocampus (Monyer et al. 1992). The 2B subunit is expressed first and predominates in immature neurons; later in development, its expression declines in parallel with an increase in the 2A subunit (Monyer et al. 1994; Watanabe et al. 1992). This age-related switch between 2B and 2A subunits has been well documented with biochemical and functional assays both in vitro and in vivo and is critical to normal synapse maturation and brain development (Carmignoto and Vicini 1992; Li et al. 1998; Williams et al. 1993; Zhong et al. 1994).
Recombinant iGluRs have been the preparation of choice for biophysical and pharmacological investigations because the identity of the isoform or subtype expressed can be controlled, and, thus, isoform-specific properties can be revealed and characterized. However, for AMPARs and KARs, the biophysical properties of recombinant receptors differ markedly from those recorded in native preparations. Unless recombinant AMPARs are coexpressed with transmembrane AMPAR auxiliary proteins, they have lower glutamate affinity and faster kinetics than their native counterparts (Nicoll et al. 2006; Straub and Tomita 2011). Similarly, KARs require the coexpression of neuropilin tolloid-like proteins (Neto) to recapitulate in recombinant systems the characteristically slow decay of synaptic KARs (Straub et al. 2011; Zhang et al. 2009). The recent discovery of the modulatory role of auxiliary proteins emphasizes the importance of identifying the neuronal factors that control receptor responses and delineating the reaction mechanisms of native receptors.
In addition, the biophysical properties of recombinant iGluRs can vary with the expression system used. For example, in Xenopus oocytes, N1 can produce functional channels when expressed alone. Based on these initial observations, it appeared that NRs could function as homomeric channels (Moriyoshi et al. 1991). However, in mammalian expression systems, functional receptors were observed only when both N1 and N2 subunits were coexpressed (Grimwood et al. 1995). These discrepancies between expression systems were later resolved when it was discovered that oocytes express several endogenous iGluR subunits, whose biophysical properties are, in many cases, substantially different from their mammalian counterparts (Schmidt and Hollmann 2008, 2009; Schmidt et al. 2009). With the demonstration that Xenopus oocytes express a variety of endogenous iGluR subunits, human embryonic kidney (HEK)-293 cells have become the preparation of choice for kinetic studies. We have recently developed complete kinetic mechanisms for the activation of both recombinant N1/2A and N1/2B receptor subtypes that reside in intact HEK-293 cell membranes (Amico-Ruvio and Popescu 2010; Kussius et al. 2009). Here, we used similar methods and delineated the reaction mechanisms of native NRs that reside on the soma of intact neurons dissociated from the cerebral cortex or hippocampus of rat embryos.
We found that currents recorded from young neuronal cultures [4–33 days in vitro (DIV)] had single channel kinetics that were indistinguishable from those of recombinant N1/2B receptors expressed in HEK-293 cells, whereas currents recorded from mature cultures (25–50 DIV) matched closely the behaviors described for recombinant N1/2A receptors. To gain insight into the gating mechanisms of synaptic NRs, which are difficult to access at the single channel level, we also characterized the effects of cotransfection, along with NRs, the postsynaptic density-specific proteins, postsynaptic density protein (PSD)95 or Neto1. We found that PSD95 cotransfection changed the gating kinetics of recombinant N1/2B but not N1/2A receptors, whereas cotransfection with Neto1 affected neither NR subtype.
METHODS
Dissociated Embryonic Neuronal Cultures
Primary cortical and hippocampal neuronal cultures were prepared from Sprague-Dawley rat embryos at embryonic day 18 (E18) with minor adjustments from previously described methods (Misonou and Trimmer 2005). Briefly, a pregnant rat was euthanized in a CO2 chamber and decapitated, and the uterus was surgically removed. Embryos were decapitated, and the heads were placed on ice in 100 mM PBS. Either the frontal area of the cerebral cortex or the hippocampus was removed and placed in a solution containing Puck's D1 saline, sucrose, glucose, and HEPES. Cells were dissociated using trypsin and trituration through a stereological pipette with a 40-μm strainer (BD Falcon, Franklin Lakes, NJ). Dissociated cells free of tissue debris were plated on poly-l-lysine-coated 35-mm dishes at a density of 5 × 105 cells/ml in minimal essential medium (Invitrogen, Grand Island, NY) with 10% FBS supplemented with glucose and l-glutamine. After 24 h, the medium was replaced with neurobasal medium containing a B27 supplement (Invitrogen, Grand Island, NY), glutamine, and penicillin-streptomycin. Cytosine β-d-arabinofuranoside hydrochloride (Sigma-Aldrich, St. Louis, MO) was added after 5 days in culture to inhibit the cell division of non-neuronal cells. Half of the incubated media was replaced every 5 days. Neurons were used for electrophysiological experiments between days 3 and 50 after plating. All procedures were performed in adherence with National Institutes of Health (NIH) guidelines and were approved by the Animal Care Committee of the University of Buffalo.
HEK-293 Cell Cultures and Transfections
HEK-293 cells (CRL-1573, American Type Culture Collection), a gift from A. Auerbach (University at Buffalo, Buffalo, NY), were grown in DMEM (Invitrogen, Grand Island, NY) with 10% FBS and 1% penicillin-streptomycin and were sustained in 5% CO2 atmosphere at 37°C. Cells were grown to ∼85% confluence, and passages 20–40 were used for transfections. Plasmids N1 (NR1–1a, U08261), 2B (NR2B, M91561), and 2A (NR2A M91562) were gifts from R. Wenthold (NIH), M. Salter (University of Toronto), and A. Auerbach (University at Buffalo), respectively. N1 and either of the N2 plasmids were transiently transfected along with green fluorescent protein at a 1:1:1 ratio using the calcium phosphate method using solutions that contained (in mM) 140 NaCl, 5 KCl, 0.75 Na2HPO4, 6 sucrose, 125 CaCl2, 25 HEPES-NaOH (pH 7.05), and 0.25 μg of each cDNA per 35-mm dish. Experiments with PSD95 (NP_001100841) and Neto1 (NM_144946), gifts from J. Marshall (Brown University) and S. Tomita (Yale), respectively, were cotransfected at a fivefold higher cDNA ratio. After 2 h of incubation, the medium was replaced with one that contained 2 mM MgCl2 to suppress NR activity and prevent apoptosis. Before electrophysiological measurements, cells were washed and immersed in PBS.
Electrophysiology
Single channel recordings.
Single channel currents were recorded with the cell-attached patch-clamp technique (Hamill et al. 1981). Recordings were performed with electrodes pulled in two stages with a vertical puller (PC-10, Narishige, East Meadow, NY) from borosilicate glass capillaries (BF150-86-10, Sutter Instrument, Novato, CA), fire polished to a final resistance of 12–25 MΩ, and filled with an extracellular solution containing (in mM) 150 NaCl, 2.5 KCl, 10 HEPBS, 1 EDTA, 1 glutamate, and 0.1 glycine adjusted to pH 8.0 (NaOH). While neurons were being recorded, the pipette solution also contained 20 μM 6-cyano-7-nitroquinoxaline (CNQX; Sigma-Aldrich) to inhibit AMPAR and KAR currents. Fresh solutions were prepared daily from a DMSO stock (final DMSO concentration: ∼0.2%) stored at −80°C. NR activity was recorded as inward Na+ fluxes after the application of +100 mV through the recording pipette. Currents were amplified and analog filtered at 10 kHz (Axopatch 200B, 4-pole Bessel) and digitally sampled at 40 kHz (NiDAq, PCI-6229, M Series Card, National Instruments, Austin, TX) into digital files with QUB software (www.QUB.buffalo.edu, University at Buffalo).
To measure resting membrane potentials for HEK-293 cells and cultured neurons, after obtaining a high-resistance cell-attached seal and subsequently breaking through the membrane into whole cell voltage clamp, we switched the recording configuration to current clamp and noted the voltage (VR); average values were computed for HEK-293 cells and for neurons. Single channel conductance (g) was calculated using the on-cell single channel amplitude (i) measured at a given applied voltage (Va), and the following relationship was calculated: g = i/V, where V = VR + Va.
Macroscopic recordings.
Whole cell currents were recorded using electrodes pulled from borosilicate glass capillaries (BF150-110-10, Sutter Instrument), fire polished to a final resistance of 3–8 MΩ, and filled with an intracellular solution containing (in mM) 135 CsF, 33 CsOH, 2 MgCl2, 1 CaCl2, 10 HEPES, and 11 EGTA adjusted to pH 7.4 (CsOH). Extracellular solutions contained (in mM) 150 NaCl, 2.5 KCl, 0.5 CaCl2, 0.01 EDTA, 10 HEPBS, and 0.1 glycine adjusted to pH 8.0 (NaOH) with or without 1 mM glutamate, as indicated. Cells were held at −70 mV and perfused with lightly pressurized extracellular solutions (BPS-8, ALA Scientific Instruments, Westbury, NY). Inward currents were amplified and filtered at 2 kHz (Axopatch 200B, 4-pole Bessel), sampled at 5 kHz (Digidata, 1322A), and digitally recorded using pCLAMP 10.2 software (Molecular Devices, Sunnyvale, CA).
Data Analysis
Recorded currents were analyzed as previously described in detail (Kussius et al. 2009). Microscopic recordings that contained more than one active channel, as indicated by simultaneous openings, were discarded, and the selected one-channel files were processed, idealized, and fitted to kinetic models in QUB. Occasional noise spikes were erased by setting the corresponding region to either a closed or an open current level; baseline drift was corrected by resetting the baseline to the zero-current level. Preprocessed data were then idealized with the segmental k-means algorithm, at 150-μs resolution, and modeled with a maximum interval log-likelihood algorithm. The number of closed and open states in the final model was determined incrementally adding closed and open states to an initial 1C1O model (where C is closed and O is open) and setting the increase in log likelihood to an arbitrary threshold of 10 units/added state (two additional rate constants). Values calculated for kinetic parameters in each record were averaged for each data set and are reported as means ± SE.
Macroscopic traces (3–10 traces/cell) were averaged and evaluated by measuring peak current (Ipk) and equilibrium [steady state current (Iss)] amplitudes and by fitting a monoexponential function to the decay phase of the current (Clampfit 10.2 software, Molecular Devices). These analyses provided metrics for macroscopic desensitization as a time constant (τD) and as the Iss-to-Ipk ratio (Iss/Ipk). Values are reported as means ± SE and were obtained for 6–15 cells/condition.
Simulated macroscopic responses were generated in QUB as previously described (Kussius et al. 2009). The models derived from stationary single channel recordings were expanded to include two sequential glutamate-binding steps leading into the C3 state of each model; glutamate association and dissociation rate constants were considered equal to those reported for recombinant N1/2A (Popescu et al. 2004) and N1/2B (Amico-Ruvio and Popescu 2010) receptors. Long (5 s) glutamate applications were modeled as instantaneous concentrations jumps between 0 and 1 mM glutamate.
The significance of observed differences between means was evaluated with a two-tail Student's t-test; the significance of observed differences in variance was evaluated with a two-tailed F-test. Differences were considered significant for P values of <0.05.
RESULTS
Experimental Recording Conditions of Native and Recombinant NRs
Equilibrium single channel activities of recombinant and native NRs were measured using the cell-attached patch-clamp technique as inward Na+ fluxes (Fig. 1A). Recordings were made in saturating concentrations of the physiological agonists glutamate (1 mM) and glycine (0.1 mM) and under conditions specifically selected to minimize inhibition by extracellular protons (pH 8.0) and divalent cations, such as magnesium and zinc (1 mM EDTA). Native receptors were investigated in neurons dissociated from the cerebral cortixes or hippocampi of rat embryos (E18; n = 34); the VR value of these neurons was −62 ± 2 mV (n = 54) and was not significantly different across neuronal preparations or culture age (P > 0.05). Recombinant receptors were expressed in HEK-293 cells; the measured VR value for these cells was significantly smaller, −15 ± 2 mV (n = 19, P < 0.001). These values are close to those reported previously for HEK-293 cells and cultured central neurons (Evans et al. 1998; Johansson et al. 1992; Voigt et al. 2001). In addition, to control for possible effects of CNQX, an AMPAR and KAR inhibitor included in all our recordings from neurons, we obtained a set of single channel currents from recombinant N1/2A receptors expressed in HEK-293 cells and included CNQX in the extracellular solution. CNQX is widely used to isolate NR-specific glutamate responses in native tissues, but under certain conditions, it can act as a weak competitive antagonist for the glycine site (Honore et al. 1988; Kessler et al. 1989). At the high glycine concentrations used in this study, this antagonism is negligible (Lester et al. 1989). Consistent with previous reports, we found that CNQX had no effect on the properties of single channel currents recorded from recombinant N1/2A receptors expressed in HEK-293 cells (Table 1). Having accomplished these preliminary measurements, we next examined the behavior of native NRs.
Fig. 1.
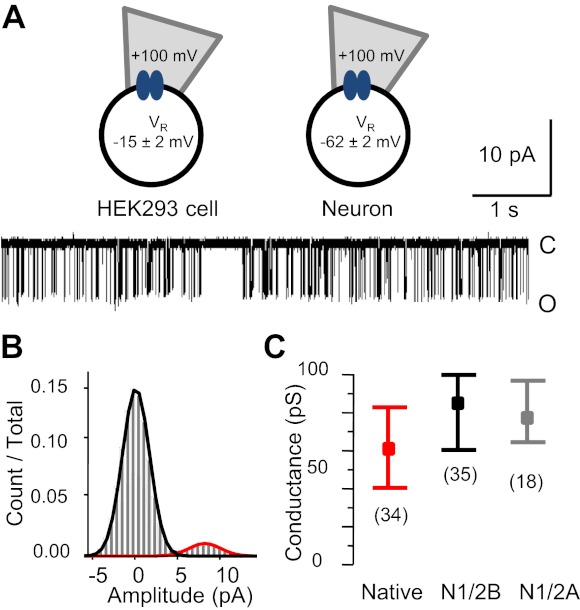
N-methyl-d-aspartate receptor (NR) unitary conductance. A: cell-attached patch-clamp recording configuration used in this study (top) and representative single channel current trace recorded from a native receptor [29 days in vitro (DIV); bottom]. HEK-293 cell, human embryonic kidney-293 cell; VR, recorded voltage. B: event amplitude histogram for the entire record illustrated in A (21,507 events, 14 min) overlaid with the probability density function calculated for a two-state model. Values are means ± SD. Components are in black [closed (C), 0.01 ± 1.56 pA] and red [open (O), 8.9 ± 1.9 pA]. C: summary of unitary conductance results showing the means (boxes) and ranges (whiskers) for the three conditions indicated.
Table 1.
Single channel parameters of native and recombinant NRs
| NR | Modulator | Amplitude, pA | Conductance, pS | Open Probability | Mean Open Time, ms | Mean Closed Time, ms | Number of Cells Studied | Duration, min | Events Analyzed |
|---|---|---|---|---|---|---|---|---|---|
| Native | 9.8 ± 0.3 | 61 ± 2 | 0.17 ± 0.03 | 5.1 ± 0.3 | 62 ± 12 | 34 | 367 | 1.5 × 106 | |
| 2B | 9.9 ± 0.3 | 86 ± 3 | 0.16 ± 0.02 | 4.4 ± 0.3 | 56 ± 11 | 35 | 686 | 2.1 × 106 | |
| Early neurons | 10.3 ± 0.4 | 63 ± 3* | 0.07 ± 0.01* | 4.3 ± 0.3 | 87 ± 15 | 23 | 250 | 6.6 × 105 | |
| 2A | 9.0 ± 0.3 | 78 ± 2 | 0.50 ± 0.03 | 7.1 ± 0.6 | 6.9 ± 0.7 | 18 | 756 | 6.3 × 106 | |
| Late neurons | 8.9 ± 0.5 | 55 ± 3* | 0.39 ± 0.02* | 6.4 ± 0.5 | 9.5 ± 0.9 | 11 | 117 | 9.6 × 105 | |
| 2A | PSD95 | 8.3 ± 0.3 | 73 ± 2 | 0.45 ± 0.05 | 6.4 ± 0.7 | 8.7 ± 1.5 | 8 | 369 | 3.3 × 106 |
| 2B | PSD95 | 9.0 ± 0.3 | 79 ± 2 | 0.20 ± 0.02† | 4.7 ± 0.5 | 20 ± 2† | 11 | 290 | 1.5 × 106 |
| 2A | Neto1 | 10.2 ± 0.7 | 89 ± 6 | 0.49 ± 0.04 | 5.7 ± 0.7 | 6.0 ± 0.6 | 6 | 133 | 1.4 × 106 |
| 2A | CNQX | 10.0 ± 0.4 | 87 ± 3 | 0.49 ± 0.06 | 6.7 ± 1.5 | 6.5 ± 0.9 | 5 | 200 | 1.9 × 106 |
Values are means ± SE for amplitude, open probability, and mean open and closed times. NRs, N-methyl-d-aspartate receptors; PSD95, postsynaptic density protein 95; Neto1, neuropilin tolloid-like protein 1; CNQX, 6-cyano-7-nitroquinoxaline.
P < 0.05 relative to the mean of the recombinant receptor type above (by Student's t-test);
P < 0.05 relative to the variance in the absence of modulator (by F-test).
Unitary Current Amplitude and Channel Conductance
NR currents recorded from neurons had large uniform amplitudes that were very similar to those previously reported for recombinant N1/2A and N1/2B receptors expressed in HEK-293 cells (Fig. 1B). Given that we measured markedly different VR values for neurons and HEK-293 cells, this observation indicated that native and recombinant NRs may differ in their single channel conductances. We calculated the unitary conductance for native and recombinant receptors as the ratio of mean single channel amplitudes and the respective driving potentials during cell-attached recordings (V), which were estimated as the sum of measured VR and VA (V = VR + −100 mV). The unitary conductance of neuronal NRs (61 ± 2 pS) was significantly smaller than that calculated for recombinant N1/2B or N1/2A receptors (86 ± 3 or 78 ± 2 pS, respectively, P < 0.05; Table 1 and Fig. 1C) but was larger than values previously reported (∼50 pS) for the main large conductance of native NRs (Ascher and Nowak 1988; Gibb and Colquhoun 1992; Howe et al. 1991; Stern et al. 1992, 1994). However, note that these previous measurements were all performed on excised membrane patches with extracellular Ca2+ concentrations of 1–2 mM. The single channel Na+ conductance is ∼15% larger in cell-attached patches relative to excised patches and has been shown to increase ∼34% when the Ca2+ concentration is reduced from 1 to 0 mM and (Clark et al. 1997; Jahr and Stevens 1993). Thus, our results are consistent with previous literature and also with reports showing that native receptors have a lower conductance than recombinant receptors (Auerbach and Zhou 2005; Clark et al. 1997; Jahr and Stevens 1993; Premkumar et al. 1997).
Kinetic Characterization of Individual Neuronal NRs
Developmental shifts in the kinetics of neuronal NRs correlate well with age-dependent changes in the N2 subunit expressed (Carmignoto and Vicini 1992; Monyer et al. 1994; Williams et al. 1993). In both the cortex and hippocampus, the 2B subunit dominates early in development and declines slowly as neurons mature; in contrast, the 2A subunit is increasingly expressed during development and becomes dominant in adult neurons. This subunit switch has been well documented in vivo and is recapitulated in cultures (Li et al. 1998; Thomas et al. 2006; Williams et al. 1993; Zhong et al. 1994). We expected that as neurons were cultured for longer periods, we would observe changes in the kinetic behaviors of recorded neuronal NR currents and that these changes would be indicative of changes in subunit expression. The gating mechanisms of the two major NR subtypes expressed in the principal cells of the cerebral cortex and hippocampus, N1/2A and N1/2B, have been characterized in detail in recombinant systems, and their kinetic parameters are distinct. Overall, the N1/2A subtype has a higher open probability (PO) due to a longer mean open time (MOT) and a shorter mean closed time (MCT) compared with the N1/2B subtype (Amico-Ruvio and Popescu 2010; Banke and Traynelis 2003; Chen et al. 1999; Popescu and Auerbach 2003). We used these overall kinetic parameters to evaluate the activity recorded from native receptors.
The PO values we measured for native receptors segregated into two distinct populations, and these correlated with culture age: receptors with low PO values (0.07 ± 0.01, n = 23) were observed in early neurons (ENs; 4–33 DIV), whereas receptors with significantly higher PO values (0.39 ± 0.02, n = 11, P < 10−17) were observed in late neurons (LNs; 25–50 DIV; Table 1 and Fig. 2A). Within a 7-day window, between 25 and 33 DIV, we observed both types of activity. Notably, however, recordings from this period did not have intermediate PO values; instead, each recording could be clearly assigned to one type of behavior, low or high PO. We also noted that native receptors, whether recorded from ENs or LNs, had significantly lower PO values relative to those of recombinant N1/2B or N1/2A receptors, respectively (P < 0.05), but their mean open and closed durations were not statistically different (P > 0.05; Table 1). Markedly, the characteristically wide range of mean closed durations previously reported for recombinant N1/2B receptors (Amico-Ruvio and Popescu 2010) was also apparent for the recombinant N1/2B receptors recorded in this study (7–306 ms) and for native receptors in early cultures (15–290 ms; Fig. 2B).
Fig. 2.
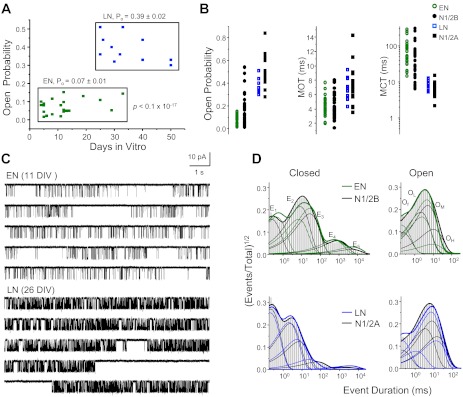
Single channel properties of native NRs. A: NR activities recorded from dissociated neurons segregate into two open probability (PO) ranges, which correlated roughly with culture age: early neurons (ENs; 4–33 DIV, n = 23) and late neurons (LNs; 25–50 DIV, n = 11). B: Scatterplot of single channel kinetic parameters for native (EN and LN) and recombinant (N1/2B and N1/2A) receptors. C: representative single channel current traces recorded from one EN receptor (top; 11 DIV) and one LN receptor (bottom; 26 DIV). D: histograms of closed (left) and open (right) event durations for the records illustrated in C (EN: green and LN: blue) overlaid with those measured in records from one recombinant N1/2B receptor (top) or N1/2A receptor (bottom; black). Overall probability distributions (thick solid lines) and individual closed (E1–E5) and open [fast (Of), low (OL), medium (OM), and high (OH)] kinetic components (thin solid lines) are also shown.
Before we proceeded to characterize in more detail the kinetics of native receptors, we examined the calculated conductances after classifying native NRs by their PO value as occurring in ENs (63 ± 3 pS) or LNs (55 ± 3 pS; Table 1). In early cultures, NRs had ∼13% larger single channel conductance than in late cultures; however, in our study, this difference was not statistically significant (P > 0.06). A previous report also noted that recombinant N1/2B receptors tended to produce larger unitary currents than N1/2A receptors, but these differences were not statistically significant (Amico-Ruvio and Popescu 2010). The unitary conductance we calculated in this study for recombinant N1/2B receptors was ∼10% larger than that of recombinant N1/2A receptors (P < 0.047). Independent measurements of slope NR conductances for recombinant NRs also found N1/2B receptors to have slightly higher Na+ conductance than N1/2A receptors (Maki et al. 2012). We conclude that, overall, subunit-dependent differences in conductance are relatively small and are probably obscured in on-cell recordings by cell-to-cell variations in membrane potential. However, relative to recombinant N1/2B and N1/2A receptors, both EN and LN receptors had smaller Na+ conductances (∼27% and ∼29%, respectively). This suggests that in neurons, whether composed of 2B or 2A subunits, NRs may have smaller conductances than recombinant subtypes expressed in HEK-293 cells.
The activity patterns recorded from neurons in early and late cultures were sufficiently different as to be qualitatively detectable by direct visual inspection (Fig. 2C). For a quantitative assessment, we examined the closed and open interval distributions present in individual recordings. It is well established that both native and recombinant NRs visit multiple closed and open states during receptor activation and gating (Gibb and Colquhoun 1991; Howe et al. 1991; Stern et al. 1992). We also observed multiple-component distributions in all records analyzed. Dwell-time histograms and corresponding kinetic components for both the closed and open distributions were nearly identical when ENs were compared with recombinant N1/2B and LNs were compared with recombinant N1/2A receptors (Fig. 2D). Single channel data recorded from either early or late cultures were well described with five closed components and two to four open components, similar to data from recombinant receptors. We observed significant differences only for the shortest closed time constant between EN and N1/2B receptors (0.27 ± 0.01 vs. 0.22 ± 0.01 ms) and only for the longest closed time constant, which reflects a desensitized state, between LN and N1/2A receptors (1.6 ± 0.2 vs. 2.7 ± 0.3 s). Based on these observations, we conclude that native NRs have kinetics that are largely determined by the N2 subtype expressed, 2B or 2A, and these properties are not substantially different in cultured neurons and HEK-293 cells.
Gating Mechanism of Neuronal NRs
NR currents reflect the gating transitions experienced by receptors while active, and these can be functionally assigned to receptor activation, receptor desensitization, and modal gating. At the single channel level, activations appear as bursts of openings interrupted by short closures; desensitization transitions appear as long silent periods that separate bursts, and modal transitions cause infrequent but obvious changes in overall gating pattern, which can be analytically detected as changes in mean open durations. Based on these findings, a detailed description of the NR gating mechanism should include, at a minimum, 15 closed states and 6 open states (Popescu 2012). However, in practice, not all states can be readily resolved, even in single channel data. Instead, an average scheme was found to be more readily accessible (Kussius et al. 2009; Popescu and Auerbach 2003). Here, we used this operational model to evaluate the gating mechanism of native NRs. When we compared the reaction mechanism of ENs and recombinant N1/2B receptors, we found no significant difference between any of the gating rates explicit in this model; this similarity was also apparent when we compared LNs with recombinant N1/2A receptors (Fig. 3). Considered together with the large body of evidence supporting a developmental switch between 2B and 2A subunit expression in neurons, our results demonstrate practically identical gating mechanisms for these two receptor subtypes in neurons and in HEK-293 cells.
Fig. 3.
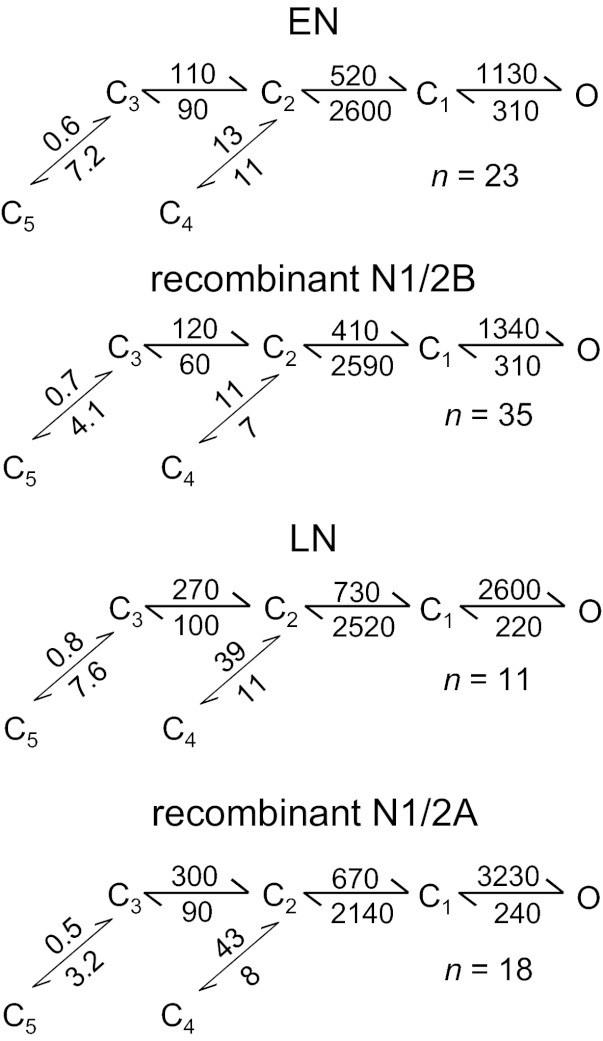
NR gating mechanisms. Rates (in s−1) are given for each transition as the rounded average across all records in each set. Differences were not significant between EN and N1/2B receptors or between LN and N1/2A receptors for any of the transitions estimated (P > 0.05 by Student's t-test).
The only open state included in the operational model used above stands in for all open states observed in each record and, thus, aside from activation gating, it also aggregates modal gating. Modal gating is defined by stochastic changes in a receptor's single channel kinetics that occur abruptly even though experimental conditions are held constant (Magleby 2004). In several cases where modal gating has been investigated in detail, it was found to arise from changes in either MOTs or MCTs, and its molecular basis was correlated with changes in the receptor's phosphorylation state(s), inter- or intradomain interactions, and association with cellular proteins (Popescu 2012). For recombinant N1/2A and N1/2B receptors expressed in HEK-293 cells, modal gating has been characterized quantitatively and was proposed to control the biphasic deactivation of synaptic receptors; however, the molecular basis for this behavior remains elusive (Amico-Ruvio and Popescu 2010; Popescu and Auerbach 2003; Popescu et al. 2004; Zhang et al. 2008). Early observations of currents generated by NRs native to primary cortical and hippocampal cultures have clearly illustrated the existence of modal gating in neuronal receptors (Jahr and Stevens 1987; Kleckner and Pallotta 1995). Modal gating appears absent when recombinant NRs are expressed in Xenopus oocytes (Auerbach and Zhou 2005).
In our recordings, the presence of multiple components in the open dwell-time histograms of native NRs was a definitive indication that these receptors displayed modal gating (Fig. 2D). Using the component durations as a guide, and noting that low-, medium-, and high-duration components were present in recordings obtained from both ENs and LNs, we conclude that in these preparations, three kinetic modes were present, as previously described for recombinant receptors (Fig. 4). Furthermore, open components in ENs were indistinguishable from those observed for recombinant N1/2B receptors (low: 2.4 ± 0.2 vs. 2.3 ± 0.1 ms, medium: 5.0 ± 0.5 vs. 5.1 ± 0.2 ms, and high: 11 ± 1 vs. 11 ± 1 ms). Also, we observed no significant differences in open components between LN and recombinant N1/2A recordings (low: 2.5 ± 0.4 vs. 3.4 ± 0.3 ms, medium: 6.7 ± 0.5 vs. 7.8 ± 0.4 ms, and high: 15 ± 1 vs. 16 ± 1 ms). The relative areas of open components were not significantly different for either LNs or ENs compared with their recombinant counterparts. These data are further evidence showing that modal gating is not only preserved but occurs with similar frequencies in both native NRs and recombinant N1/2B and N1/2A receptors expressed in HEK-293 cells. These results support the conclusion that the mechanism of modal gating is an inherent property of NRs and are consistent with the proposal that mechanisms that control the kinetics of modal transitions may represent important regulators of NR-mediated synaptic currents.
Fig. 4.
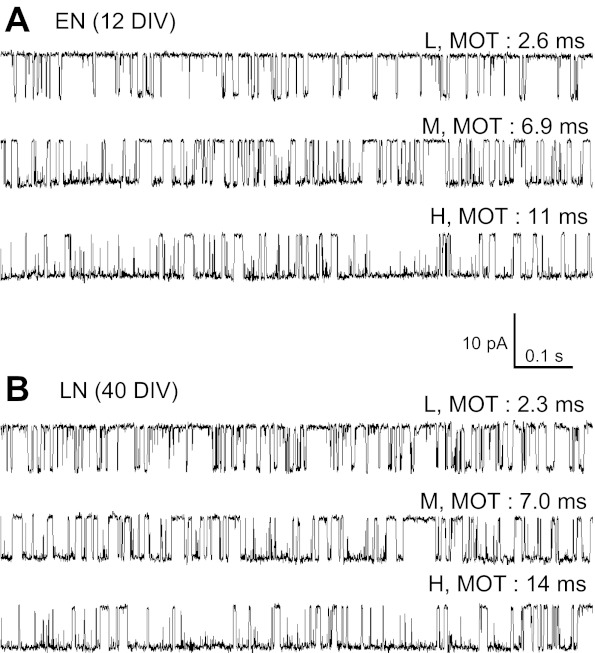
Gating modes of native NRs. Three separate 1-s periods were selected from one EN recording (A) and one LN recording (B). In A and B, traces differ by their mean open durations [mean open time (MOT)] and illustrate activity in one of the low (L), medium (M), and high (H) gating regimes adopted randomly by NRs.
Cotransfection With PSD95 But Not Neto1 Altered Macroscopic Desensitization of Recombinant NRs
At synapses, iGluRs interact directly with proteins specific to the postsynaptic density. These interactions are critical to synaptic localization and stability and also influence the inherent biophysical properties of synaptic receptors (Kim and Sheng 2004). Having determined that in HEK-293 cells, N1/2A and N1/2B receptors have kinetic behaviors that closely match those measured at the soma of neurons, we next investigated whether these behaviors are altered when plasmids encoding PSD95 or Neto1 are cotransfected along with N1 and N2 subunits in HEK-293 cells. We first characterize the kinetic effects of cotransfection of PSD95 and Neto1 on NR activity by measuring ensemble currents with the whole cell patch-clamp configuration. HEK-293 cells were cotransfected with N1 and 2B or N1 and 2A, and with PSD95 or Neto1, at a 1:1:5 ratio. Macroscopic current decay (macroscopic desensitization) was evaluated after currents were elicited with a long (5 s) pulse of 1 mM glutamate (Fig. 5A). Cotransfection with PSD95 significantly decreased the extent (Iss/Ipk) and slowed the rate (τD) of macroscopic desensitization for both N1/2B and N1/2A receptors (Fig. 5B). These findings are in line with a previous report showing that PSD95 reduces the extent of macroscopic desensitization for both recombinant N1/2B and N1/2A receptors (Sornarajah et al. 2008). In contrast, cotransfection with Neto1 had no discernible effects on either N1/2B or N1/2A macroscopic desensitization parameters. This result may indicate a lack of effect for the putative interaction, a lack of interaction, or simply a lack of proper Neto1 expression in our experimental conditions. We did not investigate this aspect further.
Fig. 5.
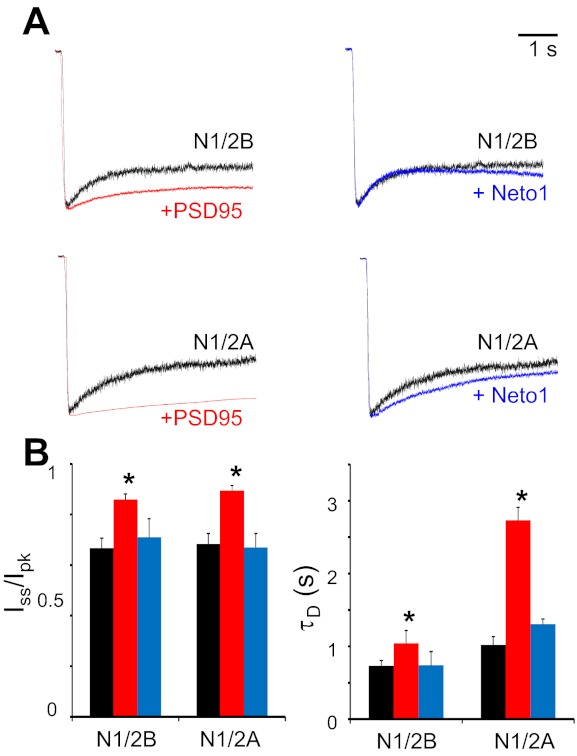
Effects of postsynaptic density protein (PSD)95 and neuropilin tolloid-like protein (Neto)1 on macroscopic responses of recombinant NRs. A: whole cell currents were elicited with 5-s applications of 1 mM glutamate from HEK-293 cells transfected with N1 and 2B (top) or with N1 and 2A (bottom) and in combination with PSD95 (left) or Neto1 (right). B: summary of changes in macroscopic desensitization kinetics observed in HEK-293 cells cotransfected NR subunits alone (black) and in conjunction with PSD95 (red) or Neto1 (blue). Iss/Ipk, steady-state (equilibrium) current-to-peak current; τD, time constant of macroscopic desensitization. *P < 0.05 relative to NR subunits alone (by Student's t-test).
PSD95 Alters the Single Channel Properties of N1/2B But Not N1/2A Receptors
Next, we determined how cotransfection with PSD95 affected the mean single channel properties of recombinant NRs by examining on-cell single channel currents recorded from cotransfected HEK-293 cells. To our surprise, the overall kinetic parameters were not significantly different between the two conditions for either N1/2A or N1/2B preparations (Table 1). However, we noted that cotransfection with PSD95 reduced the wide range of PO and MCT characteristics of N1/2B receptors observed in both HEK-293 cells and neurons (P < 0.05 by F-test; Fig. 6).
Fig. 6.
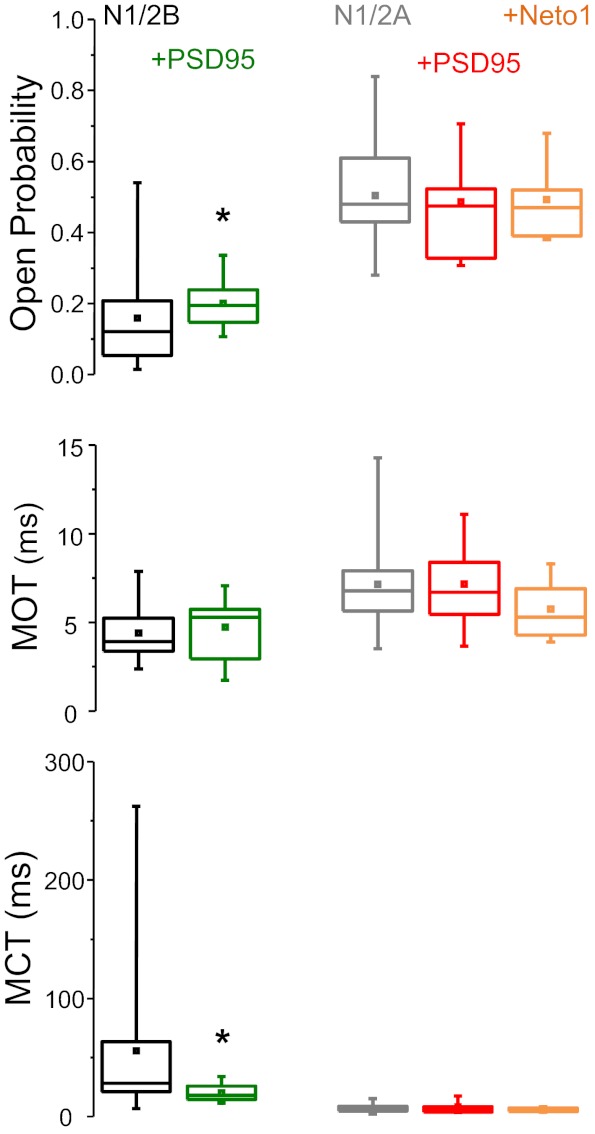
Effects of PSD95 and Neto1 on recombinant NR single channel kinetic parameters. Statistical graphs show the means (box), medians (line), and ranges (whiskers) of values measured for N1/2B (black), N1/2B + PSD95 (green), N1/2A (gray), N1/2A + PSD95 (red), and N1/2A + Neto1 (orange). MCT, mean closed time. *Significant differences in the variance of these parameters observed in cells transfected with NR subunits alone relative to those observed in cells cotransfected with PSD95 (P < 0.05 by F-test).
However, at the levels of dwell-time distributions and of rate constants, we observed several differences in recombinant NR kinetics during cotransfection with PSD95. For N1/2B receptors, cotransfection with PSD95 reduced the closed but not open component durations. Specifically, the closed time components τE2, τE3, and τE4 were all significantly shorter compared with values obtained for N1/2B receptors in the absence of PSD95 (Fig. 7A). These differences were reflected in the stationary gating reaction as changes in C3-C2, C2-C1, and C4-C2 transitions; however, these differences did not materialize in measurable kinetic differences of the simulated macroscopic trace (Fig. 7A). For N1/2A receptors, cotransfection with PSD95 reduced only one of the closed time constants (τE1) and changed only one transition rate (C2 → C1; Fig. 7B). Simulations with the model deduced from cells cotransfected with PSD95 produced N1/N2A currents that were very similar to those simulated with models deduced from cells transfected with N1/N2A alone. Finally, cotransfection of cells with N1/2A and Neto1 produced single channel currents that had similar PO, MOT, and MCT (Table 1) and kinetics that were indistinguishable from those produced by N1/N2A in the absence of Neto1 (data not shown).
Fig. 7.
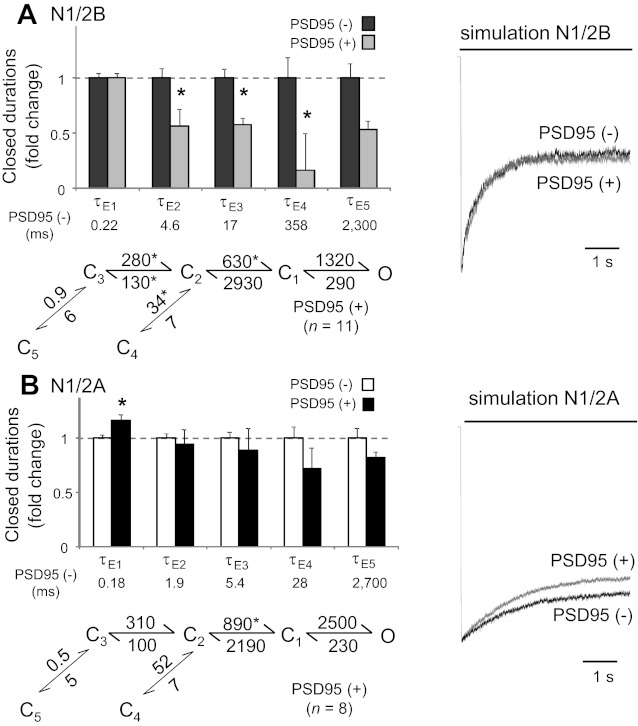
Effects of PSD95 on recombinant NR kinetics. A and B: summary of changes in closed component durations of single channel records obtained from cells expressing N1/2B (A) or N1/2A (B) receptors alone [PSD95(−), with mean durations below] and in conjunction with PSD95 [PSDD95(+)] as well as the reaction mechanism deduced from single channel records. All rates are in s−1; mean rate constants for NRs alone are shown in Fig. 3. *Significant differences in means relative to cells expressing NRs alone (P < 0.05 by Student's t-test). Macroscopic traces were simulated with the models shown in Fig. 3 (black) and overlaid with traces simulated with the models given at the left (gray) for each receptor subtype.
DISCUSSION
In this study, we examined the single channel behavior of NRs expressed endogenously at the soma of neurons dissociated from rat embryonic cortices or hippocampi during in vitro development, and we compared these directly with those observed for recombinant NRs expressed in the HEK-293 cell line. We found that native receptors had slightly lower Na+ single channel conductance and displayed a clear change in single channel kinetics, which correlated with time in culture and also with the well-established developmentally regulated 2B to 2A switch in subunit expression. Importantly, our results showed that neuronal somatic NRs and recombinant receptors expressed in HEK-293 cells gate with essentially similar mechanisms: during the first ∼10 days in culture, neurons express NRs that closely match the kinetic behaviors of N1/2B recombinant receptors, whereas receptors expressed in neurons cultured for more than ∼22 days closely match the kinetics reported for N1/2A recombinant receptors. Finally, we found no changes in the kinetics of NRs expressed in HEK-293 cells that were cotransfected with Neto1, whereas cells cotransfected with PSD95 expressed NRs with small but significant changes in gating, which were subunit dependent.
Recent computational advances have afforded the development of high-resolution kinetic schemes for several recombinant NR subtypes and have identified the mechanistic bases for the observed differences in response time courses (Dravid et al. 2008; Erreger et al. 2005; Kussius et al. 2009; Vance et al. 2012). Comparable advances for native NRs have been hindered primarily by the inability to unambiguously determine the molecular composition of neuronal receptors. In addition, responses from recombinant and endogenous receptors may differ due to a multitude of factors, including differences in posttranslational modification, signaling cascades, and neuronal specific proteins as well as in sensitivity to allosteric modulators. To gain insight into the basic gating mechanism of neuronal receptors, we examined their single channel behavior under conditions designed to minimize confounding factors: we examined two relatively well-studied neuronal preparations, cortical and hippocampal neurons, and we observed these for several weeks during their development in culture and recorded their activity in the virtual absence of allosteric modulators. This approach afforded a direct comparison between the behaviors we observed and those of previously well-characterized recombinant receptors observed under identical conditions. However, despite the definitive insights that our results have provided, it will be useful to keep in mind several limitations inherent in our experimental methods.
First, our study was limited to somatic receptors and thus provides no information regarding possible kinetic differences for receptors residing elsewhere, including the vital synaptic receptors or the abundant dendritic receptors. However, extrasynaptic receptors, of which somatic receptors represent a significant fraction, represent important pharmacological targets in a number of neurodegenerative diseases, and our study clearly identifies recombinant receptors as an adequate model for pharmacological studies of this nature. Second, our study pertains only to NRs containing the most abundant N2 subtypes, 2A and 2B, and thus provides no information regarding possible differences in the kinetics for other subtypes, including those incorporating 3A subunits. Importantly, the gating mechanism of 2A- and 2B-containing receptors appears insensitive to the N1 splice variant with which they associate. N1 subunits are expressed as eight splice variants and have been shown to modulate various channel properties. For example, N1-b splice variants incorporate a highly charged peptide encoded by the exon 5 and substantially alter the sensitivity of NRs to extracellular proton concentrations (Traynelis and Cull-Candy 1990; Traynelis et al. 1995). Relevant to this issue, a recent report showed that depending on exon 5 expression, N1 subunits significantly alter the kinetics of recombinant 2D-containing receptors (Vance et al. 2012). The recombinant receptors examined in this study contained the N1–1a splice variant. This lacks the exon 5-encoded peptide; it is the most abundant N1 splice variant expressed in both the cortex and hippocampus (Laurie and Seeburg 1994; Monyer et al. 1994) and produces receptors that are highly sensitive to proton inhibition. Because our study was done in very low concentrations of protons (pH 8), we have no information regarding possible differences in proton sensitivity between the two preparations examined. However, the strong correlation we observed between the activity of recombinant and native receptors suggests that the majority of native somatic NRs were composed of the N1–1a splice variant or a variant that, under the conditions used here, produced receptors with the same basic gating kinetics of the recombinant isoforms studied here. Finally, although we did not find evidence for native NRs with kinetic behaviors intermediate between those of recombinant 2A- and 2B-containing receptors, we cannot exclude the possibility that triheteromeric N1/2A/2B assemblies are expressed in the preparations investigated here. The existence of triheteromeric NRs is well documented in vivo; however, their physiological roles and channel properties, in particular their single channel behaviors, remain to be fully delineated (Chazot and Stephenson 1997; Hatton and Paoletti 2005).
The limitations noted above notwithstanding, we sought to determine whether and if so how possible associations with synaptic proteins may affect NR gating when coexpressed jointly in HEK-293 cells. PSD95 is an abundant postsynaptic protein that binds directly to the intracellular domains of NRs (Kim and Sheng 2004). Intracellular binding of PSD95 can serve to anchor signaling proteins in the immediate vicinity of synaptic receptors to facilitate an immediate response (Husi et al. 2000; Kornau et al. 1995); it prevents NR internalization and can increase receptor surface expression in both neurons and recombinant systems (Roche et al. 2001). Finally, cotransfection of PSD95 along with recombinant NRs decreases their sensitivity to glutamate, increases channel opening rates of N1/2B receptors, and reduces high-affinity zinc inhibition of N1/2A receptors (Lin et al. 2006; Rutter and Stephenson 2000; Yamada et al. 1999, 2002). In agreement with previous studies, we observed that cotransfection of PSD95 in HEK-293 cells decreased the extent of macroscopic desensitization for both recombinant N1/2B and N1/2A receptors (Sornarajah et al. 2008). Because our measurements included EDTA, a strong divalent cation chelator, we can unambiguously attribute these effects to changes in gating, thus separating them from possible effects on zinc-mediated desensitization. However, to our surprise, we did not observe substantial changes in single channel parameters of either N1/2B or N1/2A receptors upon cotransfection with PSD95. We noted, however, that the large variance in PO and MCT characteristics of recombinant N1/2B receptors, which we also observed in somatic neuronal receptors expressed in early cultures, was significantly reduced. This decrease in variance was also observed for macroscopic traces; however, the models we derived from on-cell single channel recordings were largely invariant and thus could not explain the difference in macroscopic desensitization we recorded. This result may indicate that PSD95 had no effect on channel gating per se but influenced how other intracellular signaling systems impinged on NRs. This hypothesis is consistent with a large body of knowledge on PSD95 effects on NR-mediate processes; however, it clearly requires more detailed investigation.
We also sought to determine whether cotransfection of recombinant receptors with Neto1 modifies channel kinetics. Neto proteins directly modulate the activity of KARs in both recombinant and in vivo systems and have been designated as bona fide auxiliary subunits for this receptor (Straub et al. 2011; Tang et al. 2011; Zhang et al. 2009). Conflicting findings on Neto1 interactions with and effects on NRs have left unanswered the question of whether Neto1 modifies NR channel gating (Ng et al. 2009; Straub et al. 2011). Our whole cell results showed no evidence for an effect of Neto1 cotransfection on either the macroscopic time course or single channel mechanisms for either 2B- or 2A-containing receptors. These results may simply reflect inadequate Neto1 expression or surface trafficking in our experimental conditions, a lack of interaction between NRs and Neto1 in HEK-293 cells, or more specifically the absence of effects on gating. However, we did not investigate these possibilities further.
A major impetus in completing the studies reported here has been the heterogeneity in the experimental conditions used in the literature to define NR kinetic behavior and the resulting inability to adequately compare results obtained in distinct cellular preparations, patch-clamp configurations, and receptor milieu. This lack of continuity across literature was especially problematic in delineating possible differences between recombinant and native receptors. Here, we systematically compared recombinant N1/2B and N1/2A receptors with native somatic NRs during in vitro development. By selecting experimental conditions that focused our investigations specifically on the core gating mechanisms of native and recombinant receptors, we were able to ascertain that in contrast to what is currently known about AMPARs and KARs, N1/2B and N1/2A receptors expressed in a mammalian cell line faithfully reproduced the gating behaviors of native receptors. These results support the continued use of HEK-293 cells for detailed investigations of NR mechanisms and also underscore the need for quantitative investigation into neuron-specific mechanisms of NR modulation.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Research Grants F31-NS-071782 (to W. F. Borschel) and R01-NS-052669 (to G. K. Popescu) and American Heart Association Grant EIA9100012 (to G. K. Popescu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.F.B., E.M.K., N.M.G., L.M.N., and G.K.P. conception and design of research; W.F.B., J.M.M., and T.P.S. performed experiments; W.F.B., T.P.S., and G.K.P. analyzed data; W.F.B. and G.K.P. interpreted results of experiments; W.F.B. and G.K.P. prepared figures; W.F.B. and G.K.P. drafted manuscript; W.F.B. and G.K.P. edited and revised manuscript; W.F.B. and G.K.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank T. K. Aman for assistance with neuronal culture preparation along with T. J. Ruffino for sharing single channel recordings from recombinant N1/2B receptors.
Present address of J. M. Myers: Department of Physiology and Biophysics, School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY.
Present address of T. P. Smith: Department of Chemistry, University of Wisconsin-Madison, Madison, WI.
Present address of N. M. Graziane: Department of Molecular Pharmacology, Physiology and Biotechnology, Brown University, Providence, RI.
REFERENCES
- Amico-Ruvio SA, Popescu GK. Stationary gating of GluN1/GluN2B receptors in intact membrane patches. Biophys J 98: 1160–1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-d-aspartate responses of mouse central neurones in culture. J Physiol 399: 247–266, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci 25: 7914–7923, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6: 144–152, 2003 [DOI] [PubMed] [Google Scholar]
- Bowie D. Ionotropic glutamate receptors & CNS disorders. CNS Neurol Disord Drug Targets 7: 129–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258: 1007–1011, 1992 [DOI] [PubMed] [Google Scholar]
- Chazot PL, Stephenson FA. Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem 69: 2138–2144, 1997 [DOI] [PubMed] [Google Scholar]
- Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci 19: 6844–6854, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Farrant M, Cull-Candy SG. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. J Neurosci 17: 107–116, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-d-aspartate receptor. Neuron 7: 605–613, 1991 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327–335, 2001 [DOI] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol 586: 4425–4439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 563: 345–358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MS, Collings MA, Brewer GJ. Electrophysiology of embryonic, adult and aged rat hippocampal neurons in serum-free culture. J Neurosci Methods 79: 37–46, 1998 [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Activation of N-methyl-d-aspartate receptors by l-glutamate in cells dissociated from adult rat hippocampus. J Physiol 456: 143–179, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc Biol Sci 243: 39–45, 1991 [DOI] [PubMed] [Google Scholar]
- Grimwood S, Le Bourdelles B, Whiting PJ. Recombinant human NMDA homomeric NR1 receptors expressed in mammalian cells form a high-affinity glycine antagonist binding site. J Neurochem 64: 525–530, 1995 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46: 261–274, 2005 [DOI] [PubMed] [Google Scholar]
- Hirai H, Kirsch J, Laube B, Betz H, Kuhse J. The glycine binding site of the N-methyl-d-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3-M4 loop region. Proc Natl Acad Sci USA 93: 6031–6036, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron 10: 943–954, 1993 [DOI] [PubMed] [Google Scholar]
- Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science 241: 701–703, 1988 [DOI] [PubMed] [Google Scholar]
- Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol 432: 143–202, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3: 661–669, 2000 [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Calcium permeability of the N-methyl-d-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci USA 90: 11573–11577, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature 325: 522–525, 1987 [DOI] [PubMed] [Google Scholar]
- Johansson S, Friedman W, Arhem P. Impulses and resting membrane properties of small cultured rat hippocampal neurons. J Physiol 445: 129–140, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Baudry M, Lynch G. Quinoxaline derivatives are high-affinity antagonists of the NMDA receptor-associated glycine sites. Brain Res 489: 377–382, 1989 [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781, 2004 [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Pallotta BS. Burst kinetics of single NMDA receptor currents in cell-attached patches from rat brain cortical neurons in culture. J Physiol 486: 411–426, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269: 1737–1740, 1995 [DOI] [PubMed] [Google Scholar]
- Kussius CL, Kaur N, Popescu GK. Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J Neurosci 29: 6819–6827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, et al. Molecular diversity of the NMDA receptor channel. Nature 358: 36–41, 1992 [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8: 413–426, 2007 [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron 18: 493–503, 1997 [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci 14: 3180–3194, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Quarum ML, Parker JD, Weber E, Jahr CE. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-d-aspartate receptor-associated glycine binding site. Mol Pharmacol 35: 565–570, 1989 [PubMed] [Google Scholar]
- Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci 10: 1704–1715, 1998 [DOI] [PubMed] [Google Scholar]
- Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci USA 103: 19902–19907, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321: 519–522, 1986 [DOI] [PubMed] [Google Scholar]
- Magleby KL. Modal gating of NMDA receptors. Trends Neurosci 27: 231–233, 2004 [DOI] [PubMed] [Google Scholar]
- Maki BA, Aman TK, Amico-Ruvio SA, Kussius CL, Popescu GK. C-terminal domains of NMDA (N-methyl-D-aspartic acid) receptor modulate unitary channel conductance and gating. J Biol Chem. First published September 4, 2012; doi:10.1074/jbc.M112.390013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Trimmer JS. A primary culture system for biochemical analyses of neuronal proteins. J Neurosci Methods 144: 165–173, 2005 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256: 1217–1221, 1992 [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature 354: 31–37, 1991 [DOI] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol 7: e41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science 311: 1253–1256, 2006 [DOI] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci 6: 476–483, 2003 [DOI] [PubMed] [Google Scholar]
- Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature 430: 790–793, 2004 [DOI] [PubMed] [Google Scholar]
- Popescu GK. Modes of glutamate receptor gating. J Physiol 590: 73–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Qin F, Auerbach A. Subconductance states of a mutant NMDA receptor channel kinetics, calcium, and voltage dependence. J Gen Physiol 109: 181–189, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802, 2001 [DOI] [PubMed] [Google Scholar]
- Rutter AR, Stephenson FA. Coexpression of postsynaptic density-95 protein with NMDA receptors results in enhanced receptor expression together with a decreased sensitivity to l-glutamate. J Neurochem 75: 2501–2510, 2000 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Hollmann M. Apparent homomeric NR1 currents observed in Xenopus oocytes are caused by an endogenous NR2 subunit. J Mol Biol 376: 658–670, 2008 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Hollmann M. Molecular and functional characterization of Xenopus laevis N-methyl-d-aspartate receptors. Mol Cell Neurosci 42: 116–127, 2009 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Klein C, Hollmann M. Xenopus laevis oocytes endogenously express all subunits of the ionotropic glutamate receptor family. J Mol Biol 390: 182–195, 2009 [DOI] [PubMed] [Google Scholar]
- Sornarajah L, Vasuta OC, Zhang L, Sutton C, Li B, El-Husseini A, Raymond LA. NMDA receptor desensitization regulated by direct binding to PDZ1–2 domains of PSD-95. J Neurophysiol 99: 3052–3062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci 250: 271–277, 1992 [DOI] [PubMed] [Google Scholar]
- Stern P, Cik M, Colquhoun D, Stephenson FA. Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J Physiol 476: 391–397, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci 14: 866–873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol 22: 488–495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci 31: 10009–10018, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95: 1727–1734, 2006 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-d-aspartate receptors in cerebellar neurons. Nature 345: 347–350, 1990 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268: 873–876, 1995 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Hansen KB, Traynelis SF. GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J Physiol 590: 3857–3875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD. Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci 21: 8895–8905, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3: 1138–1140, 1992 [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-d-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 11: 37–49, 2005 [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 10: 267–278, 1993 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Chochi Y, Takamiya K, Sobue K, Inui M. Modulation of the channel activity of the ε2/ζ1-subtype N-methyl-d-aspartate receptor by PSD-95. J Biol Chem 274: 6647–6652, 1999 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Iwamoto T, Watanabe Y, Sobue K, Inui M. PSD-95 eliminates Src-induced potentiation of NR1/NR2A-subtype NMDA receptor channels and reduces high-affinity zinc inhibition. J Neurochem 81: 758–764, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat Neurosci 11: 1373–1375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61: 385–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Russell SL, Pritchett DB, Molinoff PB, Williams K. Expression of mRNAs encoding subunits of the N-methyl-d-aspartate receptor in cultured cortical neurons. Mol Pharmacol 45: 846–853, 1994 [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci 18: 306–313, 1995 [DOI] [PubMed] [Google Scholar]


