Abstract
N-methyl-d-aspartate (NMDA) receptors are of critical importance for locomotion in the developing neonatal spinal cord in rats and mice. However, due to profound changes in the expression of NMDA receptors in development between the neonatal stages and adulthood, it is unclear whether NMDA receptors are still an important component of locomotion in the adult rodent spinal cord. To shed light on this issue, we have taken advantage of recently developed preparations allowing the intracellular recording of adult motoneurons that control the tail in the sacrocaudal spinal cord of adult mice and rats. We show that in the adult sacrocaudal spinal cord, NMDA induces rhythmic activity recorded on the ventral roots, often coordinated from left to right, as in swimming motions with the tail (fictive locomotion). The adult motoneurons themselves are intrinsically sensitive to NMDA application. That is, when motoneurons are synaptically isolated with TTX, NMDA still causes spontaneous bursts of rhythmic activity, depending on the membrane potential. We show that these bursts in motoneurons depend on an NMDA-mediated persistent inward current and are terminated by the progressive activation of a persistent outward current. These results indicate that motoneurons, along with the central pattern generator, can actively participate in the production of swimminglike locomotor activity in adult rodents.
Keywords: spinal cord, fictive locomotion, persistent inward current, persistent outward current
n-methyl-d-aspartate (NMDA) receptors (NMDARs) are a family of glutamate receptors widely expressed in the central nervous system. NMDARs exert a central role in numerous physiological processes, such as synaptic plasticity. In the spinal cord, NMDARs are likely involved in locomotion, as NMDA is a critical compound in the drug cocktails used to initiate fictive locomotion in isolated neonatal mouse or rat spinal cords. Locomotion requires a precise spatiotemporal activation of muscle groups to produce the right sequence of movements. The generation of locomotion in vertebrates is mainly attributed to a population of spinal interneurons called the central pattern generator (Burke et al. 2001; Grillner and Wallen 1985; Kiehn and Butt 2003; Rossignol 1996; Wallen and Grillner 1985), the role of which is to generate the basic rhythm and to send a synaptic drive to the various motor pools (McCrea and Rybak 2008). The drugs used to elicit locomotor activity in vitro (fictive locomotion) likely act on these interneurons (Goulding et al. 2002; Jessell 2000; Kiehn and Butt 2003; Lanuza et al. 2004; Wenner et al. 2000). Yet, several studies have shown that, in lamprey, frogs, turtles, and neonatal rodents, NMDA can have a direct effect on the motoneurons themselves (del Negro et al. 1999; Guertin and Hounsgaard 1998; Hsiao et al. 2002; Kim and Chandler 1995; MacLean et al. 1997; Rioult-Pedotti 1997; Wallen and Grillner 1985). In these animals, NMDA can trigger slow oscillations of the membrane potential independently of synaptic inputs onto motoneurons.
The role of NMDARs in adult mammalian motoneurons remains, however, uncertain. In rodents, NMDARs are abundant during the 1st postnatal week throughout the spinal cord but are dramatically reduced thereafter (Kalb et al. 1992; Palecek et al. 1999). For example, the monosynaptic Ia excitation to motoneuron transitions from having a significant NMDA component in the neonate to virtually none in the adult (Engberg et al. 1993). Nonetheless, some NMDARs are still present in the adult spinal cord (Monyer et al. 1994; Piehl et al. 1995; Tolle et al. 1993; Wee et al. 2008). Moreover, in the brain stem, administration of NMDA has been shown to depolarize motoneurons that control eye movements (Durand 1991, 1993). The goal of this paper is to investigate the direct action of NMDARs on adult motoneurons of rodents. To do so, we have taken advantage of the recently developed in vitro sacral cord preparation (Bennett et al. 2001; Jiang and Heckman 2006), which allowed us to perform intracellular recordings of the motoneurons innervating the tail muscles of both adult mice and rats.
MATERIALS AND METHODS
Intracellular recordings were made from motoneurons in the sacrocaudal spinal cord of adult mice (age 30–50 days old) and rats (age 80–120 days). All experimental procedures were approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee (rat experiments) and the Northwestern University Institutional Animal Care and Use Committee (mouse experiments). Procedures were identical in rat and mouse experiments.
Surgery.
Animals were deeply anesthetized with urethane (0.18 mg/100 g), and the spinal cord caudal to the T12 vertebrae was transferred to a dissection dish containing oxygenated modified artificial cerebral spinal fluid (mACSF) at room temperature (20–21°C). Following a 1-h resting period in mACSF, the cord was transferred to a recording chamber, where it was submerged in normal ACSF flowing at 3–6 ml/min and maintained at 25°C. The cord was loosely supported on a nappy paper mesh and secured by passing insect pins through lateral vasculature and connective tissue and into a silicone elastomer (Sylgard) base below the nappy paper. For intracellular recording, and not ventral root recording, the ventral surface was oriented upward and the dorsal surface of the cord was glued (with cyanoacrylate) to the nappy paper to stabilize further the cord to enable long-term intracellular recordings.
Solutions.
The normal ACSF had the composition, in mM, 122 NaCl, 24 NaHCO3, 3 KCl, 2.5 CaCl2, 1 MgSO4, and 12 glucose in distilled water, bubbled with 95% O2-5% CO2, pH 7.4. An mACSF was used during dissection and recovery to prevent excitotoxic injury. The mACSF composition was, in mM, 118 NaCl, 24 NaHCO3, 3 KCl, 1.5 CaCl2, 1.3 MgSO4, 25 glucose, 1.4 NaH2PO4, 5 MgCl2, and 1 kynurenic acid (McQuiston and Madison 1999). NMDA (Sigma-Aldrich) and tetrodotoxin (TTX-citrate; Alomone) were added to the ACSF as described in the text.
Intracellular and extracellular recording.
Intracellular recordings were obtained using sharp intracellular electrodes filled with either 3 M KCl or a mixture of 1 M K-acetate and 1 M KCl and beveled to a resistance of 25–30 MΩ using a rotary beveller (BV-10; Sutter Instruments). A Stepper Motor (Model 2660 Micropositioner; David Kopf Instruments) was used to advance the electrodes vertically into the ventral horn, and intracellular recordings from motoneurons were made with an AxoClamp 2B intracellular amplifier (Axon Instruments) running in discontinuous current clamp (switching rate 5–8 kHz, output bandwidth 10 kHz) or discontinuous single-electrode voltage clamp (gain 0.8–2.5 nA/mV) modes and sampled at 6.7 kHz with a Clampex system (Axon Instruments) or at 20 kHz with a Spike2/1401plus system (Cambridge Electronic Devices). The ventral roots were wrapped around Ag/AgCl wire electrodes and sealed with grease, which allowed for antidromic stimulation identification of motoneurons. Motoneurons with a resting potential below −60 mV and antidromic spike overshoot >0 mV were considered healthy and used for recording. In some animals, we also recorded from the ventral roots on the Ag/AgCl electrodes (ventral root extracellular recording).
RESULTS
NMDA-induced bursting.
As a first step, we examined whether bath application of NMDA could alter the activity of adult mouse and rat motoneurons by recording the global activity of the motor pools in the ventral roots S2–S4 that innervate the tail. As illustrated in Fig. 1, administration of NMDA (30–80 μM in both mice and rats) usually induced a regular bursting in the sacral ventral roots that began within a few minutes and persisted for several minutes (n = 9/13 mouse spinal cords; n = 9/12 rat cords). In all cases, whether or not bursts could be observed, a significant increase of tonic activity was observed on all ventral roots recorded, and oscillations superimposed on this tonic activity could sometimes be seen lasting from minutes to hours. Washout of NMDA eliminated this regular bursting and tonic activity within a few minutes (data not shown). Bursting activity was often organized in an alternating left-to-right manner (Fig. 1B), which, in the intact animal, would move the tail side-to-side in a swimming motion, as seen in the neonatal rat (Delvolve et al. 2001). This organized bursting became less clear once the tonic activity emerged (Fig. 1B, right).
Fig. 1.
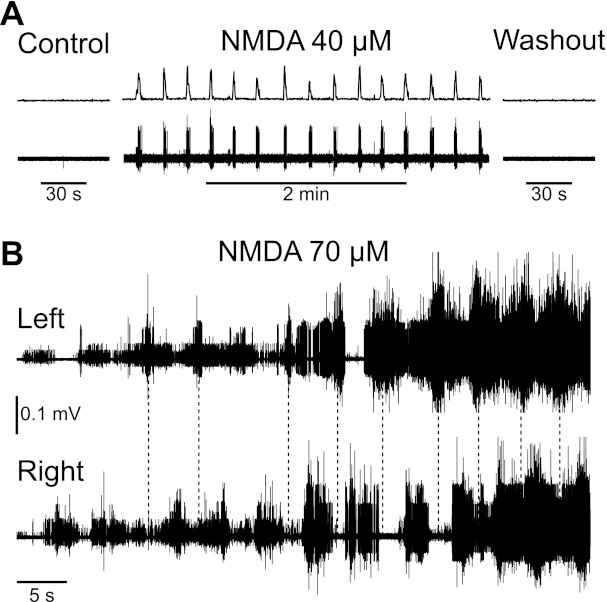
N-methyl-d-aspartate (NMDA)-induced bursting activity on ventral roots. A: root recording from a mouse spinal cord showing the rectified and integrated activity on the right ventral root S2 (top trace) and the raw electrical activity recorded on ventral root S2 (bottom trace). The left part of the trace illustrates the control condition with no NMDA present in the bath. Little to no spontaneous activity was visible in those conditions. The middle part shows the bursts that appeared on the ventral root S2 when NMDA was applied to the bath. When the NMDA was washed out (right part), the activity returned to its initial quiescent state. B: recordings from the left and right S4 ventral roots of a rat spinal cord. In presence of NMDA (75 μM), bursts of activity could be observed on the roots, alternating from left to right (vertical dashed lines demark leftside bursts) and increasing with time after application (application 3 min before recording). Eventually, tonic synchronous activity also emerged (right of plot).
Bursts are intrinsic to motoneurons.
To investigate the role of motoneurons in NMDA-induced bursts, we performed intracellular recordings in 11 mouse and 15 rat sacrocaudal motoneurons. A typical example is illustrated in Fig. 2. In this mouse motoneuron, bath application of NMDA (75 μM) induced, within 1 min, a steady depolarization that brought the motoneuron to threshold. After a minute of slow, long, repeated bursts of firing, the motoneuron began firing tonically. Using a bias current to drive the membrane potential to a more hyperpolarized value, we observed shorter bursts ∼1 s in duration, each of which started spontaneously. These bursts repeated about every 6 s, as long as the cell was held hyperpolarized (Fig. 2, right). Overall, we observed similar spontaneous bursts in 8 of the 11 mouse and 14 of 15 rat motoneurons (73 and 93%, respectively) recorded. However, they were sometimes masked by the global depolarization of the motoneuron and required various amounts of hyperpolarizing current to be revealed. Generally, we found that motoneuron bursting required NMDA doses >30 μM, similar to bursting in ventral roots.
Fig. 2.
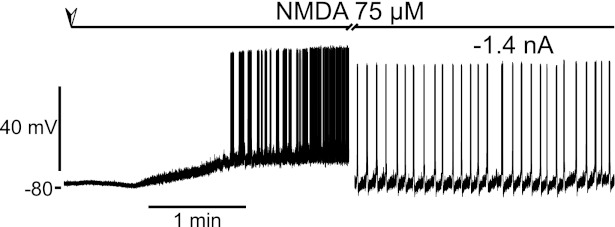
NMDA-induced bursting in a motoneuron recorded intracellularly. Intracellular recording was from a mouse motoneuron. Bath perfusion of NMDA was started at the point indicated by the top arrow and lasted for the whole duration of the recording. The cell depolarized and then burst repeatedly for a minute followed by tonic firing. The rightmost part of the trace was obtained with a hyperpolarizing bias current of −1.4 nA, and the cell produced repeated short bursts of firing.
Two opposite hypotheses can be proposed to explain these bursts. Either they are created by premotor interneurons impinging on the motoneurons that become rhythmically active in presence of NMDA or the bursts are intrinsic to the motoneurons. In the former hypothesis, bursts of synaptic activity should remain when the motoneuron is hyperpolarized below the activation threshold for the NMDARs, and the bursts should disappear in presence of TTX. In the latter hypothesis, hyperpolarization of the membrane should inactivate the intrinsic current(s) responsible for the bursts, and, in presence of TTX, the underlying depolarization should remain visible.
We found that increasing hyperpolarization progressively reduced the burst frequency and duration until bursts disappeared completely at the most hyperpolarized levels (n = 7/7 mouse cells and 15/15 rat cells). The motoneuron illustrated in Fig. 3 showed spontaneous bursts at rest (without bias current). Hyperpolarizing the motoneuron with −0.4 nA of bias current slightly decreased the frequency and duration of the bursts. Further hyperpolarization decreased the frequency and duration of the bursts even more. For bias currents more than −1.5 nA, the bursts disappeared completely, and no subthreshold oscillations, indicative of bursts of synaptic activity, could be observed. This observation is consistent with an intrinsic origin for the NMDA-induced bursts in motoneurons. Importantly, these intracellular data were collected from cords that had glue on their dorsal surface (unlike during ventral root recording) to hold the cord to the bottom of the dish (nappy paper) to stabilize mechanically the intracellular recordings. This likely did not favor interneuron-mediated NMDA bursting because of the poorer oxygenation of the glued dorsal surface. Indeed, when we did not use the glue on the cord, then subthreshold oscillations could be seen in NMDA under hyperpolarized conditions (n = 4/4 cords; data not shown), suggesting additional involvement of interneurons, although the recordings did not last long because of mechanical instability (see discussion).
Fig. 3.
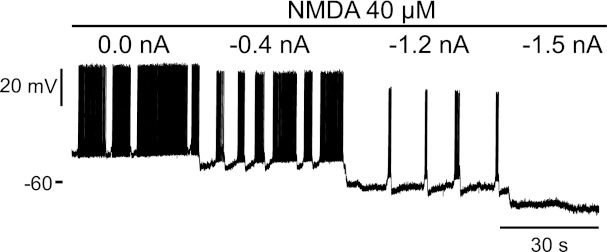
Effect of hyperpolarization on the NMDA-induced bursts. Intracellular recording was from a mouse motoneuron. On continuous bath application of NMDA 40 μM (top line), this motoneuron was driven to more and more hyperpolarized potentials with a bias current (intensity written on top of the trace). As the motoneuron got more and more hyperpolarized, the frequency and duration of the bursts decreased, and they even stopped completely at −1.5 nA of bias current.
To confirm the involvement of motoneurons in bursting, we applied TTX (1 μM) after the initiation of the NMDA bursts to see whether they persisted. A typical example is shown in Fig. 4. In this motoneuron, bath application of NMDA (30 μM) initiated spontaneous bursts of activity. Adding TTX (1 μM) in addition to the NMDA blocked the spikes, as expected, but failed to block the underlying depolarizations: waves of spontaneous depolarization remained, followed a few seconds later by a sharp return to baseline. The same behavior was observed in three of the four mouse cells tested with TTX and six of the six rat cells tested.
Fig. 4.
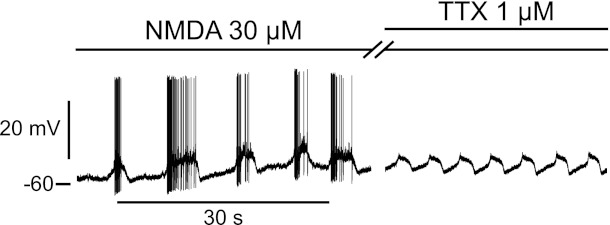
NMDA-induced bursts persist in presence of TTX. This mouse motoneuron exhibited spontaneous bursts of activity in presence of NMDA (2nd line from the top). Adding TTX (1 μM; top line) in addition to the NMDA stopped the firing but not the underlying depolarizations causing the bursting, as indicated by the waves of depolarization still visible on the right.
Ionic origin of the NMDA-induced oscillations.
To investigate the origin of the NMDA-induced bursting, we performed voltage-clamp experiments in 15 adult rat motoneurons in the presence of TTX. With TTX, both the synaptic input to motoneurons (spike mediated) and the sodium component of the persistent inward current (Na PIC) were blocked, but there remained a Ca PIC. This Ca PIC was seen in voltage clamp during a slow ramp to produce a downward deflection in current (thick line in Fig. 5) relative to the extrapolated leak current (thin line). This Ca PIC was activated at −55 ± 7 mV (Von; n = 6), near the usual sodium spike threshold but significantly above the resting potential (−70 ± 7 mV, n = 6; P < 0.05), as previously described (Harvey et al. 2006; Li et al. 2004). On average, the Ca PIC amplitude was −0.8 ± 0.4 nA (measured at initial peak in current). Importantly, the Ca PIC remained activated even when the voltage was ramped back down below where it was initiated (Von) and thus produced a clear hysteresis, characteristic of this current.
Fig. 5.
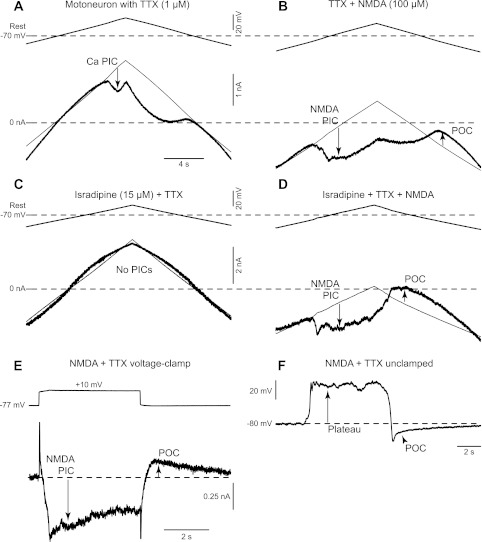
Currents responsible for the NMDA-induced bursting. A: Ca persistent inward current (PIC) in a rat motoneuron recorded in presence of TTX (1 μM), activated by slowly increasing the membrane potential under voltage clamp (top trace), and quantified at its initial peak, where it produced a downward deflection in the recorded current (at arrow) relative to the leak current (thin line). B: voltage-clamp recording of a rat motoneuron in presence of NMDA (100 μM) and TTX (1 μM). Same organization as A. In these conditions, the Ca PIC is hidden by a larger NMDA PIC (downward arrow), and, at the end of the voltage ramp, a persistent outward current (POC; upward arrow) becomes visible. C: voltage-clamp recording of a rat motoneuron in presence of TTX (1 μM) and isradipine (15 μM). Same organization as A. Isradipine is used to eliminate Ca PIC and NMDA PIC interactions by blocking the Ca PIC. As a consequence, the voltage ramp gave a linear current response. D: voltage-clamp recording of a rat motoneuron in presence of TTX (1 μM), isradipine (15 μM), and NMDA (100 μM). Same organization as A. Even when the Ca PIC is blocked by isradipine, clear NMDA PIC and POC are visible on this recording. E: response (bottom thick trace) of a rat motoneuron to a 10-mV voltage step from −77 to −67 mV (top trace). The NMDA PIC is initially visible as a downward deflection in the current trace (downward arrow). The net inward current then decreased continuously after this peak due to the progressive activation of the POC (upward arrow). F: current-clamp recording of the same motoneuron as in E showing the NMDA-mediated depolarization (plateau), which spontaneously turns off and reveals an hyperpolarization phase due to the POC.
After application of NMDA (75–100 μM), a large and completely different PIC emerged that we term the NMDA PIC (Fig. 5B). This current was initiated at a very hyperpolarized potential (−76 ± 7 mV, Von NMDA, n = 6), approximately at the resting membrane potential before NMDA (not significantly different from rest) and significantly lower than the Ca PIC onset voltage before NMDA (P < 0.05). With NMDA present, the motoneurons had to be held with a negative bias current of about −2 nA to prevent the NMDA PIC activation because NMDA significantly reduced the overall PIC onset current (Ion) from +1.0 ± 0.7 nA before NMDA (Ca PIC) to −2.1 ± 1.1 nA in NMDA (NMDA PIC onset current). The NMDA PIC amplitude was −2.2 ± 0.9 nA (initial peak PIC), nearly three times larger than the Ca PIC before NMDA (P < 0.05). Unlike the Ca PIC, the NMDA PIC tended to inactivate with time and lacked inward current hysteresis. That is, a few seconds after the NMDA PIC was activated, it slowly decreased and reversed to a significant persistent outward current (POC; +0.6 ± 0.3 nA, measured relative to the leak line after PIC end as in Fig. 5B), which remained activated as the voltage was ramped back to rest. Before NMDA, no significant POC existed (0.1 ± 0.2 nA, measured at the same voltage). These NMDA PICs and POCs were readily distinguished from the Ca PIC in most motoneurons because they typically were much larger and lower threshold than the Ca PICs and indeed usually masked any sign of Ca PIC activation in the presence of NMDA (Fig. 5, A and B). However, in 1 cell, we saw the Ca and NMDA PICs come on at separate potentials (as seen by 2 negative slope regions in the current response on the upward ramp; data not shown).
To eliminate Ca PIC and NMDA PIC interactions, we investigated the action of NMDA in isolation in five rat motoneurons that had the Ca PIC blocked with isradipine (15 μM) and synaptic input and Na PIC blocked with TTX (Fig. 5C). In these motoneurons, before NMDA application, a voltage ramp gave a linear current response, confirming the block of the Ca and Na PICs (n = 5/5 cells), as previously detailed (Li and Bennett 2003). Application of NMDA (75–100 μM) produced a low-threshold and large PIC (NMDA PIC; Fig. 5D) with the same asymmetric characteristics followed by a POC, as seen before the Ca PIC block. That is, the NMDA PIC was activated at −71 ± 5 mV, again near the predrug resting potential (−69 ± 10 mV), and required a negative bias current to prevent activation (activation current −0.9 ± 1.5 nA). This NMDA PIC was on average −1.7 ± 0.9 nA (initial peak) and not significantly different from the NMDA PIC measured without the Ca PIC block, confirming that the NMDA PIC dominates over the Ca PIC. Again, the NMDA PIC decreased slowly with time and was replaced by significant net outward current (POC) that could always be seen on the downward ramp of the voltage-clamp protocol, which was on average +0.7 ± 0.3 nA.
To investigate further the timing of these NMDA currents, we initially held the motoneurons at a potential below the resting potential to prevent the NMDA PIC activation and then applied steps in voltage (Fig. 5E; n = 5). Steps over the resting potential initiated the NMDA PIC with a time to peak current of ∼0.5 s. The net inward current then decreased continuously after this peak, suggesting the onset of a POC that increased with time. This POC could be seen in isolation when the voltage step was terminated, and then lasted >2 s, in all cells tested (n = 5/5). It was clear that these currents caused plateau potentials and membrane oscillations when the cells were not under voltage-clamp control (Fig. 5F). That is, in these same cells, releasing the voltage clamp and removing all bias current led to a rapid depolarization (plateau), mediated by the NMDA PIC (at its activation threshold). When a small negative bias current was applied, the membrane potential oscillated slowly. That is, a plateau was again activated but then slowly decreased to a point where there was a sudden termination of the plateau and the onset of an afterhyperpolarization that lasted for a few seconds, which was mediated by the POC (at same voltage). At the end of this POC-mediated afterhyperpolarization, the plateau was reactivated and the whole process continued, leading to the observed spontaneous oscillations (Fig. 6A; n = 5/5 cells). When additional depolarizing bias current was applied during these oscillations, the NMDA plateau lasted longer (Fig. 6B), thus explaining the longer bursts of firing seen at depolarized levels without TTX (Fig. 3).
Fig. 6.
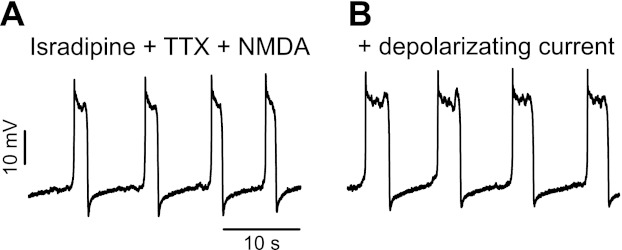
Voltage dependence of the NMDA-induced plateaus. Current-clamp recording of a rat motoneuron in presence of isradipine (15 μM), TTX (1 μM), and NMDA (100 μM) is shown. This motoneuron exhibited spontaneous NMDA plateaus at rest, which terminated and restarted repeatedly. When the cell was depolarized by injection of a bias current (0.5 nA), the plateaus lasted longer.
DISCUSSION
We demonstrate here that in the adult rodent spinal cord, the motoneurons innervating the muscles of the tail are sensitive to application of NMDA. NMDA induces intrinsic bursts of activity in these motoneurons, which suggests that they can take an active role in the production of the rhythmic activity required for swimming. In general, our results support the possibility that locomotion in the adult state shares the NMDA-driven behavior seen in the neonatal preparation (Kiehn et al. 2000; MacLean et al. 1997; Schmidt et al. 1998; Wallen and Grillner 1987).
Ionic conductances responsible for the bursts.
We showed that the bursts and the underlying waves of depolarization can be elicited in absence of all synaptic activity since they remained in presence of TTX. They are therefore partly due to an intrinsic property of the motoneurons. The bursts are reminiscent of plateau potentials classically described in other motoneurons and caused by Ca PICs. However, Ca PICs are not very strong in our experimental conditions where the cords are acutely removed from normal rats and mice, and thus levels of serotonin and norepinephrine are low (M. Manuel, S. M. ElBasiouny, and C. J. Heckman, unpublished observations; Li and Bennett 2003; Murray 2010), and, furthermore, NMDA-induced bursting persisted in a block of Ca PICs and Na PIC. Thus these bursts are likely mediated by an NMDA current from the NMDARs themselves.
The NMDA current, because of its magnesium block, has a voltage dependence and thus behaves like the Ca and Na PICs described in motoneurons (Flatman et al. 1983; MacDonald et al. 1982; Mayer et al. 1984; Nowak et al. 1984). Voltage-clamp experiments presented here show that after NMDA application, motoneurons exhibit a new PIC that is readily distinguishable from the Na and Ca PICs. This PIC (NMDA PIC) is the current sustaining the depolarizing phase of the slow waves of depolarization (NMDA Plateau). Activation of the NMDA PIC is followed by the activation of an outward current (POC), which is the current responsible for the spontaneous termination of the NMDA plateaus. We suggest that this POC was produced by the calcium entering the cell through the NMDAR and slowly activating a calcium-activated current (SK) that persisted until the calcium was sequestered, as this was proven to be the mechanism responsible for burst termination in the lamprey (el Manira et al. 1994; Wallen and Grillner 1987), although we cannot rule out the role of Na+-K+-ATPase pump currents (del Negro et al. 1999; Hsiao et al. 2002; Kim and Chandler 1995).
Functional consequences.
Our results show that, under the right set of conditions, activation of NMDARs on adult motoneurons can lead to their direct recruitment, including production of rhythmic bursts of activity. The motoneurons of the sacrocaudal region of the spinal cord are connected to the muscles of the tail (Brink and Pfaff 1980; Grossman et al. 1982; Masson et al. 1991), and although we did not identify whether the recorded motoneurons were connected to extensor or flexor muscle groups, it has been shown that bursts of activity in sacrocaudal motoneurons of neonatal rats are able to elicit left-to-right abductions of the tail, similar to during swimming (Delvolve et al. 2001). Thus our results are consistent with the NMDA-mediated input to motoneurons occurring during locomotion. A fundamental role of NMDAR activation on motoneurons in the adult state during rhythmic activity is supported by the recent study of Enríquez Denton et al. (2012). This study showed that block of NMDARs markedly suppressed respiratory oscillations in phrenic motoneurons. Although this study was done using an in vivo preparation (decerebrate cat), this block was achieved via injection of the drug from an intracellular microelectrode, showing that the NMDARs blocked were highly likely to be on the motoneurons themselves. There may, however, also be sensory inputs that activate NMDARs on motoneurons. Ia monosynaptic excitatory postsynaptic potentials (EPSPs) are not mediated by NMDARs to any significant degree (Engberg et al. 1993), but Brownstone et al. (1994) demonstrated that EPSPs generated by stimulation of flexion reflex afferents undergo a voltage-dependent amplification in an anesthetized preparation where there is unlikely to be any contribution of amplification from Ca and Na PICs (Hultborn et al. 2003; Lee and Heckman 2000; Svirskis and Hounsgaard 1998). Thus the voltage-dependent amplification of this sensory input may involve NMDAR activation, but this possibility has not yet been tested.
Although our data clearly demonstrate that NMDA can induce rhythmic alternation in motoneurons, the relative contribution of NMDA oscillations in motoneurons compared with interneurons is harder to assess. Considering that motoneurons are not electrically or chemically coupled in adults rodents (Chang et al. 1999; Kandler and Katz 1995; Walton and Navarrete 1991), it is likely that any intrinsic oscillatory activity in a single motoneuron will not be synchronized with the activity of other motoneurons, and thus NMDA oscillations in motoneurons cannot by themselves initiate the organized locomotor-like activity of motoneurons pools seen in our ventral root recordings. Thus interneuron oscillations must initiate the synchronous activation of a given motoneuron pool (say, leftside) and coordinate the transition to other pool activity (left-to-right alternations). The NMDA PIC currents in the motoneurons would then contribute to amplifying and prolonging this underlying interneuron drive to the motoneuron pools. Likewise, NMDA POCs in motoneurons would help terminate bursts during locomotor behavior. Also, excess NMDA would lead to tonic activation of NMDA PICs on motoneurons and explain the tonic activity seen to follow the rhythmic activity in motoneuron pools.
Our finding that NMDA-induced oscillations in motoneurons can sometimes be eliminated by hyperpolarizing a motoneuron does not rule out the involvement of interneurons because the experimental conditions that we used to make stable intracellular motoneuron recordings do not favor interneuron activity, as the spinal cord was glued on its dorsal surface, unlike during ventral root recordings. We have found that interneuron-mediated NMDA oscillations can be seen in motoneurons under hyperpolarized conditions when we reduce or eliminate the glue, although recordings are not stable, thus verifying this experimental limitation.
The electrical behavior of the adult motoneuron has proven to be unexpectedly complex. At least three distinct states have now been demonstrated. First, for many years, the properties of motoneurons were assumed to conform to their behavior in anesthetized preparation when driven by ionotropic inputs (reviewed by Binder et al. 1996). Perhaps this relatively unexcitable state can be considered the base state of the motoneuron.
The discovery of Ca PICs (Schwindt and Crill 1977) and their neuromodulation by the brain stem via axons that release either serotonin or norepinephrine (Holstege and Kuypers 1987; Hounsgaard et al. 1988; Hounsgaard and Kiehn 1989) identified a new and highly excitable state of motoneurons controlled by neuromodulators (2nd state, neuromodulatory state). Work in several laboratories established that PICs are likely to be a standard component of motoneuron behavior in normal behavior in both animals and humans (reviewed in Binder 2002; Heckmann et al. 2005; Hornby et al. 2002). Serotonin and norepinephrine also depolarize resting membrane potentials and hyperpolarize spike thresholds (Binder et al. 1996; Powers and Binder 2001) so that these brain-stem neuromodulatory systems have the capacity to alter greatly the net input-output gain of motoneurons (Heckman et al. 2008b, 2009; Hultborn et al. 2004). The PIC effects are in fact so strong that a major role of inhibitory inputs may be to control excess motoneuron excitability by deactivating the PIC, providing a focused control to oppose the diffuse and widespread effects of the brain-stem neuromodulatory system (Bennett et al. 1998; Heckman et al. 2008a; Hyngstrom et al. 2007). Inhibition of the PIC may often be coupled to excitation in a push-pull fashion to achieve this goal (Bennett et al. 1998; Johnson et al. 2012). Equally important, strong neuromodulatory inputs originate within the spinal cord (Power et al. 2010), further contributing to this neuromodulatory state, with for example the motoneuron afterhyperpolarization controlled by the cholinergic inputs activating the large “C” synaptic boutons on motoneurons (Miles et al. 2007; Zagoraiou et al. 2009).
The NMDA-driven motoneuron activity demonstrated in the present paper is yet another state of the motoneuron (3rd state). This NMDA state is inherently oscillatory and thus seems well-suited to locomotion. The input-output properties of motoneurons thus have the potential to be reconfigured for different motor behaviors, depending on the mixture of inputs from brain-stem neuromodulatory centers, and spinal neuromodulatory and NMDA systems. Many questions remain about how these systems interact in generating the wide range of normal motor behaviors. One particularly important question is how these systems change in pathological states. There is remarkable recovery of Ca PIC activity after spinal cord injury via constitutive activity of receptors for 5-HT and NE (Murray et al. 2010, 2011; Rank et al. 2011), but changes in NMDA inputs require further study.
GRANTS
M. Manuel was supported by the Fondation pour la Recherche Médicale and the Milton Safenowitz Post Doctoral Fellowship for ALS Research (ALS Association). S. M. ElBasiouny was supported by the Tim E. Noël Fellowship from the ALS Society of Canada. This work was financed by Canadian Institutes of Health Research and National Institutes of Health National Institute of Neurological Disorders and Stroke Grants NS-034382, NS-071951, and NS-047567.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M. Manuel and S. M. ElBasiouny collected and analyzed the mouse data. Y. Li, A. Griener, K. Murray, and D. J. Bennett collected and analyzed the rat data. M. Manuel, C. J. Heckman, and D. J. Bennett wrote the article.
ACKNOWLEDGMENTS
We thank Dr. Jack Miller, Rochelle Bright, and Leo Sanelli for technical assistance.
REFERENCES
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001 [DOI] [PubMed] [Google Scholar]
- Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev 40: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996 [Google Scholar]
- Brink EE, Pfaff DW. Vertebral muscles of the back and tail of the albino rat (Rattus norvegicus albinus). Brain Behav Evol 17: 1–47, 1980 [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp Brain Res 102: 34–44, 1994 [DOI] [PubMed] [Google Scholar]
- Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 86: 447–462, 2001 [DOI] [PubMed] [Google Scholar]
- Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J Neurosci 19: 10813–10828, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Negro CA, Hsiao CF, Chandler SH. Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol 81: 1478–1485, 1999 [DOI] [PubMed] [Google Scholar]
- Delvolve I, Gabbay H, Lev-Tov A. The motor output and behavior produced by rhythmogenic sacrocaudal networks in spinal cords of neonatal rats. J Neurophysiol 85: 2100–2110, 2001 [DOI] [PubMed] [Google Scholar]
- Durand J. NMDA actions on rat abducens motoneurons. Eur J Neurosci 3: 621–633, 1991 [DOI] [PubMed] [Google Scholar]
- Durand J. Synaptic excitation triggers oscillations during NMDA receptor activation in rat abducens motoneurons. Eur J Neurosci 5: 1389–1397, 1993 [DOI] [PubMed] [Google Scholar]
- el Manira A, Tegner J, Grillner S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J Neurophysiol 72: 1852–1861, 1994 [DOI] [PubMed] [Google Scholar]
- Engberg I, Tarnawa I, Durand J, Ouardouz M. An analysis of synaptic transmission to motoneurones in the cat spinal cord using a new selective receptor blocker. Acta Physiol Scand 148: 97–100, 1993 [DOI] [PubMed] [Google Scholar]
- Enríquez Denton M, Wienecke J, Zhang M, Hultborn H, Kirkwood PA. Voltage-dependent amplification of synaptic inputs in respiratory motoneurones. J Physiol 590: 3067–3090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman JA, Schwindt PC, Crill WE, Stafstrom CE. Multiple actions of N-methyl-d-aspartate on cat neocortical neurons in vitro. Brain Res 266: 169–173, 1983 [DOI] [PubMed] [Google Scholar]
- Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol 12: 508–515, 2002 [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P. The ionic mechanisms underlying N-methyl-d-aspartate receptor-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neurosci Lett 60: 289–294, 1985 [DOI] [PubMed] [Google Scholar]
- Grossman ML, Basbaum AI, Fields HL. Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms). J Comp Neurol 206: 9–16, 1982 [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. NMDA-induced intrinsic voltage oscillations depend on l-type calcium channels in spinal motoneurons of adult turtles. J Neurophysiol 80: 3380–3382, 1998 [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586: 1225–1231, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120: 2040–2054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience 23: 809–821, 1987 [DOI] [PubMed] [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve 25: 632–648, 2002 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Wu N, Levine MS, Chandler SH. Development and serotonergic modulation of NMDA bursting in rat trigeminal motoneurons. J Neurophysiol 87: 1318–1328, 2002 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552: 945–952, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007 [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000 [DOI] [PubMed] [Google Scholar]
- Jiang MC, Heckman CJ. In vitro sacral cord preparation and motoneuron recording from adult mice. J Neurosci Methods 156: 31–36, 2006 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Hyngstrom AS, Manuel M, Heckman CJ. Push-pull control of motor output. J Neurosci 32: 4592–4599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb RG, Lidow MS, Halsted MJ, Hockfield S. N-methyl-d-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc Natl Acad Sci USA 89: 8502–8506, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Opin Neurobiol 5: 98–105, 1995 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol 70: 347–361, 2003 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull 53: 649–659, 2000 [DOI] [PubMed] [Google Scholar]
- Kim YI, Chandler SH. NMDA-induced burst discharge in guinea pig trigeminal motoneurons in vitro. J Neurophysiol 74: 334–346, 1995 [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004 [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Porietis AV, Wojtowicz JM. l-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res 237: 248–253, 1982 [DOI] [PubMed] [Google Scholar]
- MacLean JN, Schmidt BJ, Hochman S. NMDA receptor activation triggers voltage oscillations, plateau potentials and burstinq in neonatal rat lumbar motoneurons in vitro. Eur J Neurosci 9: 2702–2711, 1997 [DOI] [PubMed] [Google Scholar]
- Masson RL, Jr, Sparkes ML, Ritz LA. Descending projections to the rat sacrocaudal spinal cord. J Comp Neurol 307: 120–130, 1991 [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261–263, 1984 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity induces an afterdepolarization in a subpopulation of hippocampal CA1 interneurons. J Neurosci 19: 5703–5710, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105: 731–748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465, 1984 [DOI] [PubMed] [Google Scholar]
- Palecek JI, Abdrachmanova G, Vlachova V, Vyklick L., Jr Properties of NMDA receptors in rat spinal cord motoneurons. Eur J Neurosci 11: 827–836, 1999 [DOI] [PubMed] [Google Scholar]
- Piehl F, Tabar G, Cullheim S. Expression of NMDA receptor mRNAs in rat motoneurons is down-regulated after axotomy. Eur J Neurosci 7: 2101–2110, 1995 [DOI] [PubMed] [Google Scholar]
- Power KE, McCrea DA, Fedirchuk B. Intraspinally mediated state-dependent enhancement of motoneurone excitability during fictive scratch in the adult decerebrate cat. J Physiol 588: 2839–2857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Rank MM, Murray KC, Stephens MJ, D'Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol 105: 410–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS. Intrinsic NMDA-induced oscillations in motoneurons of an adult vertebrate spinal cord are masked by inhibition. J Neurophysiol 77: 717–730, 1997 [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, p. 173–216 [Google Scholar]
- Schmidt BJ, Hochman S, MacLean JN. NMDA receptor-mediated oscillatory properties: potential role in rhythm generation in the mammalian spinal cord. Ann NY Acad Sci 860: 189–202, 1998 [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res 120: 173–178, 1977 [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol 79: 45–50, 1998 [DOI] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci 13: 5009–5028, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen P, Grillner S. The effect of current passage on N-methyl-d-aspartate-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neurosci Lett 56: 87–93, 1985 [DOI] [PubMed] [Google Scholar]
- Wallen P, Grillner S. N-methyl-d-aspartate receptor-induced, inherent oscillatory activity in neurons active during fictive locomotion in the lamprey. J Neurosci 7: 2745–2755, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J Physiol 433: 283–305, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee KS, Zhang Y, Khanna S, Low CM. Immunolocalization of NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord. J Comp Neurol 509: 118–135, 2008 [DOI] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ, Matise MP. Topographical and physiological characterization of interneurons that express engrailed-1 in the embryonic chick spinal cord. J Neurophysiol 84: 2651–2657, 2000 [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


