Abstract
Recent work has investigated the link between motor learning and sensory function in arm movement control. A number of findings are consistent with the idea that motor learning is associated with systematic changes to proprioception (Haith A, Jackson C, Mial R, Vijayakumar S. Adv Neural Inf Process Syst 21: 593–600, 2008; Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. J Neurosci 30: 5384–5393, 2010; Vahdat S, Darainy M, Milner TE, Ostry DJ. J Neurosci 31: 16907–16915, 2011). Here, we tested whether motor learning could be improved by providing subjects with proprioceptive training on a desired hand trajectory. Subjects were instructed to reproduce both the time-varying position and velocity of novel, complex hand trajectories. Subjects underwent 3 days of training with 90 movement trials per day. Active movement trials were interleaved with demonstration trials. For control subjects, these interleaved demonstration trials consisted of visual demonstration alone. A second group of subjects received visual and proprioceptive demonstration simultaneously; this group was presented with the same visual stimulus, but, in addition, their limb was moved through the target trajectory by a robot using servo control. Subjects who experienced the additional proprioceptive demonstration of the desired trajectory showed greater improvements during training movements than control subjects who only received visual information. This benefit of adding proprioceptive training was seen in both movement speed and position error. Interestingly, additional control subjects who received proprioceptive guidance while actively moving their arm during demonstration trials did not show the same improvement in positional accuracy. These findings support the idea that the addition of proprioceptive training can augment motor learning, and that this benefit is greatest when the subject passively experiences the goal movement.
Keywords: human motor learning, proprioception, arm movements, reaching, sensorimotor plasticity
a number of recent studies have tested the degree to which motor learning directly influences sensory perception. Visual perception of object motion changes after motor adaptation to a novel force field (Brown et al. 2007). Increased visual sensitivity can also develop near the functional end of learned tools (Brown et al. 2011). The perception of movement curvature and movement symmetry can be changed through the provision of altered visual feedback (Cressman and Henriques 2009; Malfait et al. 2008).
There is also evidence that proprioception is affected by recent motor learning. The sense of hand position changes after visuomotor adaptation (Cressman and Henriques 2009; Haith et al. 2008). A similar sensory change has been observed after adaptation to novel forces. Learning to reach in the presence of a directional force field results in systematic changes to the perception of one's hand position (Ostry et al. 2010). Specifically, the sensed hand position becomes biased in the direction of the learned load. This change in perceptual bias is not observed when subjects merely experience the same trajectories passively, and therefore seems to occur directly as part of a sensorimotor learning process.
Distinct from perceptual bias, the sense of hand position can also be tuned to greater precision. Learning to generate accurate movements also results in improvements in sensory acuity (Wong et al. 2011), and the improved sense of limb position is spatially localized to the area of training. It thus might be hypothesized that the learning process includes both sensory and motor changes that together mediate new behavior (Vahdat et al. 2011).
Here we tested the hypothesis that proprioceptive training can improve motor learning. Subjects were provided with the task of reproducing a specific hand trajectory, either a circle at constant velocity or a handwritten word (see Figs. 1 and 8). Throughout the training period subjects were regularly provided with a visual demonstration of the desired movement. Experimental subjects were additionally provided with proprioceptive information of the desired trajectory. The hand was moved by a robot precisely through the desired time-varying positions in concert with visual presentation of the hand's desired location. Subjects who experienced this additional proprioceptive information were better able to learn the desired movement.
Fig. 1.

Experimental apparatus and learning task. A: subjects performed arm movements while grasping a robotic manipulandum and attempted to draw a perfect circle. B: position trace of the circle in space (x and y coordinates) and as functions of time for example subject: final baseline movement, shown in black, relative to desired movement, in gray.
Fig. 8.

Cursive writing experiment. In this experiment subjects were required to copy the written proper noun “Liz,” shown from an overhead view (left) and by x (top right) and y (bottom right) coordinates as functions of time. One example subject, final baseline movement, is shown in black relative to the desired movement in gray.
MATERIALS AND METHODS
Subjects.
Seventy subjects between 17 and 38 yr of age (38 women, 32 men; mean age = 22.51 yr) were randomly assigned to one of four groups. All subjects reported no history of visual, neurological, or musculoskeletal disorder. Twelve subjects were assigned to each of the passive (PASS) and control (CTRL) groups (of both circle and cursive writing tasks; see results). Eleven subjects were assigned to each of the additional reverse (REV) and active (ACT) groups. REV subjects controlled for the possibility that proprioceptive information about any movement (and not proprioceptive information about the desired movement itself) might result in learning benefits (see Proprioceptive specificity). ACT subjects tested whether proprioceptive information during active movement results in even further benefits to motor learning (see Active proprioceptive training). Finally, 12 subjects were assigned to each of two additional movement groups, writing-passive (wPASS) and writing-control (wCTRL), which were used to test whether proprioceptive training benefits more complex movements. Written informed consent was obtained from each subject prior to participation. The University of Western Ontario Research Ethics Board approved all procedures.
Apparatus.
Subjects performed reaching movements while grasping the handle of a robotic manipulandum (InMotion Technologies) in the right hand. A six-axis force transducer (ATI Industrial Automation, Apex, NC; resolution: 0.05 N), located inside the handle, measured forces applied by the hand. All subjects were seated at a desk and interacted with the experimental robot in the horizontal plane at shoulder height (see Fig. 1). A custom air-sled, placed beneath the subject's right elbow, supported the arm against gravity and minimized friction between the arm and the desk. During motor learning, visual information was displayed via a mirror and LCD monitor display system (Wong et al. 2011). The horizontal mirror was placed just below chin height and occluded the subject's view of his/her arm. Visual feedback of hand position was provided on the mirror in real time with the LCD display.
Experimental protocol.
The experiment occurred over three consecutive days, taking ∼25 min per day. At the beginning of the experiment and after a brief 10-movement introduction to the experimental apparatus and task goal, a set of 20 baseline movement trials were recorded. After this baseline, subjects began proprioceptive training, during which active movement trials were interleaved with demonstration trials. We manipulated the information provided during demonstration trials depending on subject group.
Movement task.
The complete set of 240 training movements (+ 80 demonstration trials) after baseline were divided into blocks of 30 movements. The goal movement was a perfect circle, radius 10 cm. This movement was chosen for several reasons. First, the movement is challenging: performance of the desired trajectory at sufficient speed does not result in asymptotic performance after very brief practice trials, and subjects continue to improve performance over >100 trials and across multiple days. A perfect circle is also complex to perform because it involves alternating patterns of joint torques and joint reversals. Second, the movement is naturalistic, featuring a constant tangential velocity (Gribble and Ostry 1996; Lacquaniti et al. 1983). Finally, because subjects must learn a reaching movement of constant radius, there is a clear means by which errors in the position of the hand can be analyzed independently from errors in movement velocity.
There were two kinds of trials in each block: training trials and demonstration trials. Training trials were identical for all subject groups and consisted of attempts to replicate the desired movement. Demonstration trials, and the sensory information contained about desired movement, were different depending on subject group.
In each block, subjects from all groups were first shown two visual demonstrations of the desired circle. The complete circle was shown as a red line, and a white cursor moved counterclockwise around the circle at constant tangential velocity (between brief 0.2-s cosine ramps; average velocity of 36 cm/s, duration of 1.67 s). The subject's hand was held fixed at the starting location (at 12 o'clock) during these two visual demonstrations. In training trials, subjects were asked to “replicate, as best as possible, the position and velocity of the demonstrated perfect circle.” No visual circle was displayed during training trials; only a cursor representing hand position was displayed. Training and demonstration trials were interleaved at a ratio of 2:1 throughout each movement block.
For experimental subjects (PASS, ACT, REV), the remaining demonstration trials featured the robotic manipulandum guiding their hand through the perfect circle in concert with the movement of the visual cursor (the robot was controlled with a PD controller, 2,000 N·m−1, 20 N·s·m−1). For all demonstration trials, visual information was the same across subject groups.
Postbaseline training on days 1, 2, and 3 consisted of 60, 90, and 90 training trials, respectively. Subjects were provided brief breaks every 30 movements to avoid fatigue.
Data analysis.
We used several measures to characterize changes to kinematics over the course of learning. Cross correlation index (CCI) was calculated for movements by computing the correlation between the desired and produced signals in both x and y, as functions of time. The mean of the two x-y correlations was used as one dependent measure of motor learning.
Positional error was calculated by measuring the absolute distance between the produced radial distance of the hand and the desired (10 cm) radial distance, averaged over the entire circle.
Average velocity was measured for each trial to determine how well subjects approximated the desired (constant angular) velocity of the circle.
Statistical analysis of changes in kinematic measures was performed with analysis of variance and Tukey post hoc tests. Violations of sphericity were tested for, and Greenhouse-Giesser corrections were employed to correct for any violations of sphericity.
RESULTS
We measured motor learning over the course of 3 days of training. Several kinematic variables were measured to characterize how subjects improved motor performance relative to baseline. We compared performance of subjects receiving visual demonstration of the desired movement (CTRL subjects) to that of subjects who were moved through the desired trajectory by the robot, together with visual presentation of the cursor movement (PASS subjects).
Cross correlation.
To assess whether the addition of proprioceptive training on the desired movement improves motor learning, we measured subjects' ability to generate the desired positions [x,y] of the hand over time. As subjects achieve greater skill at generating the desired circle, the produced x and y positions as a function of time should become more correlated with the desired x and y position signal, across the training period. Signal correlation is affected by both errors made in the position of the hand as well as velocity matching error, and is thus in some sense a net measure of movement learning.
Figure 2A shows the CCI for both experimental (PASS) and control (CTRL) subjects. This dependent variable is an error measure, with a score of 0 indicating no deviation between desired and actual position signals. Both subject groups clearly show learning over the 3 days of motor learning. Notably, large improvements are observable relative to baseline on day 1 for PASS subjects receiving passive proprioceptive training. At baseline, CTRL subjects demonstrated CCI of mean ± SD = 0.39 ± 0.18, and mean performance for the remainder of day 1 was 0.34 ± 0.15. PASS subjects demonstrated similar performance at baseline (0.45 ± 0.22) but showed larger improvement immediately on day 1, reducing CCI to 0.21 ± 0.06. To test for reliable differences in CCI over the course of learning, an analysis of variance was performed with one within-subjects measure (4 levels: baseline and each day of learning; Fig. 2B) and one between-subjects measure (groups: CTRL and PASS). A significant interaction was found (P < 0.001); post hoc tests showed that PASS subjects had smaller error on day 1 relative to baseline (P < 0.01), while CTRL subjects did not demonstrate this day 1 performance improvement relative to baseline (P > 0.4). On subsequent days 2 and 3, both subject groups showed improved performance (P < 0.01). These data support the idea that passive proprioceptive demonstration trials improve the rate of motor learning.
Fig. 2.
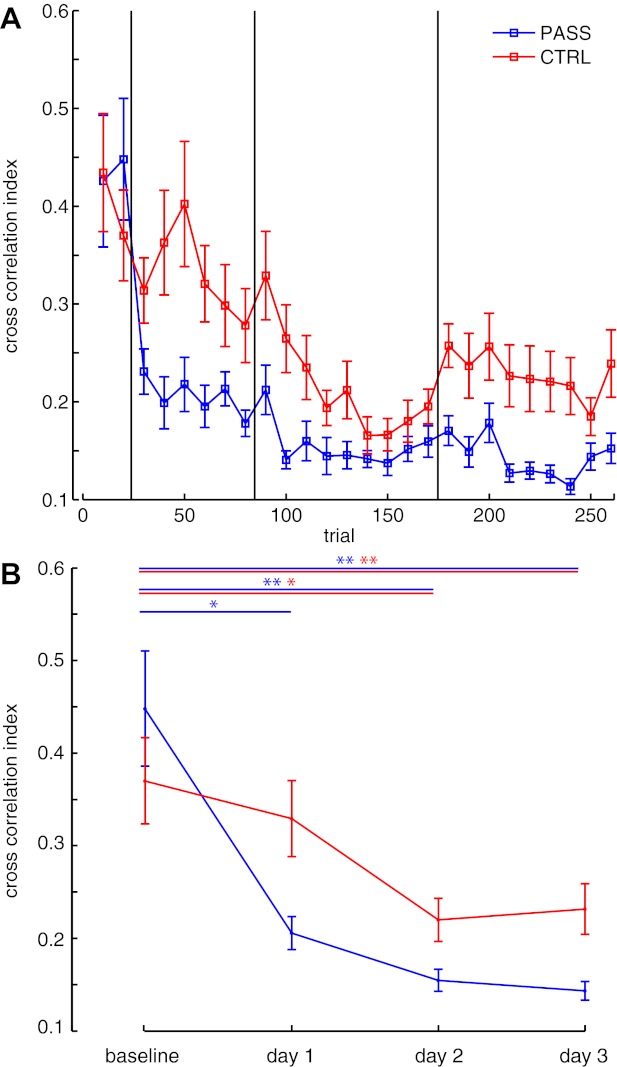
Motor learning. A: cross correlation index: mean (±SE) cross correlation index of performed movement to desired movement, averaged in bins of 10 movements. An index of 0 indicates no difference between the produced and desired movement trajectories. Data are from passive (PASS) subjects and control (CTRL) subjects. B: mean cross correlation index averaged across training days. Statistical significance: *P < 0.05; **P < 0.01.
We also quantified signed movement error (+/− indicating hand positions that were greater/smaller than the desired 10-cm radius) to determine whether the overall size of performed circles changed as a function of training. No changes were observed in pairwise comparisons between any conditions (P > 0.05 in all cases).
Movement velocity.
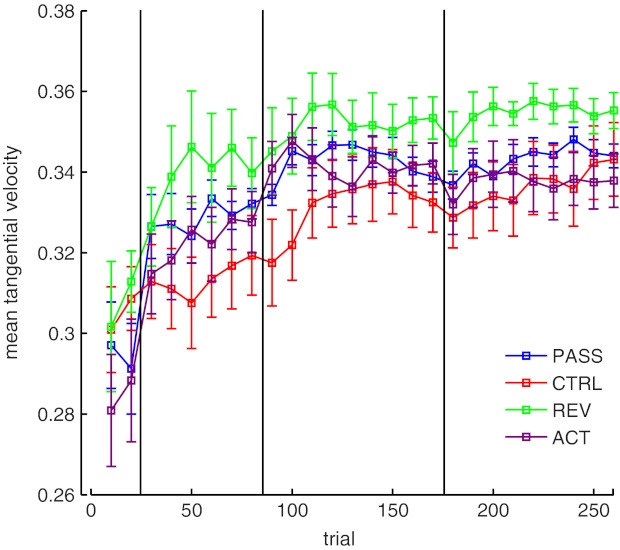
We also assessed the ability of subjects to perform the desired average velocity. Figure 3 shows average movement velocity across the training period. Clear increases in movement speed toward the desired speed are observed relative to baseline for PASS subjects upon presentation of the desired trajectory, while both subject groups asymptote to similar levels. Analysis of variance [1 within-subject factor (time, 4 levels), 1 between-subjects factor (group)] demonstrated an interaction between training time and group (P < 0.05); post hoc tests showed that average movement velocity was significantly higher for PASS subjects on day 1 (P < 0.05); CTRL subjects did not increase movement speed on day 1 (P > 0.05). By training end, subjects had achieved an increase in movement velocity relative to baseline (P < 0.05 for both groups). These data also support the idea that passive proprioceptive demonstration trials improve the rate of motor learning.
Fig. 3.

Mean tangential velocity. A: mean (±SE) tangential velocity for subjects in the PASS group and the CTRL group, averaged in 10 movement bins. Subjects learn to generate movements close to the average velocity profile (shown by dashed line). B: averaged across training days (*P < 0.05).
Positional error.
To determine whether subjects were able to reduce the positional error of their movement, independent of any speed information, we measured the radial error—the deviation of the hand's position from the 10-cm radius—around the entire circle. We observed that subjects who received the passive training were also better at replicating the positions of the circle. Figure 4 shows the (absolute) positional error averaged across the entire movement trajectory, across the training period. Subjects in both groups reduce average radial error over the training period. Another analysis of variance with one within-subject measure (time, 4 levels: baseline, training days 1, 2, 3) and one between-subjects measure (group: control, experimental) found a significant effect of training (P < 0.02). Post hoc comparisons showed that the two groups do not differ reliably at baseline (P > 0.4). On days 2 and 3 PASS subjects performed better than baseline (P < 0.01), while CTRL subjects showed reduced positional error on day 3. These results support the idea that the passive proprioceptive training specifically improves the motor system's ability to generate the desired positions of the hand. These results are consistent with the idea that the demonstration trials with proprioception of desired hand position improved the subject's ability to reduce movement error. These positional error reductions are particularly striking because PASS subjects have also shown great increases in movement velocity (as noted above) and thus are moving more accurately without compromising movement speed.
Fig. 4.

Positional error. A: mean (±SE) absolute radial error throughout learning for PASS and CTRL subject groups, averaged over 10 movement bins. Subjects demonstrate reduction of this error over the course of learning. B: averaged across training days (**P < 0.01).
Proprioceptive specificity.
We next investigated the degree to which this benefit of passive proprioceptive training was specifically due to the experienced movement trajectory. It might be noted that when subjects are passively moved through the desired trajectory, they are also given task-relevant information independent of the path itself. Timing information like overall movement duration is provided by a salient start and stop of the robotic manipulandum. In addition, it might be argued that passive demonstration movements also cause subjects to dedicate more attention to their hand during these demonstrations. Either or both of these aspects of proprioceptive demonstration might confound the role of proprioception itself in causing observed improvements for PASS subjects. To control for these factors, we provided a new group of subjects (n = 11, hereafter REV subjects) with the same experience of training and passive presentation trials, but in this case we manipulated the passive presentation trials such that the hand was moved through the opposite, clockwise, circle. As a result of this training, the magnitude of hand tangential velocity and the range of joint angles experienced were identical to those of passive subjects; movement duration is constant, and the task includes similar attentional demands on the subject as in the main experiment. Clearly, however, the sequence of hand positions is different. On the active movement trials subjects in this control were asked to reproduce the circular trajectory in the counterclockwise direction, the same as subjects in the main experiment and opposite to the direction observed during their passive proprioceptive training.
Figure 5 shows the changes to CCI for these subjects. Subjects show similar improvements to CCI (as subjects in the main experiment). CCI increased from 0.471 ± 0.19 to 0.324 ± 0.175 on day 1 and maintained the improvement over the following days. An analysis of variance was performed to assess differences between these additional groups of subjects (see statistics below: Active proprioceptive training). Post hoc tests found that these subjects did not demonstrate the early day 1 improvement to CCI that PASS subjects demonstrated on day 1 (P > 0.05). By days 2 and 3, REV subjects had significantly improved CCI relative to baseline (P < 0.05), similar to CTRL subjects. Similar results were observed in measures of movement velocity (Fig. 6).
Fig. 5.
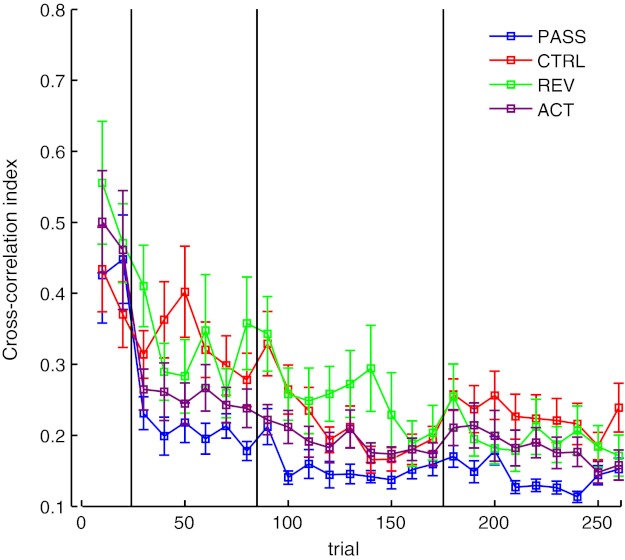
Cross correlation index: data as shown in Fig. 2 (means ± SE), with the addition of reverse (REV) subjects and active (ACT) subjects. Again, data were averaged over 10 movement bins.
Fig. 6.
Mean tangential velocity: data as shown in Fig. 3 (means ± SE), with the addition of REV subjects and ACT subjects. Data were averaged over 10 movement bins.
Figure 7 shows mean positional error over the training phase. Unlike in the CCI, no improvements are seen over the entire learning phase. An analysis of variance (see statistics below: Active proprioceptive training) confirmed that these subjects did not demonstrate reliable improvements to their positional error at any point during training.
Fig. 7.
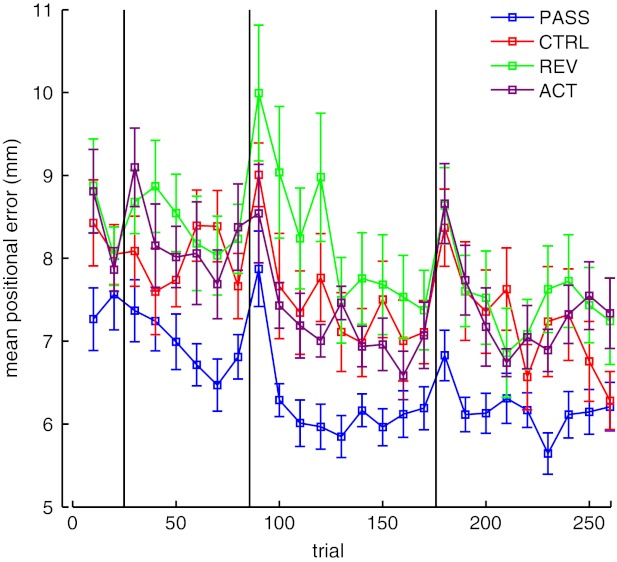
Positional error: data as shown in Fig. 2 (means ± SE), with the addition of REV subjects and ACT subjects. Data averaged over 10 movement bins.
Active proprioceptive training.
It might be proposed that passive displacement of the arm is suboptimal for providing valuable sensory information. In particular, it might be noted that the state of the arm, while relaxed, contains muscle states (length and associated time derivatives) significantly different from those required during active movement. It thus might be that some form of active motor learning—where the subject receives guidance through the desired trajectory while also generating active movement—would be more beneficial (see Marchal-Crespo and Reinkensmeyer 2009 for review). To test this hypothesis we provided an additional group of subjects (n = 11, hereafter ACT subjects) with the same experimental protocol as the main experiment, with one change to instructions: instead of keeping their arm passive, subjects were asked to move actively with the robot during demonstrations of the desired circle. Thus this experience might be described as “augmented” or robot-“assisted” control. The PD controller's coefficients were kept the same as those used for PASS subjects.
Figure 5 shows CCI error measured throughout learning. These subjects show improvements similar to those in the passive group of subjects. To test for reliable changes in performance from each day, an analysis of variance was performed with one between-subjects measure (4 levels: PASS, CTRL, REV, and ACT groups) and one within-subject measure (4 levels: baseline and days 1–3). A main effect of training was found (P < 0.001). Post hoc tests found that, similar to PASS subjects, these ACT subjects improved CCI on day 1 relative to baseline (P < 0.05) and maintained this performance throughout the learning period.
Figure 7 shows positional error over the course of learning. An analysis of variance [with 1 within-subject factor (time, 4 levels), 1 between-subjects factor (group, 4 levels)] found a reliable factor of training time (P < 0.01). Post hoc comparisons found no reliable reductions in positional error for ACT subjects, relative to baseline, for the training period. Together, these data suggest that actively moving the arm during proprioceptive demonstration trials helps to improve motor performance but does not admit the same fine improvements to positional accuracy.
The positional data (Fig. 7) appear to suggest that the first movements on each day were significantly poorer than subsequent performance. To determine whether these initial movements were affecting our statistical analyses we re-ran statistical tests without the first block of movements on each day, and these statistical conclusions were identical, thereby ruling out this concern.
This inability of active movement during proprioceptive training to provide reduction in positional errors is surprising, and we sought to further understand the bases of this effect. One possible explanation for this result is that active demonstration trials may have been uninformative if active movement against the handle did not result in positional errors. If this is true, then we may expect that variations in force at the handle during demonstration trials may not be accompanied by positional error.
We examined mean force at the handle for all subjects, during all demonstration trials. It was observed that the variance of force during demonstration trials was indeed higher for ACT subjects than PASS subjects (SD = 2.01 ± 0.95 PASS vs. 3.02 ± 1.58; P < 0.05). Because the manipulandum commanded tight control about the desired position, these varying forces at the handle did not result in reliable differences in positional deviation of the manipulandum during demonstration trials (means: 1.81 ± 0.5 mm PASS, 0.9 mm ± 0.53 ACT; P > 0.05). Thus active demonstration trials did not provide information about the relationship between muscle activation and motor error.
Other complex movements.
We were also interested to see whether other movements might similarly be improved by passive guidance through the desired trajectory. To do this we tested a new task: cursive writing of the short proper noun “Liz.” This movement requires an even longer trajectory (∼3.5 s) and features a complex velocity profile and higher peak velocities than the circular movement tested in the first experiment (Fig. 8).
Figure 9 shows CCI for two new groups of subjects (wPASS and wCTRL). Clear immediate improvements are observable for wPASS subjects, in contrast to wCTRL subjects. A mixed analysis of variance found a main effect of training (P < 0.001) and a weak interaction (P = 0.073). Post hoc tests showed that wPASS subjects improved relative to baseline on day 1 (P < 0.05); while again wCTRL subjects did not show significant improvement relative to baseline until day 3. These results support the notion that passive presentation of desired movement provides a benefit for learning.
Fig. 9.

Cross correlation index of produced to desired hand position for subjects in the PASS and CTRL groups on the cursive writing task across the 3 training days.
DISCUSSION
We measured the effect on motor learning of adding proprioceptive training to visual presentation of a goal movement and found that passive presentation of the desired trajectory results in faster motor learning. Subjects who experienced passive proprioceptive demonstration trials showed an improved learning rate—these subjects were immediately better at the task on day 1—and maintained this improvement over the subsequent days. Control subjects did not show the same early improvements to CCI, velocity, or position error. These data suggest that passive presentation of desired trajectories may be a useful method for augmenting motor learning. These improvements were not seen for control subjects who received passive demonstration of circles matched for speed but opposite in direction. Interestingly, we observed that proprioceptive presentation of desired movement in concert with active movement did not afford the same improvements to positional accuracy. This result suggests a specific role for purely passive proprioceptive demonstration in the improvement of positional accuracy during motor learning.
By providing new controls with a passive circle in the opposite direction, we attempted to determine whether the improvements to learning could be attributed to the movement trajectory itself. It might otherwise be argued that any arm motion would direct more attention to the arm. Similarly, it might be argued that certain gross characteristics of movement—such as movement duration—are simply more salient during robot-guided proprioceptive demonstration trials. These reverse-circle control subjects received a matched range of joint motion, identical magnitude of hand velocity, and cognitive information about movement timing. Analysis of motor learning showed that these subjects had delayed improvements to CCI similar to control subjects and did not show the same improvements to purely positional accuracy. Since the learning benefits were not observed for these subjects, the results are consistent with the idea that the benefits to motor learning conferred by passive proprioceptive guidance can be attributed to the presentation of the trajectory itself.
Previous studies have shown that in some cases the addition of another source of information about a task can improve learning. This has been shown in reaching movements by providing visual information about desired movement (Brown et al. 2009, 2010; Mattar and Gribble 2005), during the visual perception of gait patterns by providing haptic guidance of the subject through the movements themselves (Casile and Giese 2006), and in grasping movements where the integration of visual and haptic information has been shown to be statistically optimal (Ernst and Banks 2002). Thus the findings of the present study add to a body of literature exploring the diversity of sensory signals that can be integrated to augment learning.
The fact that speed and timing information was conferred from both passive and active demonstration trials better than for purely visual demonstration trials is consistent with other experiments involving active haptic guidance (Feygin et al. 2002; Milot et al. 2010). Previous work involving active demonstration—movements with the aid of a robotic manipulandum—has also reported little or no benefit to positional accuracy (Bluteau et al. 2008; Feygin et al. 2002; Liu et al. 2006). While subjects in the present study performed 240 training movements, the length of the training regime for motor learning has also varied significantly across previous experiments, ranging between 15 and 63 training movements, partly because the analysis of the effect of training type—haptic guidance and visual demonstration versus visual demonstration only—was performed within-subject. The present study also selected a task with considerably different kinematic parameters, with average velocity being more than five times that reported in the above studies. Given Fitts' law, this relatively high task difficulty may have provided greater likelihood for observing benefits to positional variability.
Here we have used the term “motor learning” to denote subjects' improved ability to produce the desired trajectory. It is unclear what aspects of learning are modulated from this experience. One possibility is that the addition of proprioceptive training allows better representation of the desired movement. It also could be that execution errors are better perceived when the desired performance in sensory space is provided. Alternatively, proprioceptive training might change how signals to muscles are computed. Our experiment cannot test between these hypothesized underlying mechanisms, but future modeling and empirical studies may examine these different aspects of learning.
Previous studies have found that proprioception of remembered active movement is better than passive movement (Marteniuk 1973), but only when the movement is self-defined and not externally determined (Stelmach et al. 1976). These results might speak to the present finding that positional error was reduced for PASS subjects and not ACT subjects. The previous literature might expect no benefit for active movement for movements that are externally defined, such as those in the present study.
Somatosensory afferent signals have been observed to be attenuated during movement (Brooke et al. 1997; Jones et al. 2001; Prochazka 1989). Somatosensory evoked potentials have been shown to be gated during both active (Cohen and Starr 1985, 1987) and passive (Staines et al. 1996) movement and greater for movements (passive or active) with higher movement velocity (Rauch et al. 1985). It is therefore interesting that despite these previous observations of downregulated somatosensory signals, they clearly provide additional information to result in a motor learning benefit greater than the visual controls.
To determine the variability of subjects' application of force to the handle, we measured change in the force transducer signal across trials and observed active subjects having reliably greater variability. The recording of electromyographic signals during demonstration movements is another measure of arm activity, and would be a useful way of collecting such passive-subject data in the future explorations of proprioceptive training on motor learning. Additionally, while our data show that PASS subjects behaved differently than ACT subjects during demonstration trials, we cannot rule out the possibility that, against explicit provided instructions, PASS subjects were not completely passive during demonstration trials and instead were moving with the robot. If this is the case, it might be argued that PASS and ACT subjects are performing better than control subjects because they are receiving essentially extra practice. This does not, however, explain the benefits to positional error observed for passive subjects compared with ACT subjects.
In a recent study that examined movement adaptations within task-relevant versus irrelevant dimensions (Diedrichsen et al. 2010), it was observed that the motor system adjusts motor commands to replicate movement kinematics of previous trials, including subtle deviations caused by passive robotic guidance. The authors term such motor adaptations “use-dependent,” and discuss how such adaptations are restricted to task-irrelevant dimensions. It may be that a similar mechanism is at work in this study, although in the present study replication of the demonstrated circle is task-relevant given that it is the explicit goal for subjects.
The specific neurophysiological basis for the ability of passive movements to influence motor behavior has not been determined. Recent studies have attempted to examine how afferents signaling muscle length are modulated depending on movement context. One recent study found that human spindle afferent signals may change based on movement context. Spindle reflexes from stabilizing ankle muscles show increased amplitude during quiet standing at an elevated ledge compared with standing at ground height (Horslen et al. 2011). Since no changes in either H-reflex magnitude or tonic muscle activation were observed, these data suggest that spindle sensitivity can be independently modulated in humans based on conditions of stress. These behavioral results support earlier findings of modulations to spindle afferent signals when subjects are required to actively attend passive joint rotation (Hospod et al. 2007). Taken together, these results support the general notion that peripheral sensory signals may be modulated in a context-specific manner. To our knowledge no studies have investigated the manner in which spindle behavior changes during the acquisition of a novel motor task.
The observed improvement in motor learning that results from passive proprioceptive demonstration is presumably based on changes in motor cortical regions. It may be that this results from direct cortico-cortico connections between proprioceptive and motor cortices, a network that has been shown to be altered during motor learning at short timescales (Vahdat et al. 2011). Other studies have reported rapid changes in motor cortical representations following motor practice (Classen et al. 1998; Pascual-Leone et al. 1995). As far as we know, the present study is the first demonstration that passive proprioceptive training can result in reduced positional movement error during the learning of natural movements.
Considerable research has been performed on the benefits of haptic assistance in movement recovery in both clinical (Lo et al. 2010; see Marchal-Crespo and Reinkensmeyer 2009 for review) and healthy (Marchal-Crespo et al. 2010; Reinkensmeyer and Patton 2009 for review) subpopulations, where most often a robot is used to assist active movement. The present study may contribute to this growing body of research by detailing the specific benefits of passive sensory training for motor learning.
GRANTS
This work was supported by the National Institutes of Health (NIH), the Canadian Institutes of Health Research (CIHR), and the Natural Sciences and Engineering Research Council of Canada (NSERC). D. A. Kistemaker is supported by the European Community's Seventh Framework Programme (CORDIS FP7-PEOPLE-2011-CIG-303849).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.W., D.A.K., and P.L.G. conception and design of research; J.D.W. and A.C. performed experiments; J.D.W. analyzed data; J.D.W., D.A.K., and P.L.G. interpreted results of experiments; J.D.W. prepared figures; J.D.W. drafted manuscript; J.D.W., D.A.K., A.C., and P.L.G. edited and revised manuscript; J.D.W., D.A.K., A.C., and P.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Elizabeth Wilson and Nicholas Cothros for their helpful comments.
REFERENCES
- Bluteau J, Coquillart S, Payan Y, Gentaz E. Haptic guidance improves the visuo-manual tracking of trajectories. PLoS One 3: e1775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol 51: 393–421, 1997 [DOI] [PubMed] [Google Scholar]
- Brown LE, Doole R, Malfait N. The role of motor learning in spatial adaptation near a tool. PLoS One 6: e28999, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Goodale MA, Gribble PL. Motor force field learning influences visual processing of target motion. J Neurosci 27: 9975–9983, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Gribble PL. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci 21: 1013–1022, 2009 [DOI] [PubMed] [Google Scholar]
- Brown LE, Wilson ET, Obhi SS, Gribble PL. Effect of trial order and error magnitude on motor learning by observing. J Neurophysiol 104: 1409–1416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casile A, Giese MA. Nonvisual motor training influences biological motion perception. Curr Biol 16: 69–74, 2006 [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998 [DOI] [PubMed] [Google Scholar]
- Cohen LG, Starr A. Localization, timing and specificity of gating of somatosensory evoked potentials during active movement in man. Brain 110: 451–467, 1987 [DOI] [PubMed] [Google Scholar]
- Cohen LG, Starr A. Vibration and muscle contraction affect somatosensory evoked potentials. Neurology 35: 691–698, 1985 [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102: 3505–3518, 2009 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci 30: 5159–5166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002 [DOI] [PubMed] [Google Scholar]
- Feygin D, Keehner M, Tendick F. Haptic guidance: experimental evaluation of a haptic training method for a perceptual motor skill. Proceedings 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems. IEEE, 2002, p. 40–47 [Google Scholar]
- Gribble PL, Ostry DJ. Origins of the power law relation between movement velocity and curvature: modeling the effects of muscle mechanics and limb dynamics. J Neurophysiol 76: 2853–2860, 1996 [DOI] [PubMed] [Google Scholar]
- Haith A, Jackson C, Mial R, Vijayakumar S. Unifying the sensory and motor components of sensorimotor adaptation. Adv Neural Inf Process Syst 21: 593–600, 2008 [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R, Carpenter MG. Stretch reflex amplitudes increase with both the likelihood and consequence of falling (Abstract). 41st Annu Mtg Soc Neurosci 2011: 923–19, 2011 [Google Scholar]
- Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci 27: 5172–5178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo A. Proprioceptive feedback is reduced during adaptation to a visuomotor transformation: preliminary findings. Neuroreport 12: 4029–4033, 2001 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Terzuolo C, Viviani P. The law relating the kinematic and figural aspects of drawing movements. Acta Psychol (Amst) 54: 115–130, 1983 [DOI] [PubMed] [Google Scholar]
- Liu J, Cramer SC, Reinkensmeyer DJ. Learning to perform a new movement with robotic assistance: comparison of haptic guidance and visual demonstration. J Neuroeng Rehabil 3: 20, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT, Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 362: 1772–1783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Henriques DY, Gribble PL. Shape distortion produced by isolated mismatch between vision and proprioception. J Neurophysiol 99: 231–243, 2008 [DOI] [PubMed] [Google Scholar]
- Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil 6: 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal-Crespo L, McHughen S, Cramer SC, Reinkensmeyer DJ. The effect of haptic guidance, aging, and initial skill level on motor learning of a steering task. Exp Brain Res 201: 209–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteniuk R. Retention characteristics of motor short-term memory cues. J Mot Behav 5: 249–259, 1973 [DOI] [PubMed] [Google Scholar]
- Mattar AA, Gribble PL. Motor learning by observing. Neuron 46: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- Milot MH, Marchal-Crespo L, Green CS, Cramer SC, Reinkensmeyer DJ. Comparison of error-amplification and haptic-guidance training techniques for learning of a timing-based motor task by healthy individuals. Exp Brain Res 201: 119–131, 2010 [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995 [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989 [DOI] [PubMed] [Google Scholar]
- Rauch R, Angel RW, Boylls CC. Velocity-dependent suppression of somatosensory evoked potentials during movement. Electroencephalogr Clin Neurophysiol 62: 421–425, 1985 [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Patton JL. Can robots help the learning of skilled actions? Exerc Sport Sci Rev 37: 43–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines WR, Brooke JD, Angerilli PA, McIlroy WE. Phasic modulation of somatosensory potentials during passive movement. Neuroreport 7: 2971–2974, 1996 [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Kelso JA, McCullagh PD. Preselection and response biasing in short-term motor memory. Mem Cognit 4: 62–66, 1976 [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci 31: 16907–16915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JD, Wilson ET, Gribble PL. Spatially selective enhancement of proprioceptive acuity following motor learning. J Neurophysiol 105: 2512–2521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


