Abstract
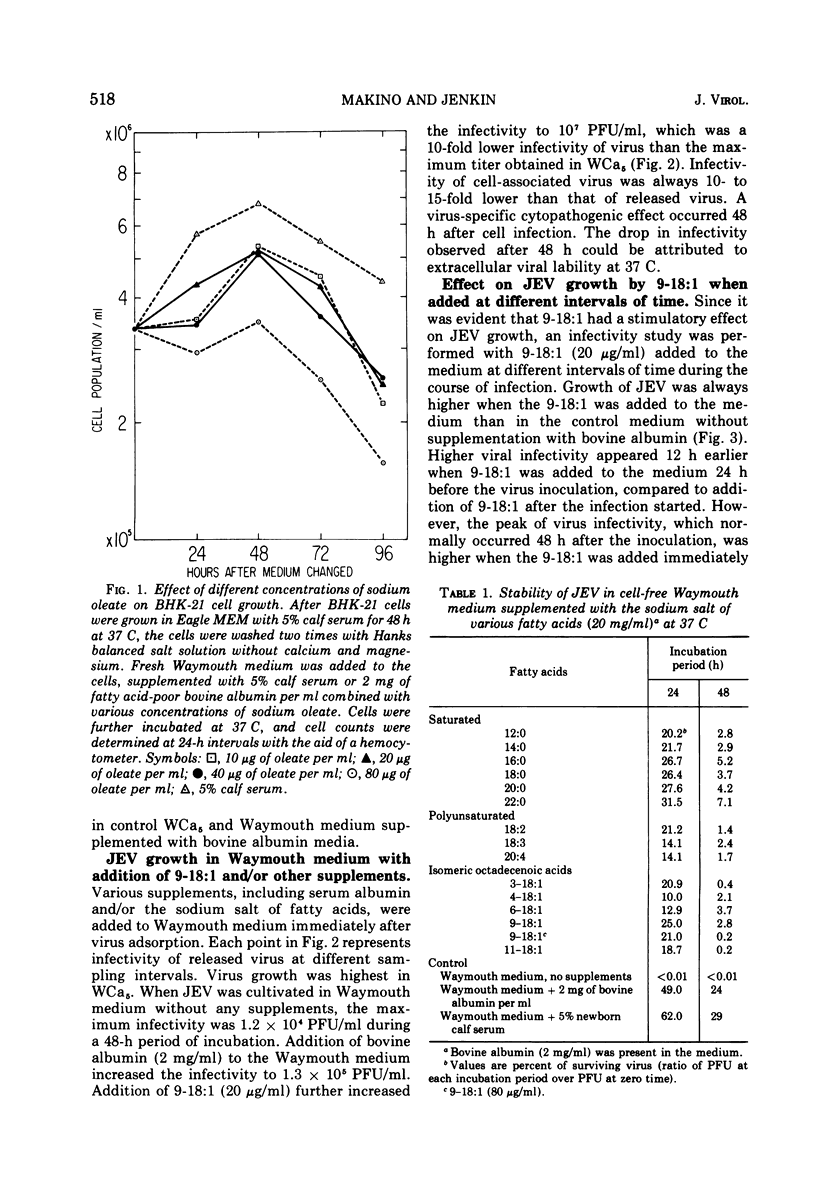
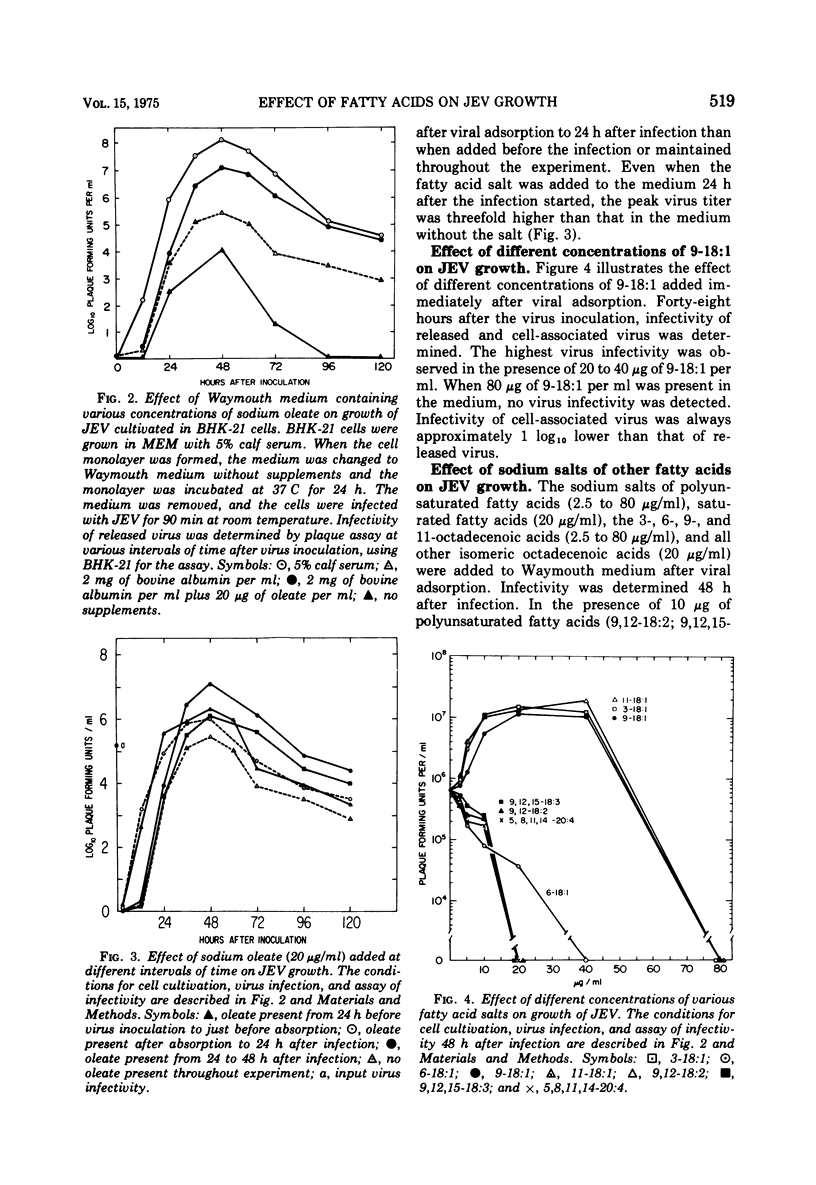
Growth of Japanese encephalitis virus (JEV) in BHK-21 cells was stimulated in the presence of 20 to 40 mug of the sodium salt of oleic acid (cis-9-octadecenoic acid, 9-18:1) per ml supplemented in Waymouth medium. The stimulatory effect of the salt was highest when 9-18:1 was added after adsorption of the virus. Study of the effect of other fatty acids on growth of JEV showed the following results: the longer the chain length of the saturated fatty acid salt, the higher the stimulatory effect on viral growth. In contrast, polyunsaturated fatty acids had an inhibitory effect on viral growth. The effect of isomeric cis-octadecenoic acids on viral growth was variable, depending upon the position of the double bond. The cis-6-octadecenoic acid had the highest inhibitory effect on growth of JEV compared to other isomeric octadecenoic acids. The sodium salt of (1-14C) cis-9-octadecenoic acid (9-18:1, 20 mug/ml) was rapidly incorporated into control and JEV-infected cells. Specific radioactivity in phosphatidylcholine dropped 12 to 24 h after virus inoculation, whereas synthesis of phosphatidylethanolamine increased 12 to 24 h after virus inoculation in infected cells compared to uninfected cells. Results from these studies suggest that phospholipid metabolism of infected cells is markedly changed, which can be associated with altered fatty acid metabolism when using labeled 9-18:1 fatty acid as a marker.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON S. G., ADA G. L. The action of phospholipase A and lipid solvents on Murray Valley encephalitis virus. J Gen Microbiol. 1961 Jul;25:451–458. doi: 10.1099/00221287-25-3-451. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Cumming R. B., Walton M., Snyder F. Lipid metabolism in cells grown in tissue culture: O-alkyl, O-alk-I-enyl, and acyl moieties of L-M cells. Biochim Biophys Acta. 1969 Apr 29;176(3):491–501. doi: 10.1016/0005-2760(69)90216-1. [DOI] [PubMed] [Google Scholar]
- Artom C. Methylation of phosphatidyl monomethylethanolanine in liver preparations. Biochem Biophys Res Commun. 1964 Mar 26;15(3):201–206. doi: 10.1016/0006-291x(64)90146-9. [DOI] [PubMed] [Google Scholar]
- BAILEY J. M. LIPID METABOLISM IN CULTURED CELLS. V. COMPARATIVE LIPID NUTRITION IN SERUM AND IN LIPID-FREE CHEMICALLY DEFINED MEDIUM. Proc Soc Exp Biol Med. 1964 Mar;115:747–750. doi: 10.3181/00379727-115-29026. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Menter J. Lipid metabolism in cultured cells VII. Linoleic acid content of cells grown on lipid-free synthetic medium. Proc Soc Exp Biol Med. 1967 May;125(1):101–105. doi: 10.3181/00379727-125-32024. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Pastan I. Nature and function of the structural phospholipids of an arbovirus. J Mol Biol. 1969 Feb 28;40(1):107–115. doi: 10.1016/0022-2836(69)90299-x. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., WILSON J. D., UDENFRIEND S. The enzymatic conversion of phospholipid ethanolamine to phospholipid choline in rat liver. J Biol Chem. 1961 Mar;236:673–679. [PubMed] [Google Scholar]
- Grossberg S. E., O'Leary W. M. Hyperlipaemia following viral infection in the chicken embryo: a new syndrome. Nature. 1965 Dec 4;208(5014):954–956. doi: 10.1038/208954a0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydrick F. P., Wachter R. F., Hearn H. J., Jr Host influence on the characteristics of Venezuelan equine encephalomyelitis virus. J Bacteriol. 1966 Jun;91(6):2343–2348. doi: 10.1128/jb.91.6.2343-2348.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E., Holman R. T., Ismail I. A., Gunstone F. D. Effect of isomeric cis-octadecenoic acids on the growth of Leptospira interrogans serotype patoc. J Bacteriol. 1969 Jun;98(3):1026–1029. doi: 10.1128/jb.98.3.1026-1029.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E., Holman R. T., Ismail I. A., Gunstone F. D. The effect of isomeric cis-octadecenoic acids on the growth of monkey kidney cells (LLC-MK2). Exp Cell Res. 1970 Jan;59(1):1–5. doi: 10.1016/0014-4827(70)90615-4. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E. The effect of oleic acid on the growth of monkey kidney cells (LLC-MK2). Exp Cell Res. 1970 Jan;59(1):6–10. doi: 10.1016/0014-4827(70)90616-6. [DOI] [PubMed] [Google Scholar]
- LEE H. W., SCHERER W. F. The anamnestic antibody response to Japanese encephalitis virus in monkeys and its implications concerning naturally acquired immunity in man. J Immunol. 1961 Feb;86:151–164. [PubMed] [Google Scholar]
- Makino S., Fujita N., Aoki H., Takehara M., Hotta S. Biologic and antigenic variation of Japanese encephalitis viruses. Kobe J Med Sci. 1971 Jun;17(2):75–84. [PubMed] [Google Scholar]
- Makino S., Jenkin H. M., Yu H. M., Townsend D. Lipid composition of Chlamydia psittaci grown in monkey kidney cells in defined medium. J Bacteriol. 1970 Jul;103(1):62–70. doi: 10.1128/jb.103.1.62-70.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C., Nomura M., Kitaoka M. Comparative studies on the structure and properties of two selected strains of Japanese encephalitis virus. Jpn J Med Sci Biol. 1968 Feb;21(1):1–10. doi: 10.7883/yoken1952.21.1. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Reitz R. C., el-Sheikh M., Lands W. M., Ismail I. A., Gunstone F. D. Effects of ethylenic bond position upon acyltransferase activity with isomeric cis-octadecenoyl coenzyme A thiol esters. Biochim Biophys Acta. 1969 Apr 29;176(3):480–490. doi: 10.1016/0005-2760(69)90215-x. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Käräinen L., Simons K., Gahmberg C. G. The lipid class composition of Semliki forest virus and plasma membranes of the host cells. Virology. 1971 Nov;46(2):318–326. doi: 10.1016/0042-6822(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H. Lipid metabolism in tissue culture cells. Adv Lipid Res. 1969;7:135–163. [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- SNYDER F. RADIOASSAY OF THIN-LAYER CHROMATOGRAMS: A HIGH-RESOLUTION ZONAL SCRAPER FOR QUANTITATIVE C14 AND H3 SCANNING OF THIN-LAYER CHROMATOGRAMS. Anal Biochem. 1964 Oct;9:183–196. doi: 10.1016/0003-2697(64)90102-2. [DOI] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., John K., Fletcher J. E. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969 Jan;10(1):56–67. [PubMed] [Google Scholar]
- Spector A. A. Lipids, hormones, and atherogenesis. The transport and utilization of free fatty acid. Ann N Y Acad Sci. 1968 Nov 21;149(2):768–783. doi: 10.1111/j.1749-6632.1968.tb53834.x. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Caesar H. Der Stoffwechsel der ungesättigten Fettsäuren. V. Zur beta-Oxydation der Mono- und Polyenfettsäuren. Der Mechanismus der enzymatischen Reaktionen an delta-2-cis-Enoyl-CoA-Verbindungen. Hoppe Seylers Z Physiol Chem. 1965;341(1):76–83. [PubMed] [Google Scholar]
- TAKEHARA M., HOTTA S. Effect of enzymes on partially purified Japanese B encephalitis and related arbor viruses. Science. 1961 Dec 8;134(3493):1878–1880. doi: 10.1126/science.134.3493.1878-a. [DOI] [PubMed] [Google Scholar]
- THEILER M. Action of sodium desoxycholate on arthropod-borne viruses. Proc Soc Exp Biol Med. 1957 Nov;96(2):380–382. doi: 10.3181/00379727-96-23483. [DOI] [PubMed] [Google Scholar]
- Tsao S. S., Cornatzer W. E. Biosynthesis of phospholipids in subcellular particles from cultured cells of human tissue. Lipids. 1967 Sep;2(5):424–428. doi: 10.1007/BF02531858. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. Rapid proliferation of sublines of NCTC clone 929 (strain L) mouse cells in a simple chemically defined medium (MB 752/1). J Natl Cancer Inst. 1959 May;22(5):1003–1017. doi: 10.1093/jnci/22.5.1003. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Phospholipid synthesis in Sindbis virus-infected cells. J Virol. 1970 Nov;6(5):637–643. doi: 10.1128/jvi.6.5.637-643.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA S. [JAPANESE B ENCEPHALITIS VIRUS. 6. THE LIPID FRACTION IN THE ALCOHOL-PROTAMINE PURIFIED VIRUS PARTICLE]. Igaku To Seibutsugaku. 1965 Jan 10;70:19–23. [PubMed] [Google Scholar]



